Abstract
Diabetic neuropathic pain remains an unmet clinical problem and is poorly relieved by conventional analgesics. N-methyl-D-aspartate (NMDA) receptors play an important role in central sensitization in neuropathic pain. Although NMDA antagonists are highly effective in reducing neuropathic pain, these agents cause severe side effects at therapeutic doses, which limit their clinical uses. Neramexane and memantine are uncompetitive NMDA antagonists with minimal side effects at therapeutic doses. Here we determined the antinociceptive effect of chronic administration of neramexane and compared its effect with that of memantine and gabapentin in a rat model of diabetic neuropathic pain. Mechanical hyperalgesia was measured with a noxious pressure stimulus, and tactile allodynia was assessed with von Frey filaments in diabetic rats induced by streptozotocin. Compared with vehicle-treated rats, treatment with neramexane (12.3, 24.6, and 49.2 mg/kg/day) for 2 weeks via an osmotic minipump produced dose-dependent and sustained effects on mechanical hyperalgesia and allodynia. Administration of memantine (20 mg/kg/day) or gabapentin (50 mg/kg/day) for 2 weeks also produced significant and persistent antinociceptive effects on mechanical hyperalgesia and allodynia. The magnitude of the antinociceptive effect produced by the intermediate and high doses of neramexane was comparable to that of gabapentin and memantine. The plasma level achieved by neramexane at 12.3, 24.6, and 49.2 mg/kg/day was 0.26 ± 0.04, 0.50 ± 0.05, and 1.21 ± 0.16 μM, respectively. These data suggest that neramexane at therapeutically relevant doses attenuates diabetic neuropathic pain. Our study provides valuable information about the therapeutic potential of chronic administration of neramexane and memantine for painful diabetic neuropathy.
Introduction
Diabetic neuropathy is one of the most common causes of chronic neuropathic pain. The neuropathic pain symptoms are often intractable because they are poorly relieved by conventional analgesics (Brown and Asbury, 1984; Chen and Pan, 2003; Clark and Lee, 1995). Pain associated with diabetic neuropathy can occur spontaneously or as a result of exposure to mildly painful stimuli (hyperalgesia) or to stimuli not normally perceived as painful (allodynia). In addition to the changes of primary afferent nerves, central sensitization plays an important role (Chen and Pan, 2002; Daulhac et al., 2006; Khan et al., 2002; Wang et al., 2007). Although the cellular and molecular mechanisms underlying chronic pain in diabetic neuropathy are not fully known, increased glutamatergic input and N-methyl-D-aspartate (NMDA) receptor activity contribute importantly to central sensitization in painful diabetic neuropathy (Chen and Pan, 2002; Wang et al., 2007). Persistent over-stimulation of NMDA receptors is essential for the long-term plastic changes in the spinal dorsal horn and the development of diabetic neuropathic pain (Calcutt and Chaplan, 1997; Daulhac et al., 2006; Tomiyama et al., 2005). The NMDA receptor antagonists, such as ketamine and dextromethorphan, are effective in reducing various types of neuropathic pain symptoms in patients (Correll et al., 2004; Eide et al., 1995; Max et al., 1995; Sang et al., 2002). However, these agents also cause severe side effects at therapeutic doses including hallucinations, dysphoria, and impairment of cognitive and motor function, which limit their clinical uses (Cvrcek, 2008; Max et al., 1995; Sang et al., 2002). Thus, development of new NMDA antagonists with a reduced side-effect profile is much needed.
Neramexane and memantine are uncompetitive NMDA receptor antagonists with moderate affinity, strong voltage-dependency, and rapid unblocking kinetics, which could explain their minimal side effects at the doses within the therapeutic range (Danysz et al., 2002; Johnson and Kotermanski, 2006; Parsons et al., 1999a; Parsons et al., 1999b; Rogawski and Wenk, 2003). Both drugs are presently used clinically to treat Alzheimer's disease (Danysz et al., 2002). It has been shown that acute administration of memantine has a potent inhibitory effect on the hypersensitivity of spinal dorsal neurons in animal models of neuropathic pain (Carlton et al., 1998; Suzuki et al., 2001). Although neramexane is well tolerated in patients, its therapeutic actions on diabetic neuropathic pain are uncertain.
