Abstract
The objective of this review is to describe self-administration procedures for modeling addiction to cocaine, cannabis and heroin in the human laboratory, the benefits and pitfalls of the approach, and the methodological issues unique to each drug. In addition, the predictive validity of the model for testing treatment medications will be addressed. The results show that all three drugs of abuse are reliably and robustly self-administered by non-treatment-seeking research volunteers. In terms of pharmacotherapies, cocaine use is extraordinarily difficult to disrupt either in the laboratory or in the clinic. A range of medications has been shown to significantly decrease cocaine’s subjective effects and craving without decreasing either cocaine self-administration or cocaine abuse by patients. These negative data combined with recent positive findings with modafinil suggest that self-administration procedures are an important intermediary step between pre-clinical and clinical studies. In terms of cannabis, a recent study suggests that medications that improve sleep and mood during cannabis withdrawal decrease the resumption of marijuana self-administration in abstinent volunteers. Clinical data on patients seeking treatment for their marijuana use are needed to validate these laboratory findings. Finally, in contrast to cannabis or cocaine dependence, there are three efficacious Food and Drug Administration-approved medications to treat opioid dependence, all of which decrease both heroin self-administration and subjective effects in the human laboratory. In summary, self-administration procedures provide meaningful behavioral data in a small number of individuals. These studies contribute to our understanding of the variables maintaining cocaine, marijuana and heroin intake, and are important in guiding the development of more effective drug treatment programs.
Keywords: Addiction, dependence, marijuana, opioid, reinforcement, validity
INTRODUCTION
Self-administration is a procedure for directly characterizing drug reinforcement by requiring that individuals make a behavioral response in order to receive a dose of drug. The procedure has several features that make it essential for understanding abuse liability, for characterizing the compulsive nature of drug addiction and for evaluating medications to treat this disorder. In fact, one of the most important features of the self-administration procedure is its validity. First, addiction comprises drug-taking behavior, which is precisely what is modeled by self-administration, thereby demonstrating face validity. Second and more important is predictive validity. There is a high degree of consistency between the drugs that are abused by humans and the drugs that non-human animals will self-administer, demonstrating that the mechanisms mediating drug reinforcement are largely conserved across species. Thus, the technique is indispensable for determining whether a novel compound or candidate treatment medication is likely to have abuse potential (Brady et al. 1987; Balster 1991; Lile & Nader 2003). In addition, self-administration procedures are predictive when used to evaluate potential treatment medications for drug dependence.
Studies of cocaine pharmacotherapy are the most illustrative for evaluating the predictive validity of laboratory models. Over 60 medications have proven ineffective for the treatment of cocaine dependence in double-blind, placebo-controlled clinical trials, which are the standard by which a new medication is assessed (see Vocci & Ling 2005). These negative clinical data therefore highlight which preliminary studies predicted medication effects in cocaine-dependent patients and which did not. Approximately 20% of the medications used in clinical trials were tested in human models of cocaine self-administration where they also failed to shift cocaine taking. By contrast, as discussed below, many of these same medications significantly decreased ratings of cocaine craving or its subjective effects. Given that clinical trials take several years to complete, require hundreds of patients and are costly and potentially risky (see Fischman & Foltin 1998), it is essential to use experimental models that accurately predict clinical outcome.
Construct validity, like face validity, is arguably less relevant than predictive validity for developing potential treatment medications. Construct validity refers to the similarity in mechanisms underlying drug taking in the laboratory and drug taking in the natural ecology. Self-administration may or may not have construct validity, but as discussed by Epstein and colleagues, few pre-clinical models of psychiatric disorders and their pharmacological treatment do (see Epstein et al. 2006). Construct validity may be highly relevant when characterizing the neurobiological basis of addiction, yet within the context of medications development, the most relevant issue is predictive validity.
The first objective of this review is to describe procedures for measuring cocaine, cannabis and heroin self-administration procedures in the human laboratory. The overall benefits and pitfalls of self-administration and methodological issues unique to each drug will be discussed. Given that clinical outcome for drug treatment is characterized by high rates of relapse, human laboratory studies attempting to model this critical component of addiction will also be described. Finally, the review will address the predictive validity of the models for testing treatment medications for cocaine, cannabis and heroin dependence.
METHODS
Self-administration procedures: overall
There are numerous ways by which self-administration has been assessed in humans (see Rocha et al. 2008), but most studies described below measure operant responding, in which participants make a behavioral response, such as pressing a spacebar, in order to receive drug. Volunteers may be required to emit a specified number of responses (fixed-ratio schedule), or a progressive-ratio schedule may be utilized, in which successive choices for one reinforcer requires progressively greater responding (increased response cost). The frequency by which drugs are available for self-administration within a laboratory session typically approximates the pattern by which the drug is abused. For example, smoked cocaine may be made available for self-administration every 15 minutes, while the inter-dose interval for intravenous heroin might be 6 hours. In addition to measures of reinforcement, ratings of drug and mood effects are also obtained in order to provide information on drug craving, and the subjective experience of the drug, as well as medication side effects.
For ethical reasons, the research volunteers enrolled in cocaine, heroin or cannabis self-administration studies are currently abusing the drug under investigation but express no interest in receiving treatment for their drug use. Lighter drug users (e.g. ‘chippers’) are usually not enrolled in studies attempting to model drug dependence because medication effects on drug taking in this population may not model medication effects in heavier users; drug taking in occasional users would likely be easier to disrupt with a medication than drug use in daily or near-daily drug users. Although research volunteers may abuse drugs other than the one under investigation, they cannot meet criteria for dependence on any other drug (except nicotine). Depending on the research question being asked, participants may be studied under conditions where they manifest symptoms of withdrawal, or they may be allowed to self-administer sufficient quantities of drug to prevent the onset of withdrawal symptoms. Research volunteers are typically excluded if they meet criteria for a current major Axis 1 psychiatric disorder (schizophrenia, major depression, bipolar disorder, suicidality).
These characteristics of research volunteers may differ from the characteristics of some clinical patients, who are not only seeking treatment, but who may also have psychiatric co-morbidities as well as other drug dependencies. Nonetheless, the data presented below suggest that studies conducted in non-treatment seekers predict outcome in clinical patients overall. As recently reviewed (Haney & Spealman 2008), an effective model need not be identical to behavior in the natural ecology to predict behavior in the natural ecology. Thus, as demonstrated most clearly by heroin self-administration studies, medications that decrease heroin’s direct reinforcing effects in the human laboratory accurately predict clinical outcome, despite the fact that the research volunteers may not be identical to clinical patients.
