Summary
E2A-encoded proteins, key transcriptional regulators in B lineage specification and commitment, have been shown to decrease in B cell precursors in old age. E2A regulates genes encoding the surrogate light chain proteins λ5 and VpreB. In old age, B cell precursors express less surrogate light chain and this results in compromised pre-B cell receptor function and diminished expansion of new pre-B cells in senescence. Herein, we show that aged bone marrow has increased Hardy Fraction A (CD19- B220+) cells, including NK cells, that can inhibit both E47 (E2A) protein and surrogate light chain protein expression in B cell precursors. In vitro, NK associated inhibition of E47 protein is contact independent and partially reversed by neutralization of TNFα. In vivo, depletion of NK cells in aged mice by treatment with anti-asialo GM1 antibody led to restoration of surrogate light chain protein levels to that typical of young B cell precursors. These studies suggest that NK cells, within the CD19- B220+ bone marrow cell fraction, may contribute to a bone marrow microenvironment that has the potential to negatively regulate E47 (E2A) as well as surrogate light chain levels in B cell precursors in old age.
Keywords: E2A, B cell precursors, bone marrow microenvironment, NK cell
Introduction
B lymphopoiesis is regulated by the sequential expression of a series of transcriptional regulators, including E2A, EBF, and Pax-5 (Zhuang, et. al., 1994, Zhuang, et. al., 1996, Busslinger & Urbanek 1995, Kee, et. al., 2000, Kee & Murre 1998, O'Riordan & Grosschedl 1999). Early specification of cells into the lymphoid pathway involves E2A expression; EBF and Pax-5 expression are associated with commitment to the B lineage pathway (Zhuang, et. al., 1996, Busslinger & Urbanek 1995, Kee, et. al., 2000, Kee & Murre 1998, O'Riordan & Grosschedl 1999). E2A encoded proteins have been observed to promote expression of multiple B lineage genes, including IL-7Rα, EBF, RAGs, and the surrogate light chains λ5 and VpreB (Kee, et. al., 2000, Kee, et. al., 1998, O'Riordan & Grosschedl 1999, Sigvardsson, et. al., 1997). Reduced expression of E2A, and in particular E47, results in dramatic inhibition of B lymphopoiesis (Zhuang, et. al., 1994, Zhuang, et. al., 1996, O'Riordan & Grosschedl 1999). Although expression and function of E2A proteins are of key importance to normal B lymphopoiesis within the bone marrow, local mechanisms that regulate E2A expression and that of E2A targets, e.g., surrogate light chains, are only beginning to be elucidated.
An important function of E2A is to promote the expression of the surrogate light chains and facilitate formation of the pre-B cell receptor (preBCR). We have previously demonstrated reduced expression of the surrogate light chains in B cell precursors from aged mice as well as diminished numbers of B cell precursors (Riley, et. al., 1991; Sherwood, et. al., 1998; Sherwood, et. al., 2000; Alter-Wolf, et. al., 2009). This coincides with reduced E2A encoded proteins in developing B cell precursors (Sherwood, et. al., 2000, Frasca, et. al., 2003, Van der Put, et. al., 2003). While E2A is important in the regulation of surrogate light chain expression, as described above, Early B cell Factor (EBF) also is critical to surrogate light chain expression and other transcription factors, e.g., interferon regulatory factors 4 and 8 acting via induction of Ikaros and Aiolos, act to down-regulate surrogate light chains and consequently preBCR signaling in B lineage precursors (Ma, et. al., 2008).
The mechanisms that regulate events at the preBCR checkpoint are important to the proliferative expansion of pre-B cells, the selection of the μ Ig heavy chain variable region repertoire (Ten Boekel, et. al., 1997), and the antibody specificity of new B cells. In senescent mice, compromise of the preBCR checkpoint, associated with down-regulation of surrogate light chain, likely contributes to diminished B lyimphopoiesis as well as changes in the read-out of the nascent B cell antibody repertoires (Alter-Wolf, et. al., 2009). To date, it is not known whether particular bone marrow cell elements influence the expression of key regulatory molecules (e.g., E2A, surrogate light chains) necessary for normal maintenance of B lymphopoiesis or cause the abnormal B lymphopoiesis seen in old age.
That the bone marrow microenvironment may play an important role in the deficiency of B lymphopoiesis in old age is suggested by the studies of Stephan, et al., (1997) who have shown that the stromal cells within the bone marrow microenvironment of aged mice are less capable of supporting B lineage development in vitro. In particular, Labrie, et al., (2004, 2005) have demonstrated that the senescent bone marrow microenvironment plays an important role in limiting RAG enzyme expression and Ig variable gene recombination in B cell precursors. However, it is also likely that intrinsic deficits in hematopoietic stem cell function may contribute to deficiencies in aged mice, (Rossi, et. al., 2005; Guerrettaz, et. al., 2008).
NK cells in the periphery have been shown to interact with B cells, e.g., by initiating class switch recombination (Yuan, 2004; Gao, et. al., 2005). NK cells also function as a barrier to allogeneic bone marrow transplant engraftment (George, et. al., 1997) and in “hybrid resistance” in bone marrow transplantation (Kumar, et. al., 1997). However, participation of NK cells in a regulatory fashion during normal B lymphopoiesis, and alterations commiserate with old age, have not been reported. In assessing the stages of B lineage development altered in old age, we have previously noted that, while numbers of pro-B and pre-B cells are reduced, Hardy Fraction A is increased (Van der Put, et. al., 2003). This heterogeneous bone marrow cell fraction includes NK lineage cells, both precursors and mature cells, in addition to early B cell precursors (Hardy 2003).
Our observations indicate that a subset of Hardy Fraction A cells, with characteristics of activated NK cells, found in young adult bone marrow and with increased frequency in aged bone marrow, can affect the expression of E47 (E2A) as well as surrogate light chain proteins in B cell precursors. This is underscored by the finding that depletion of NK cells in vivo increased levels of surrogate light chain proteins among pro-B cells of young adult mice and, importantly, restored the suppressed levels of surrogate light chain in aged B cell precursors back to young adult levels. These findings suggest that the bone marrow microenvironment of aged mice possesses increased populations of NK cells that contribute to negatively regulate expression of key molecules (E2A; surrogate light chains) that control the expression and function of the preBCR during B lymphopoiesis.
Results
Aged bone marrow has increased frequencies of CD19- B220+ NK cells
Hardy (2003) determined that early B cell progenitors were present in a heterogenous bone marrow population termed Fraction A. This population was characterized by expression of surface CD43 and CD45R (B220) (Hardy 2003). Further studies indicated that Fraction A cells did not express the pan-B lineage surface protein CD19, and in addition to having B cell progenitors, also contained NK cell precursors expandable in IL-2 (Rolink, et. al., 1996). NK cells have also been shown to be capable of B220 expression; B220 levels increase during NK cell activation (Vosshenrich, et. al., 2007).
As shown in Figure 1, Fraction A CD19- B220+ cells were increased in the bone marrow of aged BALB/c mice by both number (Fig 1A) and percent (Fig 1C). In contrast, Fraction D (IgM- CD43- B220+) late-stage pre-B cells were decreased in aged mice, as expected (Van der Put 2003). Fraction A possessed a large population of plasmacytoid dendritic cells (pDC) characterized by surface CD11c, B220, Ly6C and lacking CD19 and DX5 expression (Figs. 1B and 1C). While the proportions of pDC in Fraction A from young adult and aged BALB/c mice remained relatively constant (e.g., ∼65%), the numbers of pDC in the bone marrow was elevated in aged mice by about twofold (Fig. 1B).
Figure 1. CD19- B220+ cells are increased in aged mice and subsets include plamacytoid dendritic cells and NK lineage cells.