To determine the therapeutic effect of analgesics on chronic pain in animals, it is important to evaluate the actions of the agents administered at a constant rate for a prolonged period of time. This will allow for the assessment of how drug effects, at a clinically relevant dosage, change over time in the presence of ongoing pain. Therefore, the first aim of the present study was to investigate the dose-response effect of systemic chronic administration of neramexane on mechanical allodynia and hyperalgesia in a rat model of diabetic neuropathic pain. The anticonvulsant, gabapentin, has been effectively used to treat patients with chronic pain caused by diabetic neuropathy and postherpetic neuralgia (Backonja et al., 1998; Rowbotham et al., 1998) and is commonly used as a comparator in animal studies in which the efficacy of new compounds are evaluated for the treatment of chronic pain. The second aim of this study was to compare the antinociceptive effect of neramexane on diabetic neuropathic pain with that produced by memantine and gabapentin.
Materials and Methods
Animal model of diabetic neuropathic pain
Male rats (Harlan Sprague-Dawley) weighing 225 to 250 g were used in this study. The surgical preparation and experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas M. D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals. All efforts were made to minimize both the suffering and number of animals used. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ, 50 mg/kg; Sigma, St. Louis, MO) freshly dissolved in 0.9% sterile saline. Five days later, diabetes was confirmed by measuring the glucose level (> 350 mg/dl) in the blood obtained from the tail vein using Sigma diagnostic glucose reagents. Previous studies have demonstrated that after STZ injection, most rats display a reproducible mechanical allodynia and hyperalgesia within 3 weeks, lasting for at least 7 weeks (Chen et al., 2001; Chen and Pan, 2002; Courteix et al., 1993). The dose of STZ and the body weight of the rats before STZ treatment are two major factors to be considered when attempting to induce neuropathic pain but not profound illness in diabetic rats. Thus, the experimental conditions used to induce diabetes in the current study (STZ dose of 50 mg/kg; body weight of 225 - 250 g) were employed to minimize the effects of STZ administration on the overall health of the animal (Chen et al., 2001; Chen and Pan, 2002; Chen and Pan, 2003). This model of neuropathic pain mimics the symptoms of neuropathy in diabetic patients, with alterations in pain sensitivity and poor responses to μ opioid administered systemically or intrathecally (Chen and Pan, 2002; Malcangio and Tomlinson, 1998; Zurek et al., 2001). Only diabetic rats with evident hyperalgesia and allodynia were included in the study.
Implantation of osmotic minipumps
Alzet osmotic minipumps (model 2ML2; DURECT Corporation, Cupertino, CA) were used to administer drug solutions continuously at ∼5 μl/h for 14 days. The pumps were filled with the compound solution under sterile condition according to the manufacturer's instruction. They were implanted subcutaneously at the back under 2-3% isoflurane anesthesia 3 weeks after STZ injection. Briefly, a small incision was made in the skin (at the mid-scapular region). Using a blunt end scissor, a small pocket was formed by spreading apart the subcutaneous connective tissues. The pump was inserted into the pocket with the flow moderator pointing away from the incision site so that the delivery of the solution will have minimal interaction with the healing wound. The skin incision was then closed with stainless steel wound clips. After pump implantation, penicillin (200 mg/kg) was injected subcutaneously everyday for three days. The animals were carefully monitored each day over a treatment period of 2 weeks. To minimize the tissue damage at the site of pump implantation, the pump was gently moved around within the subcutaneous pocket every 2-3 days. Behavioral testing using the pressure stimulus and von Frey filaments was performed every 2-3 days after the pump implantation.
Behavioral test of mechanical hyperalgesia
The nociceptive mechanical withdrawal thresholds, expressed in grams, were measured with the Randall-Selitto test using Ugo Basil Analgesimeter (Varese, Italy). The test was performed by applying a noxious pressure to the hindpaw. By pressing a pedal that activated a motor, the force increased at a constant rate on the linear scale. When the animal displayed pain by withdrawal of the paw or vocalization, the pedal was immediately released and the nociceptive threshold read on the scale. The cutoff of 400 g was used to avoid potential tissue injury (Chen et al., 2001; Chen and Pan, 2003). Both hindpaws were tested in each rat, and the mean value was used as the withdrawal threshold in response to the noxious pressure. Motor function was also evaluated by testing the animals' ability to stand and ambulate in a normal posture and to place and step with the hindpaw (Chen et al., 2000). We assessed the motor function in a simple manner by grading the ambulation behavior of rats as the following: 2 = normal, 1 = limping, and 0 = paralyzed. All the behavioral tests were performed between 9 and 11 am on the test day.
Assessment of tactile allodynia
Rats were individually placed in suspended chambers on a mesh floor. After an acclimation period for 30 min, a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) were applied perpendicularly to the plantar surface of both hindpaws with sufficient force to bend the filament for 6 s. Brisk withdrawal or paw flinching was considered as a positive response. In the absence of a response, the filament of the next greater force was applied. Following a response, the filament of the next lower force was applied. The tactile stimulus producing a 50% likelihood of withdrawal response was calculated by using the “up-down” method, as described previously (Chaplan et al., 1994; Chen et al., 2000; Chen et al., 2001).
