Abstract
Kruppel-like factor 2 (KLF2) is a central regulator of endothelial and monocyte/macrophage gene expression and function in vitro. While the composite effects of KLF2 in these two cell types predict that it likely inhibits vascular inflammation, the role of KLF2 in this process in vivo is uncharacterized. In this study, we provide evidence that hemizygous deficiency of KLF2 increased diet-induced atherosclerosis in apolipoprotein E (ApoE) deficient mice. Our studies highlight an important role for KLF2 in primary macrophage foam cell formation via the potential regulation of the key lipid binding protein adipocyte Protein 2 (aP2)/fatty-acid binding protein 4 (FABP4). These novel observations establish that KLF2 is an atheroprotective factor.
Keywords: atherosclerosis, Kruppel-like factor 2, endothelium, macrophage, adipocyte Protein 2
Introduction
Kruppel-like factors (KLFs) are members of the zinc finger family of transcription factors that have been implicated in the regulation of cellular growth and differentiation. KLF2 is a key regulator of endothelial and monocyte/macrophage pro-inflammatory action 1, 2. In endothelial cells, KLF2 expression is induced by laminar shear stress and inhibited by pro-inflammatory cytokines. Sustained overexpression of KLF2 results in marked induction of endothelial nitric oxide synthase (eNOS) and thrombomodulin (TM) expression while reducing cytokine-mediated activation of proinflammatory genes such as vascular cell adhesion molecule-1 (VCAM-1). Similarly, previous studies indicate that KLF2 inhibits monocyte pro-inflammatory gene expression and phagocytosis. Importantly, KLF2 expression in peripheral blood monocytes of patients with established atherosclerotic disease is reduced by ~ 30% 2. On the basis of these observations KLF2 has been viewed as a candidate atheroprotective factor. However, in vivo evidence in support of this hypothesis has been lacking.
Herein, we provide evidence that hemizygous deficiency of KLF2 significantly increases diet-induced atherosclerotic lesion formation in ApoE-deficient (ApoE−/−) mice. Our results also underscore the importance of KLF2 in macrophage lipid uptake and foam cell formation via effects on the lipid binding protein aP2/FABP4.
Materials and Methods
Animals and diets
KLF2+/− mice (generously provided by J. Leiden) were generated as previously described 3. KLF2+/− mice of mixed background (calculated to be ~75% C57Bl/6) were mated to ApoE−/− mice 4 on the C57Bl/6 background (Jackson Laboratories) to generate KLF2+/−/ApoE−/− and KLF2+/+/ApoE−/− mice. All studies were performed with strict age-matched, sex-matched, littermate controls. Mice were weaned at 4 weeks, fed a normal rodent chow diet (4.5% fat by weight, 14% kcal, Lab Diet P3000), and at 6–8 weeks of age were initiated on a high fat (12.5% by weight, 40% kcal), high cholesterol (1.25% by weight) diet (Research Diets D-12108) for a total of 20 weeks. Animal care and procedures were performed according to the NIH guidelines.
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Results
Enhanced atherosclerotic lesion formation in ApoE-deficient mice
Previous studies demonstrate that systemic knockout of KLF2 is embryonic lethal 3, 5. However, hemizygous deficient (KLF2+/−) mice are viable and fertile. KLF2+/− mice were crossed to ApoE−/− mice to generate KLF2+/−/ApoE−/− and KLF2+/+/ApoE−/− mice. At six weeks of age, male mice (n=12–14 per group) were initiated on a high fat, high cholesterol diet for 20 weeks. Plasma lipid analysis reveals that there was no significant difference in lipid profiles between mice at baseline (data not shown) or after an atherogenic diet (Online Table I).
After 20 weeks of an atherogenic diet mice were sacrificed and aortas harvested for analysis of atherosclerotic lesion area by Sudan IV staining. KLF2+/−/ApoE−/− mice exhibited a significant 31% increase in atherosclerotic lesion area when compared to KLF2+/+/ApoE−/− littermate controls (35.9±10.2% vs. 27.3±9.9%; p=0.04) (Figure 1A–B). Similarly, quantitative analysis using an en face preparation revealed a 37% increase in atherosclerotic lesion area (24.0±6.3% vs. 17.6±5.4%; p=0.0099) (Figure 1C–D).