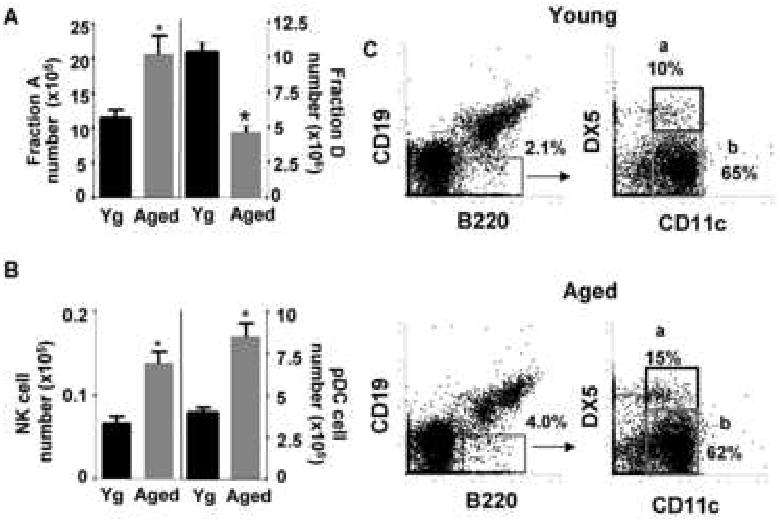
A, Bone marrow from young and aged BALB/c mice were harvested and surface stained for CD19, B220, analyzed by flow cytometry and numbers of CD19- B220+ cells were calculated based on total bone marrow cell numbers. Additionally, bone marrow from young and aged Balb/c mice was surface stained for B220, CD43 and IgM and analyzed by flow cytometry. Numbers of Fraction D early pre-B cells (B220+ CD43- IgM-) were calculated based on total bone marrow cell numbers. (n=10) *p≤0.05 by Student's t-test. B, Bone marrow from young and aged BALB/c mice were harvested and surface stained for CD19, B220, CD11c, Ly6C and DX5 and cell numbers of CD19- B220+ CD11c+ DX5+ (NK cells, n=4) and CD19- B220+ CD11c+ Ly6C+ (pDCs, n=10) in the bone marrow were calculated based on total bone marrow cell number *p≤0.05 by Student's t-test for young vs. aged groups. C, Freshly isolated bone marrow cells from young and aged BALB/c were surface stained for CD19, B220, CD11c, and DX5 and analyzed by flow cytometry (a: NK cells, b: pDCs). Data are representative of 4 individual pairs of young and aged mice.
Further analysis indicated that Fraction A cells, in both young adult and aged BALB/c bone marrow, contained a subset of cells staining for both DX5 and CD11c surface antigens in addition to B220 (Fig. 1C). Typically, the Fraction A population accounted for 2% (young) or 4% (aged) of bone marrow cells and within this fraction the CD19- B220+ CD11c+ DX5+ cells were 10-15%. In analogous studies with C57BL/6 mice, CD19- B220+ CD11c+ DX5+ cells also stained uniformly for NK1.1 antigen and in BALB/c young and aged mice, were Ly6C low/- (data not shown). Therefore, these cells have a phenotype consistent both with NK cells and a recently described NK-like cell type, the interferon producing killer dendritic cells (IKDC). The latter have been suggested to be activated NK cells (Vosshenrich, et. al., 2007); we will include these in our classification as NK cells.
CD19- B220+ bone marrow cells negatively regulate E47 protein levels in B cell precursors in vitro
Since we have previously observed that E47 protein levels are reduced in aged B cell precursors and that this correlated with reduced surrogate light chain levels in B cell precursors (Sherwood, et. al., 2000; Frasca, et. al., 2003; Van der Put, et. al., 2004), we asked whether Fraction A CD19- B220+ bone marrow cells from young adult and aged mice would affect the amount of E47 protein in B cell precursors in vitro. Co-culture of CD19+ bone marrow B lineage cells with freshly isolated bone marrow CD19- B220+ cells from young adult BALB/c mice resulted in at least a two-fold reduction in E47 protein levels by B cell precursors (Fig. 2). Similar results were also obtained when CD19- B220+ cells were isolated from aged BALB/c mice and co-cultured with young B cell precursors (Fig. 2). Aged B cell precursors were also inhibited in E47 protein levels when co-cultured with either young or aged CD19- B220+ cells (Fig. 2). Note that the aged mice utilized had moderate to severe deficits in B lymphopoiesis as measured by a decline in pre-B cells (6-10% of young control pre-B levels) (data not shown, Van der Put, et. al., 2003). Typically, these aged mice have B cell precursors with ∼50-70% reduction in E47 protein level (Sherwood, et. al., 2000; Van der Put, et. al., 2004).
Figure 2. CD19- B220+ cells from young and aged mice inhibit E47 protein in CD19+ B cell precursors from young and aged mice.
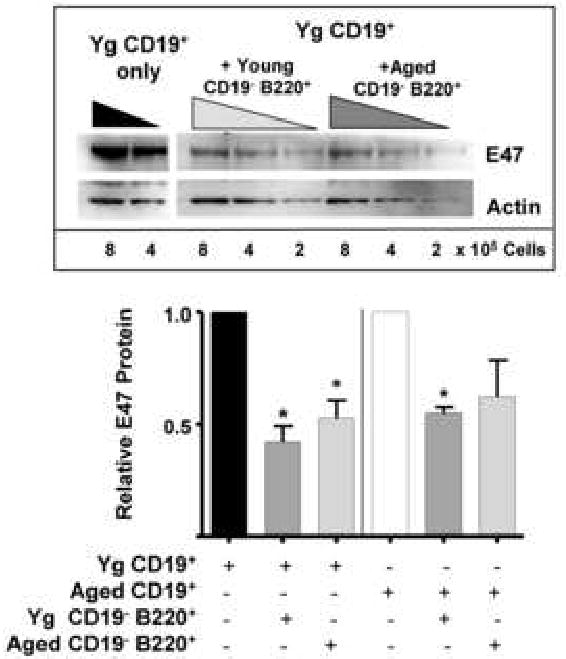
CD19+ cells were magnetically bead separated from bone marrow of young BALB/c mice and co-cultured with magnetically sorted CD19- B220+ cells from young and aged mice at a 1:10 ratio (CD19- B220+:CD19+ cells) with IL-7 for 7 days. Non-adherent cells were harvested and E47 and actin proteins detected by Western blot. Shown in the graph below are cumulative data from 3 experiments where young and aged CD19+ cells were co-cultured with CD19- B220+ cells from young and aged mice. Aged CD19+ cells alone used in these experiments exhibited a 57% loss in E47 protein levels relative to young control CD19+ cells (data not shown). E47 values were normalized to their respective loading controls and then to young or aged CD19+ values in each experiment with young or aged CD19+ E47 values each set to 1. (*p≤0.05 by Student's t-test for all groups vs. young CD19+ cells alone based on loading control normalized values).
Importantly, the cell populations harvested after the co-culture were predominantly (∼95%) CD19+ pro-B/pre-B cells (<5% surface IgM+ B cells), indicating that the reduction seen in E47 protein levels in the cultured cells was not the result of dilution due to the added CD19- B220+ cells. Input CD19- B220+ cells were also observed in numbers similar to those at inception of culture suggesting that these cells did not expand significantly during the culture period.
As indicated above and in Figures 1 and 2, the Fraction A cells from young and aged mice were similar in both cell composition and, importantly, both young and aged Fraction A cells were comparable in their capacities to inhibit E47 levels in B cell precursors. The spectrum of aged phenotypes, from young-like B lymphopoiesis to poor B lymphopoiesis (Van der Put, et. al., 2003), presented a challenge to sorting the required NK containing bone marrow populations from old mice. In order to further define the mechanisms by which Fraction A cells accomplished this regulation, we utilized young adult mice, thereby circumventing the often extensive mouse-to-mouse variability seen in aged mice.
