Abstract
Genetic variation may influence initial sensitivity to nicotine (i.e. during early tobacco exposure), perhaps helping to explain differential vulnerability to nicotine dependence. This study explored associations of functional candidate gene polymorphisms with initial sensitivity to nicotine in 101 young adult nonsmokers of European ancestry. Nicotine (0, 5, 10 μg/kg) was administered via nasal spray followed by mood, nicotine reward (e.g. “liking”) and perception (e.g. “feel effects”) measures, physiological responses, sensory processing (pre-pulse inhibition of startle), and performance tasks. Nicotine reinforcement was assessed in a separate session using a nicotine vs. placebo spray choice procedure. For the dopamine D4 receptor (DRD4 VNTR), presence of the 7 repeat allele was associated with greater aversive responses to nicotine (decreases in “vigor”, positive affect, and rapid information processing; increased cortisol) and reduced nicotine choice. Individuals with at least one DRD4 7-repeat allele also reported increased “feel effects” and greater startle response, but in men only. Also observed in men but not women were other genetic associations, such as greater “feel effects” and anger, and reduced fatigue, in the dopamine D2 receptor (DRD2 C957T SNP) TT versus CT or CC genotypes. Very few or no significant associations were seen for the DRD2/ANKK1 TaqIA polymorphism, the serotonin transporter promoter VNTR or 5HTTLPR (SLC6A4), the dopamine transporter 3’ VNTR (SLC6A3), and the mu opioid receptor A118G SNP (OPRM1). Although these results are preliminary, this study is the first to suggest that genetic polymorphisms related to function in the dopamine D4, and perhaps D2, receptor may modulate initial sensitivity to nicotine prior to the onset of dependence and may do so differentially between men and women.
Keywords: nicotine, sensitivity, genetics, dopamine, reward, reinforcement
INTRODUCTION
Although nearly three-quarters of U.S. adults have experimented with cigarettes, less than half of those ever exposed go on to become nicotine dependent (Anthony et al., 1994). Understanding this differential vulnerability to dependence is a major emphasis of research on smoking initiation and prevention (e.g., Audrain-McGovern et al., in press). Substantial research supports the heritability of smoking onset, intensity, and persistence (Vink et al. 2005), but the specific genes that are responsible remain uncertain.
Prior research suggests that genetic factors potentially related to neurotransmitter function may influence the onset and progression of smoking in adolescents. The A1 allele of the DRD2/ANKK1 TaqIA polymorphism may interact with other vulnerability characteristics, such as depression, to accelerate smoking progression (Audrain-McGovern et al. 2004), and with protective factors, such as sports participation, to deter smoking progression (Audrain-McGovern et al. 2006). The short allele of the promoter variant in the serotonin transporter gene (SLC6A4 5HTTLPR VNTR) is associated with onset of smoking in teens (Gerra et al., 2005). Other studies suggest that the dopamine transporter (SLC6A3) gene (rs27072) A allele is associated with increased risk of early smoking onset (Ling et al. 2004). It should be noted, however, that these other findings often have not been replicated (see Munafo et al., 2004 for a meta-analysis).
There is also evidence for associations of these and other specific genetic variants with smoking behavior or clinical outcome in smoking cessation trials with medications in adults (Comings et al. 1996; Shields et al 1998; Bierut et al. 2000; Lerman et al. 2003; 2004; 2006; Munafo et al., 2004; 2006; Swan et al. 2007; David et al. in press; Yudkin et al. 2004), although lack of replication is a concern in this literature as well (e.g., Munafo et al. 2004; 2006). Also, the mu opioid receptor polymorphism (OPRM1) has been related to acute preference for nicotine via smoking in women but not men (Ray et al. 2006), suggesting some genetic influences may be moderated by subject sex.
The mechanisms by which specific genes may increase risk of onset of nicotine dependence are not clear. Because risk of dependence may be enhanced in those with greater initial sensitivity to nicotine, i.e. upon early exposure, genes may influence risk by enhancing that initial sensitivity. Pomerleau (1995) has proposed a “sensitivity” model, in which teens at greater risk for nicotine dependence experience greater positive, and possibly aversive, responses to nicotine upon first exposure to smoking, compared to those at lower risk. The rationale is that greater responses to early exposure may increase the likelihood of repeated smoking exposures, fostering smoking escalation and dependence. Empirical evidence is limited, but adults who currently smoke retrospectively report having had greater pleasant sensations the first time they ever smoked, compared to adults who never smoked regularly but had some exposure (Pomerleau et al., 2004; O'Connor et al., 2005; Hu, et al., 2006). Similarly, among teens who currently smoke, retrospective reports of greater feelings of “relaxed”, “high”, and “dizziness” from their first cigarette are associated with a more rapid escalation of smoking (Hirschman, et al., 1984; Blitstein, et al., 2003). This model follows from animal research showing genetic or other individual differences in initial sensitivity to nicotine (Marks et al., 1991; Schechter et al. 1995). Some rat strains that are more sensitive than others to nicotine upon initial exposure may show greater subsequent acquisition of nicotine reinforcement (Shoaib et al. 1997; Le et al., 2006). (Note that the “exposure” model, an alternative view based on observations in the alcohol literature, makes the opposite prediction, that attenuated initial sensitivity to drugs increases vulnerability to dependence; Schuckit and Smith, 1996.)
Thus, although many other mechanisms for heritability of nicotine dependence are possible, genetic factors may alter risk of nicotine dependence by influencing initial sensitivity to the acute rewarding and reinforcing effects of nicotine. In this study, we examined the association of specific genetic polymorphisms with initial sensitivity to acute nicotine administration in young adult nonsmokers. Because the specific effects of nicotine that promote reinforcement in humans are not known, we examined a wide variety of acute nicotine responses. Candidate genes and SNPs were selected based on the following criteria: (1) genes in pathways implicated in the neurobiology of nicotine, including the dopamine, serotonin and endogenous opioid pathway; (2) within those pathways, genes that have been linked to smoking initiation or nicotine dependence phenotypes in prior research (Lerman et al., 2007); and (3) within these genes, polymorphisms with documented functional effects (in vitro or in vivo) and minor allele frequencies >0.10). Based on these criteria, we selected the following polymorphisms: dopamine D4 receptor (DRD4 VNTR), dopamine D2 receptor (DRD2 C957T SNP and DRD2/ANKK1 TaqIA SNP), the dopamine transporter (SLC6A3 VNTR), the mu opioid receptor exon 1 SNP (OPRM1 A118G), and the serotonin transporter promoter variant (SLC6A4 5HTTLPR VNTR).