Figure 1.
KLF2 heterozygous mice develop more atherosclerosis. Male littermate ApoE−/− and KLF2+/−/ApoE−/− mice at 6 weeks of age were fed a high fat, high cholesterol diet for 20 weeks. Aortas were harvested and Sudan IV stained for lipid. A, Two representative pairs of fixed and stained whole aortas. B, Percent lesion area of the entire isolated aorta area was calculated. ApoE−/− 27.3±9.9% (n=12), and KLF2+/−/ApoE−/− 35.9±10.2% (n=14). C, Two representative pairs of fixed and stained aortas en face. D, Percent lesion area of the entire aorta surface area was calculated. ApoE−/− 17.6±5.4% (n=12), and KLF2+/−/ApoE−/− 24±6.3% (n=14). K2+/− = KLF2+/−.
Effect of KLF2 deficiency on endothelial gene expression
KLF2 is known to induce eNOS and inhibit VCAM-1 expression in cultured endothelial cells 1. However, analysis of aortic sections demonstrated no appreciable difference in the expression of endothelial eNOS or VCAM-1 (Online Figure IA–B). Consistent with this result, quantitative PCR analysis for eNOS, TM, and VCAM-1 revealed no significant change in KLF2+/− mice compared to littermate controls (data not shown).
A recent study indicates that the Kruppel family member KLF4 is capable of conferring gene regulatory effects similar to KLF2 in endothelial cells 6. Therefore, we reasoned that a compensatory increase in KLF4 in KLF2+/− mice might account, in part, for the absence of any clear effect on the KLF2 endothelial targets assessed. Indeed, tissues from KLF2+/− mice demonstrated a 39±4% increase in KLF4 expression (p=0.0033) (Figure 2A).
Figure 2.
Effect of KLF2 deficiency on endothelial gene expression and macrophage recruitment. A, QPCR analysis was performed on RNA from isolated lung tissue harvested from KLF2+/− and wild-type littermate control mice, (n=3). Expression levels in KLF2+/− mice (K2 0.44±0.08, K4 1.39±0.04) were normalized to wild-type. B, Aortic arch sections of male littermate ApoE−/− and KLF2+/−/ApoE−/− mice fed an atherogenic diet for 20 weeks were stained for Mac-3 (n=8). Representative slides are shown. C, Mac-3 staining was quantified as percent staining area of the entire lesion area. KLF2+/−/ApoE−/− 3.13±1.70%, ApoE−/− 2.32±1.16%, (n=9).
Enhanced lipid uptake and aP2/FABP4 expression in KLF2 hemizygous deficient macrophages
We next sought to assess the effect of KLF2 hemizygosity on macrophage recruitment and function. Immunohistochemical assessment using the macrophage specific antibody Mac-3 revealed a non-significant trend towards increased macrophage area staining in the aortic arch of KLF2+/−ApoE−/− mice (3.13±1.70% vs. 2.32±1.16%; p=0.176) (Figure 2B–C). Thus, while KLF2 deficiency leads to increased atherosclerotic burden (Figure 1), this cannot be explained simply by an increase in macrophage number.
