Abstract
Background
Prior work documented racial and ethnic disparities in incidence of stroke, stroke risk factors, and use of carotid endarterectomy(CEA). Less is known about disparities in outcomes and appropriateness of CEA, or reasons for such inequalities.
Methods
This was a population-based cohort of CEA performed in Medicare beneficiaries in NY. Clinical data were abstracted from medical charts to assess sociodemographics, clinical indication for CEA, disease severity, comorbidities, and deaths and strokes within 30 days of surgery. Appropriateness was based on validated criteria from a national expert panel. Differences in patients, providers, outcomes and appropriateness were compared using chi square tests. Differences in risk-adjusted rates of death or non-fatal stroke were compared using multiple logistic regression accounting for patient, physician, and hospital level risk factors.
Results
Overall, 95.3% of CEA patients were White, 2.5% Black, and 2.2% Hispanic (N=9093). Minorities had more severe neurological disease and more comorbidities, and were more likely to be cared for by lower volume surgeons and hospitals(p<.0001). Rates of 30 day death/stroke were higher in Hispanics(9.5%) and Blacks(6.9%) than Whites(3.8%;p<.0001). Multivariable analyses which adjusted for pre-surgical patient risk and provider characteristics found that Blacks no longer had significantly worse outcomes(OR=1.37;CI, 0.78-2.40), though the higher risk of death/stroke in Hispanics persisted(OR=1.87; CI,1.09-3.19). Minorities had higher rates of inappropriate surgery (Hispanics 17.6%, Black 13.0%, and White 7.9%,P<.0001) largely due to higher comorbidity.
Conclusions
Minorities had worse outcomes and higher rates of inappropriate surgery. Differences in underlying pre-surgical risk factors and provider characteristics explained the higher risk of complications in Blacks, but not Hispanics.
Keywords: carotid endarterectomy, racial and ethnic disparities, outcomes, complications, appropriateness
INTRODUCTION
Over the past decade, there have been expanding research activities and national policy initiatives focusing on racial and ethnic disparities in the quality and outcomes of health care.1, 2 While access to care, particularly adequate insurance, is a major driver of disparities, there are often inequalities even among well-insured populations. Racial and ethnic variations in the quality and outcomes of care may also be mediated in part by differences between minority and majority patients in: underlying sociodemographic characteristics, severity of disease, and comorbid illness burden, underuse of effective care, and lower quality, experience and cultural competency of the physicians and hospitals that care for them, among others.1-3 Identifying how various patient, physician, and hospital level factors may contribute to disparities has important implications for the design of clinical and health policy strategies for reducing them.
The burden of cerebrovascular disease is higher in minorities, with Blacks having higher rates of stroke incidence, severity, mortality, and recurrence.4-6 Carotid endarterectomy (CEA) is the primary procedure used to reduce the risk of stroke or death due to atherosclerotic plaque in the carotid artery. CEA is a model procedure for investigating disparities in the outcomes and quality of care for several reasons. First, it is one of most common major vascular surgical procedures in the US. Second, the evidence base for CEA is very strong with several large randomized controlled trials (RCTs) confirming its efficacy.7-10 Third, for over a decade, there have been national practice guidelines outlining appropriate patient selection and benchmarks for selecting providers.11, 12 Fourth, the procedure is largely performed on elderly patients with Medicare insurance so differences in use and outcomes would reflect more subtle patient and provider contributors to disparities, not primary insurance effects.
Some prior studies have shown worse outcomes of CEA among Blacks (compared to Whites), 13-17while others have not.18-23 The validity of most of these studies are limited by their reliance on claims data which does not permit robust adjustment for the key patient prognostic factors like the clinical indication for surgery, severity of carotid disease, neurological disability, and total comorbid illness burden. This is important because Blacks are more likely to have stroke as the indication for CEA, be operated on emergently, and have more pre-surgical comorbidities than Whites.15, 17, 21, 22, 24--all factors known to increase the chance of perioperative complications. Most prior studies also focused solely on mortality or in-hospital complications, or used claims data to ascertain post-operative outcomes. Less is known about disparities in the most important clinical outcome of death or post-operative stroke within 30 days of CEA (the primary combined endpoint in the RCTs). Even less is known about differences in CEA outcomes among Hispanics versus others.
If there are disparities in risk-adjusted rates of perioperative death and stroke, the extent to which they are explained by the characteristics of the surgeons and hospitals who care for them is critically important in developing approaches to reduce disparities. Minorities are more likely to have CEA in low volume hospitals15, 16, 24, 25 which may partly explain higher rates of adverse events.26 Prior investigations of disparities in CEA outcomes lack the combination of detailed clinical information on a large number of patients and providers to rigorously control for patient, surgeon and hospital level effects.
Finally, most studies of health disparities focus on underuse of effective care. Despite the higher burden of cerebrovascular disease, minorities are less likely to undergo CEA compared to Whites, even when clearly indicated.13, 27-30 Theoretically, lower overall rates of procedures among minorities would manifest as both more underuse and less overuse of surgery. However, no prior work has examined disparities in overuse of CEA.
This study used clinically detailed data from a large, population-based cohort study of CEA to assess racial and ethnic disparities in: 1) Clinical indications for CEA and pre-surgical patient risk; 2) the surgeons and hospitals who care for minorities; 3) Risk-adjusted death or stroke within 30 days of CEA; and 3) rates of inappropriate surgery. We also sought to understand whether disparities in outcomes and inappropriateness were explained by differences in pre-surgical patient factors, and/or the characteristics of their providers.
METHODS
Study Population
We used the New York Carotid Artery Surgery (NYCAS) database, a population-based, retrospective cohort study of all Medicare beneficiaries (fee-for-service and managed care) who underwent CEA (ICD code 38.12) between January 1, 1998 and June 30, 1999 in New York (NY) State.31 Details about the assembly of the NYCAS cohort have been reported previously, but briefly it involved a two-step process of identifying all CEAs using Medicare Part A, NY State hospital discharge, and Medicare eligibility databases, followed by eligibility screening and in-depth clinical chart review by trained Island Peer Review Organization research nurses.31 The parent NYCAS study has data on 9588 cases. For the present study, we excluded patients who had combined CEA and coronary artery bypass graft surgery (N=280) and those with other or missing race information (N=215). The data presented here are based on 9093 cases. The study was approved by the Mount Sinai Institutional Review Board.
