Abstract
An α-galactosyl ceramide (α-GalCer) 2 was synthesized and evaluated for its ability to stimulate iNKT-cell proliferation and elicit T-helper cytokines, IL-4 and IFNγ. Compound 2 combines the acyl chain of the potent, Th2 biasing α-GalCer 1 with a sphingoid base of the same length as that found in OCH, which also exhibits Th2 skewing, Such complementation may enhance cytokine bias, which is thought to be important for therapeutic applications of iNKT cell stimulation. Two related α-GalCers, 3 and 4, with saturated acyl chains were prepared for comparison.
CD1d has been shown to present lipid antigens for recognition by the T cell receptors (TCRs) of a specialized subset of lymphocytes known as invariant natural killer T (iNKT) cells. Over the past decade CD1d activated iNKT cells have been demonstrated to elicit a range of immune responses with potential implications for treating viral and bacterial infections, cancer and a variety of autoimmune conditions.1–8 KRN7000, an α-galactosyl ceramide (α-GalCer) identified by the Kirin brewery company in SAR studies around glycolipids isolated from the Agelus genus of marine sponge,9 has emerged as the key lipid for probing CD1d activation of iNKT cells.
In addition to the induction of a rapid expansion of iNKT cells, KRN7000 elicits a burst of both T helper 1 (Th1) and Th2 cytokines (IFNγ and IL-4, respectively, are the ones generally monitored). Th1 cytokines support cell-mediated immunity, important for the elimination of viruses and other intracellular pathogens. Th2-type cytokines provide humoral immunity, which induces antibody production. Autoimmune conditions, such as diabetes and multiple sclerosis, are characterized by a Th1 bias involving high Th1/Th2 cytokine ratios. Conversely, many types of cancer are characterized by predominant Th2 cytokines. It is believed that the anti-tumor, antiviral and antibacterial effects of KRN7000 are mediated by the high level of Th1 cytokines it elicits, while the ability of KRN7000 to prevent the onset of autoimmune conditions is thought to arise from its induction of Th2 cytokines. It is now apparent that the opposing effects induced by Th1 and Th2 cytokines would preclude the therapeutic use of KRN7000,10 as it induces high levels of both types of cytokines.
One way to overcome the opposing activities seen with KRN7000 would be to identify compounds selective for Th1 or Th2 cytokines. That this is possible was demonstrated in 2001, when Experimental Autoimmune Encephalomyelitis (EAE), a prototypical autoimmune disease, was prevented in B6 mice by a single injection of OCH, a truncated analog of KRN7000.11 This remarkable result appeared to be mediated by the enhanced relative production of IL-4 to IFNγ by OCH in comparison to KRN7000. OCH, however, is not potent, and it is thought that a more active compound with a similar Th2 bias would be needed for therapeutic applications. In looking at the effect of variations in the acyl chain on the ability of α-GalCers to stimulate NKT cells and elicit various cytokines, we identified 1 as a potent, Th2 biasing α-GalCer.12,13 In light of the enhanced potency of 1, we decided to evaluate its truncated sphingoid base analog 2 to see if the potency was maintained and/or the Th2 skewing was enhanced. We chose to synthesize a sphinganine-containing α-GalCer, rather than one with a phytosphingosine (as in OCH) since sphinganines, aminodiol sphingoid bases, are more easily prepared than phytosphingosines, which are aminotriols. Moreover, sphinganine-containing α-GalCers provide similar activities and cytokine profiles to those of phytosphingosine-containing α-GalCers in standard murine assays.14 The saturated analog 3 of 2, as well as its phytosphingosine counterpart 4, were prepared for comparison purposes.
Compounds 2–4 were prepared by approaches we have previously used.15 For the preparation of 2 we employed our recently reported glycosylated sphinganine-containing sphingoid base 5, prepared in seven steps from serine-derived Weinreb amide 6.16 Acylation of 5 with the p-nitrophenyl ester of eicos-11,14-dienoate (10% yield) provided 2. The low yield of the coupling was likely related to the solubility of 5 under the reaction conditions, but this was not further explored, since a sufficient quantity of 2 was obtained for these preliminary studies.