When the CD19- B220+ cells from young mice were further restricted by cell sorting to those that expressed CD11c (e.g., pDCs and NKs), again marked loss of E47 protein levels was apparent upon co-culture with CD19+ B lineage cells (Fig. 3A,B). Importantly, similar results were obtained when CD19- B220+ CD11c+ cells were cultured with CD19+ B lineage cells, but separated by a semi-permeable membrane (Fig. 3B open symbols). This precluded cell-cell contact during the experiment; the comparable inhibition seen in the absence of cell-cell contact implicates soluble factors in the regulatory mechanism.
Figure 3. CD19- B220+ CD11c+ cells inhibit E47 protein in B cell precursors through a soluble factor.
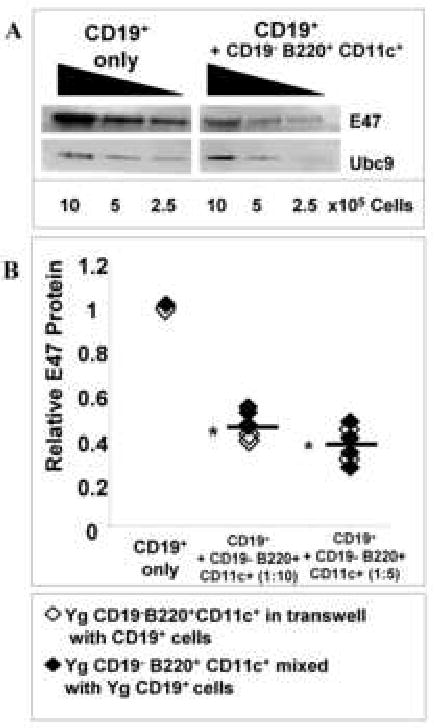
A, Young CD19+ cells were sorted by magnetic beads and CD19-CD11c+ B220+ cells sorted from bone marrow by fluorescence flow cytometry and mixed at a 1:10 effector:target ratio of CD19- B220+ CD11c- to CD19+ cells, and cultured with IL-7 for 7 days. E47 and Ubc9 (loading control) proteins were detected by Western blot. B, CD19+ cells were co-cultured at varying ratios with CD19- B220+ CD11c+ cells, together with IL-7, for 7 days, either as cell mixtures or separated via transwells. Relative E47 levels of CD19+ cells as E47 to Ubc9 ratios were calculated.* p≤0.05 by Student's t-test for CD19+ only vs. CD19+ cells + inhibitory cells (n=5).
DX5+ bone marrow cells inhibit E47 protein expression in B cell precursors
As indicated above, the majority of CD19- B220+ cells were plasmacytoid dendritic cells (pDC) and a minority had NK/IKDC phenotypes. Both pDC and total dendritic cell populations (e.g., DX5- CD11c+ B220+ pDC and DX5- CD11c+ myeloid dendritic cells plus pDCs) were sorted from bone marrow and their capacities to affect E47 protein expression in B cell precursors were tested. Neither pDC nor total dendritic cells altered E47 protein levels in cultured B cell precursors (Fig. 4 A,C). In contrast, when DX5+ NK cells were isolated from bone marrow, these cells consistently reduced E47 protein expression in B cell precursors (Fig. 4 B,C). DX5 can be expressed by some T cells, including NK-T cells; however, CD19- B220+ cells contained very low proportions of CD3+ cells (<3%). Co-culture of CD19+ B cell precursors with isolated CD4+/CD8+ Thy1hi DX5+ cells at levels comparable to those CD3+ contaminants failed to inhibit E47 levels (data not shown). Furthermore, isolation of CD3- CD19- B220+ DX5+ cells, devoid of T cells, resulted in inhibition of E47 expression by B cell precursors (see below). Therefore, the reduction of E47 seen in B cell precursors is likely induced by the NK population in Fraction A.
Figure 4. DX5+ bone marrow cells, but not dendritic cells, inhibit E47 expression and increase pERK expression in B cell precursors.
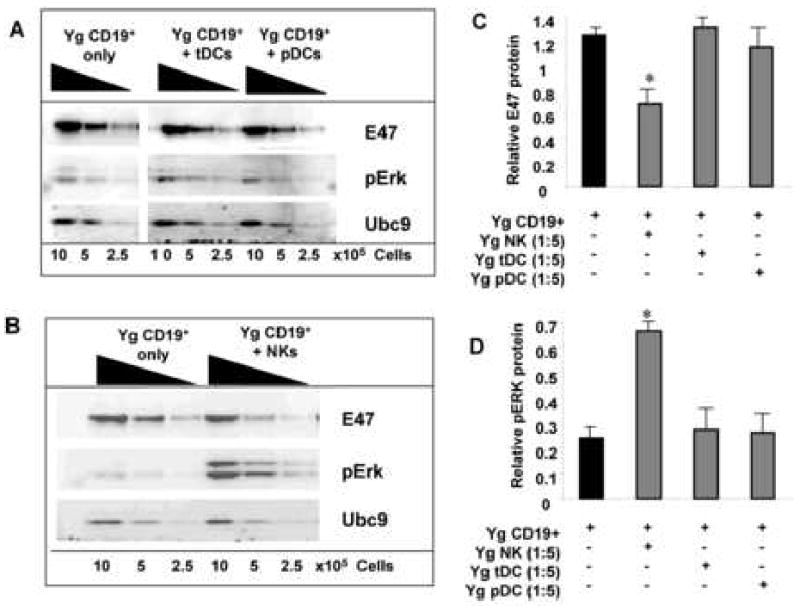
A and B, B cell precursors (CD19+) from young BALB/c mice were sorted by flow cytometry and co-cultured with the following sorted bone marrow cell subsets from young mice and placed in transwells: total DC (CD19- CD11c+); pDCs (CD19- B220+ CD11c+); or NK cells (DX5+) at 1:5 ratios. Cultures also contained 5ng/ml IL-7 and were harvested at 7 days and Western blots performed for E47, pERK and the loading control Ubc9. Cumulative results for E47 protein levels for 3 experiments are shown in C and for phospho-ERK protein in D normalized to loading controls. * p≤0.05 by Student's t-test for CD19+ vs. CD19+ cells + NK, tDC, or pDC groups. Data for E47 protein was normalized to loading control values [E47/Ubc9 ratio or pErk/Ubc9 ratio] in Western blots.
We have previously described that activation of the Ras-MEK-Erk MAPK pathway is associated with increased turnover of E47 protein in cultured B cell precursors (King, et. al., 2007). Therefore, we tested whether activation of this MAPK pathway was affected in B cell precursors upon co-culture with DX5+ bone marrow cells. As shown in Figure 4 A, D, culture of B lineage cells with DX5+ bone marrow cells, but not dendritic cells, caused reduction in E47 protein levels in B cell precursors; reciprocally, this was associated with 2-3-fold increases in the activated, phosphorylated form of Erk proteins (Fig. 4 B,D). Since these experiments used B lineage cells separated from the DX5+ bone marrow cells by semi-permeable membranes, both the loss of E47 and rise in phospho-Erk levels in B cell precursors resulted from soluble factors secreted by the DX5+ bone marrow cells.
A unique population of DX5+ cells has been recently described in bone marrow that protects immature B cells from B cell receptor induced apoptosis (Sandel, et. al., 2001). However, unlike the DX5+ cells we describe that inhibit E47 protein expression in B cell precursors, the anti-apoptotic DX5+ cells in bone marrow fail to express either B220 or CD11c. Moreover, they are retained in the bone marrow from either RAG knockout or IL2Rγ knockout mice, the former devoid of T, NK-T, and B cells and the latter devoid of NK and likely IKDC cells (Ranson, et. al., 2003; Vosshenrich, et. al., 2005). Bone marrow CD19- B220+ Fraction A cells from double defective RAG-2/γc knockout mice showed significantly reduced capacity to inhibit E47 protein expression in B cell precursors (BCP) (70% + 12% E47 protein reduction in BCP co-cultured with wild-type C57BL/6 CD19- B220+ cells vs. 30% + 10% E47 protein reduction in BCR co-cultured with RAG-2/γc KO CD19- B220+ cells, p<0.05, data not shown). This is consistent with a role for bone marrow NK cells in reducing E47 protein expression in B cell precursors.