Determination of the drug plasma levels
Following the last behavioral measurement blood was collected by cardiac puncture, under isoflurane anesthesia, using heparinized syringes. The blood samples were centrifuged at 2,000 g at 4°C for 10 min, and the plasma transferred to Eppendorf tubes and stored at -70°C until bioassay. The osmotic pumps were also removed at the end of the experiment, and the residue drug solution within the pump was obtained to confirm the appropriate concentrations of active compounds. The compound concentrations in the plasma and the pumps were analyzed by using liquid chromatography-mass spectrometry (LC-MS) methods. Samples were extracted by liquid-liquid (neramexane and memantine) or solid-phase extraction (gabapentin) and analyzed by LC-MS/MS with a selected reaction monitoring positive ion mode (Danysz et al., 1994; Zoladz et al., 2006). Peak height ratios of the analyte to the respective internal standard were used for the quantification of the analytes in unknown samples. The assays for neramexane, memantine, and gabapentin were linear in the range of 10 to 5,000 ng/ml, 50 to 2,000 ng/ml, and 50 to 5,000 ng/ml for neramexane, memantine, and gabapentin, respectively. During the analysis of study samples, the accuracy of the standard and quality control samples for neramexane, memantine and gabapentin were within ± 9.7%, ± 1.7%, and ± 14.4%, respectively.
Drugs and treatment groups
Neramexane mesylate (1-amino-1,3,3,5,5-pentamethyl-clohexane; Forest Research Institute, Farmingdale, NY) and gabapentin (1-(aminomethyl)cyclohexaneacetic acid; Spectrum Chemicals, New Brunswick, NJ) were dissolved in 0.9% sterile saline. Memantine HCl (1-amino-3,5-dimethyl-adamantane; Forest Research Institute, Farmingdale, NY) was prepared in water because the solubility of memantine was limited in 0.9% saline. Drug treatment groups include neramexane (12.3, 24.6, and 49.2 mg/kg/day), gabapentin (50 mg/kg/day), and memantine (20 mg/kg/day). The doses of neramexane and memantine chosen for this study were determined in pilot experiments and previous studies using nondiabetic rats (Danysz et al., 2002; Hesselink et al., 1999b; Zoladz et al., 2006). The dose of gabapentin selected for this study is sufficient to express its representative pharmacological activity produced using minipump administration in normal rats (Bruins Slot et al., 2002). In the preliminary study, we found that using 0.9% saline or water in the minipump had no significant effects on allodynia and hyperalgesia in diabetic rats. Thus, we reported only data using saline as the vehicle control in this study.
Statistical analysis
All the data are presented as means ± SEM. The effect of STZ treatment on the paw withdrawal threshold was determined using a paired t-test. The area under the behavioral response curve (AUC; calculated from the baseline to treatment day 14) was determined and used to compare the antinociceptive effect of each drug with the vehicle group. The data were first grouped into 3 families and pair-wise comparisons made (1) between the neramexane treatment groups, (2) between the neramexane and the other active treatment groups, and (3) between each drug treatment group and the vehicle (saline) control group. A nonparametric statistical method (Wilcoxon rank-sum test) was used for the between-group comparisons. The effect of each drug treatment on the paw withdrawal threshold on each test day was also compared with the respective baseline by using repeated measures analysis of variance followed by Dunnett's post hoc test. P < 0.05 was considered to be statistically significant.
Results
Most rats developed hyperglycemia (blood glucose levels greater than 350 mg/dl) and other clinical diabetic symptoms within 5 days of a single injection of STZ. The diabetic rats exhibited a robust reduction in the paw withdrawal thresholds in response to application of von Frey filaments and a noxious pressure stimulus (tactile allodynia and mechanical hyperalgesia, respectively) three weeks after the injection of STZ. There were no significant differences in the reduction of the paw pressure and tactile thresholds among the 6 experimental groups (Figure 1). Rats (n = 12) that did not exhibit hyperglycemia or an increase in the response to tactile and mechanical stimuli after STZ treatment were not included in the experiments. All the diabetic rats remained relatively healthy, although their growth rate was reduced (body weight gain: ∼5 g/week in the diabetic group vs. ∼20 g/week in the nondiabetic group).
Figure 1.