METHODS
Participants
Participants were 101 young adult nonsmokers (36 m, 65 f) of European ancestry aged 21–39, with ≤ 10 lifetime tobacco exposures and no use in the prior 3 years. Lifetime tobacco use was assessed twice (for reliability), during an initial telephone screening and at a subsequent in-person interview. Current problem alcohol use was an exclusion criterion, determined by self-report of more than 24 drinks per week. Mean ± SEM age was 25.0±0.4 yrs, 58.4% were college graduates, and 80.2% were single. About half (n=52) had prior tobacco experience (mean of 2.5 lifetime exposures), with the first exposure occurring at 15.7±0.4 years of age, but they had had no tobacco exposure in the past 8.2±0.6 years (range = 3–20 yrs).
Dependent measures
The dependent measures in this study were self-reported reward-related and mood items, physiological responses, sensory processing and attention (startle response, and pre-pulse inhibition of the startle response), task performance, and a nicotine choice reinforcement measure.
Self-report
Spray ratings of nicotine reward, incentive salience, and perception were assessed using visual-analog scale (VAS) items rated 0–100 (anchored by “not at all” and “extremely”). Items tapping nicotine “reward”, or its hedonic value (Everitt and Robbins, 2005), were “liking” and “satisfying.” Incentive salience was assessed by the item of “want more”, adapted from a similar item shown to be sensitive in smokers to duration of smoking abstinence and to individual differences (e.g., Evans et al. 1999). Perception of the nicotine content in sprays was assessed by “feel the effects” and “how much nicotine”. These items have been shown in other research to be sensitive to nicotine administration and to individual differences in sensitivity to nicotine intake via smoking (e.g., Perkins et al. 2006). In addition, a VAS item assessing nasal “irritation” determined the degree of sensory irritation due to the spray, for use as a covariate.
Mood measures included a) the Positive And Negative Affect Scale (PANAS; Watson et al. 1988), with positive and negative affect subscales; b) the Profile of Mood States (POMS; McNair et al. 1971), with subscales labeled tension, anger, fatigue, vigor, depression; and c) a series of 12 VAS items (rated 0–100): comfortable, satisfied, pleasant, relaxed, buzzed, jittery, anxious, tired, sedated, alert, stimulated, and nausea. These and similar measures have been used in many prior studies of acute nicotine effects in smokers and nonsmokers (e.g., Perkins et al. 2001a; 2001b; Kalman and Smith 2005). POMS scale scores were converted to 0–100 scores to simplify comparison with VAS items.
Physiological responses
Heart rate (HR, in beats per minute), systolic and diastolic blood pressure (BP, in mmHg), were obtained by Dinamap blood pressure recorder (Critikon Inc., Tampa FL). Cortisol was obtained by saliva sample using a Sarstedt salivette (dental swab) that was analyzed by Salimetrics, LLC (State College, PA, www.salimetrics.com).
Sensory processing
Greater magnitude of eyeblink response to a loud acoustic stimulus (i.e. startle) is associated with negative affect (Filion et al., 1998). The degree to which this response is attenuated by a mild acoustic stimulus immediately preceding the startle probe (pre-pulse inhibition, PPI) provides an index of attention to sensory stimuli (Swerdlow et al., 1992). Cigarette smoking has been shown to acutely enhance PPI (Duncan et al. 2001), suggesting that nicotine can improve attention and sensory gating, but other studies indicate that smoking may impair PPI (Hutchison et al. 2000). Effects of smoking on startle response are unclear (Duncan et al. 2001; Hutchison et al. 2000). Startle and PPI were assessed by presenting short (50 msec) bursts of rapid onset loud (106 dB) acoustic stimuli either alone (to measure startle) or preceded 120 msec by milder (84 dB) 20-msec bursts (to assess PPI), with background white noise of 75 dB. During each of the 3 dose trials per session, we presented 6 startle and 6 PPI probes in random order, with an inter-trial interval ranging from 9–23 sec (mean of 15 sec). The raw EMG signal was amplified and then filtered using Biopac AcqKnowledge software (Biopac, Goleta CA) to produce a characteristic eye-blink maximum (“max”) amplitude and area under the curve (“AUC”) for each measure (startle, PPI) and each dose trial. PPI values were expressed as a percent of the startle response obtained during the same trial; smaller values indicate greater inhibition of startle, or greater sensory gating (Swerdlow et al., 1992).
Performance tasks
Performance tasks assessed after each dose administration included finger-tapping speed, handsteadiness, Sternberg rapid information-processing, and memory recall. Nicotine dose effects on finger-tapping speed (increasing), handsteadiness (worsening), and memory recall (curvilinear) have been shown in nonsmokers as well as smokers (Perkins et al. 1994a; 2001a). Most of these tasks are described elsewhere in more detail (Perkins et al. 2001a). For the Sternberg rapid information processing task, subjects were given one or five “target” letters to retain in short-term memory. They then were to respond as quickly as possible to a series of letter pairs, indicating whether the given letter pair did (“hits”) or did not (“correct rejections”) contain a target letter. The difference in reaction time in msec between the one- and five-letter trials (“D-prime”) on items requiring correct rejection (involving processing of all target letters) was the primary measure of memory scanning speed (information processing; Schneider and Shiffrin, 1977). Responding has been shown to be improved (i.e. faster) by nicotine in smokers under distracting conditions involving auditory presentation of non-target letters while attempting to process target letters presented visually on a computer monitor (Grobe et al. 1998). This task was presented in the current study under both distracting and non-distracting conditions, in random order, following each dose administration.
Nicotine Reinforcement
The relative reinforcing effects of nicotine were determined by the number of nicotine (1.25 μg/kg/spray) versus placebo sprays selected in a choice procedure, described below. Greater nicotine choice via this procedure has been related to greater pleasurable responses to nicotine in nonsmokers, as well as smokers (Perkins et al. 2001b). Nicotine choice also increases in smokers with overnight abstinence (Perkins et al. 1996), predicts greater withdrawal severity and faster relapse in smokers trying to quit (Perkins et al. 2002), and is sensitive to individual differences, including obesity status (Blendy et al. 2005) and genetic variation (Ray et al. 2006).
Nicotine dosing
Nicotine was administered via a nasal spray procedure developed by us and used in many prior studies (e.g. Perkins et al. 1986; 1994a; 2001a). Each participant received 0, 5, and 10 μg/kg doses, with dose administration spread over 8 sprays (2 @ 30 secs). The relatively low 5 and 10 μg/kg doses, which produce plasma nicotine levels comparable to about 1/4 and 1/2 typical cigarette, respectively (Perkins et al. 1994a; 2001a), were selected because these are typical of amounts naive individuals are likely to absorb in initial experimentation with smoking (Eissenberg and Balster, 2000), which we were trying to simulate in these assessments. A blood sample was obtained by venipuncture at the end of each session and analyzed for plasma nicotine concentration by gas chromatography with nitrogen-phosphorus detection using 5-methylnicotine as the internal standard (Jacob et al. 1981). Mean (±SEM) plasma nicotine levels following the 5 and 10 μg/kg dose sessions were 2.3±0.1 and 3.4±0.2 ng/ml, respectively.