Data Collection and Measurement
Detailed clinical information was abstracted from hospital charts including: age, gender, race/ethnicity, admission source, neurological, medical and surgical history, admission neurologic exam, functional status, laboratory values, medications, and diagnostic imaging test results. Patients were classified as White, Black, Hispanic, or other/missing race based on race/ethnicity data recorded in the physicians’ admission history and physical and daily progress notes (supplemented by administrative admission data if these data were missing from the physician notes). CEA is performed in both patients with and without neurological events (stroke and TIA). Patients with a carotid distribution stroke or TIA in the 12 months prior to surgery were considered symptomatic and classified into the following indications for CEA: acute syndromes (stroke-in-evolution or crescendo TIA), stroke, or carotid TIA. Asymptomatic patients were divided into those with and without a distant history of stroke or TIA (>12 months before surgery). Overall, neurological acuity was divided into five clinical indication risk strata (acute syndromes, stroke, TIA, asymptomatic with distant history of stroke or TIA, or asymptomatic and no history of distant stroke or TIA.32 The percent stenosis of the operated and non-operated internal carotid artery was abstracted from all imaging tests and pre-operative notes.
NYCAS also contains data on individual comorbid conditions, the Revised Cardiac Risk Index,33 and neurological disability (modified Rankin score). The degree of comorbidity was defined by a national expert panel (and later validated) as: 1) High (≥3 Revised Cardiac Risk Index risk factors (cerebrovascular disease, coronary artery disease, heart failure, renal insufficiency, or diabetes on insulin),33 severe disability (Rankin score 4 or 5), or severely limited life expectancy (metastatic cancer, end stage disease); 2) Moderate (two cardiac risk factors), or 3) Low (≤1 risk factors).34
Clinical Outcomes
The medical record of the index admission and all readmissions within 30 days of CEA were reviewed by trained research nurses to identify perioperative deaths, strokes and TIAs (as potentially misclassified strokes). The nurses noted any mention of complications in the admission, operative, or daily progress notes, discharge summaries, and brain imaging tests. Cases with a post-operative death, stroke, or TIA were independently reviewed and confirmed by two study physicians (including a neurologist). Initial agreement was 95%; disagreements were resolved by consensus. The primary clinical outcome was death or non-fatal stroke within 30 days of surgery—the composite endpoint in the RCTs and national clinical guidelines.7, 8, 12
Appropriateness
The appropriateness of surgery was based on expert ratings of 1557 mutually exclusive clinical indications for CEA as described elsewhere.31, 34 Briefly, these ratings were generated using the RAND appropriateness process (a validated group judgment process) by a national panel of experts in vascular surgery, neurosurgery, neurology, internal medicine, radiology, and vascular medicine.35, 36 Surgery was considered “appropriate” when the benefits exceeded the risks, “uncertain” when the benefits equaled the risks, and “inappropriate” when the risks outweighed the benefits. Reasons for inappropriate surgery included: high comorbidity in asymptomatic patients, operating in setting of a very recent or severe disabling stroke, minimal stenosis, operating contralateral to symptoms, or on an occluded artery.
Analysis Plan
The primary independent variable was race/ethnicity defined as: White, Black, or Hispanic. Differences in patient, surgeon and hospital characteristics, clinical outcomes, and rates of inappropriateness between White, Black and Hispanic patients were compared using chi square, students T, and Wilcoxon rank sum tests, as appropriate. Surgeon and hospital volume was stratified into quintiles. Similar results were obtained when modeling volume by tertiles and quartiles.
To examine whether there were race/ethnic differences in 30 day death or stroke, we performed a nested series of hierarchical logistic regression models using generalized estimating equations to account for clustering of cases among surgeons and hospitals. First, we compared unadjusted rates of perioperative death and stroke for Black, Hispanic, and White patients. Next, we compared rates of perioperative complications adjusted for whether a patient was symptomatic or asymptomatic—the single most important clinical distinction according to the RCTs and guidelines (Model 1). Model 2 used a CEA-specific risk adjustment model that includes: age ≥80 years old, admission from the Emergency Department (ED), admitted from home, neurologic acuity, severe disability, contralateral stenosis ≥50%, coronary artery disease, or diabetes on insulin.32 Model 3 analyzed the incremental impact of surgeon factors (annual volume, specialty, and years since graduation from medical school) on risk-adjusted outcomes from Model 2. Model 4 examined the contribution of hospital level factors (annual volume, teaching status, and size of city it was located in) to patient risk factor adjusted outcomes (Model 2). Model 5 is the multivariable model with all patient, surgeon, and hospital level factors from each of the prior models. Additional multivariable analyses that also controlled for Medicaid status (as a proxy for income) or other clinical variables that were imbalanced in the three racial/ethnic groups produced similar results to those shown. Secondary analyses of the alternate clinical outcome of ‘all strokes’ within 30 days of surgery (fatal and non-fatal) produced similar results (data not shown). We also examined risk-adjusted outcomes of patients who were operated by surgeons or in hospitals with differing proportions of minority cases. All analyses consider two-sided p values of .05 as statistically significant and were performed using SAS statistical software version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics and Clinical Indications for CEA
Table 1 displays the sociodemographic and clinical characteristics of the 9093 cases. White patients were about two years older than Blacks and Hispanics on average. Blacks and Hispanics had more comorbidities compared with Whites including: cerebrovascular disease, coronary artery disease, heart failure, hypertension, diabetes, and renal insufficiency (p<.05). Ten percent of Whites had high comorbidity compared with 15.6% of Blacks and 21.0% of Hispanics (p<.0001). Blacks and Hispanics also had more severe neurological disease according to several indicators (Table 1 and 2) including higher rates of: symptomatic carotid disease (particularly stroke) as the reason for CEA, past cerebrovascular disease, severe disability, and admission from the ER. Non-Whites had modestly higher proportion of patients with moderate or low grade ipsilateral stenosis (though rates overall were low). Among minorities, Hispanics had more heart failure and coronary artery disease than Blacks, however Blacks had more hypertension, renal insufficiency, and diabetes. Rates of Medicaid insurance (in addition to their Medicare coverage) were similar in Blacks and Hispanics.
Table 1.