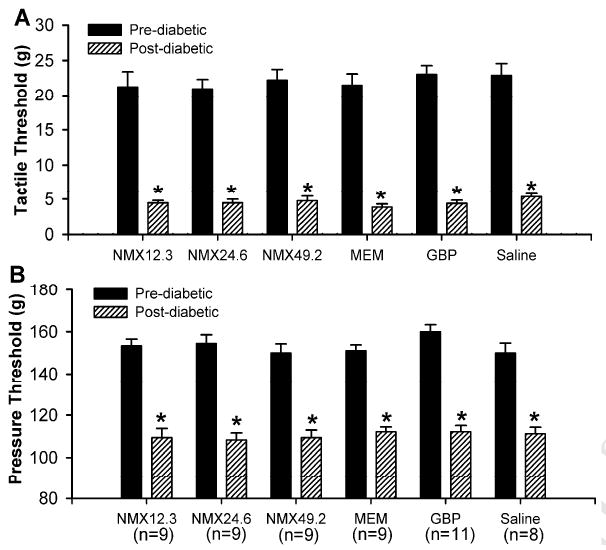
Changes in the paw withdrawal thresholds in diabetic rats in 6 experimental groups relative to pre-diabetic control values. A, reduction in the tactile threshold of the hindpaw in response to von Frey filaments. B, reduction in the nociceptive threshold of the hindpaw in response to a noxious stimulus. * P < 0.05 compared with respective control values. NMX, neramexane; MEM, memantine; GBP, gabapentin.
Drug effects on tactile allodynia in diabetic rats
Administration of neramexane (12.3, 24.6, and 49.2 mg/kg/day) for 2 weeks increased the tactile withdrawal threshold in a dose-dependent manner (Figure 2). Each escalating dose produced a significantly greater antiallodynic effect over the duration of treatment (P < 0.005). Compared with the respective baseline withdrawal threshold, a significant effect of neramexane was observed by day 3 of treatment (the earliest time point tested after the start of drug administration), and this effect remained over the entire treatment duration (Figure 2). Comparisons of the areas under the time-response curve (AUC, baseline – treatment day 15) revealed a significant increase in the paw withdrawal threshold for each drug dose compared with the vehicle group (n = 8 rats). The low dose of neramexane (12.3 mg/kg/day, n = 9) produced a small, but significant, antiallodynic effect. Administration of higher doses of neramexane (24.6 and 49.2 mg/kg/day, n = 9 in each group) resulted in a much greater increase in the paw withdrawal threshold to tactile stimuli compared with the vehicle group (P < 0.001). However, treatment with the vehicle had no significant effect on the paw withdrawal threshold, measured with von Frey filaments.
Figure 2.

Time course of the effects of treatment with different doses of neramexane (12.3, 24.6, and 49.2 mg/kg/day, n = 9 in each group), memantine (20 mg/kg/day, n = 9), gabapentin (50 mg/kg/day, n = 11), and saline (n = 8) for 2 weeks on the tactile threshold in diabetic rats. The tactile threshold was tested by application of von Frey filaments to the hindpaw. * P < 0.05 compared with respective baseline values. NMX, neramexane; MEM, memantine; GBP, gabapentin.
Continuous administration of memantine (20 mg/kg/day, n = 9) for 2 weeks also produced a significant effect on the tactile withdrawal threshold compared with the baseline and the vehicle group. This effect became evident within 3 days after drug administration and lasted during the duration of treatment. There were no significant differences in the effect on the tactile threshold between memantine and higher doses of neramexane (24.6 and 49.2 mg/kg/day) or gabapentin (50 mg/kg/day, n = 11) over the 2-week treatment period. While treatment with memantine produced an effect that was numerically greater than that of an equimolar dose of neramexane (24.6 mg/kg/day), the AUC over the treatment period indicated that this difference was not significant (P = 0.07). In general, gabapentin (50 mg/kg/day, the positive control group) produced a similar antiallodynic effect compared with that produced by the medium and high doses of neramexane (24.6 and 49.2 mg/kg/day, respectively). Notably, in all treatment groups, the drug effects were somewhat reduced toward the end of the experiments.
Drug effects on mechanical hyperalgesia in diabetic rats
Similar to the effects of drug treatment observed for tactile allodynia, administration of neramexane (12.3, 24.6, and 49.2 mg/kg/day) increased the pressure withdrawal threshold in a dose-dependent and long-lasting manner, compared with the baselines (Figure 3). Pair-wise comparisons of the AUCs for the behavioral response curves over the duration of the treatment period (baseline to treatment day 15) revealed a significant effect for all drug treatment groups versus the vehicle group (P < 0.005).
Figure 3.
