Abstract
Tibia fracture in rats initiates a syndrome resembling the complex regional pain syndrome type I. Accumulating evidence indicates that IL-1β is involved in the modulation of nociceptive information and it acts as an intermediate inflammatory mediator via up-regulation of NGF. We hypothesized that IL-1β signaling might mediate the development of the CRPS-like changes after tibial fracture, either directly or by stimulating NGF expression. Rats underwent distal tibia fracture and casting for 4 weeks and were chronically treated with an IL-1 receptor antagonist (IL-1ra). Nociceptive testing and assessment of edema and hindpaw warmth were performed at baseline and after cast removal. Bone microarchitecture was evaluated by microcomputed tomography. Confocal immunofluorescence and in situ hybridization techniques were used to evaluate changes in the cutaneous expression of IL-1β at 4 weeks post-fracture. The nociceptive and vascular effects of intraplantar IL-1β injections were evaluated in intact rats at different time-points after injection. We found that: (1) IL-1ra reduced fracture-induced nociceptive sensitization, but did not decrease hindpaw edema or warmth, (2) fracture chronically up-regulated IL-1β mRNA and protein expression in hindpaw skin keratinocytes, (3) IL-1β intraplantar injection induced mechanical allodynia in a dose-dependent manner and stimulated keratinocyte NGF expression in the hindpaw skin, (4) intraplantar injection of NGF induced nociceptive sensitization. Collectively, these results indicate that cutaneous IL-1β signaling can contribute to chronic regional nociceptive sensitization after fracture, possibly by stimulating NGF over-expression in keratinocytes. Our data also highlight the importance of the keratinocyte as the primary source of post-traumatic IL-1β over-expression.
1. Introduction
CRPS type I is a frequent sequelae of distal tibia [33] and radius fractures [3]. Recently we described a distal tibia fracture model in rats that exhibits chronic unilateral hindlimb warmth, edema, facilitated spontaneous protein extravasation, allodynia, unweighting, and periarticular osteoporosis, a constellation of nociceptive, vascular, and bone changes closely resembling CRPS [13]. We also observed elevated levels of interleukin 1β (IL-1), interleukin 6 (IL-6), and tumor necrosis factor α (TNF) in the hindpaw skin of the fractured limb [31, 38]. Patients with CRPS also exhibit increased inflammatory cytokine levels in the affected limb [12, 17, 18, 25]. Pharmacological investigations in the rat fracture model suggest that cytokine mediators support the enhanced nociception seen after trauma. For example, chronic administration of a TNF antagonist (sTNF-R1) attenuated the development of nociceptive sensitization the tibia fracture CRPS model [30], and the phosphodiesterase inhibitor pentoxifylline prevented fracture-induced cytokine up-regulation in hindpaw skin and reduced pain behavior and paw warmth [38].
Based on these data we postulated that IL-1β signaling could contribute to the CRPS inflammatory response. IL-1β is a key cytokine in the inflammatory cascade, functioning as a peripheral mediator of allodynia and hyperalgesia [8, 10, 32]. Blocking IL-1β signaling pathways has proven to be highly effective in reducing inflammatory pain sensitization in animal models [8, 32, 36]. Intraplantar TNF injection increases IL-1β expression and intraplantar IL-1β injection increases NGF expression in the paw skin of control rats [32, 40]. After intraplantar injection of complete Freund's adjuvant in rats there is a sequential increase in cutaneous levels of the inflammatory mediators TNF, IL-1β, and nerve growth factor (NGF), and blocking any of these inflammatory mediators reduces nociceptive sensitization [32, 40]. Based on these studies it has been proposed that tissue injury, infection, or autoimmune responses can initiate a TNF IL-1β NGF signaling cascade in the skin leading to NGF induced nociceptive sensitization. Recently we observed that NGF expression was also enhanced in the rat fracture model and that this NGF signal mediated the development of nociceptive sensitization in the injured hind limb [31]. Base on these data we postulated that the cytokine-NGF signaling pathway plays a critical role in the development of regional post-traumatic sensitization after fracture.