Characteristics of Patients Undergoing CEA (N=9093)
| Patient Characteristics | White (%) (N=8662) |
Black (%) (N=231) |
Hispanic (%) (N=200) |
P Value |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age (yrs) | ||||
| Mean age (sd) | 74.7 (6.7) | 72.0 (7.8) | 72.6 (7.2) | <.0001 |
| < 60 | 1.9 | 6.5 | 3.5 | |
| 60-70 | 20.4 | 30.3 | 29.0 | |
| 70-79 | 53.6 | 45.9 | 53.5 | |
| ≥ 80 | 24.1 | 17.3 | 14.0 | <.0001 |
| Male | 56.0 | 46.1 | 52.3 | .008 |
| Admitted from the ER | 9.6 | 21.7 | 18.5 | <.0001 |
| Transferred from another hospital | 3.4 | 6.1 | 4.0 | .08 |
| Medicaid insurance | 3.2 | 16.9 | 18.0 | <.0001 |
| Revised Cardiac Risk Index Score | ||||
| 0 | 19.5 | 14.7 | 17.0 | <.0001 |
| 1 | 44.8 | 42.9 | 33.0 | |
| 2 | 27.8 | 30.7 | 34.0 | |
| ≥ 3 | 7.9 | 11.7 | 16.0 | |
| Comorbidity class | ||||
| None | 19.2 | 13.8 | 15.0 | <.0001 |
| Low | 43.2 | 41.1 | 32.0 | |
| Moderate | 27.1 | 29.4 | 32.0 | |
| High | 10.5 | 15.6 | 21.0 | |
| Comorbid conditions | ||||
| Cerebrovascular disease | 43.7 | 52.8 | 49.5 | .007 |
| Coronary artery disease | 60.9 | 54.6 | 66.0 | .048 |
| Congestive heart failure | 9.4 | 10.0 | 15.0 | .02 |
| Atrial fibrillation | 9.3 | 8.2 | 8.0 | .71 |
| Hypertension | 77.8 | 87.0 | 83.5 | .0007 |
| Diabetes mellitus | 28.8 | 48.5 | 42.5 | <.0001 |
| Insulin-dependent diabetes | 7.0 | 14.3 | 15.5 | <.0001 |
| Creatinine >2 preoperatively | 4.6 | 10.4 | 6.5 | .0001 |
| COPD | 19.7 | 10.0 | 12.5 | <.0001 |
| Severe disability (Rankin 4 or 5) | 2.4 | 5.2 | 5.0 | .003 |
| Ipsilateral carotid artery stenosis* | .006 | |||
| 100% | 0.9 | 0.9 | 1.0 | |
| 70-99% | 93.7 | 91.3 | 90.0 | |
| 50-69% | 4.0 | 5.2 | 4.0 | |
| 0-49% | 0.6 | 1.3 | 3.0 | |
| Contralateral carotid stenosis | .88 | |||
| 100% | 5.1 | 4.8 | 4.5 | |
| 70-99% | 23.6 | 21.6 | 22.0 | |
| 50-69% | 10.3 | 10.4 | 12.5 | |
| 0-49% | 61.0 | 63.2 | 61.0 | |
| Neurologic Acuity | <.0001 | |||
| Asymptomatic: No distant CVD | 56.3 | 47.2 | 50.5 | |
| Asymptomatic: Yes distant CVD | 15.6 | 16.0 | 15.0 | |
| Carotid TIA | 19.0 | 19.5 | 18.5 | |
| Stroke | 8.8 | 16.9 | 16.0 | |
| Acute Syndromes | 0.3 | 0.4 | 0.0 |
Ipsilateral stenosis refers to the operated carotid artery for asymptomatic cases and the symptomatic artery for symptomatic ones.
Table 2.
Clinical Indications for CEA in White, Black and Hispanic Patients (N=9093)
| Specific Clinical Indication for Surgery | White (%) (N=8662) |
Black (%) (N=231) |
Hispanic (%) (N=200) |
P Value |
|---|---|---|---|---|
| Carotid TIAs or amaurosis fugax: | 19.0 | 19.5 | 18.5 | <.0001 |
| Crescendo carotid TIAs | 0.2 | 0.0 | 0.0 | |
| Stroke-in-evolution | 0.2 | 0.4 | 0.0 | |
| Minor stroke | 7.4 | 13.8 | 13.5 | |
| Major stroke | 1.5 | 3.0 | 2.5 | |
| Asymptomatic carotid disease | 71.9 | 63.2 | 65.5 | |
|
Major Clinical Indication Category for Surgery |
<.0001 | |||
| Asymptomatic | 71.9 | 63.2 | 65.5 | |
| Carotid TIA | 19.0 | 19.5 | 18.5 | |
| Stroke | 8.8 | 16.9 | 16.0 | |
| Acute Syndromes | 0.3 | 0.4 | 0.0 |
Row % are rounded to nearest tenth, and may not exactly add up to 100%.
Surgeon and Hospital Characteristics
The CEAs were performed by 481 surgeons in 166 hospitals. Overall, Blacks and Hispanics were operated on by lower volume surgeons compared to Whites (Table 3; mean 31.7 v. 29.0 v. 50.5 cases/year, p<.0001). Nearly half of Blacks and Hispanics (47.6% and 42.5%) were operated on by the lowest quintile volume surgeons compared to Whites (20.4%, p<.0001). Hispanics were more likely to have their surgery done by a very junior surgeon (≤10 years since graduation from medical school, p<.05). A little more than half of all cases were performed by vascular surgeons and this did not vary by group. However, Non-Whites were less likely to be operated on by general surgeons and more likely to have neurosurgeons, likely due to their higher prevalence of stroke as the reason for surgery among minorities.
Table 3.