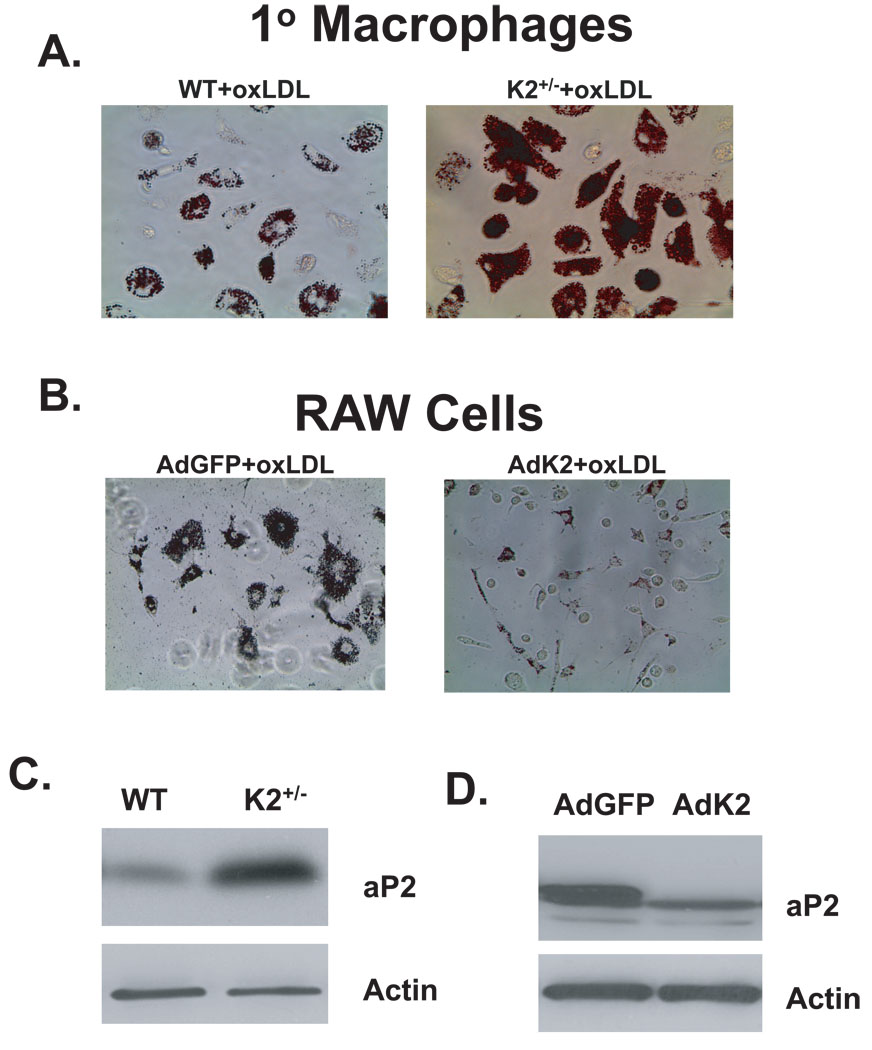
Because previous studies indicated that KLF2 inhibits phagocytosis 2, we reasoned that enhanced lipid uptake in macrophages may account for the increase in atherosclerosis. Remarkably, peritoneal macrophages from KLF2+/− mice treated with oxidized LDL demonstrated a 40% increase in lipid uptake when compared to wild-type macrophages (Figure 3A and Online Figure IIA). Conversely, adenoviral overexpression of KLF2 in a mouse macrophage cell line (RAW 264.7 cells) strongly inhibited oxidized LDL uptake (Figure 3B and Online Figure IIB).
Figure 3.
KLF2 regulates lipid uptake and macrophage aP2 levels. A and B, Peritoneal macrophages isolated from KLF2+/− and male wild-type littermate control mice (A) or RAW cells adenovirally infected with KLF2 (AdK2) or control virus (AdGFP) (B) were treated with oxidized LDL (oxLDL) (50µg/mL) for 72hours and stained for lipid uptake with oil red O. Representative results are shown. C and D, Whole cell lysates were harvested from isolated peritoneal macrophages (n=4) (C) or RAW cells (n=12) (D) and assessed for aP2 levels by western blotting. A representative blot is shown.
While lipid accumulation within cells is a complex process, recent studies support a key role for the lipid chaperone aP2/FABP4 in macrophage lipid accumulation and atherogenesis 7, 8. Indeed, KLF2+/− macrophages expressed higher protein levels of aP2 (Figure 3C). Conversely, overexpression of KLF2 in RAW cells strongly inhibited aP2 expression (Figure 3D). These results are specific as the expression of other factors involved in lipid accumulation such as CD36 were unaffected (data not shown). Taken together, these results indicate that reduced lipid uptake in KLF2+/− macrophages is mediated in part by KLF2’s regulation of aP2.
Discussion
Atherosclerosis is an extremely complex disease process with a number of important cellular contributors including endothelial cells, smooth muscle cells, and immune cells (monocyte and T cells). Because KLF2 has been shown to play a particularly potent role in inhibiting endothelial cell and monocyte/macrophage activation, we focused our mechanistic studies on these two cell types.