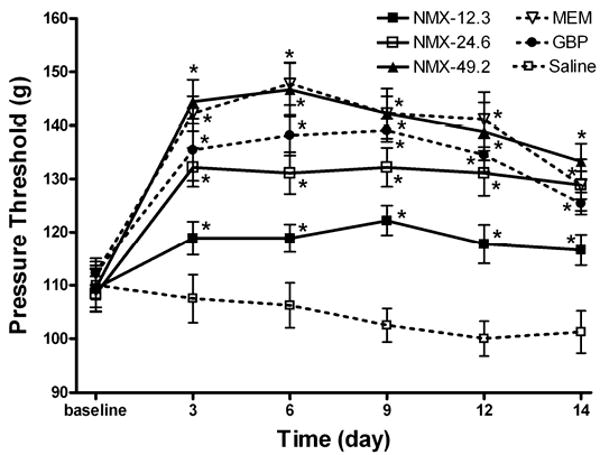
Time course of the effects of treatment with different doses of neramexane (12.3, 24.6, and 49.2 mg/kg/day, n = 9 in each group), memantine (20 mg/kg/day, n = 9), gabapentin (50 mg/kg/day, n = 11) and saline (n = 8) for 2 weeks on the mechanical nociceptive threshold in diabetic rats. The nociceptive threshold was tested by application of a noxious pressure stimulus to the hindpaw. * P < 0.05 compared with respective baseline values. NMX, neramexane; MEM, memantine; GBP, gabapentin.
Treatment with memantine (20 mg/kg/day) also produced a significant effect on the pressure withdrawal threshold that was significantly greater than the low dose of neramexane (12.3 mg/kg/day). However, the magnitude of the effect produced by memantine was not statistically different than that produced by an equimolar dose of neramexane (24.6 mg/kg/day). Furthermore, gabapentin (50 mg/kg/day) produced an effect comparable to that produced by the medium and high doses of neramexane (24.6 and 49.2 mg/kg/day, respectively) and by memantine (Figure 3).
The motor function, based on the placing/stepping reflex and the righting reflex, appeared normal in all treatment groups. All rats tested received a score of 0 during the course of drug treatments.
Drug plasma levels after chronic treatment using minipumps
Subcutaneous administration of neramexane at doses of 12.3, 24.6, and 49.2 mg/kg/day for 2 weeks produced dose-proportional steady-state plasma levels, measured on the last day of treatment (Figure 4). Notably, administration of equimolar doses of neramexane (24.6 mg/kg/day) and memantine (20 mg/kg/day) produced comparable plasma level. The desired drug concentrations were confirmed using the residue drug solutions removed from the pump (data not shown).
Figure 4.
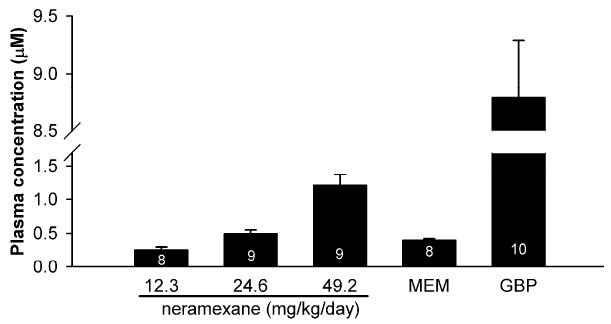
Plasma levels measured after the last behavioral test following a 2 week subcutaneous administration (minipump) of neramexane (12.3, 24.6, and 49.2 mg/kg/day), memantine (MEM, 20 mg/kg/day), and gabapentin (GBP, 50 mg/kg/day) in diabetic rats. Numbers in the bar column denote the number of animals from which blood samples were taken and the plasma levels measured were above the Limit of Quantification.
Discussion
Our study provides valuable information about the therapeutic potential of neramexane and memantine, two clinically tolerated, low-affinity NMDA receptor antagonists for the treatment of diabetic neuropathic pain. Although NMDA receptor antagonists are clearly effective in the control of chronic pain in patients (Correll et al., 2004; Cvrcek, 2008; Fisher et al., 2000; Max et al., 1995; Sang et al., 2002), unwanted side effects have limited the clinical use of this class of drugs. Neramexane belongs to a group of uncompetitive NMDA receptors known as the amino-alkyl-cyclohexanes (Danysz et al., 2002) and has a marked analgesic effect on acute pain evoked by intradermal capsaicin injection in humans (Klein et al., 2008). Like memantine, neramexane exhibits moderate affinity, fast blocking/unblocking kinetics, and strong voltage dependence at the NMDA receptor, attributes that are thought to contribute to the drug's efficacy and minimal side effect profile (Parsons et al., 1999a; Parsons et al., 1999b). Memantine has been approved for the treatment of moderate-to-severe Alzheimer's disease. It has also been widely used to treat Parkinson's disease, and dementia with minimal side effects at the therapeutic doses (Danysz et al., 2002; Ditzler, 1991; Gortelmeyer and Erbler, 1992; Kornhuber et al., 1994). It also has the best therapeutic indices against maximal electroshock-induced convulsions (Parsons et al., 1995) and enhances the long-term spatial memory in adult rats (Zoladz et al., 2006). Both neramexane and memantine act in an uncompetitive ‘use-dependent’ manner, meaning that they only blocks the channel in the open state (Foster and Wong, 1987; Huettner and Bean, 1988). Therefore, this class of antagonists may be particularly useful when over-activation of NMDA receptors, such as the state of diabetic neuropathic pain, are expected. In the present study, we found that neramexane produced a dose-dependent effect on both hyperalgesia and allodynia induced by diabetic neuropathy. Also, such effects were sustained during chronic systemic administration in rats with diabetic neuropathy. Thus, our data strongly suggest that neramexane has a potent therapeutic effect on painful diabetic neuropathy.