Characteristics of Surgeons and Hospitals Caring for White, Black and Hispanic Patients Undergoing CEA
| Provider Characteristics | White (%) (N=8662) |
Black (%) (N=231) |
Hispanic (%) (N=200) |
P Value |
|---|---|---|---|---|
| Surgeon Characteristics | ||||
| Surgeon CEA volume (cases/year) | 50.5 | 31.7 | 29.0 | <.0001 |
| Quintile 1: (1-17) | 20.3 | 47.6 | 42.5 | <.0001 |
| Quintile 2: (18-31) | 19.7 | 16.4 | 19.0 | |
| Quintile 3: (32-54) | 19.9 | 6.5 | 14.0 | |
| Quintile 4: (55-99) | 19.6 | 15.6 | 13.0 | |
| Quintile 5: (100-434) | 20.6 | 13.8 | 11.5 | |
| Board specialty | <.0001 | |||
| Vascular surgery | 51.9 | 55.0 | 53.5 | |
| General surgery | 34.8 | 30.3 | 25.5 | |
| Neurosurgery | 4.9 | 4.8 | 10.5 | |
| Cardiothoracic surgery | 7.1 | 7.4 | 4.5 | |
| Missing | 1.3 | 2.6 | 6.0 | |
| Years since graduation from medical school |
23.5 (9.2) | 23.9 (9.6) | 21.8 (8.3) | .08 |
| ≤ 10 | 7.5 | 7.0 | 14.2 | .004 |
| 11-20 | 34.0 | 33.8 | 36.0 | |
| 21-30 | 35.1 | 35.5 | 34.5 | |
| ≥ 31 | 23.5 | 23.6 | 15.2 | |
| Hospital Characteristics | ||||
| Hospital CEA volume (cases/year) | 154.7 (123.1) |
111.5 (108.8) |
112.3 (104.4) |
<.0001 |
| Quintile 1: (1-48) | 20.0 | 37.2 | 33.2 | <.0001 |
| Quintile 2: (50-82) | 20.0 | 16.4 | 20.6 | |
| Quintile 3: (83-151) | 21.7 | 20.4 | 17.1 | |
| Quintile 4: (152-263) | 18.8 | 16.9 | 20.6 | |
| Quintile 5: (264-433) | 19.6 | 9.1 | 8.5 | |
| Teaching hospital | 36.6 | 55.0 | 65.8 | <.0001 |
| Hospital location (Metropolitan statistical area city size) |
<.0001 | |||
| <500,000 residents | 16.2 | 6.1 | 3.0 | |
| 500,000-1,000,000 | 18.8 | 10.8 | 5.0 | |
| 1 to 2.5 million | 21.0 | 17.8 | 7.0 | |
| >2.5 million | 44.0 | 65.4 | 84.9 | |
| Proportion of Black/Hispanic CEA patients at operating hospital |
<.0001 | |||
| <10% | 91.9 | 62.3 | 46.7 | |
| 10-19% | 6.8 | 14.7 | 32.7 | |
| ≥20% | 1.3 | 22.9 | 20.6 |
Blacks and Hispanics were more likely to have surgery in the lowest volume hospitals and less likely to have surgery in the highest volume centers (p<.0001). However, Non-Whites were more likely to be treated in hospitals that were teaching institutions, located in the largest cities, and cared for larger proportions of minority CEA patients (p<.0001).
Disparities in Perioperative Outcomes
Overall, 305 patients had a stroke (3.28%) and 106 died (1.14%) within 30 days of surgery. The combined rate of perioperative death or non-fatal stroke was 3.99%. Among those who died, 33 died after a stroke, 13 died after a myocardial infarction, and 7 died after both. The cause of the remaining 53 deaths is not readily known, but occurred on the median seventh post-operative day--most likely due to fatal stroke or myocardial infarction.
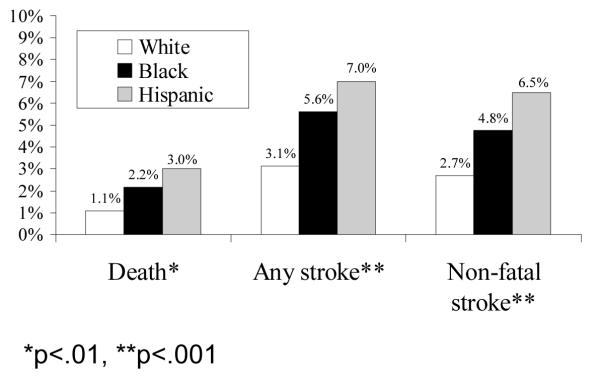
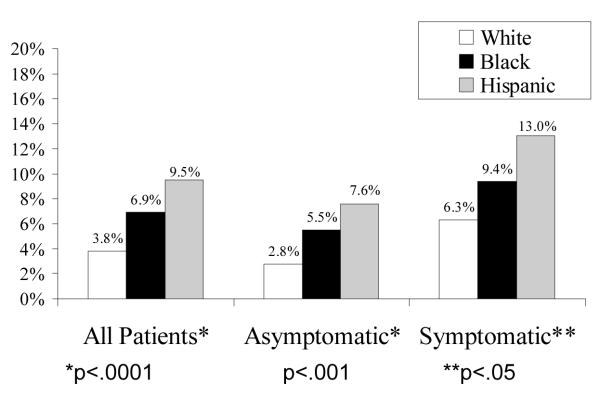
Rates of perioperative death, any stroke (fatal and non-fatal), and combined death or non-fatal stroke were highest in Hispanics, intermediate in Blacks and lowest in Whites (Figure 1-A). The unadjusted odds of death or stroke were higher in Blacks (OR=1.89; CI, 1.12-3.17) and Hispanics (OR=2.52; CI, 1.53-4.16) compared to Whites (Table 4). This pattern of Blacks and Hispanics having worse outcomes persisted after stratifying for the clinical indication for surgery (asymptomatic v. symptomatic, Figure 1-B). Table 4 shows the results of the nested risk adjustment models. Accounting for symptom status (Model 1) only minimally attenuates the difference in outcomes (as compared to the unadjusted analyses). In Model 2, which adjusts for all CEA-specific, patient risk variables, the higher risk of death or stroke in Blacks attenuates (OR=1.57; CI, 0.92-2.70) and becomes of borderline significance (p=.10). The increased risk in Hispanics persists (OR=2.14; CI, 1.27-3.60), though diminishes somewhat. Models 3, 4 and 5 demonstrate the after additionally adjusting for surgeon and hospital characteristics, the gap between Blacks and Whites further attenuated and was no longer close to statistically significance. However, odds of adverse events for Hispanics remained nearly twice that of Whites even after adjusting for all patient, surgeon, and hospital factors (Model 5; OR=1.87; CI, 1.09-3.19).
Figure 1-A: Disparities in Perioperative Complications of Carotid Endarterectomy
Figure 1-B: Disparities in Perioperative Death or Stroke by Clinical Indication
Table 4.