Procedures
All subjects provided informed consent after the nature and consequences of participation were explained. This research was approved by the Institutional Review Board of the University of Pittsburgh Medical Center. Subjects participated in four sessions, three to assess nicotine sensitivity to most responses and a fourth to assess nicotine reinforcement. Upon arrival to the lab for each session, subjects first provided expired-air carbon monoxide (CO) assessment (Vitalograph CO analyzer, Breathco, Inc., Lenexa KS), to verify absence of any recent smoking exposure (CO< 5 ppm), and breathalyzer assessment (Alco-Sensor III breathalyzer, Intoximeters Inc., St Louis MO) to verify no recent alcohol intake (BAL=0.00).
Nicotine Sensitivity Assessment
The first three sessions were virtually identical, differing only in the administered dose of nicotine spray (0, 5, 10 μg/kg). The order of doses across sessions was counter-balanced. Only one of the doses was presented on a given day, and the dose was administered three times per session, once every 30 mins. At the start of each session, subjects rested quietly, followed by a baseline assessment of mood, cardiovascular responses, sensory perception, and the performance measures, in that order. This sequence of measures was repeated for two more baseline trials, one every 30 mins, to habituate to testing. Saliva cortisol was obtained at the end of this baseline period. Then, this assessment sequence, along with the spray ratings (after mood items), was repeated another three times (3 dose trials), again once every 30 mins, with each assessment following nasal spray administration of the dose assigned for that session. A second saliva sample for cortisol analysis was obtained after the third and last spray dose trial.
Nicotine Reinforcement Assessment
At the start of the choice session, participants first engaged in two “sampling” trials. They self-administered the 0 μg/kg or 1.25 μg/kg/spray bottles 8 times each (i.e. 0 and 10 μg/kg in all, respectively), waited 20 mins, then self-administered the other bottle 8 times, in blind fashion and counterbalanced order. In the subsequent four “choice” trials, one every 20 mins, participants were instructed to choose any combination of the two sprays, such that they self-administered a total of 8 sprays during each trial. The number of times nicotine was chosen (out of 32 total opportunities) was taken as the measure of reinforcement.
Genotyping
We performed the genotyping of the SNPs using the GoldenGate Assay on the Illumina platform (Illumina, San Diego, CA) at the Genomics Core Facility at USC/Norris Comprehensive Cancer Center (Director, David Van Den Berg). The assay utilizes a multiplexed oligonucleotide ligation assay (OLA) on genomic DNA and PCR amplification with universal primers. For additional details see http://www.illumina.com/products/prod_snp.ilmn. Data from the assay array is read by the BeadArray Reader (Illumina) and genotype calling is performed in BeadStudio software (Illumina, Inc.) using the genotyping module. The system includes automated protocols for the entire genotyping process utilizing robotics, barcoding, and extensive data and process tracking to ensure high call rates and accuracy. Additionally, genotyping quality control was further monitored by the inclusion of replicates and CEPH trios. Amplification by PCR was as described elsewhere (George et al., 1993; Lesch et al., 1996; Vandenbergh et al., 2002). Results were analyzed using GeneMarker® v1.5 (SoftGenetics) software.
The coding of genotypes for analysis, based on prior literature, was as follows: 1) DRD2 C957T, rs6277 (CC vs. CT vs. TT); 2) 5HTTLPR (or SLC6A4, presence or absence of the short allele); 3) DRD4 VNTR (presence or absence of the 7-repeat allele); 4) SLC6A3, rs27072 (presence or absence of the 9-repeat allele); 5) OPRM1 A118G, rs1799971 (presence or absence of the G allele); and 6) DRD2/ANKK1, rs1800497 (TT or CT vs. CC; note: T is the less common Taq1A allele).
Data Analysis
All analyses were conducted using SPSS 15.0. For most measures, response to each dose (0, 5, 10 μg/kg) was defined by change from pre-dose baseline to the post-dose mean of responses across the three dose administrations per session. Cortisol response was taken as the difference between the baseline sample and the single post-session sample. For spray ratings of reward and perception, and for startle and PPI, responses to each dose were examined directly, rather than as changes from baseline. Reinforcement was determined simply by the number of times nicotine spray (1.25μg/kg/spray) was chosen versus placebo spray on the separate session devoted to this measure.
We first determined any influence of the order of doses (6 orders) across sessions on responses, but found no such order effects, allowing us to collapse across order. Differences in responses to nicotine dose as a function of each gene were analyzed separately for each dependent measure using analysis of covariance (ANCOVA). Gene and sex were the between-subjects factors, and dose was the within-subjects factor. Only two effects were of interest: the interactions of gene x dose, which would suggest differential sensitivity across nicotine doses associated with genotype, and gene x dose x sex, indicating that the genetic association varies between men and women. The measure of sensory irritation from the spray was included as a covariate in all analyses. Because of the numerous mood and performance measures, analyses of effects on those responses were first conducted using multivariate ANCOVA (MANCOVA), to reduce the number of comparisons. Significant MANCOVA results were followed up with univariate ANCOVAs. For the reinforcement measure, there was no dose effect (i.e. subjects chose either nicotine or placebo), and so ANCOVAs analyzed the influences of gene and gene x sex.
RESULTS
The distribution of genotypes for each gene, by sex, is presented in Table 1. Because no analyses were significant for the OPRM1 A118G SNP, this gene will not be discussed further.
Table 1.
Frequencies of alleles for each gene.
| Males | Females | Total | ||
|---|---|---|---|---|
| DRD2 C957T | TT | 10 (28.5%) | 23 (36%) | 33 |
| CT | 15 (42%) | 29 (45%) | 44 | |
| CC | 10 (28.5%) | 12 (19%) | 22 | |
| 5HTTLPR (SLC6A4) | Absence of Short | 13 (37%) | 23 (35%) | 36 |
| Presence of Short | 22 (63%) | 42 (65%) | 64 | |
| DRD4 | Absence of 7 | 24 (69%) | 37 (57%) | 61 |
| Presence of 7 | 11 (31%) | 28 (43%) | 39 | |
| SLC6A3 | Absence of 9 | 13 (37%) | 24 (37%) | 37 |
| Presence of 9 | 22 (63%) | 41 (63%) | 63 | |
| OPRM1 | AA | 30 (86%) | 47 (73%) | 77 |
| AG or GG | 5 (14%) | 17 (27%) | 22 | |
| DRD2/ANKK1 | TT or TC | 10 (29%) | 18 (30%) | 28 |
| CC | 24 (71%) | 42 (70%) | 66 |
Nicotine Reward and Perception
Nicotine reward and perception measures were associated with genetic variation, but only in men. The interaction of 5HTTLPR x dose x sex was significant for “liking”, F (2,190) = 3.20, p = .043. As shown in Figure 1, compared to placebo, 5 μg/kg (but not 10 μg/kg) nicotine decreased liking more in men in the absence versus presence of the short allele. The nicotine perception measure of “feel the effects” was associated with the interactions of DRD2 C957T x dose, F (4,184) = 2.49, p = .045, DRD2 C957T x dose x sex, F (4,184) = 2.55, p = .041, and DRD4 x dose x sex, F (2,190) = 4.26, p = .015. As also shown in Figure 1, “feel the effects” was increased in men by 5 μg/kg nicotine more among those with the DRD2 C957T TT versus CT or CC genotype, and by 10 μg/kg nicotine more among those with the DRD2 C957T TT or CT versus CC genotype. Nicotine also increased “feel the effects” in men more in the presence versus absence of the DRD4 7 allele (Figure 1). Neither gene was associated with this response in women.