The objectives of the current study were to test the nociceptive, vascular and NGF generating effects of local IL-1β injection, to identify the cellular source for the increase in IL-1β expression in hindpaw skin after tibia fracture, and to evaluate the effects of an IL-1 receptor antagonist on the development of nociceptive, vascular, and skeletal changes in the tibia fracture model of CRPS.
2. Materials and methods
These experiments were approved by our Institutional Animal Care and Use Committee and followed the animal subjects guidelines of the IASP [46]. Adult (9-month-old) male Sprague Dawley rats (Simonsen Laboratories, Gilroy, CA) were used in all experiments. The animals were housed individually in isolator cages with solid floors covered with 3 cm of soft bedding and were fed and watered ad libitum. During the experimental period the animals were fed Lab Diet 5012 (PMI Nutrition Institute, Richmond, IN), which contains 1.0% calcium, 0.5% phosphorus, and 3.3 IU/g of vitamin D3, and were kept under standard conditions with a 12-h light dark cycle.
2.1 Surgery
Tibia fracture was performed under isoflurane anesthesia as we have previously described [13]. The right hindlimb was wrapped in stockinet (2.5 cm wide) and the distal tibia was fractured using pliers with an adjustable stop (Visegrip, Petersen Manufacturing, Dewitt, NE) that had been modified with a 3-point jaw. The hindlimb wrapped in casting tape (Delta-Lite, Johnson & Johnson, Raynham, MA) so the hip, knee and ankle were flexed. The cast extended from the metatarsals of the hindpaw up to a spica formed around the abdomen. The cast over the paw was applied only to the plantar surface; a window was left open over the dorsum of the paw and ankle to prevent constriction when post-fracture edema developed. To prevent the animals from chewing at their casts, the cast material was wrapped in galvanized wire mesh. The rats were given subcutaneous saline and buprenorphine immediately after procedure (0.03 mg/kg) and on the next day after fracture for post-operative hydration and analgesia. At 4 weeks the rats were anesthetized with isoflurane and the cast removed with a vibrating cast saw. All rats used in this study had union at the fracture site after 4 weeks of casting.
2.2 Drug treatments
Nociceptive and vascular responses to intraplantar injection of IL-1β (Sigma, St. Louis, MO) were evaluated in control rats. The cytokine was diluted in sterile phosphate buffered saline (PBS) and injected at three different doses (10pg/50μl, 100pg/50μl, and 1000pg/50μl) chosen on the basis of preliminary dose-response studies demonstrating hyperalgesic effects. A similar study was performed using rat recombinant nerve growth factor beta (NGF, Sigma), which was diluted in sterile PBS and injected in three different doses (0.1μg/50μl, 1μg/50μl, and 10μg/50μl) chosen on the basis of preliminary studies.
A naturally occurring IL-1 receptor antagonist (IL-1ra) modulates the activity of IL-1 in vivo [9]. anakinra, a recombinant met-human IL-1ra (r-metHuIL-1ra, Kineret, Amgen) that binds to the IL-1 Type I receptor (IL-1 RI), effectively inhibits IL-1 signal transduction.[27] To test the hypothesis that IL-1β signaling mediates the vascular, bony and nociceptive changes observed after tibia fracture in rats, osmotic pumps (model 2ML4, Alzet) were used for delivering the anakinra IL-1ra. The pumps were inserted subcutaneously over the rat's back at the time of fracture. Based on previous studies using IL-1ra in rodents [19, 22, 39] and discussions with lead scientists from Amgen it was decided to administer 10 mg/kg/day of IL-1ra subcutaneously for 28 days after tibia fracture.
2.3. Hindpaw nociception
To measure mechanical allodynia in the rats an up–down von Frey testing paradigm was used as we have previously described [13, 20]. To accomplish these measurements, rats were placed in a clear plastic cylinder (20 cm in diameter) with a wire mesh bottom and allowed to acclimate for 15 min. The von Frey hair was applied against the hindpaw plantar skin at approximately midsole, taking care to avoid the tori pads. The fibers were presented according to the up–down method of Dixon to generate six responses in the immediate vicinity of the 50% threshold.