Racial and Ethnic Disparities in Death or Stroke within 30 Days of Carotid Endarterectomy (N=9086)
| Type of statistical adjustment comparing outcomes |
White (N=8656) |
Black (N=231) |
Hispanic (N=199) |
||
|---|---|---|---|---|---|
| Odds Ratio (CI) | P Value | Odds Ratio (CI) | P Value | ||
| Unadjusted Odds of Death/Stroke | 1.0 | 1.89 (1.12 – 3.19) | .02 | 2.52 (1.53 – 4.16) | .0003 |
| Model 1: Adjusted for symptomatic indication for surgery |
1.0 | 1.76 (1.04 – 2.97) | .03 | 2.40 (1.44 – 4.00) | .0008 |
| Model 2: Adjusted for all patient CEA risk factors |
1.0 | 1.57 (0.92 – 2.70) | .10 | 2.14 (1.27 – 3.60) | .004 |
| Model 3: Adjusted for all patient and surgeon factors |
1.0 | 1.42 (0.82 – 2.46) | .21 | 1.97 (1.16 – 3.36) | .01 |
| Model 4: Adjusted for all patient and hospital factors |
1.0 | 1.45 (0.84 – 2.51) | .18 | 1.95 (1.16 – 3.29) | .01 |
| Model 5: Adjusted for all patient, surgeon, and hospital factors |
1.0 | 1.37 (0.78 – 2.40) | .26 | 1.87 (1.09 – 3.19) | .02 |
| Type of statistical adjustment comparing outcomes |
White (N=8656) |
Black (N=231) |
Hispanic (N=199) |
||
|---|---|---|---|---|---|
| Odds Ratio | OR additionally force in Gender |
Odds Ratio | OR Additionally force in Gender |
||
| Unadjusted Odds of Death/Stroke | 1.0 | 1.89 (1.12-3.19) | 1.87* | 2.52 (1.53-4.16) | 2.53* |
| Model 1: Adjusted for symptomatic indication for CEA |
1.0 | 1.74 (1.03 – 2.94) | 1.74* | 2.40 (1.44 – 4.00) | 2.40* |
| Model 2: Adjusted for all patient CEA risk factors |
1.0 | 1.57 (0.92 – 2.70) P=.10 |
1.56, p=.10 | 2.14 (1.27 – 3.60) | 2.13* |
| Model 3: Adjusted for all patient and surgeon factors |
1.0 | 1.42 (0.82 – 2.46) P=.21 |
1.40, p=.22 | 1.97 (1.16 – 3.36) | 1.96* |
| Model 4: Adjusted for all patient and hospital factors |
1.0 | 1.45 (0.84 – 2.51) P=.18 |
1.43, p=.19 | 1.95 (1.16 – 3.29) | 1.94* |
| Model 5: Adjusted for all patient, surgeon, and hospital factors |
1.0 | 1.37 (0.78 – 2.40) P=.26 |
1.36, p=.23 | 1.87 (1.09 – 3.19) | 1.85* |
CI=95% Confidence Interval
Model 1: Adjusted for whether patient had a symptomatic v asymptomatic indication for CEA
Model 2: Adjusted for age, admission from the ER, neurologic acuity (acute syndrome, stroke, TIA, asymptomatic with distant TIA/stroke, asymptomatic with no distant TIA/Stroke), severe disability, contralateral stenosis ≥50%, coronary artery disease, diabetes on insulin
Model 3: Model 2 + surgeon volume, surgical specialty, and years since graduation from medical school
Model 4: Model 2 + hospital volume, teaching status, hospital location (city size)
Model 5: Adjusted for all patient, surgeon and hospital factors in above models
P<.05, CI=95% Confidence Interval
Model 1: Adjusted for whether patient had a symptomatic v asymptomatic indication for CEA
Model 2: Adjusted for age, ER admission, neurologic acuity (acute syndrome, stroke, TIA, asymptomatic with distant TIA/stroke, asymptomatic with no distant TIA/Stroke), severe disability, contralateral stenosis ≥50%, coronary artery disease, diabetes on insulin
Model 3: Model 2 + surgeon volume, surgical specialty, and years since graduation from medical school
Model 4: Model 2 + hospital volume, teaching status, hospital location (city size)
Model 5: Adjusted for all patient, surgeon and hospital factors in above models
One column shows the original Odds Ratio and confidence interval. The new column to the right shows the nested multivariable models with gender forced in.
This alternate Table shows that forcing in gender, which was not a significant covariate in our previously published carotid endarterectomy complications risk model (Halm et al, Stroke, 2008), produces nearly identical results as the original Table 4**
Worse outcomes in Non-Whites were seen in both men and women. Among men, the rates of death or stroke in Whites, Blacks and Hispanics were 3.6%, 9.4% and 7.7%, respectively (p<.007). Among women, the rates of death or stroke in Whites, Blacks, and Hispanics were 4.1%, 4.8%, and 11.6% (p<.002). In the NYCAS final risk model, there were no statistically significant differences in the rates of death or stroke between men and women. 32 Secondary analyses which additionally added gender to all five nested multivariable models shown in Table 4 produced similar results (data not shown). In additional subgroup comparisons, there were no statistically significant gender differences in perioperative death or stroke by race/ethnicity (White males v. White females, 3.55% v. 4.12%, p=.17; Black males v. Black females, 9.43% v. 4.84%, p=.18; Hispanic males v. Hispanic females, 7.69% v. 11.58%; p=.35).
Nor were there any significant association between the rates of death or stroke and the proportion of minority surgical patients in that hospital (p=.56). Blacks and Hispanics had similarly elevated rates of death or stroke across all strata of hospital minority patient prevalence (p<.02). Conversely, White patients operated in hospitals with a greater proportion of minority cases, did not have worse outcomes compared to White patients operated on in hospitals that took care of predominantly White patients.
Disparities in Appropriateness of Surgery
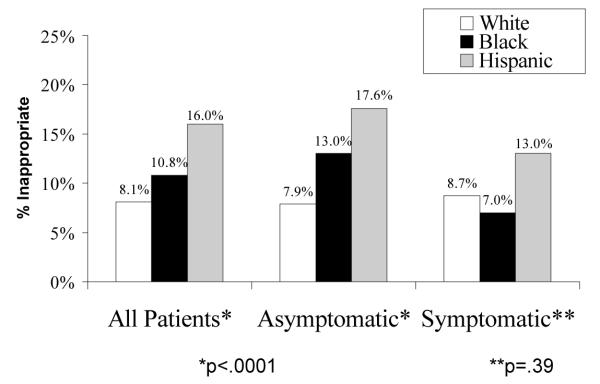
Figure 2-A shows that there were also disparities in rates of appropriateness of surgery. Non-Whites had higher rates of inappropriate surgery (White 7.9%, Black 13.0%, Hispanic 17.6%, p<.0001). Conversely minorities had lower rates of CEA being performed for appropriate indications. Stratifying by clinical indication for CEA, the disparity in inappropriateness was only seen among asymptomatic patients (Figure 2-B). The reasons for inappropriate surgery between White, Black, and Hispanic patients were also the same--predominantly high comorbidity in asymptomatic patients. Because asymptomatic Blacks and Hispanic patients had higher rates of high comorbidity, this alone explained three-quarters of the excess in inappropriateness among minorities. After controlling for high patient comorbidity, there were no statistically significant inequalities in inappropriateness (data not shown).