Isolated stromal cells and macrophages do not alter E47 levels of CD19+ B lineage cells
Bone marrow contains a variety of other cell types that may affect B lymphopoiesis, including macrophages and stromal cells. Therefore, we also tested these cell types for effects on E47 expression by B cell precursors. Unfractionated bone marrow, when placed in culture, establishes an adherent cell layer comprised primarily of macrophages (∼70%) and fibroblastic stromal cells (∼20%) (Witte et. al., 1993, Witte et. al., 1987). Adherent macrophages (CD45+) and stromal cells (CD45-) were isolated from established one week old cultures of unfractionated bone marrow by magnetic bead sorting for the leukocyte common antigen CD45; separated macrophages and stromal cells were grown in culture for an additional 3 days and purity assessed by morphology (data not shown). Co-culture of isolated bone marrow CD19+ B lineage cells on either adherent macrophages or stromal cell monolayers, with added IL-7, did not result in significant reduction in E47 protein in the B cell precursors (Fig. 5).
Figure 5. Stromal cells and macrophages from young or aged bone marrow microenvironments do not inhibit E47 expression in young CD19+ cells.
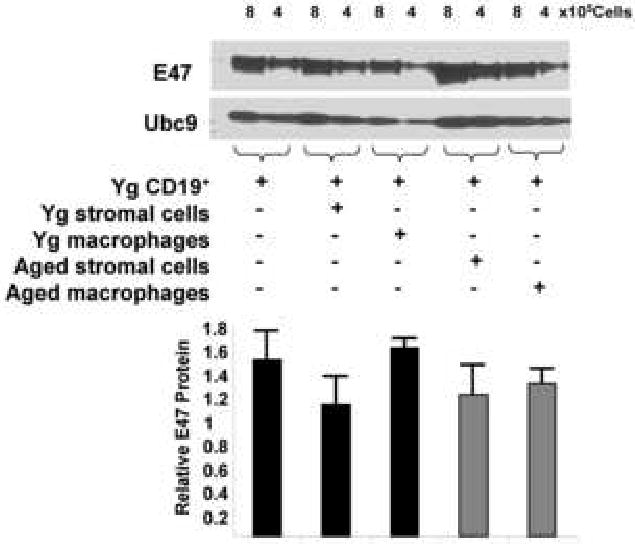
Young and aged BALB/c bone marrow cells were cultured with IL-7 for 7 days. Following the 7 day culture, adherent cells were harvested after addition of trypsin and magnetically separated based on expression of CD45. Once separated, stromal cells (CD45-) and macrophages (CD45+) from young and aged mice were cultured separately for an additional 3 days. Magnetically sorted young CD19+ B cell precursors (from separate mice) were then co-cultured with either young or aged stromal cells (CD45-) or macrophages (CD45+) for 7 days with IL-7. E47 protein was determined by Western blot and shown as E47 to Ubc9 signal ratios (n=4). No groups differed from the CD19+ alone control at the p<0.05 level by Student's t test. Data for E47 protein was normalized to loading control values [E47/Ubc9 ratio] in Western blots, not relative to the CD19+ alone control group.
Inhibition of E47 protein expression in B cell precursors is dependent upon TNFα
The negative effects of Fr. A CD19- B220+ cells on E47 protein levels in B cell precursors were shown to be mediated by soluble factors and did not require cell-cell contact. The involvement of NK lineage cells in this regulation suggested that cytokines associated with these cell types might be candidates for the inhibitory activity. NK cells produce a variety of cytokines, including TNFα and IFNγ (Welner et. al., 2007). When CD19- B220+ cells or further fractionated and T cell depleted CD3- CD19- B220+ DX5+ cells were co-cultured with B lineage cells in transwells in the presence of neutralizing antibodies to TNFα, the reduction in B cell precursor E47 levels was largely ameliorated (Fig. 6). In contrast, inclusion of neutralizing antibodies to IFNγ had no effect on E47 inhibitory capacity (Fig. 6). Therefore, TNFα secretion by cells within the CD19- B220+ and NK cell subpopulations is necessary for optimal inhibition of E47 protein expression in B cell precursors.
Figure 6. TNFα is required for Fr. A/NK inhibition of E47 protein levels in B cell precursors.
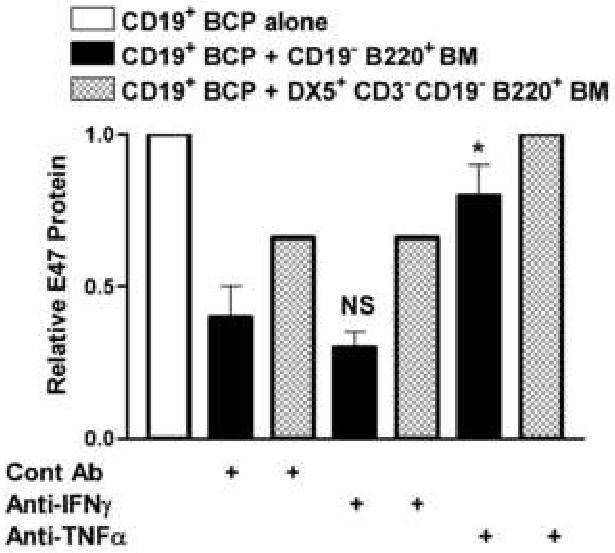
CD19+ cells were sorted from young BALB/c bone marrow and plated with CD19- B220+ (Fr. A) bone marrow cells (1:5 effector:target ratio) or cell-sorted CD3-CD19- B220+ DX5+ bone marrow cells (1:10 effector:target ratio) in transwells. Control antibody (goat IgG), anti-IFNγ goat polyclonal antibody, or anti-TNFα goat polyclonal antibody were added at the inception of culture at 5μg/ml. Post 7 days of culture with IL-7, the CD19+ cells were harvested and E47 and Ubc9 and/or actin (loading control) proteins detected by Western blot. E47 values were normalized to both loading controls and to values for CD19+ cells cultured alone. Cumulative results are shown for 4 experiments. E47 values were normalized to their respective loading controls and then to young CD19+ values in each experiment with CD19+ E47 values each set to 1. (*p≤0.05 by Student's t-test for the anti-TNFα group vs. control Ab group; NS, not significant for experimental anti-IFNγ group vs. control Ab group).
The effects of TNFα directly on CD19+ B cell precursors was tested in vitro. TNFα was added to IL-7 supplemented cultures of B cell precursors under various conditions, e.g., at concentrations from 25-100ng/ml and either at inception of culture or added daily to insure that concentrations critical to trimerization in solution were maintained. As shown in Figure 7A, TNFα treated CD19+ B cell precursors showed some reduction of E47 (E2A) protein levels as revealed by fluorescent flow cytometry and confirmed by Western blots (data not shown). However, more substantial loss was observed in λ5 protein (Fig. 7A).
Figure 7. TNFα impairs E47 and λ5 protein expression in B cell precursors and preferentially results in loss of pro-B cells in vitro.