An incapacitance device (IITC Inc. Life Science) was used to measure hindpaw unweighting. The rats were manually held in a vertical position over the apparatus with the hindpaws resting on separate metal scale plates and the entire weight of the rat was supported on the hindpaws. The duration of each measurement was 6 s and 10 consecutive measurements were taken at 60-s intervals. Eight readings (excluding the highest and lowest ones) were averaged to calculate the bilateral hindpaw weight bearing values.
2.4. Hindpaw volume
A laser sensor technique was used to determine the dorsal-ventral thickness of the hindpaw, as we have previously described [13, 14, 20]. For laser measurements each rat was briefly anesthetized with isoflurane and then held vertically so the hindpaw rested on a table top below the laser. Using optical triangulation, a laser with a distance measuring sensor was used to determine the distance to the table top and to the top of the hindpaw at the tattoo site and the difference was used to calculate the dorsal–ventral paw thickness. The measurement sensor device used in these experiments (4381 Precicura, Limab) has a measurement range of 200 mm with a 0.01 mm resolution.
2.5. Hindpaw temperature
The room temperature was maintained at 23°C and humidity ranged between 25% and 45%. The temperature of the hindpaw was measured using a fine wire thermocouple (Omega) applied to the paw skin, as previously described [13, 14, 20]. The six measurements for each hindpaw were averaged for the mean temperature.
2.6. Tissue processing and immunofluorescence confocal microscopy
Animals were euthanized and perfused with 4% paraformaldehyde (PFA) in PBS, pH 7.4, via the ascending aorta; the dorsal hindpaw skin including sub-dermal layers was removed and post-fixed in 4% PFA for 2 hours, then the tissues were treated with 30% sucrose in PBS at 4°C before embedding in OCT. Following embedding, 20-μm thick slices were made using a cryostat, mounted onto Superfrost microscope slides (Fisher Scientific), and stored at -70°C.
Frozen sections were permeabilized and blocked with PBS containing 10% donkey serum and 0.3% Triton X-100, followed by exposure to the primary antibodies overnight at 4°C in PBS containing 2% serum. Upon detection of the first antigen, primary antibody from a different species against the second antigen was applied to the sections and visualized using an alternative fluorophore-conjugated secondary antibody. Sections were then rinsed in PBS and incubated with fluorophore-conjugated secondary antibodies against the immunoglobulin of the species from which the primary antibody was generated. After three washes, the sections were mounted with anti-fade mounting medium (Invitrogen). Images were obtained using confocal microscopy (Zeiss LSM/510 Upright 2 photon; Carl Zeiss) and stored on digital media. With regard to primary antibodies, goat anti-rat IL-1β, 1:200 (R&D Systems), goat anti-rat NGF, 1:100 (R&D Systems) and monoclonal mouse anti-rat keratin, Pan Ab-1, 1:50 (clone AE1/AE3) (Fisher Scientific) were used. Double-labeling immunofluorescence was performed with donkey anti-mouse IgG (1:300) conjugated with cyanine dye 3, or donkey anti-goat IgG (1:500) conjugated with fluorescein (FITC) secondary antibodies (Jackson ImmunoResearch Laboratories), incubated with respective primary antibodies. Control experiments included incubation of slices in primary and secondary antibody-free solutions both of which led to low intensity non-specific staining patterns in preliminary experiments (data not shown).
2.7. Tissue processing and in situ hybridization
For in situ hybridization, all tissue processing was performed under RNase-free conditions. Briefly, rat dorsal hindpaw skin was washed with RNaseZAP (Sigma-aldrich, St Louis, MO, USA) followed by diethylpyrocarbamate (DEPC)-treated water, and dissected in ice-cold PBS and fixed overnight in 4% (w/v) PFA in PBS. Then tissue was infused with 0.5 M sucrose in PBS overnight, then mounted in Tissue-Tek OCT embedding compound (Sakura Finetek USA, Inc., Torrance, CA, USA), frozen on dry ice and sectioned in 10 μm slices and mounted onto Superfrost microscope slides (Fisher Scientific). All slides were stored at -70°C until use for in situ hybridization.