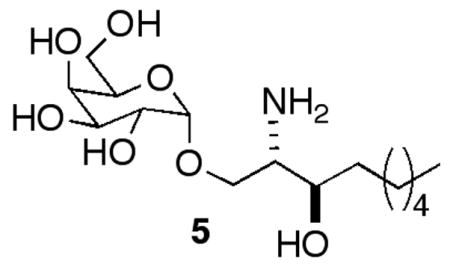
Compound 5
Compound 3 was prepared as shown in Scheme 1 from Weinreb amide 6 using methodology we had developed to prepare the sphinganine analogs of KRN7000 and OCH.14 Because of a prior issue with silyl group migrations we decided to evaluate the utility of a MOM protecting group in the protocol. As previously described, treatment of 6 with two equivalents of a sacrificial base, followed by reaction with n-hexylmagnesium bromide and MOM protection of the OH provided ketone 7. Diastereoselective reduction and benzylation were highly efficient, and one-pot cleavage of the MOM and Boc groups gave an aminoalcohol. Acylation with the p-nitrophenyl ester of eicosanoic acid provided ceramide 8. Coupling with 2,3,4,6-tetra-O-benzyl-β-D-galactopyranosyl fluoride and hydrogenolysis provided the saturated analog 3 of diene 2.
Scheme 1.
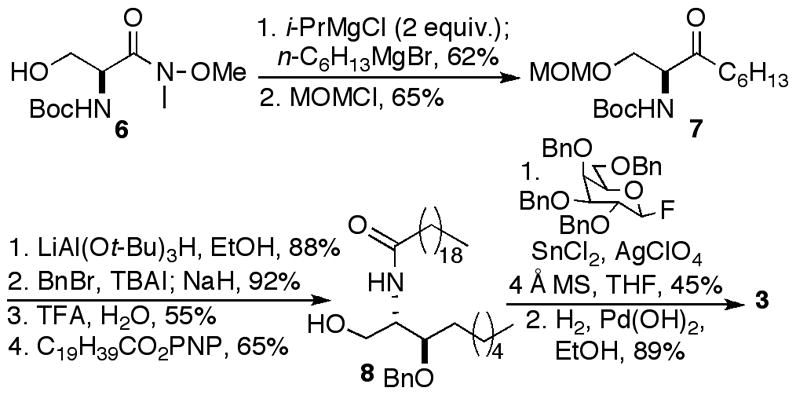
The synthesis of phytosphingosine analog 4 began with C9-ribo-phytosphingosine 9, synthesized in our previously reported preparation of OCH (Scheme 2).14 Trisilylation of 9 resulted in concomitant cleavage of the Boc group. Acylation and selective removal of the primary silyl group provided ceramide 10. Glycosylation with 2,3,4,6-tetra-O-benzyl-β-D-galactopyranosyl fluoride, cleavage of the silyl groups and hydrogenolysis provided 4.
Scheme 2.
Since our goal was to compare how shortening the sphingoid base moiety of 1 impacted activity and cytokine biasing, analogs 2–4 were evaluated in parallel with 1 and OCH. As can be seen in Figure 3, although 2–4 stimulated proliferation in mouse splenocytes, as measured by tritiated thymidine incorporation,17 none was nearly as potent as 1. Moreover, there appeared to be no advantage conferred by the presence of double bonds in the acyl chain. The profile of 2 paralleled that of OCH, and analogs 3 and 4 were slightly more stimulatory. The outcome with 2 vs. 3/4 was in contrast to the effect seen with 1 in comparison to its fully saturated analog, where the former was much more potent.12
Figure 3.
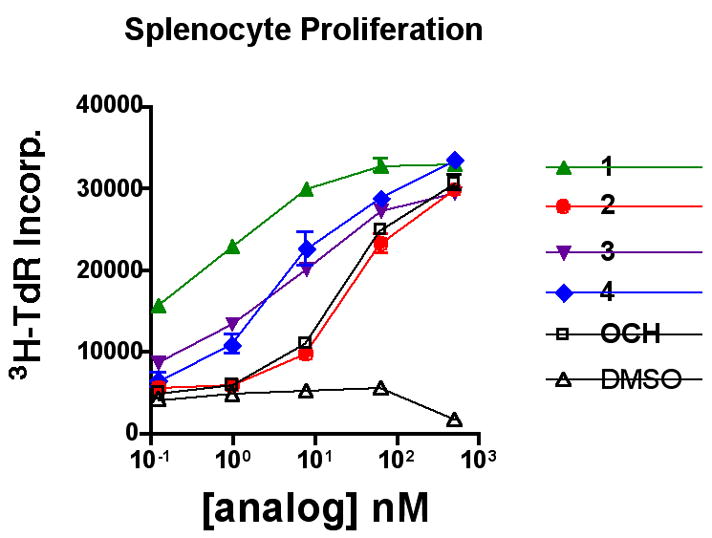
Splenocyte proliferation measured by tritiated thymidine incorporation. Means of duplicate wells are shown.