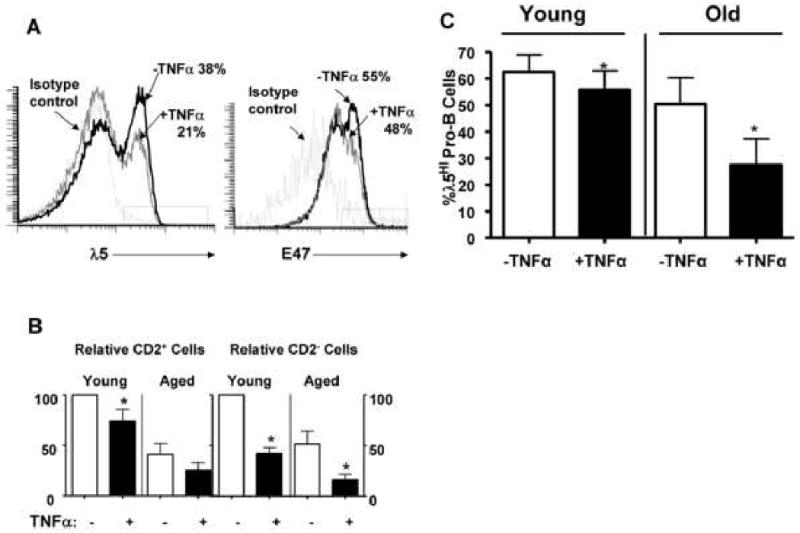
A, CD19+ B cell precursors were co-cultured with recombinant murine TNFα (100ng/ml) added daily. E47 and λ5 protein expression was determined by fluorescence flow cytometry at day 7 of culture. Proportions of cells with high λ5 levels, above that of the isotype control, are indicated. B, Pro-B cells (CD2-) and pre-B cells (CD2+) from young and aged BALB/c mice in IL-7 supplemented cultures were assessed at day 7 after TNFα (100ng/ml) treatment. Since the expansion of B cell precursors differed in control cultures in individual experiments, the data on pro-B and pre-B cell numbers has been normalized to that of the analogous cells in control cultures in each experiment (control=100% expansion). TNFα effects on both young and aged B cell precursors are shown for experiments using 12 young and 4 aged BALB/c individuals. C, CD2- pro-B cells from young and aged mice were cultured with TNFα (100ng/ml) and proportions of cells expressing high λ5 levels (above isotype control staining) are indicated as determined by cytoplasmic fluorescence flow cytometry. In all fluorescence flow cytometry analyses, only live cells were assayed as determined by lack of Annexin V staining. *, p<0.05 for −TNFα vs. +TNFα groups in each panel.
Given that NK cells, in a TNFα dependent manner, reduced E47 protein in B cell precursors (see above), it was surprising that recombinant TNFα produced less evident changes in E47 protein levels in B cell precursors. However, we also noted that TNFα altered the composition of B cell precursors in vitro. In particular, pro-B cells (CD2-) were reduced by approximately one-half in cultures while early pre-B cells (CD2+) were less affected (Fig. 7B). This served to increase the relative proportions of pre-B cells in culture. Similar effects of TNFα were also seen in the culture of B cell precursors from aged mice (Fig. 7B). Pro-B cells have been shown to have increased levels of E47 and λ5 protein relative to early pre-B cells (Quong et al., 2004). Consequently, the lower levels of E47 proteins in B cell precursors co-cultured with TNFα-producing NK cells may result primarily from changes in the cellular composition in vitro. While loss of λ5 proteins in B cell precursors co-cultured with NK cells could result from a similar mechanism, it is notable that, as shown in Fig. 7C, TNFα also inhibited λ5 protein levels in CD2- pro-B cells from young and aged mice in culture. Therefore, loss of λ5 protein in TNFα treated B cell precursors resulted from both direct and indirect effects.
NK cells inhibit surrogate light chain expression in B cell precursors in vivo
While NK cells within Hardy Fraction A were capable of inhibiting E47 protein expression in B cell precursors in vitro, it remained necessary to demonstrate a role for NK cells in regulating B lymphopoiesis in vivo, particularly in aged mice. In addressing this issue, we treated aged and young adult BALB/c mice with rabbit antibodies to asialo-GM1, a procedure that has been used successfully in a variety of protocols to selectively deplete NK cells in vivo over the last two decades (Alba, et. al., 2008; Winkler-Pickett, et. al., 2008; Yang, et. al., 1986). While the neutral glycosphingolipid asialo-GM1 is present on multiple cells, including macrophages and T cells, it is expressed at particularly high levels on NK cell surfaces and in vivo administration of anti-asialo-GM1 reduces NK activity while having no effect on either cytotoxic macrophages or cytotoxic T cell functions (Wiltrout, et. al., 1985; Stein-Streilein and Guffe, 1986).
We have previously reported that mice of comparable chronologic old age demonstrated heterogeneity in B lymphopoiesis as well as E47 and λ5 expression (Van der Put, et. al., 2003; Sherwood, et. al., 1998; Sherwood, et. al., 2000; Frasca, et. al, 2003). Therefore, for these in vivo studies, it was necessary to ensure that both control and anti-asialo GM1 treated old mouse groups would be comparably compromised in B lymphopoiesis prior to in vivo NK depletion. This was accomplished by measuring the proportions of immature B lineage cells (AA4.1+ IgD- CD19+) within the total B lineage cell compartment in peripheral blood (Alter-Wolf, S., Frasca, D., Blomberg, B.B., and Riley, R.L., manuscript in preparation). This assay proved capable of identifying BALB/c aged mice with poor B lymphopoiesis, but was less reliable for aged C57BL/6 mice, possibly due to the lower B cell precursor levels normally seen in this strain.
The aged BALB/c mice chosen for inclusion in either the NK depleted or control groups had levels of immature B lineage cells in peripheral blood that were comparable and predicted levels of pre-B/immature B cells that were at least 70% lower than young adult controls. As we have previously reported, this extent of compromised B lymphopoiesis is associated with significant reductions in the surrogate light chain protein λ5 (Sherwood, et. al., 2000).
Treatment of young adult mice with rabbit asialo-GM1 antibodies, but not control rabbit IgG, resulted in partial depletion of NK cells as measured in spleen (∼ 80% depletion) and bone marrow (∼ 50% depletion). Overall proportions and numbers of pro-B and pre-B cells did not change in young adult bone marrow and were not increased in the normally pro-B/pre-B cell deficient bone marrow of aged mice upon NK cell depletion (data not shown). This likely reflects the relatively short time of NK depletion (e.g., 7 days maximum) in these aged mice; whether longer periods of NK depletion can restore B lymphopoiesis remains to be determined.
Importantly, however, NK cell depletion resulted in a small, but significant increase in expression of the λ5 surrogate light chain by pro-B cells in young adult mice as determined by cytoplasmic fluorescent flow cytometry (Fig. 8A,C). E47 protein levels were not significantly altered (data not shown); this may reflect maintenance of pro-B/pre-B cell ratios in the bone marrow or the more limited capacity of fluorescence flow cytometry to assess quantitative differences as compared to Western blot. The latter method was impractical due to the low numbers of pro-B cells (1-3%) present in the bone marrow of individual aged mice.
Figure 8. Depletion of NK cells in aged mice increases λ5 protein expression in pro-B cells in vivo.
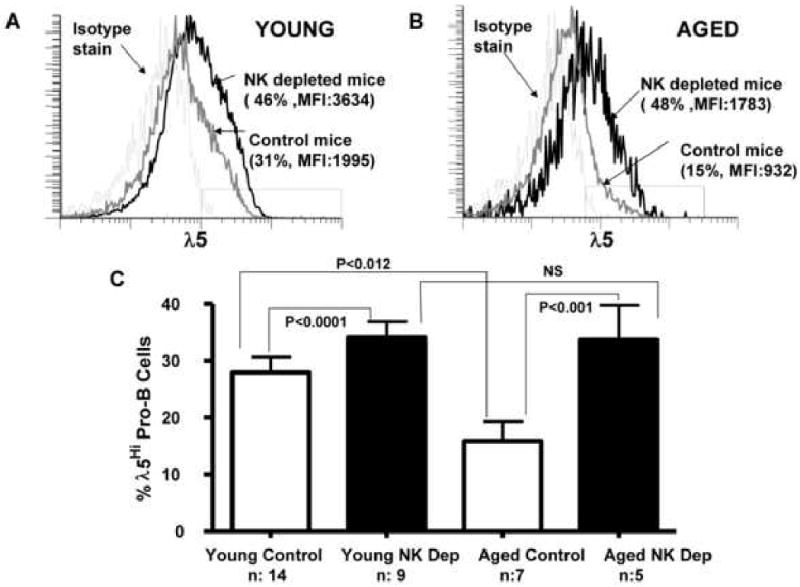
BALB/c young adult (3 mo.) and aged (24 mo.) mice were administered 200μg rabbit anti-asialo GM1 antibody or control antibody i.p. daily for 5 days. On day 7, bone marrow pro-B cells were enumerated as IgM- CD2- CD19+ B220+ cells and cytoplasmic λ5 protein levels assessed by fluorescence flow cytometry.