Cocaine
Modeling self-administration
Procedures
Cocaine, whether abused by the intranasal, intravenous or smoked route of administration, is rarely taken in single doses but is used in a binge pattern, in which multiple doses are administered in quick succession. Once a binge of cocaine use has ended, a period of abstinence ensues that may last for several days (e.g. Gossop et al. 1994). Since the probability is high that once cocaine use is initiated, more cocaine will be used shortly thereafter, research volunteers are given the opportunity to repeatedly self-administer doses of cocaine within a single session. Given that even cocaine-dependent individuals may go several days without cocaine after a binge, laboratory models do not necessarily incorporate daily self-administration sessions into their design. This pattern contrasts with studies testing drugs that produce a more marked withdrawal than cocaine. For example, in-patient studies in opioid-dependent volunteers often administer opioids at regular intervals to prevent the onset of withdrawal symptoms. However, cocaine withdrawal is a subtle phenomenon when measured in a hospital setting among non-treatment seekers (Foltin & Fischman 1997a,b; Haney et al. 2001a), so this daily dosing is not necessary in cocaine studies.
As mentioned above, self-administration studies should probably enroll ‘heavy’ cocaine users rather than ‘chippers’, if the results are to be generalized to treatment seekers. In some studies, for example, research participants spend $200–$300 per week on cocaine, consistently test positive for urinary cocaine metabolites and report using cocaine for over 10 years (e.g. Haney, Foltin & Fischman 1998; Haney et al., 2001b; Donny, Bigelow & Walsh 2004). However, not all of these participants meet DSM (Diagnostic and Statistical Manual of Mental Disorders) criteria for cocaine dependence because, as non-treatment seekers, they often report no distress about their cocaine use. Participants rarely report experiencing tolerance or withdrawal (both are minimal for cocaine), they have no desire to cut down on their cocaine use, and they report no psychological or physical problems related to their cocaine use. Thus, the criterion of cocaine dependence has neither a medical nor scientific rationale in non-treatment-seeking drug users. The most relevant characteristic is ongoing patterns of cocaine use.
Issues
Cocaine is a robust reinforcer and is reliably self-administered significantly more than placebo, regardless of the route by which it is administered. In fact, a problem in developing models of cocaine self-administration is that research volunteers take near-maximal levels of any active cocaine dose that is made available to them, so self-administration is often not dose-dependent (Fischman et al. 1990; Foltin & Fischman 1992). Since only individuals who are not attempting to curtail their drug use are enrolled in laboratory studies, there is little motivation not to take every dose of cocaine made available, even low cocaine doses. Thus, a number of strategies have been employed to incorporate a choice or a ‘cost’ to self-administration so that differences between the reinforcing effects of cocaine doses emerge.
One strategy is to have participants choose between a dose of cocaine or an alternative, non-drug reinforcer, such as money or a voucher later to be exchanged for money. Outpatient studies have shown that the value of the alternative reinforcer lawfully influences cocaine choice (Higgins, Bickel & Hughes 1994). In an in-patient setting, a range of studies has assessed smoked cocaine self-administration when a $5 voucher was available as the alternative, which is a dollar amount that was over twice the rated street value of the highest cocaine dose tested. Although there are examples in which choice for cocaine over money was dose-dependent (Collins et al. 1998, 2006), in many cases, low doses of cocaine are chosen at comparable rates as higher doses (Haney et al. 1999a; Haney, Hart & Foltin 2006; Collins et al. 2003; Hart et al. 2007), suggesting that the vouchers were not consistently competitive with cocaine.
It would appear that an obvious solution to this problem would be to increase the value of the alternative reinforcer. Yet, as discussed below, studies by Walsh, Donny and colleagues have shown that once cocaine use is initiated, it is difficult to halt: even large dollar amounts do not shift high-dose cocaine self-administration. In part, this may reflect the fact that most studies have participants sample the dose to be tested immediately prior to self-administration sessions, thereby ‘priming’ further cocaine use and making it more difficult to modulate (e.g. Fischman et al. 1990; Foltin & Fischman 1992; Dudish, Pentel & Hatsukami 1996). The alternative reinforcer value appears to have more impact in a relapse model of self-administration, where choice is assessed before individuals have used any cocaine that day.
In addition to priming, another reason that cocaine choice is difficult to shift is that cocaine is available immediately, while the money alternative can not be used until the study’s conclusion, which could be days or even weeks. This delay lessens the value of the money or voucher relative to cocaine (see Petry 2001). One in-patient study, which addressed this problem by having participants choose between cocaine and tokens that provided immediate access to privileges otherwise unavailable (e.g. pizza, radio, candy), found that choice between tokens and high-dose cocaine was comparable (Foltin & Fischman 1994). An ongoing study by Vosburg, Foltin and colleagues is also attempting to address the problem of delayed reinforcement by offering a choice between cocaine and the opportunity to earn cash by playing a game of chance. In lieu of cocaine, participants can draw balls from a spinning wheel, with each ball associated with a particular dollar amount ($0–$20). The study compared one dose of smoked cocaine to 2, 4 or 6 draws from the spinning wheel. Preliminary data suggest that significantly less cocaine is self-administered when the chance to participate in the game is high (6 chances) than when it is low (2 chances). Although money earned in the game is still not available until study conclusion, the opportunity to play the game appears to be reinforcing. These data are consistent with the reported efficacy of prize-based contingency management procedures in stimulant abusers (Petry et al. 2005).
Another strategy to distinguish the reinforcing effects of cocaine doses is to use a progressive-ratio schedule of responding. For example, in one study, choice for cocaine or a voucher increased the response requirement for the chosen option by 400 presses, while the requirement for the non-chosen option did not change. This procedure engendered dose-dependent cocaine self-administration (Haney et al. 1998). A similar approach had participants accurately complete 100 arithmetic problems to earn tokens that could be exchanged for either money or cocaine. After each token was earned, the requirement to earn the next token increased by 100 math problems, so the fifth token required that the participants complete 500 math problems. This procedure also engendered dose-dependent cocaine self-administration (Dudish et al. 1996).
Overall, these studies show that cocaine is robustly self-administered and that models of cocaine self-administration need to incorporate a ‘cost’ or a valid alternative to cocaine use in order to have a procedure that better models the natural ecology, where variables such as cost or access to non-drug activities influence drug-taking behavior. Procedures that modulate cocaine taking in the laboratory include use of a progressive-ratio schedule, and access to immediate rather than delayed alternative reinforcers.
Modeling relapse
Given that cocaine use perpetuates more cocaine use, it is perhaps understandable that it has been so difficult to find a medication to disrupt this behavior: the paradigm first primes cocaine self-administration and then determines if a medication will shift choice away from an immediate reinforcer (cocaine) to one that is delayed (vouchers). An alternative to halting ongoing drug use is to decrease the likelihood that those who have been abstinent will relapse. Pre-clinical data suggest that the neural mechanisms mediating drug seeking differ from those mediating self-administration (Spealman et al. 1999; see Shaham et al. 2003), so relapse and ongoing drug taking are likely maintained by distinct factors.