Figure 1.
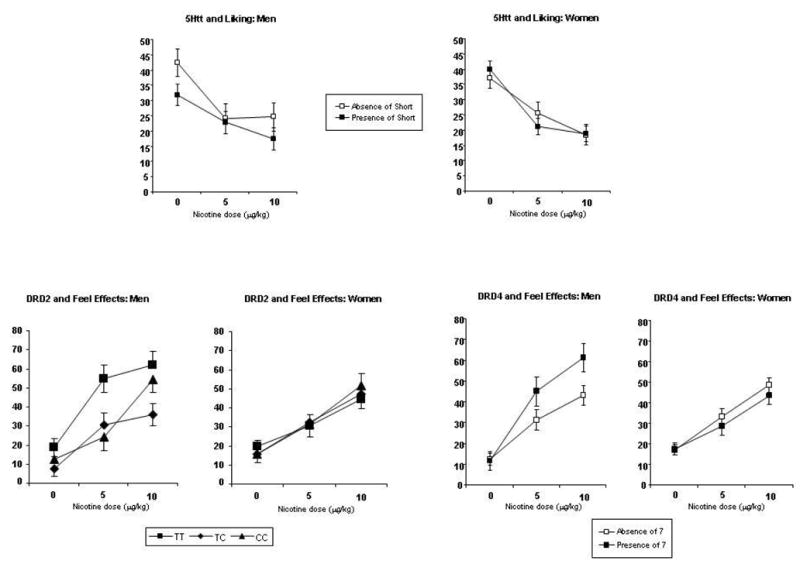
Mean (SEM) dose-response effects of nicotine (0, 5, and 10 μg/kg) on nicotine spray reward (“liking”) and perception (“feel the effects”), by sex and 5HTTLPR (SLC6A4), DRD2, and DRD4 genotypes.
Mood
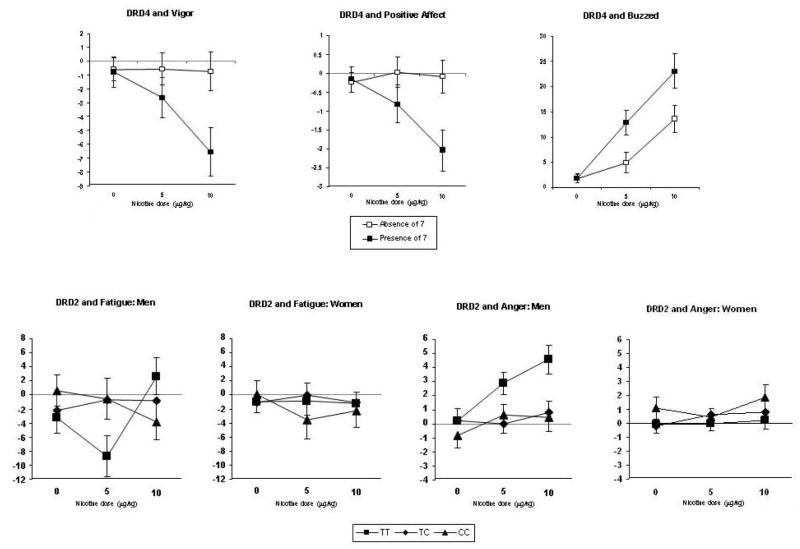
In the MANCOVAs of mood responses, DRD4 x dose, F(24,360) = 1.63, p = .033, and the interaction of DRD2 C957T x dose x sex, F(48, 704) = 1.39, p = .045, were significant. No other genetic associations with mood responses were apparent from the multivariate analyses. In follow-up univariate analyses, DRD4 x dose effects were significant for buzzed, positive affect, and vigor, F(2,190)’s= 6.15, p = .003, 4.44, p = .013, and 3.18, p = .044, respectively. As shown in Figure 2, nicotine increased buzzed and decreased positive affect and vigor more among those with the DRD4 7 allele versus those without the 7 allele. Regarding DRD2 C957T, the interaction effects of DRD2 x dose x sex were significant for fatigue and anger, F (4,184)’s = 2.56, p=.040, and 2.68, p = .033, respectively. In men, but not women, those with the TT allele showed decreased fatigue in response to 5 μg/kg nicotine and increased anger in response to both doses, compared to those with the CT or CC allele (Figure 2).
Figure 2.
Mean (SEM) dose-response effects of nicotine on mood responses, by DRD4 and DRD2 C957T genotypes and, where relevant, by sex.
Physiological
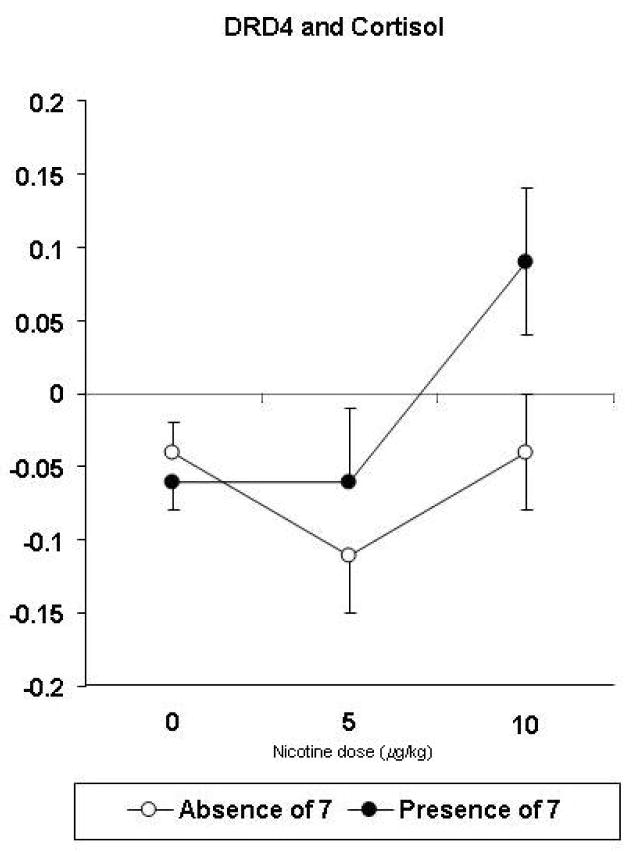
No significant effects of gene x dose, or of gene x dose x sex, were seen for the cardiovascular responses to nicotine. An interaction of DRD4 x dose, F(2,91) = 3.16, p=.047, was seen for the change in saliva cortisol concentration. As shown in Figure 3, cortisol increased in response to 10 μg/kg in those with the DRD4 7 allele, compared to those without that allele.