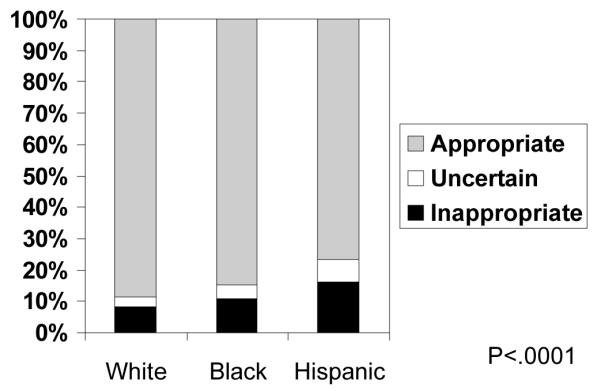
Figure 2-A: Racial and Ethnic Disparities in Rates of CEA Classified as Inappropriate, Uncertain, and Appropriate
Figure 2-B: Disparities in Appropriateness of CEA by Clinical Indication for Surgery
DISCUSSION
This study, which used clinically detailed data from a large population-based cohort study, adds to our knowledge about the extent and reasons for disparities in outcomes and use of procedures to prevent stroke in a several important areas. First, we found that both Blacks and Hispanics had higher rates of death and stroke after CEA (compared to Whites). Prior studies have shown inconsistent effects of race on outcomes of CEA, with about half reporting worse outcomes among Blacks (predominantly higher in-hospital mortality)13-17 and half finding no differences compared to Whites.18-23 Our data extends the finding of worse surgical outcomes in Blacks to the key clinical outcome of 30 day death or stroke--the main composite endpoint in the trials and national guidelines. The only two large prior studies that had clinically detailed data on outcomes found no Black-White differences in 30 day death or stroke.21, 22 Their focus on a single site22 or a Veterans Affairs population21 may explain the difference from our results which were based on a more generalizable statewide Medicare population.
Our finding that Hispanics had the highest rates of perioperative death and stroke after CEA is new. Since most prior work relied on administrative databases, they lacked the ability to accurately examine Hispanics as a separate subgroup. Trivedi et al. found a borderline excess of in-hospital deaths among Hispanics having CEA.16 In the Veterans Affairs National Surgical Quality Improvement Program study, Hispanics overall did not have worse outcomes, though the subgroup of Hispanics operated on for TIA had higher rates of death or stroke (compared to Whites).21
The reasons for the disparities in outcomes were multifactorial, were not just due to higher rates of symptomatic carotid disease among minorties, and appeared to differ in Blacks v. Hispanics. Among Blacks, the higher risk of complications attenuated to a borderline finding after procedure-specific risk adjustment indicating that differences in presurgical neurological acuity, disability, and comorbidity accounts for much, but not all of the Black-White disparity in outcomes. Further adjustments for surgeon and hospital experience further reduced the disparity suggesting that worse outcomes in Blacks are partly due to less experienced providers caring for them.
Among Hispanics, in-depth adjustment for patient, surgeon and hospital factors diminished the excess risk modestly, but Hispanics still had nearly double the risk-adjusted odds of death or stroke in the final multivariable model. This is a novel finding. Whether Hispanics had worse outcomes due to differences in other unmeasured factors related to pre-surgical patient risk, intra- or post-operative quality of care, or other more subtle surgeon, hospital, or community level influences is unknown and will require further investigation.
Contrary to what others have reported,24 the proportion of minority surgical patients at a given hospital did not influence the risk of CEA complications in either in minority or White patients. This suggests that, at least with regard to CEA, perioperative outcomes are largely driven by the individual patient’s pre-surgical risk and the skill and experience of the specific physician performing the operation, not the composition of the hospital’s patient population.
While the literature is consistent about underuse of CEA among minorities, this is the first study to examine racial and ethnic differences in overuse of CEA. Blacks and Hispanics had higher rates of inappropriate surgery, with Hispanics having the highest rates of overuse. The predominant reason for the higher rates of inappropriate surgery among minorities was that a larger proportion of them operated on for asymptomatic disease had high comorbidity. Why more minorities with asymptomatic disease and high comorbidity were selected for surgery is hard to understand and was unexpected.
Gender did not confound or explain the relationship between race/ethnicity and outcomes in either subgroup, stratified, or multivariate analyses. This is consistent with our previous report of no overall significant difference in perioperative outcomes between men and women in NYCAS.32 The literature examining the effects of gender on surgical complications of CEA is vast and has mixed findings.37, 38 Many studies, including several using similar large population-based, community practice cohorts, also reported no sex differences in perioperative death or stroke.15,23,38, 39 However, the asymptomatic RCTs found a non-significant trend suggesting a gender differential, 8, 10 and the symptomatic trials reported significantly higher surgical event rates among women.7, 9, 40
The current study had several strengths. It is the first to our knowledge to examine the step-wise impact of patient, surgeon and hospital factors as a way of understanding racial/ethnic disparities in clinically confirmed outcomes of CEA in a large, community setting with procedure specific risk-adjustment and validated measures of inappropriate care.
These strengths should be viewed in the context of a few limitations. Because NYCAS only contains data on patients who had CEA, we do not have any information about upstream decision making that would explain why certain patients were not recommended for or did not consent to surgery. Nor do we know why minorities undergoing CEA had more symptomatic disease, greater neurologic acuity, and greater comorbidity. Whether this is due to higher burden of cerebrovascular and other chronic diseases in minority elders or bias in perceived benefits and harms of CEA is unknown. Nor do we know why Non-Whites were more likely to be operated on by low volume surgeons or hospitals, even in the context of them being more likely to have surgery in teaching hospitals and large cities where high volume surgeons and centers are more readily available. Race and ethnicity were ascertained from physician clinical notes not self-report—though such determinations, while not perfect, should be more accurate than relying on claims data. Finally, while these data reflects practice in 1998-1999, operative techniques, perioperative management, and outcomes for CEA have been consistent over the intervening period, and there is little reason to believe that the association between race/ethnicity and clinical and provider risk factors would change considerably over time.