Although relapse is a defining feature of addiction, the factors influencing this behavior are poorly understood. Stressful stimuli, exposure to cocaine-paired cues and cocaine administration increase cocaine craving (Jaffe et al. 1989; Ehrman et al. 1992; Carter & Tiffany 1999; Sinha et al. 1999), yet craving does not necessarily predict self-administration (Fischman et al. 1990; Dudish-Poulsen & Hatsukami 1997; Haney et al. 1998, 2001b; Foltin & Haney 2000) or relapse to cocaine use in treatment seekers (Negrete & Emil 1992; Kosten et al. 2006). In order to develop relapse-prevention medications, it is important to demonstrate that these environmental events result in an actual return to drug use. The following laboratory studies have investigated each condition on cocaine self-administration.
Effect of cocaine
The influence of a range of alternative reinforcer values (money) on cocaine self-administration was tested in the presence and absence of a ‘priming’ dose of cocaine. Alternative reinforcer value was either high ($16) for the first choice, and became lower for each subsequent choice, or was low ($1) for the first choice and became higher for subsequent choices. The studies showed that if no cocaine was ‘on board,’ and the dollar value was high, participants would preferentially choose money. If cocaine was already on board, even a high dollar value would not decrease cocaine self-administration. Thus, priming with cocaine shifted choice to cocaine over the money alternative (Walsh et al. 2001; Donny et al. 2003, 2004).
Effect of cocaine cues
Participants experienced either a cocaine stimuli condition (cocaine video, handle cocaine-related paraphernalia) or a neutral stimuli condition (nature video, make tea). Cocaine craving was measured, and then participants had the choice to do math problems to earn tokens that could be exchanged for either cocaine or money ($2) available upon study termination. Cocaine-paired cues significantly increased cocaine craving compared to neutral cues, but the cues did not increase self-administration (Dudish-Poulsen & Hatsukami 1997). Similarly, presentation of non-drug cues that had been systematically paired with cocaine increased ratings of craving without influencing cocaine self-administration (Foltin & Haney 2000). Thus, cues were shown to increase cocaine craving but have not been shown to increase self-administration.
Effect of both cocaine cues and cocaine
In a pilot study, laboratory cues were paired with cocaine, and then cocaine self-administration was tested when participants had been: (1) administered an active dose of cocaine and exposed to the cocaine-paired cues; (2) only exposed to the cues; and (3) neither exposed to the cues or cocaine. Participants had to decide how many doses of cocaine they were going to smoke (maximum of five) immediately after the cue presentation so that the effect of the experimental manipulations could be isolated, and not confounded by self-administered cocaine. Participants were required to purchase the cocaine using their actual study earnings ($50/in-patient day), as previous studies have shown that cocaine self-administration was 40–45% lower when participants had to pay for it (Foltin & Haney 2000) compared to getting it for free (Foltin & Fischman 1992; Haney et al. 1999a). Each day, the cost of cocaine was $5, $10 or $15 for each administration, and participants were offered a $25/day bonus if no cocaine was used that day. Each cue condition was tested in combination with each cost condition.
As shown in Fig. 1, cocaine-associated cues and cocaine influenced subsequent cocaine self-administration: regardless of cost, cocaine choice was lowest when participants had not been exposed to cocaine-paired cues or cocaine (-cue, -prime). Choice increased with cues alone, and increased even further, following cocaine administration. Additionally, the cost of cocaine influenced choice. Within each of the cue conditions, choice was highest when cocaine was relatively inexpensive ($5) and lowest when cocaine was expensive ($15). These pilot data are the first to demonstrate that a cocaine cue alone increases cocaine self-administration in humans, and that the effect was enhanced when cocaine was on board. Ongoing studies will determine the influence of medications on cue- and cocaine-elicited self-administration using this model. If promising, clinical trial data focusing on relapse will be needed to confirm the model’s validity.
Figure 1.
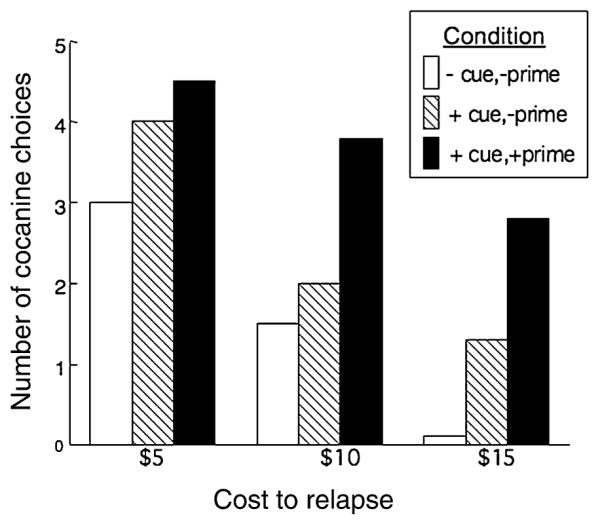
Cocaine self-administration was assessed when participants (n = 4) were exposed to: (1) cocaine-paired cues and an active dose (12 mg) of cocaine (+cue,+prime), cocaine-paired cues and placebo cocaine (+cue,−prime), or neither the cues or cocaine (−cue,−prime). Participants purchased up to five doses of cocaine (12 mg) per session using actual study earnings.Three cost conditions were tested: $5, $10, $15 per dose of cocaine.
Medications development: issues and predictive validity
The effects of medications on cocaine relapse are often not assessed either clinically (see Epstein et al. 2006) or in the laboratory. Rather, almost all clinical trials have targeted ongoing cocaine use, and almost all cocaine self-administration models have targeted this same behavior. The results from the clinic and from the human laboratory are entirely consistent. Until recently, no medication robustly shifted cocaine-taking behavior, even when these medications significantly decreased cocaine craving or intoxication. For example, desipramine resulted in a 40% decrease in ratings of cocaine craving, yet had no effect on the amount of cocaine self-administered in the human laboratory (Fischman et al. 1990) or used in the clinic (Campbell et al. 2003). Maintenance on dopamine agonists (pergolide, ABT-431), or the GABA agonist, gabapentin, significantly decreased ratings of cocaine ‘high’ but had no effect on cocaine self-administration (Haney et al. 1998, 1999a; Hart et al. 2004, 2007), and all three medications were proven to be either ineffective compared to placebo (Levin et al. 1999; Malcolm et al. 2001; Vocci & Ling 2005; Bisaga et al. 2006). Thus, medications may decrease cocaine’s subjective effects or cocaine craving but still fail in clinical trials, suggesting that the measurement of these ratings alone may not be sufficient to predict clinical response (Haney & Spealman 2008; Comer et al. 2008).