Cloning and sequencing of IL-1βbeta
IL-1β cDNAs were cloned from RNA of rat thymus by reverse transcriptase PCR. Total RNA was extracted from the thymus using TRIzol reagent (Invitrogen). Then, RNA was denaturated for 10 min at 68 °C, followed by incubation on ice for 10 min. First-strand cDNA was synthesized in a total volume of 20 μl containing 1 μg of RNA with 1 μl of random hexanucleotides (10X, Roche), 1 μl of oligo-dT12-18 (500 μg/ml) as primers, 2.5 μl of dithiothreitol (0.1 M), 1.5 μl of dNTPs (10 mM each), 1 μl of RNasin (40 units/μl), 5 μl of reaction buffer (5X) and 1 μl of Superscript II (200 units/μl) by incubation for 60 min at 42 °C. The IL-1β cDNA was then amplifed using specific primers from 1 μl of the cDNA template solution by PCR with an initial denaturation for 3 min at 94 °C, followed by 30 cycles of denaturation for 30 s at 94 °C, annealing for 30 s at 55 °C, polymerization for 45 S at 70 °C and a final polymerization step (7 min at 70 °C). The rIL-1β cDNA was amplifed with the sense primer 5′-CACCATGGCAACTGTCCCTGAACT-3′, and the antisense primer 5′-AGAGGACGGGCTCTTCTTCA-3′. A 310 bp cDNA fragment was obtained and cloned into the EcoRI site of pCR II-TOPO vector (Invitrogen) to yield pCRII-IL-1β. DNA sequences of PCR-amplified fragments were confirmed with automated DNA sequencer (ABI).
Preparation of antisense RNA and in situ hybridization
pCRII-IL-1β were linearized, and digoxigenin labeled antisense RNA probes (riboprobes) were synthesized using the DIG RNA labeling kit (SP6/T7, Roche) with appropriate RNA polymerase. Sense probes were also prepared as controls. Details of the in situ hybridization procedure have been previously described [29], except that digoxigenin labeled probe was used and signal was visualized by means of a fluorescent antibody enhancement set (Roche). Keratinocyte counter-immunostaining was subsequently performed and confocal images were acquired.
2.8. Micro computed tomography (μCT)
Ex vivo μCT scanning was performed for assessment of trabecular and cortical bone architecture (VivaCT 40, Scanco Medical AG), as previously described by our group [30, 31]. Specifically, trabecular bone architecture was evaluated at the distal femur and fourth lumbar vertebra and cortical bone morphology was evaluated at the femur midshaft. CT images were reconstructed in 1024 × 1024-pixel matrices for vertebral, distal femur, and midfemur samples and stored in 3-D arrays. The resulting grayscale images were segmented using a constrained Gaussian filter to remove noise, and a fixed threshold (25.5% of the maximal grayscale value for vertebrae and distal femur and 35% for midfemur cortical bone) was used to extract the structure of the mineralized tissue.
2.9. Serum chemistry analysis
Control and fracture rats underwent collection of 1 ml of blood at 7 am under isoflurane anesthesia by transcardial puncture 29 days after fracture. Serum was removed and stored at − 80°C. The serum concentrations of total calcium, inorganic phosphorus, creatinine, albumin, total protein, magnesium, alkaline phosphatase, alanine transaminase, and aspartate transaminase were determined using a Beckman LX 20 Analyzer.