The key observation highlighted by our study relates to the role of KLF2 in monocyte/macrophage biology. Previously, Das and colleagues reported that sustained expression of KLF2 inhibited monocyte activation and phagocytic capacity in vitro 2. In addition, Wu and colleagues have shown that KLF2−/− MEFs exhibit accelerated lipid accumulation upon adipocyte differentiation 9. Consistent with these observations, KLF2+/− macrophages exhibit enhanced lipid accumulation (Figure 3) and increased pro-inflammatory genes (G.H. Mahabeleshwar and M.K. Jain, unpublished observation). Thus, while absolute numbers of macrophages in atherosclerotic lesions of KLF2+/+/ApoE−/− and KLF2+/−/ApoE−/− mice were not significantly different, the increased lipid uptake could account for the increase in lesion size. Mechanistically, our studies identify the lipid binding protein aP2 as a KLF2 target. Work from the Hotamsigil laboratory and others have underscored the importance of aP2 in atherogenesis. Systemic and macrophage aP2 deficiency renders mice resistant to atherosclerosis 7, 8. Furthermore, aP2-deficient macrophages exhibited alterations in their ability to express inflammatory cytokines and had reduced ability to uptake lipid when stimulated by modified lipids 7. Given the results in KLF2+/− macrophages (Figure 3), it is likely that enhanced aP2 expression contributes to the increase in lipid accumulation in KLF2+/− macrophages. However, one cannot rule out the possibility that effects on other aspects of lipid metabolism (e.g. uptake or export) in macrophages or alterations in aP2 expression in other tissues (e.g. adipose) may also impact on the observed phenotype.
With respect to KLF2 effects in endothelial cells, our studies provide some important insights. Despite previous in vitro observations, we did not observe any significant effects on the expression of KLF2 targets, including eNOS and VCAM-1. Importantly, however, our findings highlight a key compensatory role for KLF4. Like KLF2, recent studies reveal that KLF4 can also induce eNOS and inhibit VCAM-1 6. Thus, the increase in KLF4 observed may compensate for KLF2 deficiency. This is an interesting finding that raises the importance of future studies to assess the effect of KLF4 or dual KLF2/KLF4 hemizygosity on atherosclerosis. Additionally, we note the possibility that other endothelial factor(s) not assessed in this study may be differentially regulated in a non-redundant fashion by KLF2 and contribute to the phenotype observed. These considerations as well as generation of inducible tissue specific KLF2 knockouts are the subject of ongoing investigation.
In sum these observations are the first in vivo evidence in support of the hypothesis that KLF2 is atheroprotective. These findings are of particular interest as they suggest that a reduction of KLF2 expression at levels that approximate those observed in human subjects with coronary artery disease is biologically relevant. Manipulation of KLF2 expression may thus be beneficial in the treatment of vascular inflammatory disorders.
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL72952, HL75427, HL76754, HL086548, HL084154, and P01 HL48743 (to M.K.J.), HL085816 and HL057506 (to D.I.S.), HL088740 (to G.B.A.), American Heart Association grants 0635579T (to Z.L.), 0725297B (to D.K.), an Alliance for Cancer Gene Therapy grant (to M.K.J.), a United Negro College Fund/Merck Science Initiative Fellowship grant (to G.B.A.), and a Robert Wood Johnson/Harold Amos Medical Faculty Development grant (to G.B.A.).
Footnotes
Disclosures: None.
References
- 1.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 2.Das H, Kumar A, Lin Z, Patino WD, Hwang PM, Feinberg MW, Majumder PK, Jain MK. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A. 2006;103:6653–6658. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 5.Wani MA, Means RT, Jr, Lingrel JB. Loss of LKLF function results in embryonic lethality in mice. Transgenic Res. 1998;7:229–238. doi: 10.1023/a:1008809809843. [DOI] [PubMed] [Google Scholar]
- 6.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg M, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007 doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 7.Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22:1686–1691. doi: 10.1161/01.atv.0000033090.81345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Srinivasan SV, Neumann JC, Lingrel JB. The KLF2 transcription factor does not affect the formation of preadipocytes but inhibits their differentiation into adipocytes. Biochemistry. 2005;44:11098–11105. doi: 10.1021/bi050166i. [DOI] [PubMed] [Google Scholar]