Although the mechanism of diabetic neuropathic pain is still unclear, the persistent increase in nociceptive glutamatergic input seems to play a key role (Daulhac et al., 2006; Wang et al., 2007). Also, blocking of NMDA receptors can reduce diabetic neuropathic pain in animal models and in patients (Amin and Sturrock, 2003; Begon et al., 2000; Calcutt and Chaplan, 1997; Carlsson et al., 2004). In diabetic neuropathy, increased levels of glutamate can act on NMDA receptors in the spinal dorsal horn, leading to neuronal hyperactivity and central sensitization (Chen and Pan, 2002; Wang et al., 2007). As a result, increased responsiveness of spinal dorsal horn neurons contributes to the reduction in pain threshold (allodynia) and amplification of pain responses (hyperalgesia). Under this condition, neramexane and memantine are expected to be more effective in blocking the NMDA receptors. We found that the magnitude of the antinociceptive effect of memantine (20 mg/kg/day) was numerically larger than that produced by an equimolar dose of neramexane (24.6 mg/kg/day) and comparable to the high dose of neramexane (49.2 mg/kg/day). These data suggest that memantine produces a greater antinociceptive effect compared to an equimolar dose of neramexane and a similar analgesic effect as a 2-fold larger dose of neramexane on an equimolar dose basis. The memantine dose tested in this study produced plasma levels that are comparable to those observed in humans administered 20 mg of memantine (Periclou et al., 2006). Gabapentin, at the dose used in this study, generally produced an antinociceptive response that was numerically less than that of memantine and the high dose of neramexane and comparable to the intermediate dose of neramexane. The plasma levels achieved in diabetic rats after a 2-week subcutaneous administration of neramexane and memantine in the current study were similar to those produced in normal rats following a 5-day treatment via minipumps (Hesselink et al., 1999a; Hesselink et al., 1999b), suggesting that drug levels were maintained throughout the entire treatment period.
Because the NMDA antagonists were administered systemically, the exact sites of drug effects on neuropathic pain in the central nervous system cannot be determined from the present study. However, it is likely that the site of the antinociceptive action of neramexane and memantine is at the spinal level. In support of this possibility, systemic treatment with memantine has a potent inhibitory effect on the hypersensitivity of spinal dorsal horn neurons induced by spinal nerve ligation of the in rats (Suzuki et al., 2001). Also, topical application of memantine to the spinal cord significantly reduces responses of spinothalamic tract neurons to cutaneous stimuli in neuropathic monkeys (Carlton et al., 1998). Furthermore, intrathecal injection of memantine produces a significant antiallodynic effect in rats subjected to spinal nerve ligation or formalin injection (Chaplan et al., 1997).
Interestingly, the effects of all three drugs on allodynia and hyperalgesia were somewhat attenuated at the last day of testing. The exact reason for this phenomenon is not clear. Because the residual drug solutions were found to be present inside the minipump at the end of the study, this phenomenon cannot be explained by drug depletion. We did observe that extensive fibrosis was formed at the site of the minipump implantation in most diabetic rats. Thus, the reduced drug effect may be due to the limited drug diffusion into the systemic circulation at the late stage of the experiments. However, the plasma drug concentrations at the end of the experiment were comparable to those observed after chronic administration of neramexane and memantine via minipumps in nondiabetic rats (Danysz et al., 1994; Hesselink et al., 1999b). This suggests that the systemic exposure in the current study may not be significantly compromised by the fibrosis. It should be recognized that the progression of the severity of neuropathic pain in the diabetic rats at the end of the study may also contribute to the “decreased” effect of drugs tested.