Figure 3.
Mean (SEM) dose-response effects of nicotine on cortisol responses (ng/ml) by DRD4 genotypes.
Sensory processing
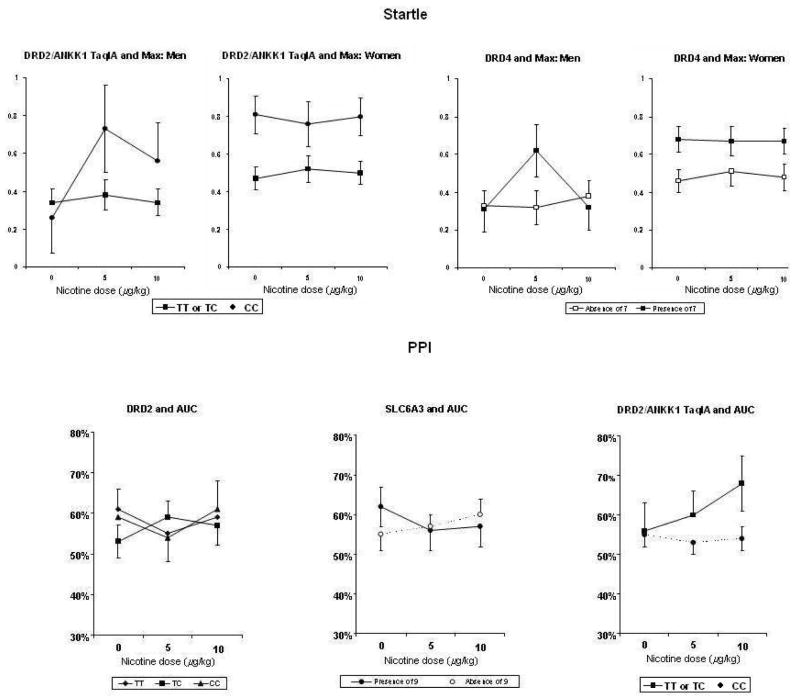
Startle response was associated with DRD4 and DRD2/ANKK1, while PPI was associated with DRD2 C957T, SLC6A3, and DRD2/ANKK1. Interactions of DRD4 x dose x sex were found for both startle measures: area under the curve (AUC), F(2,152)= 3.51, p=.032, and maximum peak (max), F(2,152)= 5.32, p=.006. Similarly, interactions of DRD2/ANKK1 x dose x sex were found for startle max, F(2,140)=3.66, p=.028. As shown in Figure 4, startle response of men to 5 μg/kg, but not 10 μg/kg (i.e. curvilinear), was greater in those with the presence of the DRD4 7 allele and in those with the DRD2/ANKK1 CC allele. (For DRD4, only max startle is shown in Figure 4, to save space. Results for AUC startle were virtually identical.)
Figure 4.
Mean (SEM) dose-response effects of nicotine on startle response (top) and on pre-pulse inhibition (PPI) of startle (bottom), by DRD4, DRD2 C957T, SLC6A3, and DRD2/ANKK1 genotypes and, where relevant, by sex. Larger values indicate greater startle (top) and reduced inhibition of startle (bottom). DRD4 effects on the AUC measure of startle (not shown) were virtually identical to those shown here for max startle. Similarly, SLC6A3 and DRD2/ANKK1 effects on the max measure of PPI (not shown) were virtually identical to those shown here for the AUC measure of PPI.
For the PPI AUC measure, interactions of gene x dose were seen for DRD2 C957T, F(4, 146) = 2.56, p=.041, SLC6A3, F(2,152) = 4.32, p=.015, and DRD2/ANKK1, F(2,140)=4.55, p=.012. Similarly, for the PPI max measure, the gene x dose interaction was significant for SLC6A3, F(2,152) = 4.96, p=.008, and DRD2/ANKK1, F(2,140)=3.01, p=.052. PPI tended to worsen (i.e. reduced inhibition of startle) in those with the DRD2 C957T CT genotype (at 5 μg/kg only), with the absence of the SLC6A3 9 allele, and with the DRD2/ANKK1 CC genotype (at 10 μg/kg only), compared to the other genotypes (Figure 4). (For SLC6A3 and DRD2/ANKK1, only the AUC measure of PPI is shown in Figure 4, to save space. Results for max PPI were virtually identical.)
Performance tasks
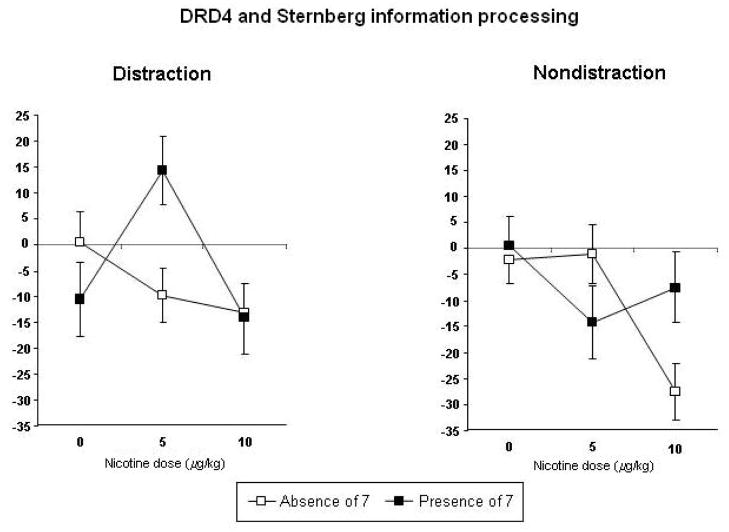
MANCOVAs showed that only DRD4 x dose effects were significant for performance task responses, F(26,354) = 1.56, p=.043. In follow-up univariate analyses, DRD4 x dose effects were significant only for Sternberg task performance (correct rejections) under either the distraction, F (2,188) = 3.37, p=.037, or non-distraction (i.e. standard) conditions, F(2,188) = 3.01, p=.052, although the pattern of results differed depending on the distraction condition. As shown in Figure 5, compared to those with the DRD4 7 allele, absence of the 7 allele was associated with better performance in response to 5 μg/kg nicotine under the distraction condition, and with better performance in response to 10 μg/kg under the non-distraction condition.
Figure 5.
Mean (SEM) dose-response effects of nicotine on Sternberg rapid information processing performance (d-prime for “correct rejections” of non-targets), under non-distraction (standard) and distraction conditions, by DRD4 genotypes. Values are change from baseline in reaction time, in msec. Decreases indicate faster information processing.