A, Representative fluorescence histograms for λ5 staining of young pro-B cells from mice treated with anti-asialo GM1 antibody or controls are shown. Proportions of λ5 high cells as well as mean fluorescent intensities are indicated. B, Representative fluorescence histograms for λ5 staining of aged pro-B cells from mice treated with anti-asialo GM1 antibody or control are shown. Proportions indicated are pro-B cells having λ5 protein levels above isotype control staining, designated “λ5Hi”. C, Cumulative data for 5-14 mice (n) used per group; abbreviations are NK depleted (NK Dep) and control (Ctrl). P values calculated by Student's t-test for the paired groups as indicated.
While the increases seen in λ5 protein in young pro-B cells after NK depletion were minimal, anti-asialo-GM1 treatment of aged BALB/c mice resulted in a marked increase in λ5 surrogate light chain expression among pro-B cells in the bone marrow (Fig. 8B,C). The levels of λ5 surrogate light chain detected in pro-B cells from NK depleted aged mice were now similar to those seen in young adult mice.
Discussion
The regulation of specification, commitment, proliferation, and developmental maturation of cells of the B lineage is highly complex. Within the bone marrow, it is clear that the B lineage developmental program is regulated by a hierarchy of transcription factors, including E2A, EBF, and Pax-5 (Zhuang et. al., 1996, Busslinger & Urbanek 1995, Kee et. al., 2000, Kee & Murre 1998, O'Riordan & Grosschedl 1999). A key event in B lineage development is expression of the preBCR, comprised of Ig μ heavy chain in association with the surrogate light chains λ5 and VpreB. This provides an efficient checkpoint governing both selection and clonal expansion of pre-B cells, the immediate precursors of new B cells. Although it is generally assumed that microenvironmental cues are involved in initiating these processes, the nature of such signals and the cells and soluble mediators involved remain to be elucidated. In this regard, our studies indicate that cells within the bone marrow of both young adult and aged mice, in particular NK cells, can have a significant impact on key molecules involved in this process, including E2A and the surrogate light chains. B lineage differentiation is likely modulated by the activities of a variety of cell subsets, in addition to those described herein with NK character, within the bone marrow microenvironment.
NK cells have been shown to affect B cell functions and, under allogeneic transplant conditions, bone marrow cell reconstitution (Yuan 2004; Gao N, et. al., 2005; George, et. al., 1997). In the former, NK cells, via cytokine production, can affect immunoglobulin isotype switch; in the latter, murine NK cells can reject allogeneic bone marrow cells. NK cells of F1 mice reject parental bone marrow cells, accounting for the phenomena of hybrid resistance (George, et. al., 1997). However, although NK cells are present in low numbers naturally in the bone marrow, their role (if any) in the regulation of normal B lymphopoiesis, in either young adults or in senescence, has not, to our knowledge, been investigated. Therefore, our findings that NK cells, in vitro and/or in vivo, can exert negative affects on the expression of key molecules (e.g., E2A, λ5) involved in B lymphopoiesis suggests that NK cells may participate as part of a regulatory cell/cytokine network within the bone marrow that affects development of new B cells. Furthermore, our studies suggest that NK cells, elevated in aged bone marrow, may contribute to impairment of the preBCR checkpoint by diminishing expression of surrogate light chains.
The inhibition of E47 expression mediated by Fraction A NK cells occurs in a contact-independent fashion, presumably mediated via soluble factors. TNFα, as well as IFNγ, are well characterized as cytokines produced by NK cells and have markedly negative affects on the proliferation and survival of B lineage precursors (Fernandez et. al., 2003, Sedger et. al., 2002). In our studies, TNFα, but not IFNγ, was involved in the inhibition of E47 expression in B cell precursors mediated by CD19- B220+ NK cells.
Although the cytokine neutralization experiments indicate that TNFα has an important role in NK-associated down-regulation of E47 in B cell precursors, studies with recombinant TNFα indicated that this cytokine likely has a limited direct effect on E47 levels. In contrast, TNFα altered the pro-B to pre-B cell ratio in vitro, favoring maintenance of early pre-B cells having lower E47 levels (Quong, et. al., 2004). It is also conceivable that other NK cytokines may act in synergy with TNFα to reduce E47 levels in B cell precursors and this will require further investigation. It is, however, clear that TNFα has a direct effect on B cell precursors to lower λ5 surrogate light chain levels. It remains to be seen whether TNFα-induced changes in transcriptional regulators for surrogate light chains, e.g., E2A, EBF, IRF-4/8, Ikaros, and/or Aiolos (Ma, et. al., 2008; Sigvardsson, et. al., 1997), either alone or in combination, are responsible for altered surrogate light chain expression.
In the context of aging, it is of interest that previous studies have shown that the senescent murine bone marrow microenvironment is not capable of supporting normal expression of RAG enzymes and Ig recombination (Labrie et. al., 2005, Labrie et. al., 2004). As suggested by Labrie, et. al., (2004, 2005) the down-regulation of RAG expression may, in part, result from lower levels of E2A proteins in developing B cell precursors. However, the mechanisms by which the bone marrow microenvironment may impact E2A expression in old age are not understood. Our studies provide a demonstration that particular bone marrow cell subsets, e.g., NK cells, but not macrophages or stromal cells, can negatively regulate E2A protein levels in B cell precursors in vitro. Moreover, in vivo depletion of NK cells, in aged mice, resulted in increased expression of the λ5 protein. Significantly, pro-B cells from aged mice, where λ5 protein expression would be predictably reduced, were rendered normal in λ5 protein expression by depletion of NK cells. These results, coupled with the finding that NK cells are increased significantly in aged bone marrow, suggests a mechanism by which alterations in the numbers of NK cells (and possibly other regulatory cells as well) within the marrow microenvironment negatively impacts B lineage development, compromises preBCR-induced pre-B cell selection and expansion, and, consequently, contributes to reduced B lymphopoiesis in old age.
Experimental Procedures
Mice
Young (2-5 mo.) and aged (19-28 mo.) BALB/c mice were purchased from the National Institutes of Aging colony at Harlan Sprague Dawley, Indianapolis, IN. Mice with obvious abdominal tumors and/or splenomegaly were eliminated from the studies.
Expansion of B cell precursors with IL-7 in vitro
Femur and tibia pairs were flushed to harvest cells from the bone marrow as previously described (Sherwood et. al., 1998, Riley et. al., 1991). Red blood cells were removed by treatment with ACK (0.15 M NH4CL, 1mM KHCO3, 0.1 mM EDTA) for 5 minutes at room temperature followed by centrifugation to remove red cell debris. Bone marrow cells were counted and resuspended at 1×106/ml in RPMI-1640 (Gibco Life Technologies, Grand Island, NY), supplemented with 10% FCS (Sigma Aldrich, St. Louis, MO) plus 1% penicillin-streptomycin, 1% L-glutamine and 2-mercaptoethanol at 2×10-5 M. Purified, recombinant mouse IL-7 (rmIL-7, Biosource International, Camarillo, CA) was added at 5ng/ml and remained in culture for 5-7 days after which non-adherent cells were harvested and used for analysis. In some experiments, CD19+ and CD19- B220+ bone marrow cells were isolated by either magnetic sorting or by flow cytometry prior to mixing and culture with IL-7 (92-96% purity).