Although the potency of 2 was low, we were also interested in whether it (or 3 and/or 4) induced an enhanced relative production of IL-4 to IFNγ. The levels of IL-4 and IFNγ in the supernatants of mouse splenocytes cultured in the presence of varying concentrations of the analogs were measured using standard sandwich ELISA (Figure 4). Although the unsaturated acyl chain of 2 elicited slightly elevated levels of both IL-4 and IFNγ in comparison to 3–4 and OCH, it is difficult to draw any definitive conclusion about relative cytokine biasing. It is noteworthy that prior studies have shown that the Th2 skewing effect elicited by some α-GalCer analogs tends to be more pronounced in vivo when serum cytokine levels are assessed after injection of the compounds.12 Compounds 1–4 and OCH were also examined in human iNKT cell clones (data not shown). Human iNKT cells respond only weakly to OCH, while 1 induces a potent response. The responses to 2–4 were weaker than that seen with OCH.
Figure 4.
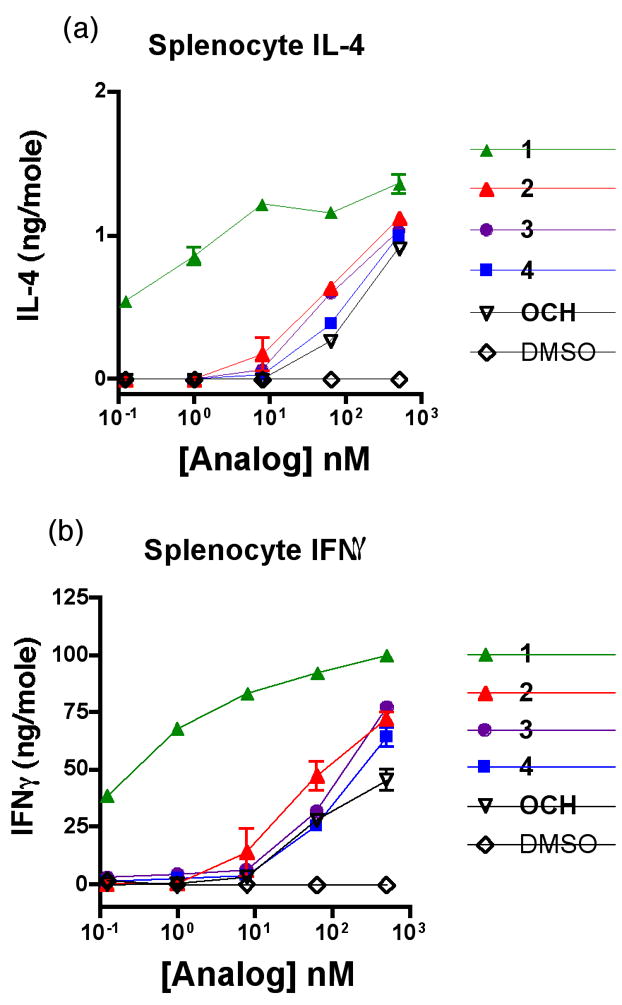
C57BL/6 splenocytes were incubated with varying doses of lipid for 48 h. Supernatant levels of (a) IL-4 and (b) IFNγ were measured by ELISA. Means of duplicate wells are shown.
In conclusion, from the results of this study it is apparent that analog 2, designed around the acyl chain of the potent Th2 biasing compound 1 and the sphingoid base of OCH, although immunostimulatory, offers no advantage over OCH. With the C9-sphingoid base there is no enhancement in activity conferred by incorporation of unsaturation in the acyl chain, in contrast to what was seen with 1, which contains a C18-sphingoid base.
Supplementary Material
Figure 1.
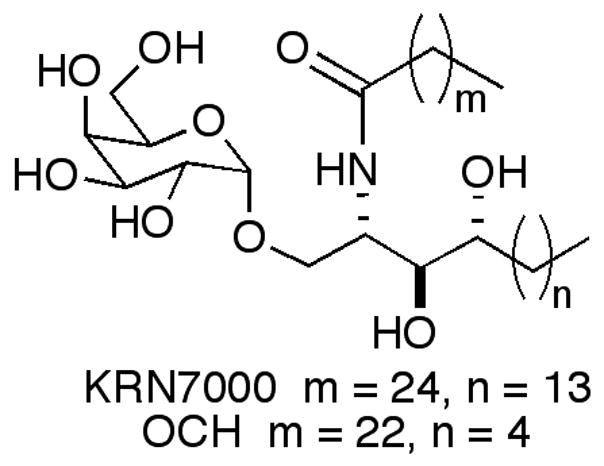
Structures of KRN7000 and its Th2 biasing analog, OCH.