Although negative self-administration data are consistent with the negative clinical outcome, a positive relationship between the laboratory and the clinic is more compelling for demonstrating predictive validity. Yet, it has been difficult to find a medication that robustly decreased cocaine use in either setting. Maintenance on baclofen produced a small decrease in low-dose cocaine self-administration (Haney et al. 2006), and showed promise in a clinical pilot trial (Shoptaw et al. 2003), but the effect is too modest to suggest that baclofen would effectively treat cocaine dependence. Modafinil, by contrast, was recently shown to decrease high-dose smoked cocaine self-administration in cocaine users (Hart et al. 2008). Modafinil also decreased ratings of cocaine ‘high’ and ‘good drug effect’ (Dackis et al. 2003; Donovan et al. 2005; Malcolm et al. 2006). These laboratory findings correspond well with pilot clinical trial data showing that modafinil significantly reduced the number of cocaine-positive urines (Dackis et al. 2005), and with a recent multi-site clinical trial, which appear to confirm modafinil’s efficacy to promote abstinence in cocaine-dependent patients, provided that patients were not alcohol dependent or did not have a history of alcohol dependence (F. Vocci, personal communication, August 2007).
Decreasing a drug’s reinforcing effects is only one component of an effective treatment medication. Another component is compliance. Human laboratory studies have shown that modafinil produces stimulant-like subjective effects (Hart et al. 2006), and is self-administered more than placebo under certain conditions (Stoops et al. 2005), suggesting modest abuse liability. However, even high doses of modafinil do not produce cocaine- or amphetamine-like discriminative-stimulus or subjective effects (Jasinski 2000; Rush et al. 2002a,b), and postmarketing surveillance indicates that modafinil misuse is low (Myrick et al. 2004). Thus, modafinil has some agonist effect, which may increase the likelihood that patients will be compliant in taking it, but it is not a medication that is likely to be abused.
One factor that appears to be important for predictive validity is the duration of medication administration prior to the assessment of cocaine’s effects. Treatment durations of just several days can reveal changes in the effects of a medication that differ from its acute effects (see Haney & Spealman 2008). For example, acute pre-treatment with the D1 antagonist, ecopipam, decreased cocaine intoxication (Romach et al. 1999), while maintenance on the same dose enhanced both cocaine intoxication and its self-administration (Haney et al. 2001a), and did not decrease cocaine use in a clinical trial (see Grabowski et al. 2000). Testing the effects of medication maintenance on the cocaine self-administration dose-response function is time-consuming, and may increase the risk of participant dropout. Yet, these procedures are more consistent with clinical trial outcome, supporting their utility as a ‘first pass’ clinical screen for new pharmacotherapies (see McCance-Katz, Kosten & Kosten 2001).
Cannabis
Modeling self-administration
Procedures
Cannabis self-administration studies, which were initiated over 30 years ago (Mendelson et al. 1976), have demonstrated that marijuana is reinforcing: active marijuana is self-administered significantly more than placebo marijuana (Mendelson & Mello 1984; Chait & Zacny 1992; Haney et al. 1997; Ward et al. 1997), and is dose-dependent, i.e. marijuana smokers choose to smoke high potency cigarettes over low potency cigarettes (Chait & Burke 1994; Kelly et al. 1997). In the natural ecology, daily marijuana users often smoke several times per day, with effects lasting approximately several hours. Thus, in in-patient studies, self-administration occurs repeatedly throughout the day. To limit the influence of individual smoking styles, cued-smoking procedures are often used to control how long participants inhale and hold marijuana smoke in their lungs (Foltin et al. 1987). Participants may smoke a pre-determined number of puffs in this manner, or cued-smoking procedures may be repeated until a specified portion of the marijuana cigarette (e.g. 70%) has been smoked (Cooper and Haney, in preparation).
Self-administration of marijuana is largely maintained because it is a positive reinforcer. Yet, in daily marijuana smokers, self-administration may also be maintained because of its negative reinforcing effects. Abstinence in daily marijuana smokers can result in withdrawal, characterized by irritability, craving, and disrupted sleep and food intake (see Budney et al. 2004; Haney 2005), which manifest after approximately 24 hours of abstinence and lasts for several weeks (Kouri & Pope 2000). In the laboratory, withdrawal is alleviated by the resumption of marijuana smoking or by the double-blind administration of Δ9-tetrahydrocannabinol (THC), the primary psychoactive cannabinoid in marijuana, demonstrating the pharmacological specificity of withdrawal (Haney et al. 1999b, 2004; Hart et al. 2002). In the clinic, marijuana smokers report using marijuana to alleviate withdrawal symptoms (Stephens, Roffman & Simpson 1993; Stephens, Roffman & Curtin 2000; Budney, Novy & Hughes 1999), supporting the idea that withdrawal contributes to relapse and the maintenance of marijuana use. Thus, in-patient studies with daily marijuana smokers need to make marijuana available nearly everyday, unless withdrawal is part of the study design.
Issues
Although dose-dependent marijuana smoking has been observed, marijuana smokers often titrate their smoking as a function of marijuana potency, which can lessen the relationship between marijuana strength and its self-administration. Even when smoking inhalation times are controlled using cued-dosing procedures, marijuana smokers adjust how deeply they inhale, inhaling harder when the potency is low than when it is high in order to control the level of intoxication. In an example from one study, the remains of marijuana cigarettes were weighed after volunteers took three controlled puffs from cigarettes of differing potencies (0.0, 1.8, 3.6% THC), and the remaining cigarette weight was 0.47 (±0.02), 0.53 (±0.03) and 0.63 (±0.02) mg, respectively, demonstrating that the weaker the marijuana, the deeper the participants inhaled (Cooper and Haney, in preparation).
An additional issue with marijuana self-administration is that, similar to cocaine, non-treatment-seekers self-administer active marijuana virtually each time it is made available, e.g. eight marijuana cigarettes per day (Kelly et al. 1997). Thus, strategies such as alternative reinforcers and progressive-ratio schedules have been used to incorporate a cost to self-administration, to see if it would vary as a function of marijuana strength. In one in-patient study, participants responded under a progressive-ratio schedule for a marijuana cigarette (0.0, 2.2 or 3.9% THC) or snack items that were otherwise unavailable. Yet, participants chose active marijuana at nearly every timepoint, so neither the alternative reinforcer (snack items) nor the progressive-ratio schedule shifted choice. Participants also chose the low potency (2.2%) marijuana as often as the higher potency marijuana (3.9%).
Money has also been tested as an alternative to marijuana. One study manipulated the amount of work required to obtain the money reinforcer: Marijuana smokers chose between marijuana (0.0, 1.8, 3.9% THC) and money ($5). To earn either reinforcer, participants had to complete a variety of tasks, and they had to achieve a performance criterion in order to obtain the reinforcer (based on individual baseline performance scores). The criterion did not vary for marijuana, but for money there were three conditions: easy, standard and hard. The results showed that when it was hard to earn money, participants chose marijuana more than money, but when earning money was easier or was as easy as earning marijuana, participants chose money over marijuana. This preference for money may partly reflect the fact that five marijuana cigarettes were available in a short period of time (3 hours), so participants may have preferentially chosen money because 1–2 marijuana cigarettes in 3 hours was sufficient. Again marijuana choice was not dose-dependent (Ward et al. 1997), which either reflects dose titration or the failure to find a competitive alternative to marijuana.