2.10 Study design
Baseline determinations were made of bilateral hindpaw temperature, thickness, mechanical nociceptive withdrawal thresholds, and weighting bearing. After baseline tests the rats underwent a right distal tibia fracture with casting. The casts were removed after 28 days and repeat bilateral testing of hindpaw temperature, thickness, mechanical nociceptive withdrawal thresholds, and weighting bearing was performed. There were three treatment groups; 1) control rats (no fracture), 2) fracture control rats underwent sham surgery for a pump insertion, but no pump was placed, and 3) fracture rats that were continuously perfused with IL-1ra (10mg/kg/day) via a subcutaneously implanted osmotic pump for 28 days post-fracture. Hindpaw temperature, thickness, and mechanical nociceptive thresholds data were analyzed as the difference between the treatment side and the contralateral untreated side. Right hindpaw weight bearing data was analyzed as a ratio between the right hindpaw weight and the sum of right and left hindpaws values ((2R/(R+L)) ×100%). Ex vivo μCT scanning was used to determine vertebral and femoral bone parameters for control rats (no fracture), fracture control rats, and IL-1ra treated fracture rats. Hindpaw cutaneous expression of IL-1β gene and protein levels were determined at 4 weeks post-fracture using in situ hybridization and immunohistochemistry in hindpaw dorsum skin samples obtained from the control and fracture control cohorts at 4 weeks post-fracture.
We also examined the nociceptive and vascular effects of intraplantar IL-1β injections in control rats. Three different doses of IL-1β (10pg/50μl, 100pg/50μl, and 1000pg/50μl in PBS) were compared to vehicle over time. Hindpaw behavioral tests were performed at baseline and at 0.5, 1, 3, 6, 24, 48, 72 and 96 hours after injection. In addition, the effects of intraplantar IL-1b injection on keratinocyte NGF levels in control rats was evaluated. The hindpaw plantar skin was collected from PBS (50μl) and IL-1β (1000pg/50μl in PBS) injected rats at 3 hours post-injection for NGF-β immunohistochemistry. The pronociceptive and inflammatory effects of intraplantar NGF injections were also determined. Three different doses of NGF (0.1ug/50μl, 1ug/50μl, and 10ug/50μl in PBS) were compared to vehicle over time. Hindpaw behavioral tests were performed at baseline and at 0.5, 1, 3, 6, 24, 48, and 72 hours after injection.
2.11 Statistical analysis
Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by post hoc Newman-Keuls multiple comparison testing to compare between control rats, fracture control rats, and IL-1ra treated fracture rats. Behavioral data collected over time after intraplantar injections of IL-1β were analyzed by a repeated measures ANOVA on data for each test time point, comparing various treatment groups (IL-1β doses), where the repeated measure was time post-injection. A Bonferroni test was used to determine the source of differences between IL-1β doses. For simple comparisons of two means, two-tailed t-testing was performed. All data are presented as the mean ± SE of the mean, and differences are considered significant at a p value less than 0.05 (Prism 4, GraphPad Software).