In summary, our findings suggest that chronic administration of neramexane reversed allodynia and hyperalgesia in a rat model of painful diabetic neuropathy. At therapeutically relevant doses, the time course and magnitude of the antinociceptive effects of neramexane were similar to those produced by memantine and gabapentin. Thus, neramexane is another NMDA antagonist that has a therapeutic potential to treat chronic neuropathic pain. Our study also provides further evidence for an important role of NMDA receptors in the maintenance of chronic pain associated with diabetic neuropathy.
Acknowledgments
This work was supported by funds from Forest Research Institute and by grants GM64830 and NS45602 from the National Institutes of Health.
List of abbreviations
- NMDA
N-methyl-D-aspartate
- STZ
streptozotocin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin P, Sturrock ND. A pilot study of the beneficial effects of amantadine in the treatment of painful diabetic peripheral neuropathy. Diabet Med. 2003;20:114–118. doi: 10.1046/j.1464-5491.2003.00882.x. [DOI] [PubMed] [Google Scholar]
- Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. Jama. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- Begon S, Pickering G, Eschalier A, Dubray C. Magnesium and MK-801 have a similar effect in two experimental models of neuropathic pain. Brain Res. 2000;887:436–439. doi: 10.1016/s0006-8993(00)03028-6. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Asbury AK. Diabetic neuropathy. Ann Neurol. 1984;15:2–12. doi: 10.1002/ana.410150103. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, Tarayre JP, Koek W, Ribet JP, Colpaert FC. Experimental conditions for the continuous subcutaneous infusion of four central analgesics in rats. Pharmacol Biochem Behav. 2002;72:943–951. doi: 10.1016/s0091-3057(02)00760-8. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Chaplan SR. Spinal pharmacology of tactile allodynia in diabetic rats. Br J Pharmacol. 1997;122:1478–1482. doi: 10.1038/sj.bjp.0701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson KC, Hoem NO, Moberg ER, Mathisen LC. Analgesic effect of dextromethorphan in neuropathic pain. Acta Anaesthesiol Scand. 2004;48:328–336. doi: 10.1111/j.0001-5172.2004.0325.x. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Rees H, Tsuruoka M, Willis WD. Memantine attenuates responses of spinothalamic tract cells to cutaneous stimulation in neuropathic monkeys. Eur J Pain. 1998;2:229–238. doi: 10.1016/s1090-3801(98)90019-2. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Chen SR, Eisenach JC, McCaslin PP, Pan HL. Synergistic effect between intrathecal non-NMDA antagonist and gabapentin on allodynia induced by spinal nerve ligation in rats. Anesthesiology. 2000;92:500–506. doi: 10.1097/00000542-200002000-00033. [DOI] [PubMed] [Google Scholar]
- Chen SR, Khan GM, Pan HL. Antiallodynic effect of intrathecal neostigmine is mediated by spinal nitric oxide in a rat model of diabetic neuropathic pain. Anesthesiology. 2001;95:1007–1012. doi: 10.1097/00000542-200110000-00033. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol. 2002;87:2726–2733. doi: 10.1152/jn.2002.87.6.2726. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Up-regulation of spinal muscarinic receptors and increased antinociceptive effect of intrathecal muscarine in diabetic rats. J Pharmacol Exp Ther. 2003;307:676–681. doi: 10.1124/jpet.103.055905. [DOI] [PubMed] [Google Scholar]
- Clark CM, Jr, Lee DA. Prevention and treatment of the complications of diabetes mellitus. N Engl J Med. 1995;332:1210–1217. doi: 10.1056/NEJM199505043321807. [DOI] [PubMed] [Google Scholar]
- Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE. Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004;5:263–275. doi: 10.1111/j.1526-4637.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain. 1993;53:81–88. doi: 10.1016/0304-3959(93)90059-X. [DOI] [PubMed] [Google Scholar]
- Cvrcek P. Side effects of ketamine in the long-term treatment of neuropathic pain. Pain Med. 2008;9:253–257. doi: 10.1111/j.1526-4637.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- Danysz W, Gossel M, Zajaczkowski W, Dill D, Quack G. Are NMDA antagonistic properties relevant for antiparkinsonian-like activity in rats?--case of amantadine and memantine. J Neural Transm Park Dis Dement Sect. 1994;7:155–166. doi: 10.1007/BF02253435. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG, Jirgensons A, Kauss V, Tillner J. Amino-alkyl-cyclohexanes as a novel class of uncompetitive NMDA receptor antagonists. Curr Pharm Des. 2002;8:835–843. doi: 10.2174/1381612024607117. [DOI] [PubMed] [Google Scholar]
- Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–1254. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- Ditzler K. Efficacy and tolerability of memantine in patients with dementia syndrome. A double-blind, placebo controlled trial. Arzneimittelforschung. 1991;41:773–780. [PubMed] [Google Scholar]
- Eide K, Stubhaug A, Oye I, Breivik H. Continuous subcutaneous administration of the N-methyl-D-aspartic acid (NMDA) receptor antagonist ketamine in the treatment of post-herpetic neuralgia. Pain. 1995;61:221–228. doi: 10.1016/0304-3959(94)00182-E. [DOI] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-D-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20:358–373. doi: 10.1016/s0885-3924(00)00213-x. [DOI] [PubMed] [Google Scholar]
- Foster AC, Wong EH. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br J Pharmacol. 1987;91:403–409. doi: 10.1111/j.1476-5381.1987.tb10295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortelmeyer R, Erbler H. Memantine in the treatment of mild to moderate dementia syndrome. A double-blind placebo-controlled study. Arzneimittelforschung. 1992;42:904–913. [PubMed] [Google Scholar]
- Hesselink MB, Parsons CG, Wollenburg C, Danysz W. Brain distribution of an uncompetitive NMDA receptor antagonist; comparison to its in vitro potency in electrophysiological studies. Naunyn Schmiedebergs Arch Pharmacol. 1999a;360:144–150. doi: 10.1007/s002109900056. [DOI] [PubMed] [Google Scholar]
- Hesselink MB, Smolders H, De Boer AG, Breimer DD, Danysz W. Modifications of the behavioral profile of non-competitive NMDA receptor antagonists, memantine, amantadine and (+)MK-801 after chronic administration. Behav Pharmacol. 1999b;10:85–98. doi: 10.1097/00008877-199902000-00008. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience. 2002;114:291–299. doi: 10.1016/s0306-4522(02)00372-x. [DOI] [PubMed] [Google Scholar]
- Klein T, Magerl W, Hanschmann A, Althaus M, Treede RD. Antihyperalgesic and analgesic properties of the N-methyl-D-aspartate (NMDA) receptor antagonist neramexane in a human surrogate model of neurogenic hyperalgesia. Eur J Pain. 2008;12:17–29. doi: 10.1016/j.ejpain.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Weller M, Schoppmeyer K, Riederer P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm Suppl. 1994;43:91–104. [PubMed] [Google Scholar]
- Malcangio M, Tomlinson DR. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain. 1998;76:151–157. doi: 10.1016/s0304-3959(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Max MB, Byas-Smith MG, Gracely RH, Bennett GJ. Intravenous infusion of the NMDA antagonist, ketamine, in chronic posttraumatic pain with allodynia: a double-blind comparison to alfentanil and placebo. Clin Neuropharmacol. 1995;18:360–368. doi: 10.1097/00002826-199508000-00008. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Bartmann A, Spielmanns P, Frankiewicz T, Hesselink M, Eilbacher B, Quack G. Amino-alkyl-cyclohexanes are novel uncompetitive NMDA receptor antagonists with strong voltage-dependency and fast blocking kinetics: in vitro and in vivo characterization. Neuropharmacology. 1999a;38:85–108. doi: 10.1016/s0028-3908(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999b;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology. 1995;34:1239–1258. doi: 10.1016/0028-3908(95)00092-k. [DOI] [PubMed] [Google Scholar]
- Periclou A, Ventura D, Rao N, Abramowitz W. Pharmacokinetic study of memantine in healthy and renally impaired subjects. Clin Pharmacol Ther. 2006;79:134–143. doi: 10.1016/j.clpt.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS Drug Rev. 2003;9:275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. Jama. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- Sang CN, Booher S, Gilron I, Parada S, Max MB. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials. Anesthesiology. 2002;96:1053–1061. doi: 10.1097/00000542-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Matthews EA, Dickenson AH. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain. 2001;91:101–109. doi: 10.1016/s0304-3959(00)00423-1. [DOI] [PubMed] [Google Scholar]
- Tomiyama M, Furusawa K, Kamijo M, Kimura T, Matsunaga M, Baba M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Brain Res Mol Brain Res. 2005;136:275–281. doi: 10.1016/j.molbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol. 2007;579:849–861. doi: 10.1113/jphysiol.2006.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Campbell AM, Park CR, Schaefer D, Danysz W, Diamond DM. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol Biochem Behav. 2006;85:298–306. doi: 10.1016/j.pbb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Zurek JR, Nadeson R, Goodchild CS. Spinal and supraspinal components of opioid antinociception in streptozotocin induced diabetic neuropathy in rats. Pain. 2001;90:57–63. doi: 10.1016/s0304-3959(00)00386-9. [DOI] [PubMed] [Google Scholar]