Thus, marijuana is robustly self-administered, but varying the dose or using progressive ratio and choice procedures has not been shown to reliably shift this behavior. However, as described below, imposing a financial cost to smoking can decrease marijuana self-administration and may be useful in differentiating the effects of marijuana doses.
Modeling relapse
Similar to the approach used for cocaine, an in-patient pilot study was conducted to test the hypothesis that high financial cost would motivate non-treatment-seeking marijuana smokers to abstain, thus modeling (not mimicking) clinical motivation to use less marijuana. A range of costs to purchase individual puffs of active marijuana throughout the day (maximum of 18 puffs/day) was tested. The first puff of marijuana self-administered each day was expensive ($10–$20) regardless of when during the day it occurred. Once the individual had ‘relapsed’ for the day, the cost of subsequent puffs was lower ($2–$4).
As predicted, marijuana self-administration decreased markedly as a function of cost: from 10.8 (±0.7) puffs/day under the $10 relapse condition, to 5.5 (±0.7) puffs/day under the $12 condition, to no self-administration under the 15 and $20 conditions (Haney et al. 2008). Thus, marijuana self-administration by abstinent daily marijuana smokers varies as a function of cost. Although participants were not treatment-seeking and were not motivated to remain abstinent, the high cost of smoking decreased marijuana use, and thus is hypothesized to model voluntary abstinence in the clinic. This procedure can be used to see if medications influence whether abstinent marijuana smokers return to marijuana use (relapse) in the laboratory. Medications that decrease marijuana use by non-treatment-seeking volunteers in the laboratory are predicted to decrease marijuana use in the clinic.
Medications development: issues and predictive validity
There are almost no clinical studies of marijuana pharmacotherapy by which to evaluate the predictive validity of the laboratory models, so the present discussion will focus on several human laboratory approaches to developing such medications. One strategy is to attenuate symptoms of withdrawal, which is a medications approach that has improved outcome for both nicotine (see Shiffman, Johnston & Khayrallah 2000) and opioid (Dole 1988) treatment. These double-blind, placebo-controlled designs, enroll non-treatment-seeking volunteers who smoke marijuana repeatedly each day, 6–7 days per week. As with cocaine, medications maintenance rather than acute pre-treatment is usually assessed, to mimic how medications would be administered clinically.
The results of these studies have shown that some medications worsen marijuana withdrawal symptoms while others lessen them (see Haney 2005). The medication regimen that most effectively attenuated withdrawal was the frequent administration of low doses (10 mg) of oral THC (dronabinol; 50 mg/day). THC selectively decreased ratings of anxiety, misery, trouble sleeping, chills and marijuana craving, and reversed the large decreases in food intake during marijuana abstinence as compared to placebo while producing no cannabinoid intoxication (Haney et al. 2004). Similar results have recently been obtained in an outpatient setting (Budney et al. 2007).
Yet, does decreasing withdrawal decrease relapse? Based on the laboratory model of relapse described above, the effects of THC (20 mg) given three times per day, and the α2-receptor agonist, lofexidine (2.4 mg/day) on marijuana withdrawal and relapse were evaluated. These medications, which have distinct mechanisms of action, were tested both when administered alone and when administered together (Haney et al. 2008). The study showed that this regimen of high-dose THC did not robustly decrease marijuana withdrawal and did not decrease marijuana relapse, in contrast to frequent administration of lower doses (Haney et al. 2004). Lofexidine alone also did not attenuate the mood symptoms of marijuana withdrawal, but did improve sleep, and did decrease marijuana relapse. The most effective medication condition was the combination of THC and lofexidine, which improved sleep and decreased marijuana withdrawal, craving and relapse in daily marijuana smokers. These data suggest that the combination of lofexidine and THC warrant further testing as a potential treatment for marijuana dependence.
The data also suggest that specifically dampening the sleep disruption during cannabis withdrawal might be a key feature of decreasing relapse. This finding contrasts with a recent study concluding that cannabis withdrawal does not predict relapse (Arendt et al. 2007). In this design, patients self-reported cannabis abstinence and withdrawal symptoms, and several years later, were asked if they had relapsed to cannabis use. The data indicated that there was no significant relationship between the severity of withdrawal and the likelihood of relapse. Yet, in addition to the retrospective data collection and the lack of objective verification of either abstinence or relapse, a primary concern with the study’s conclusion is that withdrawal is only predicted to influence relapse while symptoms are occurring. Relapse that occurs months or years after the onset of abstinence is not expected to be related to withdrawal. Given these issues, concluding that withdrawal does not influence relapse may be premature.
In terms of medications development, an important methodological consideration is whether to only enroll individuals who report experiencing symptoms of cannabis withdrawal (estimated to be over 50% of daily marijuana smokers; Budney et al. (2004, 2007). An alternative strategy is to include all daily marijuana smokers regardless of self-reported withdrawal (e.g. Haney et al. 2004, 2008). The justification for this approach is that even marijuana smokers who demonstrate a withdrawal syndrome can be unaware or unwilling to admit that their symptoms are related to marijuana. Approximately 10% of the daily marijuana smokers enrolled in in-patient studies (n = 91) by Haney and colleagues quit after 2–3 days of marijuana abstinence due to symptoms consistent with withdrawal, such as marijuana craving, anxiety, irritability, stomach upset and poor sleep. The majority attributed their mood to the discomforts of the facility (e.g. not liking their pillow or the available food) or to personal concerns (missing their family), although these issues were not overwhelming on days when active marijuana was available.
This apparent resistance to implicate marijuana may reflect the fact that cannabis withdrawal is not a well-known phenomenon: its symptoms are primarily moodrelated, and they take 1–2 days to manifest. Furthermore, attitudes toward marijuana are politically charged, and some appear motivated to defend their marijuana use as entirely benign (Haney 2005). Thus, self-awareness or acceptance of cannabis withdrawal is not universal. Even among daily cigarette smokers, a surprisingly large percentage (60%) do not report experiencing symptoms of nicotine withdrawal (Donny & Dierker 2007), yet studies developing medications to treat nicotine dependence do not distinguish among those who do and those who do not experience withdrawal. Thus, it may be that some medications would be effective even among those who do not endorse withdrawal symptoms.