Reinforcement
Nicotine choice was associated only with DRD4, F(1,95) = 5.83, p=.018. Those with an absence of the DRD4 7 allele chose nicotine more than those with presence of the 7 allele (13.4±1.3 vs 8.1±1.7 respectively). No gene or gene x sex effects were significant for the other genes examined. However, in an exploratory analysis, the interaction of 5HTTLPR x sex was marginally significant, F(1,95) = 3.02, p=.085, as men chose nicotine twice as often as did women among those homozygous for the long allele (15.9±2.9 vs 7.7±2.2), while there was no sex difference among those with the short allele (11.8±2.3 vs 11.6±1.6 for men and women, respectively).
DISCUSSION
To our knowledge, this study represents the first test of the association between genetic variation and initial sensitivity to acute nicotine responses in humans (i.e. in those essentially naïve to nicotine). Results should be considered very preliminary, given the relatively small sample and the number of genes and comparisons examined. However, our findings suggest that genetic variants purported to influence dopamine receptor function, particularly DRD4, were associated with initial sensitivity to mood and other effects of acute nicotine administration, and these effects often differed as a function of subject sex.
For DRD4, compared with those without the 7 allele, presence of the 7 allele was associated with increased sensitivity to mood (buzzed, decreased vigor and positive affect), nicotine perception (“feel effects”), and startle responses, but only in men. Regardless of sex, presence of the DRD4 7 allele was also associated with increased cortisol response and impaired rapid information processing performance. Together with the observed lower nicotine choice behavior, these findings suggest that presence of the DRD4 7 allele is associated with greater aversive responses to nicotine. Two prior studies in adult smokers have shown that the 7 allele is associated with increased risk of relapse in smoking cessation treatment (Shields et al., 1998; David et al., in press). Thus, our data suggest that greater aversive responses in the DRD4 7 group may reflect increased sensitivity to nicotine’s effects, which may be protective with regard to smoking onset in teens. However, among those who become smokers, those carrying the DRD4 7 repeat allele may become tolerant to these initially aversive responses, fostering greater subsequent risk of nicotine dependence. This notion is consistent with the “sensitivity” model (Pomerleau 1995) and with preclinical studies suggesting that rodent strains most sensitive to nicotine upon initial exposure tend to show the greatest and most rapid tolerance to such effects (Marks et al. 1991). Yet, far more research is needed before the “sensitivity” model, and DRD4 gene associations with sensitivity, can be adequately evaluated.
Associations of nicotine sensitivity with other genes believed related to dopamine function were less commonly found. In other analyses, we examined two putative functional SNPs related to the D2 dopamine receptor, DRD2 C957T and DRD2/ANKK1, and polymorphisms in the dopamine transporter, SLC6A3. Similar to DRD4, the DRD2 C957T variant was associated with anger and fatigue responses, as well as nicotine perception, across nicotine doses but only in men. The C957 allele has been associated with increased mRNA stability in vitro (Duan et al., 2003), while the T957 allele has been associated with increased receptor binding potential in vivo (Hirvonen et al. 2004). Thus, our findings appear more consistent with prior in vivo data than prior in vitro data. The second SNP (DRD2/ANKK1), located about 10kb upstream of DRD2 in the kinase gene ANKK1, was related only to startle (in men) and PPI responses to nicotine. Similarly, the dopamine transporter SLC6A3 variant was related only to PPI response.
Very few significant associations with nicotine sensitivity were seen for the genes not believed to be linked to dopamine function. The serotonin transporter 5HTTLPR variant was associated with nicotine reward (liking) in men but not in women. Similarly, in an exploratory comparison, among those homozygous for the 5HTTLPR long allele, men tended to choose nicotine more than did women, while there was no sex difference among those with the short allele. Finally, none of the genetic factors was associated with cardiovascular responses to nicotine, and none of the phenotypes examined was associated with the OPRM1 A118G SNP. While the absence of a finding for OPRM1 A118G may appear inconsistent with prior associations with smoking behavior and cessation (Lerman et al., 2003; 2004; Ray et al., 2006), these previous studies focused on nicotine dependent adults while the present study focused on nicotine sensitivity in nicotine-naïve subjects. Thus, as with our findings regarding DRD4, discussed previously, the role of these genes may be dependent on the particular smoking phenotype examined, such as vulnerability to onset of dependence versus smoking persistence after the establishment of dependence.
Our results may have implications for understanding sex differences in the discriminative stimulus effects and perhaps other effects of nicotine, as some research with dependent smokers indicates such effects are less pronounced in women compared to men (Perkins 1999; in press). We found that DRD2 and DRD4 variants influenced nicotine perception (“feel effects”), as well as some mood (“anger”, “fatigue”) responses, but only in men and not women. These observations suggest that, rather than being due simply to sex per se, many differences in nicotine responses between men and women may be mediated, or at least moderated, by particular genes. Although other research suggests that sex differences in nicotine responses of smokers may be moderated by genes (Yudkin et al. 2004; Ray et al. 2006), our results go beyond those findings to indicate that genes may moderate sex differences in responses among nicotine naïve individuals, prior to the onset of dependence.
Aside from the novelty of our study focus, genetic associations with initial nicotine sensitivity in never-smokers, many of the methods of this study were novel and constitute key strengths. One strength was our conservative lifetime tobacco use cutoff for inclusion (<10 uses), as well as exclusion of anyone with any use in the prior 3 years, to minimize the possibility of chronic adaptation to nicotine effects (i.e. tolerance or sensitization; Perkins 2002), which would alter subjects’ sensitivity across doses. Our criterion is more stringent than that used in most other research, which typically defines “ever-smoker” as those with at least 100 lifetime uses (e.g. Giovino 2002), while those with less than 100 are non- or never-smokers. Another strength of the study was the prospective assessment of nicotine responses, which included standardized measures of responses to nicotine other than self-report, as opposed to the retrospective self-report of early responses to nicotine via smoking in past studies of initial sensitivity (e.g. Pomerleau et al. 1998). The broad array of responses was also a strength, allowing us to determine whether genetic associations may be specific to some but not other nicotine effects, although the large number of responses also raises concerns about the number of comparisons (see below). Other strengths of this study included the controlled dosing of nicotine and correction of doses for body weight, inclusion of placebo and more than one dose of nicotine, and use of nasal sensory irritation as a covariate to rule out differential irritation during testing as an explanation for differences in nicotine sensitivity.