Figure 2.
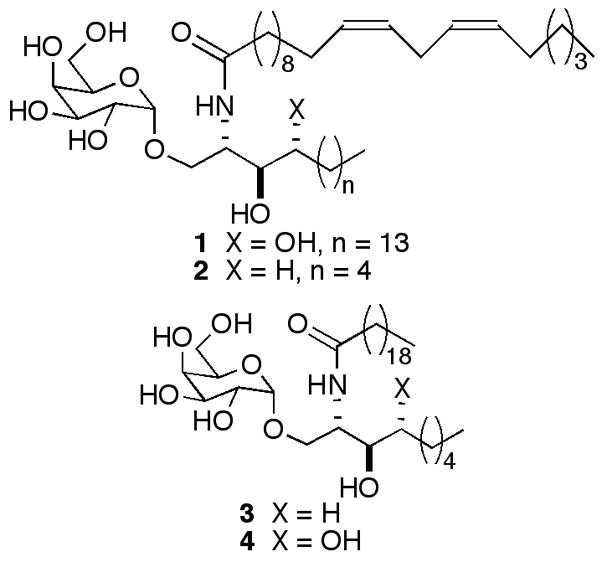
Structures of potent Th2 biasing analog 1 and related truncated sphingoid base analogs 2–4.
Acknowledgments
The authors are grateful for financial support provided by NIH NIAID: RO1AI057519 (ARH) and RO1 AI45889 (SAP). GSB acknowledges support in the form of a Personal Research Chair from Mr. James Bardrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow, the Medical Research Council and The Wellcome Trust (084923/B/08/Z).
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Meyer EH, DeKruyff RH, Umetsu DT. Annu Rev Med. 2008;59:281. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. Curr Opin Immunol. 2008;20:358. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tupin E, Kinjo Y, Kronenberg M. Nature Rev Microbiol. 2007;5:405. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 4.Cerundolo V, Salio M. Curr Top Microbiol Immunol. 2007;314:325. doi: 10.1007/978-3-540-69511-0_13. [DOI] [PubMed] [Google Scholar]
- 5.Swann JB, Coquet JMC, Smyth MJ, Godfrey DI. Curr Top Microbiol Immunol. 2007;314:293. doi: 10.1007/978-3-540-69511-0_12. [DOI] [PubMed] [Google Scholar]
- 6.Brutkiewicz RR. J Immunol. 2006;177:769. doi: 10.4049/jimmunol.177.2.769. [DOI] [PubMed] [Google Scholar]
- 7.Yu KOA, Porcelli SA. Immunol Lett. 2005;100:42. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Hammond JKL, Godfrey DI. Tissue Antigens. 2002;59:353. doi: 10.1034/j.1399-0039.2002.590501.x. [DOI] [PubMed] [Google Scholar]
- 9.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. J Med Chem. 1995;38:2176. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 10.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, Von Blomberg BM, Scheper RJ, Van Der Vliet HJ, Van Den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. Clin Cancer Res. 2002;8:3702. [PubMed] [Google Scholar]
- 11.Miyamoto K, Miyake S, Yamamura T. Nature. 2001;413:531. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 12.Yu KOA, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Proc Natl Acad Sci USA. 2005;102:3383. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forestier C, Takaki T, Molano A, Im JS, Baine I, Jerud ES, Illarionov P, Ndonye R, Howell AR, Santamaria P, Besra GS, DiLorenzo TP, Porcelli SA. J Immunol. 2007;178:1415. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 14.Ndonye RM, Izmirian DP, Dunn MF, Yu KOA, Porcelli SA, Khurana A, Kronenberg M, Richardson SK, Howell AR. J Org Chem. 2005;70:10260. doi: 10.1021/jo051147h. [DOI] [PubMed] [Google Scholar]
- 15.See supplementary data for experimentals and characterization data for compounds 2–4 and for previously unreported intermediates.
- 16.Li Q, Ndonye RM, Illarionov PA, Yu KOA, Jerud ES, Diaz K, Bricard G, Porcelli SA, Besra GS, Chang YT, Howell AR. J Comb Chem. 2007;9:1084. doi: 10.1021/cc070057i. [DOI] [PubMed] [Google Scholar]
- 17.See supplementary data for a description of the assays.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