Heroin
Modeling self-administration
Procedures
Human laboratory models have characterized both intravenous (Altman et al. 1976; Mello & Mendelson 1980; Mello et al. 1981; Mello, Mendelson & Kuehnle 1982) and intranasal (Comer, Collins & Fischman 1997; Comer et al. 1999) routes of heroin self-administration. Since heroin is not abused in a binge pattern, and has an inter-dose interval of hours rather than minutes, laboratory studies usually allow for self-administration of bolus doses a few times per day. For example, in one procedure, participants self-administered single doses of heroin every 6 hours. In the interval between dosing, participants could respond on operant tasks to earn either money or heroin (Mello et al. 1981). In another study, volunteers responded under a progressive-ratio schedule for heroin or vouchers exchangeable for money and received their choice in a bolus dose at the end of the session (Comer et al. 1997). These studies have demonstrated that heroin self-administration is dose-dependent, with both i.v. and intranasal heroin producing comparable levels of responding (Comer et al. 1999).
A range of monetary values has been tested ($10–$40) as an alternative to heroin self-administration. When $20 was the alternative, participants lawfully chose the money option for low heroin doses and the drug option at higher heroin doses. Yet, when $40 was the alternative, participants predominately chose active heroin regardless of dose (Comer et al. 1998). These data suggest that if the alternative value is high, participants may be satisfied just choosing it a few times, then devoting the rest of their choices to drug. Thus, laboratory studies with opioids need to carefully define the parameters by which heroin self-administration varies as a function of dose and alternative reinforcer value.
Issues
Physical dependence complicates laboratory measures of heroin self-administration. Heroin withdrawal is robust and includes both physical discomfort and mood symptoms (e.g. runny nose, vomiting, fatigue, insomnia, irritability, anxiety) that last from several days to weeks (see Collins & Kleber 2004). One approach that attempts to remove the confound of withdrawal from the determination of heroin’s reinforcing effects is to administer opioid agonists (Comer et al. 1997, 1999; Comer, Collins & Fischman 2001). Oral morphine, for example, can be given at doses that produce minimal subjective responses in dependent individuals. An alternative approach is to have volunteers undergo withdrawal prior to self-administration sessions. This detoxification is necessary, for example, when testing the effects of naltrexone on heroin self-administration in order to avoid precipitating withdrawal (e.g. Mello et al. 1981). Detoxification also circumvents any pharmacological confounds that agonist maintenance may present when assessing heroin self-administration. However, detoxification also increases study length, and considerably increases the risk that participants will drop out. Thus, researchers need to carefully consider how they will characterize opioid self-administration in dependent individuals.
Modeling relapse
To our knowledge, there are no laboratory models designed to specifically measure heroin relapse.
Medications development: issues and predictive validity
Perhaps the greatest success in the development of medications to treat drug addiction thus far has come from the opioid class (see Haney & Spealman 2008). In stark contrast to either marijuana or cocaine, there are three Food and Drug Administration (FDA)-approved treatment medications to treat opioid dependence (methadone, buprenorphine, naltrexone). These medications allow for the validation of the human laboratory model, and highlight at least three essential features to consider in laboratory models of medications development: medication dose, dependence and compliance.
In terms of dose, methadone effectively decreases both opioid self-administration in the laboratory (Jones & Prada 1975; Donny et al. 2005) and heroin use in the natural ecology (see Strain et al. 1999; Faggiano et al. 2003) when individuals were maintained on doses in the 100 mg/day range. Doses in the 50 mg per day range were less effective in reducing heroin use in either environment. Thus, when effective doses are tested, laboratory models of heroin self-administration predicted clinical efficacy.
Buprenorphine, either alone or in combination with naloxone, also improves clinical outcome in clinical trials compared to placebo (see Vocci & Ling 2005), and decreases the reinforcing effects of heroin in the laboratory (Comer et al. 2001 Comer, Walker & Collins 2005). Yet the effects of buprenorphine on heroin self-administration were not robust, perhaps because sublingual buprenorphine was administered 19 hours prior to the self-administration sessions. Because of this long delay, even participants on active buprenorphine reported experiencing sleep disruption and mood symptoms of opioid withdrawal and thus self-administered heroin in order to alleviate these symptoms. These data demonstrate that in order to accurately evaluate the influence of a medication on heroin reinforcement, researchers need to ensure that medication levels are adequate prior to self-administration sessions.
Regarding the issue of compliance, early laboratory studies showed that the opioid antagonist, naltrexone, was effective in virtually eliminating the reinforcing and subjective effects of heroin in the laboratory (Altman et al. 1976; Meyer et al. 1976; Mello et al. 1981). Yet clinical experience with naltrexone has been poor because most patients simply stop taking the capsules and resume heroin use (see Kirchmayer et al. 2002). This non-compliance has been addressed with the development of an injectable, sustained-release form of naltrexone, which obviates the need for daily capsule administration. The depot formulation effectively reduced both the subjective and reinforcing effects of heroin in laboratory studies, with a duration of action of up to 1 month (Comer et al. 2002; Sullivan, Vosburg & Comer 2006). Furthermore, depot naltrexone improved clinical outcome, with treatment retention rates rivaling those seen with agonist replacement therapies (Comer et al. 2006).
In terms of relapse, there have been no medication trials specifically designed to test a return to heroin use in opioid-dependent patients (see Epstein et al. 2006). Depot naltrexone does not appear to decrease relapse when it is defined as any return to drug use, as patients on naltrexone used heroin as quickly as those on placebo. Yet, those on active naltrexone reported that the heroin had no effect, and eventually their use declined. Thus, depot naltrexone appears to lessen relapse when it is defined as a return to previous patterns of opioid use, which typically manifests as dropping out of treatment, (S. Comer, personal communication, December 2007).
RESULTS AND DISCUSSION
The objective of this review was to provide an update of the procedures, issues and predictive value of human models of cocaine, cannabis and heroin self-administration, and to indicate some of the methodological issues encountered when conducting these studies. Overall, the data support the importance of the self-administration model in terms of predicting medication effects, particularly when research volunteers are maintained on medication prior to self-administration sessions. Heroin self-administration studies show that the medications that effectively treat opioid dependence increase the relative reinforcing strength of non-drug alternatives in the laboratory. These same medications also decrease heroin’s subjective effects in the laboratory, suggesting that both laboratory measures may predict clinical response.
There is however no FDA-approved medication to treat cocaine dependence. Rather, cocaine use is extraordinarily difficult to disrupt either in the human laboratory or in the clinic. Although it is reasonable to hypothesize that those medications that selectively decrease ratings of cocaine ‘high’ or cocaine craving will be efficacious clinically, this has not yet been demonstrated empirically (Comer et al. 2008; Haney & Spealman 2008). By contrast, modafinil decreased both cocaine self-administration and its subjective effects, and this decrease in the laboratory appears to mirror improved outcome in cocaine treatment. Most would agree that there should be a strong scientific rationale for testing a medication in a clinical trial prior to exposing a large number of treatment seekers to an investigational compound, and that models that amass a large number of false positives, i.e. appear positive at screening but not when tested clinically, need to be refined or abandoned (see Epstein & Preston 2003).The large bank of true negatives and the one apparent true positive (modafinil) support the use of human self-administration procedures prior to clinical trials with cocaine.