On the other hand, this study had several clear limitations. One major limitation was the relatively small sample size of 101, which produced small genetic subgroups, although this sample is larger than past research assessing responses to nicotine in nonsmokers (see Perkins 2002). This problem reflects the practical conflict between studying a limited amount of data from a large sample versus extensive phenotyping of a smaller sample (e.g., prospectively assessing many different responses to multiple nicotine doses across sessions, as in this study). As such, we may have had inadequate power to detect other genetic influences on acute nicotine responses. Somewhat in contrast, another major limitation is that some of the findings could have occurred by chance, given that we examined 6 genes, multiplying the number of comparisons for each measure of interest. To reduce the likelihood of chance findings, we focused solely on just two effects for each gene, the interactions of gene x dose and gene x dose x sex, given our interest in determining the association of genes with response to nicotine dose and how sex may moderate that association. We also limited the number of comparisons for mood and performance measures by employing multivariate analyses, conducting analyses of individual mood and performance responses only when the overall multivariate results were significant for either of these interactions involving gene x dose. The fact that many of the significant interactions involved DRD4 suggests that results for that gene were not due to chance, although the few significant effects for the other genes could have been due to chance. Given the complete lack of prior research on genetic associations with prospectively assessed initial nicotine sensitivity, this study was not intended to be definitive but rather exploratory and heuristic in nature. This study should be replicated with larger samples to verify the findings, particularly those for DRD4.
In addition, despite its advantages, our nicotine administration procedure was also a limitation of the study. We chose this nicotine nasal spray procedure to provide a fairly rapid method of delivery of controlled nicotine doses. However, sensitivity to nicotine via nasal spray may not relate to sensitivity to nicotine via cigarette smoking, although one study with smokers suggested generally comparable acute mood responses to nicotine between these methods (Perkins et al. 1994b). Even more rapid uptake of nicotine by smoking, or stimuli accompanying smoking (e.g. taste, smell) but not nasal spray, may influence genetic associations with initial sensitivity to nicotine. Thus, although our method may have allowed for assessment of initial sensitivity to nicotine per se, sensitivity to nicotine via cigarette smoke inhalation may be affected differently by genetic factors. In addition, the doses used were fairly low, and greater influences of genetic factors on nicotine sensitivity may be more apparent in acute testing with larger doses of nicotine. Finally, we recruited young adults, rather than adolescents, due to ethical and practical concerns about giving nicotine to naïve adolescents. Adolescence is the age at which those who become smokers usually experience initial nicotine exposure, and genetic factors may have more influence on nicotine sensitivity at this age.
In summary, although these findings are preliminary and require replication, they suggest that initial sensitivity to some acute responses to nicotine is associated with specific genetic variants thought to be related to dopamine function, especially in men. In particular, these findings suggest that presence of the DRD4 7 allele is associated with increased sensitivity to nicotine’s aversive effects. Gene variants thought to be linked to the function of the dopamine D2 receptor showed some association with other nicotine responses, but most of the genes examined here were not consistently associated with initial nicotine sensitivity. Also, these genetic associations may be specific to only this phenotype, initial sensitivity to nicotine, and may not be seen in other phenotypes related to tobacco exposure (e.g. persistence of smoking). Future research should examine whether these genetic factors influence initial sensitivity to nicotine via smoking, if possible to do ethically and practically in a naïve sample, and should explore whether these genes are related to risk of nicotine dependence in teens with any past exposure to tobacco. Such findings may demonstrate that variability in initial sensitivity to nicotine is a mechanism by which genetic factors promote vulnerability to onset of nicotine dependence.
Acknowledgments
This research was supported by NIDA Grants DA08578 (KAP), P50 CA/DA84718 (CL), and U01 DA20830 (NLB, CL, AWB). The authors thank David Conti, Carolyn Fonte, John Scott, Roy Chengappa, Nancy Petro, Denise Nishita, and Yungang He for their assistance.
Footnotes
Disclosure/Conflict of Interest
Dr Perkins, Ms. Coddington, Mr. Jetton, Mr. Karelitz, Dr. Wilson, Dr. Jennings, Dr. Ferrell, and Dr. Bergen have no disclosures to declare. Dr. Lerman has served as a consultant, and has received research funding unrelated to the present work, from Pfizer, GlaxoSmithKline, and AstraZeneca. Dr Benowitz has been a paid consultant to a number of pharmaceutical companies that market or are developing medications for smoking cessation. He has also been a paid expert witness in litigation against the tobacco industry in issues related to nicotine addiction.
References
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exper Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Audrain-McGovern J, Nigg J, Perkins KA. Endophenotypes for nicotine dependence risk at or before initial nicotine exposure. National Cancer Institute Monograph 22, Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence in press. [Google Scholar]
- Audrain-McGovern J, Lerman C, Wileyto EP, Rodriguez D, Shields PG. Interacting effects of genetic predisposition and depression on adolescent smoking progression. Amer J Psychiatr. 2004;161:1224–1230. doi: 10.1176/appi.ajp.161.7.1224. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Wileyto EP, Schmitz KH, Shields PG. Effect of team sport participation on genetic predisposition to adolescent smoking progression. Arch Gen Psychiatr. 2006;63:433–441. doi: 10.1001/archpsyc.63.4.433. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Rice JP, Edenberg HJ, Goate A, Foroud T, Cloninger CR, et al. Family-based study of the association of the dopamine D2 receptor gene (DRD2) with habitual smoking. Amer J Med Genet. 2000;90:299–302. doi: 10.1002/(sici)1096-8628(20000214)90:4<299::aid-ajmg7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, Lerman C. Reduced nicotine reward in obesity: cross comparison in human and mouse. Psychopharmacol. 2005;180:306–315. doi: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Blitstein JL, Robinson LA, Murray DM, Klesges RC, Zbikowski SM. Rapid progression to regular cigarette smoking among nonsmoking adolescents: interactions with gender and ethnicity. Prev Med. 2003;36:455–463. doi: 10.1016/s0091-7435(02)00041-5. [DOI] [PubMed] [Google Scholar]
- Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenet. 1996;6:73–79. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Murphy MF, Proctor M, Walton RT, Johnstone EC. Genetic variation in the dopamine D4 receptor (DRD4) gene and smoking cessation: follow-up of a randomised clinical trial of transdermal nicotine patch . Pharmacogenomics J. 8:122–128. doi: 10.1038/sj.tpj.6500447. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Human Molecular Genetics. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- Duncan E, Madonick S, Chakravorty S, Parwani A, Szilagyi S, Efferen T, et al. Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacol. 2001;156:266–272. doi: 10.1007/s002130100719. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend. 2000;59 (Suppl 1):S41–60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Fischman M, Foltin RW. Limited sex differences in response to “binge” smoked cocaine use in humans. Neuropsychopharmacol. 1999;21:445–454. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: a review. Biol Psychol. 1998;47:1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- George SR, Cheng R, Nguyen T, Israel Y, O'Dowd BF. Polymorphisms of the D4 dopamine receptor alleles in chronic alcoholism. Biochem Biophys Res Commun. 1993;196:107–114. doi: 10.1006/bbrc.1993.2222. [DOI] [PubMed] [Google Scholar]