Our results have several policy implications. First, they highlight that efforts to measure and understand disparities (including disparities report cards) will need to try to control for patient, physician, and hospital level factors. Second, surgeon characteristics (volume, board specialty, and years in practice) appeared to be more important provider factors explaining disparities in outcomes than hospital volume ones. Most initiatives to improve surgical outcomes most often promote selective referral to high volume hospitals.41 In the case of CEA, our data suggest that the optimal policy strategy for reducing disparities would be to selectively refer minorities to high volume, experienced surgeons, something that is even harder to do than steering patients to high volume hospitals. Third, our findings reveal that lower overall rates of procedures in minorities that drive underuse do not necessarily translate into lower rates of overuse. The few other studies that examined this balance found more underuse of coronary revascularization42, 43 and renal transplantation44 among Blacks compared to Whites, but also less overuse of these procedures. In the case of CEA, minorities have more underuse and more overuse.
Taken together, our findings reveal the ‘worst of all worlds’--CEA is underused, overused and results in worse clinical outcomes for minorities. Because the reasons for such disparities in the quality and outcomes of CEA care are complex and appear to be operating at the patient, physician, and hospital level, strategies for reducing these inequities will be challenging and need to target multiple factors that influence patient selection and surgical referral patterns. From a research perspective, our approach suggests that studies relying solely on administrative data may be unable to adjust for important patient, surgeon and hospital level variables that contribute to disparities in clinical outcomes. Finally, from a clinical standpoint, more evidence-based decision aides are needed to help physicians and patients more appropriately weigh the potential risks and benefits of CEA for a given individual and better inform the surgeon and hospital referral decision.
Acknowledgements
The authors acknowledge the assistance of the Island Peer Review Organization (IPRO) and the Centers for Medicare and Medicaid Services (CMS) in providing the data which made this research possible. The conclusions presented are solely those of the authors and do not represent those of IPRO, CMS or JCAHO. All authors made substantial contributions to the manuscript. We would also like to acknowledge the assistance of: Patricia Formisano, MPH, Hugh Dai, and Virginia Chan.
Funding This study was supported by the Agency for Healthcare Research and Quality (RO1 HS09754), Center for Medicare Services, Robert Wood Johnson Foundation (#020803), and the National Institute of Neurological Diseases and Stroke (R01 NSO56028).
Footnotes
Conflicts of Interest Disclosure There are no conflicts of interest or disclosures.
Contributor Information
Ethan A. Halm, Departments of Internal Medicine and Clinical Sciences University of Texas Southwestern Medical Center 5323 Harry Hines Blvd Dallas, TX 75390-8889 Tel: (214) 648-2841 Fax: (214) 648-8727 Ethan.Halm@UTsouthwestern.edu.
Stanley Tuhrim, Department of Neurology Mount Sinai School of Medicine, NY One Gustave L. Levy Place New York, NY 10029 Stanley.tuhrim@mountsinai.org.
Jason J. Wang, Department of Health Policy Mount Sinai School of Medicine, NY One Gustave L. Levy Place New York, NY 10029 Jason.wang@mountsinai.org.
Mary Rojas, Departments of Health Policy and Pediatrics Mount Sinai School of Medicine, NY One Gustave L. Levy Place New York, NY 10029 Mary.rojas@mountsinai.org.
Caron Rockman, Department of Surgery New York University School of Medicine 530 First Avenue New York, NY 10016 Caron.Rockman@nyumc.org.
Thomas S. Riles, Department of Surgery New York University School of Medicine 530 First Avenue New York, NY 10016 thomas.riles@med.nyu.edu.
Mark R. Chassin, The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) One Renaissance Boulevard Oakbrook Terrace, IL 60181 mchassin@jointcommission.org.
REFERENCES
- 1.Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. National Academies Press; Washington, D.C.: 2003. [PubMed] [Google Scholar]
- 2.Agency for Healthcare Research and Quality . National healthcare disparities report. Agency for Healthcare Research and Quality; Rockville, MD: 2003. [Google Scholar]
- 3.Zaslavsky AM, Ayanian JZ. Integrating research on racial and ethnic disparities in health care over place and time. Med Care. 2005;43(4):303–307. doi: 10.1097/01.mlr.0000159975.43573.8d. [DOI] [PubMed] [Google Scholar]
- 4.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 5.Tuhrim S. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):386–387. doi: 10.1161/01.STR.0000153060.06006.00. [DOI] [PubMed] [Google Scholar]
- 6.Sheinart KF, Tuhrim S, Horowitz DR, Weinberger J, Goldman M, Godbold JH. Stroke recurrence is more frequent in Blacks and Hispanics. Neuroepidemiology. 1998;17(4):188–198. doi: 10.1159/000026172. [DOI] [PubMed] [Google Scholar]
- 7.North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 8.Executive Committee for the Asymptomatic Carotid Atheroscelerosis Study Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421–1428. [PubMed] [Google Scholar]
- 9.European Carotid Surgery Trialst’ Collaborative Group Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351(9113):1379–1387. [PubMed] [Google Scholar]
- 10.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D, MRC Asymptomatic Carotid Surgery Trial Collaborative Group Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363(9420):1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 11.Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott T, Caplan LR, Day A, Goldstone J, Hobson RW, 2nd, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the ad hoc Committee, American Heart Association. Stroke. 1995;26(1):188–201. doi: 10.1161/01.str.26.1.188. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, Stern B, Wilterdink J. Carotid endarterectomy--an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65(6):794–801. doi: 10.1212/01.wnl.0000176036.07558.82. [DOI] [PubMed] [Google Scholar]
- 13.Hsia DC, Moscoe LM, Krushat WM. Epidemiology of carotid endarterectomy among Medicare beneficiaries: 1985-1996 update. Stroke. 1998;29(2):346–350. doi: 10.1161/01.str.29.2.346. [DOI] [PubMed] [Google Scholar]