In terms of pharmacotherapies for cannabis dependence, it is not possible to assess the predictive validity of laboratory models of marijuana self-administration and relapse because almost no medications have been tested in the clinic. The one clinical pilot study published to date with divalproex had a negative outcome (Levin et al. 2004), consistent with a laboratory study showing that divalproex worsened mood in abstinent marijuana smokers (Haney et al. 2004). More clinical marijuana research needs to be done before the validity of marijuana self-administration studies can be evaluated.
Ideally, regardless of which drug is being studied, a range of medication and drug doses should be tested in order to fully characterize their interaction. Generating these dose-response curves takes time, particularly when participants are maintained on various doses of medications, thereby increasing the likelihood that participants will drop out. As a consequence, the range of doses tested in human laboratory studies is often small, which limits their conclusions. It is also important to evaluate a range of alternative reinforcer values, as the value may be too low and not competitive, or too high, such that participants only need to choose it a few times to have earned a quantity they deem sufficient. Similarly, if the choice for the drug is too frequent, participants may choose it less than the alternative, as occurred when marijuana was offered frequently within a short period of time. Finally, an important improvement to laboratory models may be to provide an immediate, non-drug alternative in order to best characterize the choices patients encounter when deciding to return to drug use or spend their money and time on something other than drugs.
To conclude, this review demonstrates that controlled self-administration studies provide meaningful behavioral data in a relatively small number of individuals. Self-administration procedures also provide information concerning a candidate medication’s own potential for abuse or, viewed from another perspective, the medication’s acceptability by patients taking the medication. Overall, these studies contribute to our understanding of the variables maintaining cocaine, marijuana and heroin intake, and are important in guiding the development of more effective treatment and prevention programs.
Acknowledgements
The US National Institute on Drug Abuse (NIDA) supported this research (DA19239, DA10755, DA09236). We wish to thank Dr. Ziva Cooper for her thoughtful review of this manuscript, and Dr. Sandra D. Comer and Dr. Frank Vocci for their discussion of recent data.
References
- Altman JL, Meyer RE, Mirin SM, McNamee HB, McDougle M. Opiate antagonists and the modification of heroin self-administration behavior in man: an experimental study. Int J Addict. 1976;11:485–499. doi: 10.3109/10826087609056165. [DOI] [PubMed] [Google Scholar]
- Arendt M, Rosenberg R, Foldager L, Sher L, Munk-Jørgensen P. Withdrawal symptoms do not predict relapse among subjects treated for cannabis dependence. Am J Addict. 2007;16:461–467. doi: 10.1080/10550490701640985. [DOI] [PubMed] [Google Scholar]
- Balster RL. Drug abuse potential evaluation in animals. Br J Addict. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81:267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Brady JV, Griffiths RR, Hienz RD, Ator NA, Lukas SE, Lamb RJ. Assessing drugs for abuse liability and dependence potential in laboratory primates. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer Verlag; New York: 1987. pp. 45–86. [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1321. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Campbell J, Nickel EJ, Penick EC, Wallace D, Gabrielli WF, Rowe C, Liskow B, Powell BJ, Thomas HM. Comparison of desipramine or carbamazepine to placebo for crack cocaine-dependent patients. Am J Addict. 2003;12:122–136. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Cue-reactivity and the future of addiction research. Addiction. 1999;94:349–351. [PubMed] [Google Scholar]
- Chait LD, Burke KA. Preference for high- versus low-potency marijuana. Pharmacol Biochem Behav. 1994;49:643–647. doi: 10.1016/0091-3057(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 1992;107:255–262. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- Collins E, Kleber HD. Opioids: detoxification. In: Galanter M, Kleber HD, editors. Textbook of Substance Abuse Treatment. 3rd edn The American Psychiatric Publishing, Inc.; Washington, DC: 2004. pp. 265–290. [Google Scholar]
- Collins SL, Levin FR, Foltin RW, Kleber HD, Evans SM. Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug Alcohol Depend. 2006;82:158–167. doi: 10.1016/j.drugalcdep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Collins ED, Vosburg SK, Hart CL, Haney M, Foltin RW. Amantadine does not modulate reinforcing, subjective, or cardiovascular effects of cocaine in humans. Pharmacol Biochem Behav. 2003;76:401–407. doi: 10.1016/j.pbb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Collins ED, Ward AS, McDowell DM, Foltin RW, Fischman MW. The effects of memantine on the subjective, reinforcing and cardiovascular effects of cocaine in humans. Behav Pharmacol. 1998;9:587–598. doi: 10.1097/00008877-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson C-E, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Buprenorphine sublingual tablets: effects on IV heroin self-administration by humans. Psychopharmacology. 2001;154:28–37. doi: 10.1007/s002130000623. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Klever HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology. 1999;143:327–338. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Relative abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abuser. Neuropsychopharmacology. 2008;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O’Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence. Arch Gen Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Walker EA, Collins ED. Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers. Psychopharmacology. 2005;181:664–675. doi: 10.1007/s00213-005-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O’Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Dole VP. Implications of methadone maintenance for theories of narcotic addiction. JAMA. 1988;260:3025–3029. [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug Alcohol Depend. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine self-administration in humans during abstinence: effects of dose, alternative reinforcement, and priming. Psychopharmacology. 2004;172:316–323. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction. 2005;100:1496–1509. doi: 10.1111/j.1360-0443.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Dierker LC. The absence of DSM-IV nicotine dependence in moderate-to-heavy daily smokers. Drug Alcohol Depend. 2007;89:93–96. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JL, DeVane CL, Malcolm RJ, Mojsiak J, Chiang CN, Elkashef A, Taylor RM. Modafinil influences the pharmacokinetics of intravenous cocaine in healthy cocaine-dependent volunteers. Clin Pharmacokinet. 2005;44:753–765. doi: 10.2165/00003088-200544070-00006. [DOI] [PubMed] [Google Scholar]
- Dudish SA, Pentel PR, Hatsukami DK. Smoked cocaine self-administration in females. Psychopharmacology (Berl) 1996;123:79–87. doi: 10.1007/BF02246284. [DOI] [PubMed] [Google Scholar]