- Gerra G, Garofano L, Zaimovic A, Moi G, Branchi B, Bussandri M, et al. Association of the serotonin transporter promoter polymorphism with smoking behavior among adolescents. Amer J Med Genet, Part B, Neuropsychiatr Genet. 2005;135:73–78. doi: 10.1002/ajmg.b.30173. [DOI] [PubMed] [Google Scholar]
- Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- Grobe JE, Perkins KA, Goettler-Good J, Fonte C. Importance of environmental distractors in the effects of nicotine on short-term memory. Exper Clin Psychopharmacol. 1998;6:209–216. doi: 10.1037//1064-1297.6.2.209. [DOI] [PubMed] [Google Scholar]
- Hirschman RS, Leventhal H, Glynn K. The development of smoking behavior: conceptualization and supportive cross-sectional survey data. J App Soc Psychol. 1984;14:184–206. [Google Scholar]
- Hirvonen M, Laakso A, Nagren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatr. 2004;9:1060–1061. doi: 10.1038/sj.mp.4001561. Erratum in: Mol Psychiatr. 2005;10: 889. [DOI] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Amer J Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. The effects of smoking high nicotine cigarettes on prepulse inhibition, startle latency, and subjective responses. Psychopharmacol. 2000;150:244–252. doi: 10.1007/s002130000399. [DOI] [PubMed] [Google Scholar]
- Jacob P, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatography. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nic Tob Res. 2005;7:317–333. doi: 10.1080/14622200500125385. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacol. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- Lerman CE, Schnoll RA, Munafò MR. Genetics and smoking cessation: Improving outcomes in smokers at risk. Am J Prev Med. 2007;33(6S):S398–S405. doi: 10.1016/j.amepre.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH, Jr, Pinto A, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol. 2003;22:541–548. doi: 10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J. 2004;4:184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Ling D, Niu T, Feng Y, Xing H, Xu X. Association between polymorphism of the dopamine transporter gene and early smoking onset: an interaction risk on nicotine dependence. J Human Genet. 2004;49:35–39. doi: 10.1007/s10038-003-0104-5. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. J Pharmacol Exp Ther. 1991;259:392–402. [PubMed] [Google Scholar]
- McNair DM, Loor M, Droppelman LF. Profile of Mood States. San Diego CA: Educational and Testing Service; 1971. [Google Scholar]
- Munafò MR, Clark TG, Johnstone EC, Murphy MFG, Walton RT. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nic Tob Res. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC, Wileyto EP, Shields PG, Elliot KM, Lerman C. Lack of association of 5-HTTLPR genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiol Biomarkers Prev. 2006;15:398–400. doi: 10.1158/1055-9965.EPI-05-0648. [DOI] [PubMed] [Google Scholar]
- O'Connor RJ, Kozlowski LT, Vandenbergh DJ, Strasser AA, Grant MD, Vogler GP. An examination of early smoking experiences and smoking status in a national cross-sectional sample. Addiction. 2005;100:1352–1357. doi: 10.1111/j.1360-0443.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Nicotine discrimination in men and women. Pharmacol Biochem Behav. 1999;64:295–299. doi: 10.1016/s0091-3057(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nic Tob Res. 2002;4:405–422. doi: 10.1080/1462220021000018425. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Discriminative stimulus effects of nicotine in humans. In: Henningfield JE, London E, Pogun S, editors. Nicotine Psychopharmacology. New York: Springer-Verlag; in press. [Google Scholar]
- Perkins KA, Broge M, Gerlach D, Sanders M, Grobe JE, Cherry C, et al. Acute nicotine reinforcement, but not chronic tolerance, predicts withdrawal and relapse after quitting smoking. Health Psychol. 2002;21:332–339. doi: 10.1037//0278-6133.21.4.332. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula AR. Sex differences in the influence of nicotine and dose instructions on subjective and reinforcing effects of smoking. Psychopharmacol. 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Stiller R, Jennings JR, Christiansen C, McCarthy T. An aerosol spray alternative to cigarette smoking in the study of the behavioral and physiological effects of nicotine. Behav Res Meth Instr Comput. 1986;18:420–426. [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Fonte C, Wilson A. Reinforcing effects of nicotine as a function of smoking status. Exper Clin Psychopharmacol. 2001b;9:243–250. doi: 10.1037//1064-1297.9.3.243. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Sanders M, Fonte C, et al. Dissociation of nicotine tolerance from tobacco dependence in humans. J Pharmacol Exper Ther. 2001a;296:849–856. [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Fonte C, Goettler J, Caggiula AR, Reynolds WA, et al. Chronic and acute tolerance to subjective, behavioral, and cardiovascular effects of nicotine in humans. J Pharmacol Exper Ther. 1994a;270:628–638. [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Weiss D, Fonte C, Caggiula A. Nicotine preference in smokers as a function of smoking abstinence. Pharmacol Biochem Behav. 1996;55:257–263. doi: 10.1016/s0091-3057(96)00079-2. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, Reynolds WA, Grobe JE, Fonte C, Stiller RL. Comparison of acute subjective and heart rate effects of nicotine intake via tobacco smoking vs. nasal spray. Pharmacol Biochem Behav. 1994b;47:295–299. doi: 10.1016/0091-3057(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Snedecor SM, Gaulrapp S, Kardia SL. Heterogeneity in phenotypes based on smoking status in the Great Lakes Smoker Sibling Registry. Addict Behav. 2004;29:1851–1855. doi: 10.1016/j.addbeh.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet. 1995;25:161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Ray R, Jepson C, Patterson F, Strasser AA, Rukstalis M, Perkins K, et al. Association of OPRM1 Asn40Asp variant with the relative reinforcing value of nicotine in female smokers. Psychopharmacol. 2006;188:355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Meehan SM, Schechter JB. Genetic selection for nicotine activity in mice correlates with conditioned place preference. Eur J Pharmacol. 1995;279:59–64. doi: 10.1016/0014-2999(95)00139-c. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin R. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatr. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Shields P, Lerman C, Audrain J, Bowman ED, Main D, Boyd NR, Caporaso NE. Dopamine D4 receptors and the risk of cigarette smoking in African-Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:453–458. [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacol. 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ, McAfee T. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychol. 2007;26:361–368. doi: 10.1037/0278-6133.26.3.361. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Braff DL, Geyer MA. The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J Psychopharmacol. 1992;6:176–190. doi: 10.1177/026988119200600210. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Bennett CJ, Grant MD, Strasser AA, O'Connor R, Stauffer RL, et al. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: failure to replicate and finding that never-smokers may be different.[see comment] Nic Tob Res. 2002;4:333–340. doi: 10.1080/14622200210142689. [DOI] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Person Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yudkin P, Munafo M, Hey K, Roberts S, Welch S, Johnstone E, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: randomized controlled trial. Br Med J. 2004;328:989–990. doi: 10.1136/bmj.38050.674826.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]