Sorted CD19+ cells and potential regulatory cells were co-cultured in a transwell system (Corning Inc., Corning, NY, pore size 0.4μM). CD19+ cells were plated at 1×106/ml in 12 or 24 well plates with 5ng/ml IL-7. Sorted putative bone marrow regulatory cells were placed in 0.2ml transwells at a 1:5 or 1:10 ratio of regulatory cells to CD19+ cells.
In some experiments, anti-TNFα (5μg/ml) or anti-IFNγ (5μg/ml) polyclonal goat IgG antibodies from Sigma Aldrich were used to neutralize TNFα or IFNγ production in vitro. Normal goat IgG (5μg/ml) was used as a control (Jackson ImmunoResearch, West Grove, PA). In certain studies, recombinant murine TNFα (R & D Systems, Minneapolis, MN) was used at the concentrations and time periods indicated.
Magnetic cell separation of CD19+ cells and bone marrow adherent cells
CD19+ bone marrow cells were isolated by magnetic sorting prior to culture with IL-7. IL-7 cultured and expanded B cell precursors were also sorted for either CD19+ or CD19- cells by magnetic bead separation prior to analysis. Cells were first surface stained with anti-CD19 PE (1D3) (BD Bioscience, La Jolla, CA) and then secondary stained using anti-PE microbeads according to the MiniMacs protocol (Miltenyi Biotec, Auburn, CA). CD19+ cells were separated from CD19- cells on a magnetic column. From the CD19- cells, B220+ cells were further magnetically sorted using anti-B220 magnetic beads from Miltenyi Biotec.
For stromal cell and macrophage isolations, bone marrow cells were first placed in culture with IL-7 for 5-7 days. Non-adherent B cell precursors were harvested and used for other experiments while adherent cells were grown for an additional 2-3 days in the absence of IL-7 before being harvested with trypsin/EDTA. The adherent cells were stained with anti-CD45 magnetic beads (Miltenyi) and sorted according to the MACS protocol. CD45+ (macrophages) and CD45- (stromal) cells were sorted over a magnetic column. CD45+ and CD45- cells were re-plated in fresh 12 well plates and cultured for 6 hours to allow reattachment. CD19+ cells were harvested from the bone marrow, magnetic bead sorted and placed on monolayers of either macrophages or stromal cells. B cell precursors (CD19+ cells) were grown on adherent cell monolayers with 5ng/ml IL-7 for 5-7 days and before harvesting. Lysates were made from harvested B cell precursor cells and Western blots performed to assess E47 protein levels.
Western blot
For total cell lysates, cells were harvested, counted and lysed with Mammalian Protein Extraction Reagent (Pierce, Rockford, IL) at 10μl/106 cells. Protein Extraction reagent was supplemented with Halt Protease Inhibitor Cocktail (Pierce) at 10μl/ml. For P-ERK analysis, total cell lysates also contained 2mM Na3VO4. Extracts of IL-7-expanded pro-B/pre-B cells were denatured by boiling for 4 min in sample buffer, subjected to reducing conditions, and electrophoresed using SDS-PAGE 4-12% polyacrylamide gels for 50 minutes at 200V. Proteins were run out on gels, then transferred onto nitrocellulose membranes for 90 minutes at 100V. Nonspecific sites were blocked by incubation of the membranes with PBS-Tween 20 (1× PBS/0.05% Tween 20) containing 10% milk for 2 hrs at room temperature. Membranes were incubated as required with purified mouse monoclonal anti-E47 (G127-32) (BD Bioscience, La Jolla, CA); mouse monoclonal anti-actin (C-2), goat polyclonal anti-Ubc9 (N-15) (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal antibody anti-phospho- p44/42 MAPK (197G2) (Cell Signaling Technology, Danvers, MA). Following overnight incubation with the primary antibody, immunoblots were incubated with the appropriate HRP-labeled secondary antibodies for 2 h at room temperature, developed by enzyme chemiluminescence, and analyzed via an Alpha Innotech FluorChem Gel-doc system (San Leandro, CA). For quantitation, in each experiment individual blots were scanned and analyzed for desired proteins using densitometry. Densitometry values for desired proteins were normalized to that of the loading controls Ubc9 or actin. In some experiments, normalized values of experimental proteins (e.g., E47) were expressed relative to wild-type controls (set to 100% or 1).
Cell staining for flow cytometry and fluorescence activated cell sorting
For distinguishing B cell precursors, mouse bone marrow cells harvested ex vivo were stained with fluorescent antibodies for surface B220 (RA3-6B2) and CD19 (1D3) (BD Bioscience). For distinguishing plasmacytoid dendritic cells and NK-associated cells, bone marrow cells were stained for B220 (RA3-6B2), CD19 (1D3), CD11c (Integrin aX), anti-mouse pan NK marker (DX5) (e-Bioscience, San Diego, CA) and/or Ly6C (AL-21) (BD Biosciences, La Jolla, CA).
Bone marrow cells isolated from tibia and femur pairs of young and aged mice and were sorted for NK, plasmacytoid DC (pDC) and total DCs. Cells were stained for surface CD19, CD11c, CD3e, and/or DX5 (BD Biosciences and e-Biosciences) and sorted for CD19+ (B-lineage) cells, CD19- DX5+ or CD19- B220+ CD3- DX5+ (NK cells), CD19- DX5- B220+ CD11c+ (plasmacytoid DCs), or CD19- DX5- CD11c+ (total DCs) by fluorescence flow cytometry using a FACS Aria cell sorter (BD Immunocytometry, San Jose, CA) with purity ranging between 96-99%.
Pro-B and pre-B cell populations were identified based on differential staining for CD19, B220, IgM, CD43, CD2, and AA4.1 as previously described (Alter-Wolf, et. al., 2009; King, et. al., 2007). Levels of cytoplasmic λ5 and E47 proteins were determined by staining as previously described (Alter-Wolf, et. al., 2009). In some experiments, live cells only were analyzed by gating out cells that stained positive with Annexin V.
Depletion of NK cells in vivo
Both young adult and aged BALB/c mice were treated with rabbit anti-asialo GM1 antibody (Wako Chemicals, Richmond, VA) to deplete NK cells or with control rabbit antibody. In brief, mice were administered 200μg of antibody in PBS i.p. on each of 5 successive days. Two days later (day 7), mice were sacrificed and assayed for NK cells, B lineage precursors, and E47 and λ5 protein expression as described above.
Acknowledgments
We thank all members of the Blomberg and Riley laboratories for their assistance in this project. We express our appreciation to the Flow Cytometry Core Facility for assistance in both cell analysis and cell sorting.