- Dudish-Poulsen SA, Hatsukami DK. Dissociation between subjective and behavioral responses after cocaine stimuli presentations. Drug Alcohol Depend. 1997;47:1–9. doi: 10.1016/s0376-8716(97)00054-9. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology (Berl) 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;3:CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Cocaine self-administration research: implications for rational pharmacotherapy. In: Higgins ST, Katz JL, editors. Cocaine Abuse Research: Pharmacology, Behavior and Clinical Applications. Academic Press; San Diego: 1998. pp. 181–207. [Google Scholar]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther. 1990;253:760–770. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Self-administration of cocaine by humans: choice between smoked and intravenous cocaine. J Pharmacol Exp Ther. 1992;261:841–849. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of buprenorphine on self-administration of cocaine by humans. Behav Pharmacol. 1994;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. A laboratory model of cocaine withdrawal in humans: intravenous cocaine. Exp Clin Psychopharmacol. 1997a;5:404–411. doi: 10.1037//1064-1297.5.4.404. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Residual effects of repeated cocaine smoking in humans. Drug Alcohol Depend. 1997b;47:117–124. doi: 10.1016/s0376-8716(97)00093-8. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Perdroso JJ, Pearlson GD. Marijuana and cocaine interaction in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1987;28:459–464. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacol. 2000;24:24–29. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Cocaine: patterns of use, route of administration, and severity of dependence. Br J Psychiatry. 1994;164:660–664. doi: 10.1192/bjp.164.5.660. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D, Bailey R. Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J Clin Psychopharmacol. 2000;20:305–310. doi: 10.1097/00004714-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatry Rep. 2005;7:360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology. 1999a;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–112. [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology. 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW. Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology. 2006;31:1841–1821. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed S Collins, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1079-x. Epub Feb, DOI 10.1007/s00213-008-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999b;4:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001a;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Gerra G, Foltin RW. Neuroendocrine effects of d-fenfluramine and bromocriptine following repeated smoked cocaine in humans. Drug Alcohol Depend. 2001b;64:63–73. doi: 10.1016/s0376-8716(00)00232-5. [DOI] [PubMed] [Google Scholar]
- Hart C, Ward AS, Haney M, Comer SC, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral D9-tetrahydrocannabinol in humans. Psychopharmacology. 2002;154:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Collins ED, Rubin E, Foltin RW. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86:274–277. doi: 10.1016/j.drugalcdep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Gunderson E, Foltin RW. Modafinil attenuates disruptions in cognitive performance during simulated night-shift work. Neuropsychopharmacology. 2006;31:1526–1536. doi: 10.1038/sj.npp.1300991. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Human smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73:279–287. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Jones BE, Prada JA. Drug-seeking behavior during methadone maintenance. Psychopharmacologia. 1975;41:7–10. doi: 10.1007/BF00421297. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Are choice and self-administration of marijuana related to Δ9-THC content? Exp Clin Psychopharmacol. 1997;5:74–82. doi: 10.1037//1064-1297.5.1.74. [DOI] [PubMed] [Google Scholar]
- Kirchmayer U, Davoli M, Verster AD, Amato L, Ferri A, Perucci CA. A systematic review on the efficacy of naltrexone maintenance treatment in opioid dependence. Addiction. 2002;97:1241–1249. doi: 10.1046/j.1360-0443.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8:483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Brooks D, Akerele E, Donovan S, Nunes E. Pharmacotherpay for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13:21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Brooks D, Spano C, Nunes EV. Pergolide mesylate for cocaine abuse: a controlled preliminary trail. Am J Addict. 1999;8:120–127. doi: 10.1080/105504999305929. [DOI] [PubMed] [Google Scholar]
- Lile JA, Nader MA. The abuse liability and therapeutic potential of drugs evaluated for cocaine addiction as predicted by animal models. Current Neuropharmacology. 2003;1:21–46. [Google Scholar]
- Malcolm R, Herron J, Sutherland SE, Brady KT. Adverse outcomes in a controlled trial of pergolide for cocaine dependence. J Addict Dis. 2001;20:81–92. doi: 10.1300/J069v20n01_08. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N, Khan R, Mojsiak J, Myrick DL, Hedden S, Cochran K, Woolson RF. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse. 2006;32:577–587. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- McCance-Katz E, Kosten TA, Kosten TR. Going from the bedside back to the bench with ecopipam: a new strategy for cocaine pharmacotherapy development. Psychopharmacology. 2001;155:327–329. doi: 10.1007/s002130100745. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC. Buprenorphine effects on human heroin self-administration: an operant analysis. J Pharmacol Exp Ther. 1982;223:30–39. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther. 1981;216:45–54. [PubMed] [Google Scholar]
- Mendelson JH, Kuehnle JC, Greenberg I, Mello NK. Operant acquisition of marijuana in man. J Pharmacol Exp Ther. 1976;198:42–53. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Reinforcing properties of oral (9-tetrahydrocannabinol, smoked marijuana, and nabilone: influence of previous marijuana use. Psychopharmacology. 1984;83:351–356. doi: 10.1007/BF00428544. [DOI] [PubMed] [Google Scholar]
- Meyer RE, McNamee HB, Mirin SM, Altman JL. Analysis and modification of opiate reinforcement. Int J Addict. 1976;11:467–484. doi: 10.3109/10826087609056164. [DOI] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance—a review of abuse liability issues. Ann Clin Psychiatry. 2004;16:101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- Negrete JC, Emil S. Cue-evoked arousal in cocaine users: a study of variance and predictive value. Drug Alcohol Depend. 1992;30:187–192. doi: 10.1016/0376-8716(92)90025-8. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Obert J, Killeen T, Saladin ME, Cowell M, Kirby KC, Sterling R, Royer-Malvestuto C, Hamilton J, Booth RE, Macdonald M, Liebert M, Rader L, Burns R, DiMaria J, Copersino M, Stabile PQ, Kolodner K, Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Rocha B, Bergman J, Comer SD, Haney M, Spealman RD. Development of medications for heroin and cocaine addiction and regulatory aspects of abuse liability testing. In: McArthur RA, Borsini F, editors. Animal and Translational Models of Behavioral Disorders. Vol. 3: Reward Deficit Disorders. Elsevier; New York: 2008. (In press) [Google Scholar]
- Romach MK, Flue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O’Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychiatry. 1999;56:1101–1106. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002a;13:105–115. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Wooten AF. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002b;67:311–322. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwattney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–1448. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- Sinha R, Krishnan-Sarin S, Farren C, O’Malley S. Naturalistic follow-up of drinking behavior following participation in an alcohol administration study. J Subst Abuse Treat. 1999;17:159–162. doi: 10.1016/s0740-5472(98)00058-0. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Extended versus brief treatment for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Adult marijuana users seeking treatment. J Consult Clin Psychol. 1993;61:1100–1104. doi: 10.1037//0022-006x.61.6.1100. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderatevs high-dose methadone in opioid-dependent volunteers. Addiction. 1999;100:1496–1509. [Google Scholar]
- Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: antagonism of the reinforcing, subjective and physiological effects of heroin. Psychopharmacology. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Ling W. Medications development. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- Ward AS, Comer SD, Haney M, Foltin RW, Fischman MW. The effects of a monetary alternative on marijuana self-administration. Behav Pharmacol. 1997;8:275–286. doi: 10.1097/00008877-199708000-00001. [DOI] [PubMed] [Google Scholar]