- 14.Huber TS, Wheeler KG, Cuddeback JK, Dame DA, Flynn TC, Seeger JM. Effect of the Asymptomatic Carotid Atherosclerosis Study on carotid endarterectomy in Florida. Stroke. 1998;29(6):1099–1105. doi: 10.1161/01.str.29.6.1099. [DOI] [PubMed] [Google Scholar]
- 15.Dardik A, Bowman HM, Gordon TA, Hsieh G, Perler BA. Impact of race on the outcome of carotid endarterectomy: a population-based analysis of 9,842 recent elective procedures. Ann Surg. 2000;232(5):704–709. doi: 10.1097/00000658-200011000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47(2):417–424. doi: 10.1016/j.jacc.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy BS, Fortmann SP, Stafford RS. Elective and isolated carotid endarterectomy: health disparities in utilization and outcomes, but not readmission. J Natl Med Assoc. 2007;99(5):480–488. [PMC free article] [PubMed] [Google Scholar]
- 18.Brook RH, Park RE, Chassin MR, Kosecoff J, Keesey J, Solomon DH. Carotid endarterectomy for elderly patients: predicting complications. Ann Intern Med. 1990;113(10):747–753. doi: 10.7326/0003-4819-113-10-747. [DOI] [PubMed] [Google Scholar]
- 19.Estes JM, Guadagnoli E, Wolf R, LoGerfo FW, Whittemore AD. The impact of cardiac comorbidity after carotid endarterectomy. J Vasc Surg. 1998;28(4):577–584. doi: 10.1016/s0741-5214(98)70079-5. [DOI] [PubMed] [Google Scholar]
- 20.Lanska DJ, Kryscio RJ. In-hospital mortality following carotid endarterectomy. Neurology. 1998;51(2):440–447. doi: 10.1212/wnl.51.2.440. [DOI] [PubMed] [Google Scholar]
- 21.Horner RD, Oddone EZ, Stechuchak KM, Grambow SC, Gray J, Khuri SF, Henderson WG, Daley J. Racial variations in postoperative outcomes of carotid endarterectomy: evidence from the Veterans Affairs National Surgical Quality Improvement Program. Med Care. 2002;40(1 Suppl):I35–43. [PubMed] [Google Scholar]
- 22.Conrad MF, Shepard AD, Pandurangi K, Parikshak M, Nypaver TJ, Reddy DJ, Cho JS. Outcome of carotid endarterectomy in African Americans: is race a factor? J Vasc Surg. 2003;38(1):129–137. doi: 10.1016/s0741-5214(02)75455-4. [DOI] [PubMed] [Google Scholar]
- 23.Sheikh K, Jiang Y, Bullock CM. Effect of comorbid and fatal coexistent conditions on sex and race differences in vascular surgical mortality. Ann Vasc Surg. 2007;21(4):496–504. doi: 10.1016/j.avsg.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Ann Surg. 2006;243(2):281–286. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296(16):1973–1980. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 26.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 27.Gillum RF. Carotid endarterectomy in older women and men in the United States: trends in ethnic disparities. J Natl Med Assoc. 2005;97(7):957–962. [PMC free article] [PubMed] [Google Scholar]
- 28.Jha AK, Fisher ES, Li Z, Orav EJ, Epstein AM. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;353(7):683–691. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- 29.Oddone EZ, Horner RD, Monger ME, Matchar DB. Racial variations in the rates of carotid angiography and endarterectomy in patients with stroke and transient ischemic attack. Arch Intern Med. 1993;153(24):2781–2786. [PubMed] [Google Scholar]
- 30.Oddone EZ, Horner RD, Johnston DC, Stechuchak K, McIntyre L, Ward A, Alley LG, Whittle J, Kroupa L, Taylor J. Carotid endarterectomy and race: do clinical indications and patient preferences account for differences? Stroke. 2002;33(12):2936–2943. doi: 10.1161/01.str.0000043672.42831.eb. [DOI] [PubMed] [Google Scholar]
- 31.Halm EA, Tuhrim S, Wang JJ, Rojas M, Hannan EL, Chassin MR. Has evidence changed practice? Appropriateness of carotid endarterectomy after the clinical trials. Neurology. 2007;68(3):187–194. doi: 10.1212/01.wnl.0000251197.98197.e9. [DOI] [PubMed] [Google Scholar]
- 32.Halm EA, Tuhrim S, Wang J, Rockman CB, Riles TS, Chassin MR. Risk factors for perioperative death and stroke after carotid endarterectomy: Results of the New York Carotid Artery Surgery Study. Stroke. 2009;40:221–229. doi: 10.1161/STROKEAHA.108.524785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 34.Halm EA, Chassin MR, Tuhrim S, Hollier LH, Popp AJ, Ascher E, Dardik H, Faust G, Riles TS. Revisiting the appropriateness of carotid endarterectomy. Stroke. 2003;34(6):1464–1471. doi: 10.1161/01.STR.0000072514.79745.7D. [DOI] [PubMed] [Google Scholar]
- 35.Winslow CM, Solomon DH, Chassin MR, Kosecoff J, Merrick NJ, Brook RH. The appropriateness of carotid endarterectomy. N Engl J Med. 1988;318(12):721–727. doi: 10.1056/NEJM198803243181201. [DOI] [PubMed] [Google Scholar]
- 36.Shekelle PG, Chassin MR, Park RE. Assessing the predictive validity of the RAND/UCLA appropriateness method criteria for performing carotid endarterectomy. Int J Technol Assess Health Care. 1998;14(4):707–727. doi: 10.1017/s0266462300012022. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: systematic review. BMJ. 1997;315(7122):1571–1577. doi: 10.1136/bmj.315.7122.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballotta E. Female sex: a questionable risk factor for carotid endarterectomy. Stroke. 2003;34(5):1120–1125. [PubMed] [Google Scholar]
- 39.Kapral MK, Wang H, Austin PC, Fang J, Kucey D, Bowyer B, Tu JV. Sex differences in carotid endarterectomy outcomes: results from the Ontario Carotid Endarterectomy Registry. Stroke. 2003;34(5):1120–1125. doi: 10.1161/01.STR.0000066681.79339.E2. [DOI] [PubMed] [Google Scholar]
- 40.Bond R, Narayan SK, Rothwell PM, Warlow CP. Clinical and radiographic risk factors for operative stroke and death in the European carotid surgery trial. Eur J Vasc Endovasc Surg. 2002;23(2):108–116. doi: 10.1053/ejvs.2001.1541. [DOI] [PubMed] [Google Scholar]
- 41.Birkmeyer JD, Dimick JB. Leapfrog safety standards: potential benefits of universal adoption. The Leapfrog Group; Washington, DC: 2004. [Google Scholar]
- 42.Schneider EC, Leape LL, Weissman JS, Piana RN, Gatsonis C, Epstein AM. Racial differences in cardiac revascularization rates: does “overuse” explain higher rates among white patients? Ann Intern Med. 2001;135(5):328–337. doi: 10.7326/0003-4819-135-5-200109040-00009. [DOI] [PubMed] [Google Scholar]
- 43.Epstein AM, Weissman JS, Schneider EC, Gatsonis C, Leape LL, Piana RN. Race and gender disparities in rates of cardiac revascularization: do they reflect appropriate use of procedures or problems in quality of care? Med Care. 2003;41(11):1240–1255. doi: 10.1097/01.MLR.0000093423.38746.8C. [DOI] [PubMed] [Google Scholar]
- 44.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343(21):1537–1544. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]