Footnotes
Supported by NIH grants AG025256 and AI064591 to RLR and AG17618 and AG23717 to BBB.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alba A, Planas R, Clemete X, Carrillo J, Ampudia R, Puertas M, Pastor X, Tolosa E, Pujol-Borrell R, Verdaquer J, Vives-Pi M. Natural killer cells are required for accelerated type 1 diabetes driven by interferon-beta. Clin Exp Immunol. 2008;151:467–475. doi: 10.1111/j.1365-2249.2007.03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter-Wolf S, Blomberg BB, Riley RL. Deviation of the B cell pathway in senescent mice is associated with reduced surrogate light chain expression and altered immature B cell generation, phenotype, and light chain expression. J Immunol. 2009;182:138–147. doi: 10.4049/jimmunol.182.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M, Urbanek P. The role of BSAP (Pax-5) in B-cell development. Curr Opin Genet Dev. 1995;5:595–601. doi: 10.1016/0959-437x(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Knopf MA, Shankar G, McGillis JP. Calcitonin gene-related peptide indirectly inhibits IL-7 responses in pre-B cells by induction of IL-6 and TNF-[alpha] in bone marrow. Cell Immunol. 2003;226:67–77. doi: 10.1016/j.cellimm.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Frasca D, Nguyen D, Riley RL, Blomberg BB. Decreased E12 and/or E47 transcription factor activity in the bone marrow as well as in the spleen of aged mice. J Immunol. 2003;170:719–726. doi: 10.4049/jimmunol.170.2.719. [DOI] [PubMed] [Google Scholar]
- Gao N, Dang T, Dunnick WA, Collins JT, Blazar BR, Yuan D. Receptors and counterreceptors involved in NK-B cell interactions. J Immunol. 2005;174:4113–4119. doi: 10.4049/jimmunol.174.7.4113. [DOI] [PubMed] [Google Scholar]
- George T, Yu YY, Liu J, Davenport C, Lemieux S, Stoneman E, Mathew PA, Kumar V, Bennett M. Allorecognition by murine natural killer cells: lysis of T-lymphoblasts and rejection of bone-marrow grafts. Immunol Rev. 1997;155:29–40. doi: 10.1111/j.1600-065x.1997.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Guerrattaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci USA. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR. B-cell commitment: deciding on the players. Curr Opin Immunol. 2003;15:158–65. doi: 10.1016/s0952-7915(03)00012-8. [DOI] [PubMed] [Google Scholar]
- Kee BL, Murre C. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J Exp Med. 1998;188:699–713. doi: 10.1084/jem.188.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee BL, Quong MW, Murre C. E2A proteins: essential regulators at multiple stages of B-cell development. Immunol Rev. 2000;175:138–149. [PubMed] [Google Scholar]
- King AM, Van der Put E, Blomberg BB, Riley RL. Accelerated Notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased Erk MAP kinase activation. J Immunol. 2007;178:3521–3529. doi: 10.4049/jimmunol.178.6.3521. [DOI] [PubMed] [Google Scholar]
- Kumar V, George T, Yu YY, Liu J, Bennett M. Role of murine NK cells and their receptors in hybrid resistance. Curr Opin Immunol. 1997;9:52–56. doi: 10.1016/s0952-7915(97)80158-6. [DOI] [PubMed] [Google Scholar]
- Labrie J, III, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie J, III, Borghesi L, Gerstein RM. Bone marrow microenvironmental changes in aged mice compromise V(D)J recombinase activity and B cell generation. Sem Immunol. 2005;17:347–355. doi: 10.1016/j.smim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Ma S, Pathak S, Trinh L, Lu R. Interferon regulatory factors 4 and 8 induce the expression of Ikaros and Aiolos to down-regulate pre-B-cell receptor and promote cell-cycle withdrawal in pre-B-cell development. Blood. 2008;111:1396–1403. doi: 10.1182/blood-2007-08-110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L, Xu M, Vladimirova A, Sun XH. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO. 2003;21:5780–5792. doi: 10.1093/emboj/cdg567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Riordan M, Grosschedl R. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity. 1999;11:21–31. doi: 10.1016/s1074-7613(00)80078-3. [DOI] [PubMed] [Google Scholar]
- Quong M, Martensson A, Langerak AW, Rivera RR, Nemazee D, Murre C. Receptor editing and marginal zone B cell development are regulated by the helix-loop-helix protein, E2A. J Exp Med. 2004;199:1101–1112. doi: 10.1084/jem.20031180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Müller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- Riley RL, Kruger MG, Elia J. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin Immunol Immunopathol. 1991;59:301–13. doi: 10.1016/0090-1229(91)90026-7. [DOI] [PubMed] [Google Scholar]
- Rolink A, Boekel ten E, Melchers F, Fearon DT, Krop I, Andersson J. A subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors. J Exp Med. 1996;183:187–194. doi: 10.1084/jem.183.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Bryder D, Zahn J, Ahlenius H, Sonu R, Wagers A, Weissman I. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandel PC, Gendelman M, Kelsoe G, Monroe JG. Definition of a novel cellular constituent of the bone marrow that regulates the response of immature B cells to B cell antigen receptor engagement. J Immunol. 2001;166:5935–5944. doi: 10.4049/jimmunol.166.10.5935. [DOI] [PubMed] [Google Scholar]
- Sedger LM, Hou S, Osvath SR, Glaccum MB, Peschon JJ, van Rooijen N, Hyland L. Bone marrow B cell apoptosis during in vivo influenza virus infection requires TNF-α and lymphotoxin-α. J Immunol. 2002;169:6193–6201. doi: 10.4049/jimmunol.169.11.6193. [DOI] [PubMed] [Google Scholar]
- Sherwood EM, Blomberg BB, Xu W, Warner CA, Riley RL. Senescent BALB/c mice exhibit decreased expression of lambda 5 surrogate light chains and reduced development within the Pre-B cell compartment. J Immunol. 1998;161:4472–4475. [PubMed] [Google Scholar]
- Sherwood EM, Xu W, King AM, Blomberg BB, Riley RL. The reduced expression of surrogate light chain in B cell precursors from senescent BALB/c mice is associated with decreased E2A proteins. Mech Ageing Dev. 2000;118:45–59. doi: 10.1016/s0047-6374(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Sigvardsson M, O'Riordan M, Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25–36. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136:1435–1441. [PubMed] [Google Scholar]
- Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice. Decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- Ten Boekel E, Melchers F, Rolink AG. Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- Van der Put E, Frasca D, Blomberg BB, Riley RL. Aged mice exhibit distinct B cell precursor phenotypes differing in activation, proliferation, and apoptosis. Exp Gerontol. 2003;38:1137–1147. doi: 10.1016/j.exger.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Van der Put E, Frasca D, King AM, Blomberg BB, Riley RL. Decreased E47 in senescent B cell precursors is stage-specific and regulated post-translationally by protein turnover. J Immunol. 2004;173:818–827. doi: 10.4049/jimmunol.173.2.818. [DOI] [PubMed] [Google Scholar]
- Wiltrout RH, Santoni A, Peterson ES, Knott DC, Overton WR, Herberman RB, Holden HT. Reactivity of anti-asialo GM1 serum with tumoricidal and non-tumoricidal mouse macrophages. J Leukoc Biol. 1985;37:597–614. doi: 10.1002/jlb.37.5.597. [DOI] [PubMed] [Google Scholar]
- Winkler-Pickett R, Young HA, Cherry JM, Diehl J, Wine J, Back T, Bere WE, Mason AT, Ortaldo JR. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180:4495–4506. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- Vosshenrich C, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor γ-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- Vosshenrich C, Lesjean-Pottier S, Hasan M, Richard-Le Goff O, Corcuff E, Mandelboim O, Di Santo J. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welner R, Pelayo R, Garrett K, Chen X, Perry S, Sun XH, Kee B, Kincade P. Interferon-producing killer dendritic cells (IKDCs) arise via a unique differentiation pathway from primitive c-kitHiCD62L+ lymphoid progenitors. Blood. 2007;109:4825–4931. doi: 10.1182/blood-2006-08-043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte PL, Frantsve LM, Hergott M, Rahbe SM. Cytokine production and heterogeneity of primary stromal cells that support B lymphopoiesis. Eur J Immunol. 1993;23:1809–1817. doi: 10.1002/eji.1830230812. [DOI] [PubMed] [Google Scholar]
- Witte PL, Robinson M, Henley A, Low M, Stiers D, Perkins S, Fleishman R, Kincade P. Relationships between B-lineage lymphocytes and stromal cells in long-term bone marrow cultures. Eur J Immunol. 1987;17:1473–84. doi: 10.1002/eji.1830171014. [DOI] [PubMed] [Google Scholar]
- Yang JC, Mule JJ, Rosenberg SA. Murine lymphokine-activated killer (LAK) cells: phenotypic characterization of the precursor and effector cells. J Immunol. 1986;137:715–722. [PubMed] [Google Scholar]
- Yuan D. Interactions between NK cells and B lymphocytes. Adv Immunol. 2004;84:1–42. doi: 10.1016/S0065-2776(04)84001-X. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Soriano P, Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Cheng P, Weintraub H. B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2, and HEB. Mol Cell Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]