3. Results
3.1. Intraplantar IL-1β dose-dependently induced mechanical allodynia in intact rats
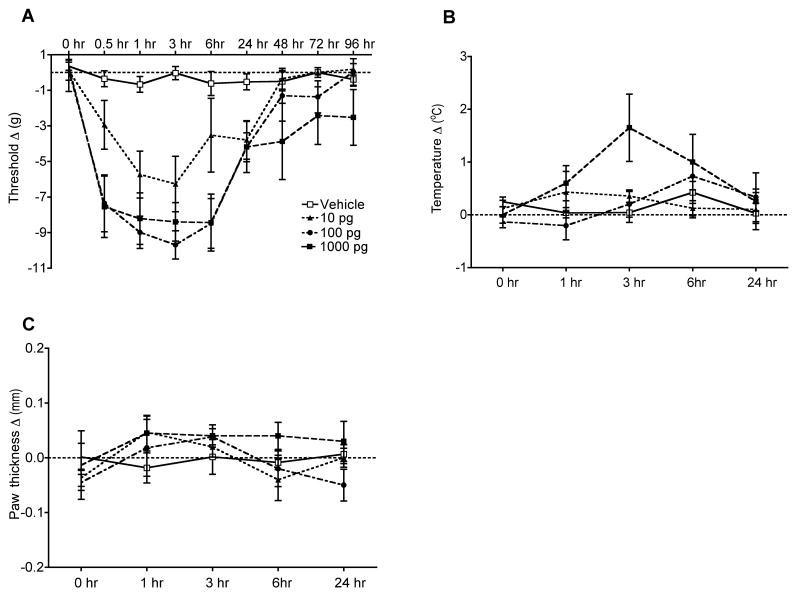
The nociceptive and vascular effects of IL-1β injection were examined in normal intact rats. Intraplantar IL-1β injection dose-dependently induced prolonged mechanical allodynia lasting between 0.5 up to 6 hours after drug administration at doses of 100pg and 1000 pg (Fig. 1A). There was also a trend towards elevation of hindpaw temperature at 3 hours post-injection with the highest dose of IL-1β (1000 pg, Fig. 1B). IL-1β injection did not change hindpaw thickness at anytime point post-injection (Fig. 1C). In addition, the ability of the rat to bear weight on the injected hindpaw was evaluated after IL-1β injection. There was no effect on hindpaw weight bearing at 6 hours post-injection, a time point when mechanical sensitization was prominent (data not shown).
Figure 1.
Changes in hindpaw von Frey thresholds, temperature, thickness, and weight bearing induced by intraplantar injection of various doses of IL-1β in normal control rats (n = 6 per injection cohort). (A) IL-1b (100 and 1000 pg) caused significant mechanical allodynia lasting between 0.5 and 6 hours after injection. (B) No significant changes in hindpaw temperature were observed after IL-1β injection, although there was a nonsignificant 1.5° increase in the paw temperature at 3 hours after the 1000 pg injection. (C) No significant changes were observed in hindpaw thickness after IL-1β injection. Measurements for (A-C) represent the difference between the fracture side and the contralateral paw, thus a negative value represents a decrease in mechanical nociceptive thresholds on the fracture side, a positive value represents an increase in thickness or temperature on the fracture side.
3.2. Fracture increased expression of IL-1β mRNA and protein in hindpaw keratinocytes
We previously observed that distal tibia fracture is accompanied by an increase in IL-1β, IL-6, and TNF mRNA and protein expression levels in the hindpaw skin at 4 weeks post-fracture [30, 31, 38]. Immunostaining in the hindpaw skin revealed no signs of immunocyte infiltration at 4 weeks post-fracture, leading us to believe that resident cells in the dermis or epidermis were responsible for the unilateral increase in inflammatory cytokine expression in the hindpaw skin after fracture [38]. Therefore, immunohistochemistry was combined with in situ hybridization to identify the skin cells expressing IL-1β at 4 weeks after fracture (Figs. 2, 3). In figure 2, sections were immunostained with an antibody directed against the rat keratin Pan-Ab1 antigen, and probed with IL-1β mRNA antisense. Keratinocytes in the epidermis stained positively for Pan-Ab1 antigen, a keratin marker. Although no IL-1β mRNA expressing cells were detected in intact control rats (Fig. 2 upper middle panel), skin sections from control fracture rats demonstrated robust IL-1β mRNA hybridization in the epidermal keratinocytes (Fig. 2, lower middle panel). Keratin and IL-1b mRNA co-staining primarily occurred in the deeper epidermal layers, including the stratum basale layer where new keratinocytes are generated. In figure 3 the co-staining of skin sections for keratin and IL-1β protein yielded similar results; IL-1β protein was strongly up-regulated in keratinocytes, especially in the deeper layers of the epidermis, after fracture. Interestingly, we also observed the transformation of the basal layer of keratinocytes, which in control animals had the morphology that is typical of resting keratinocytes (Fig. 3, upper left panel), into a thicker layer of multiple cells (Fig. 3, lower left panel) that is indicative of activated keratinocytes, with an increase in epidermal thickness after fracture.
Figure 2.
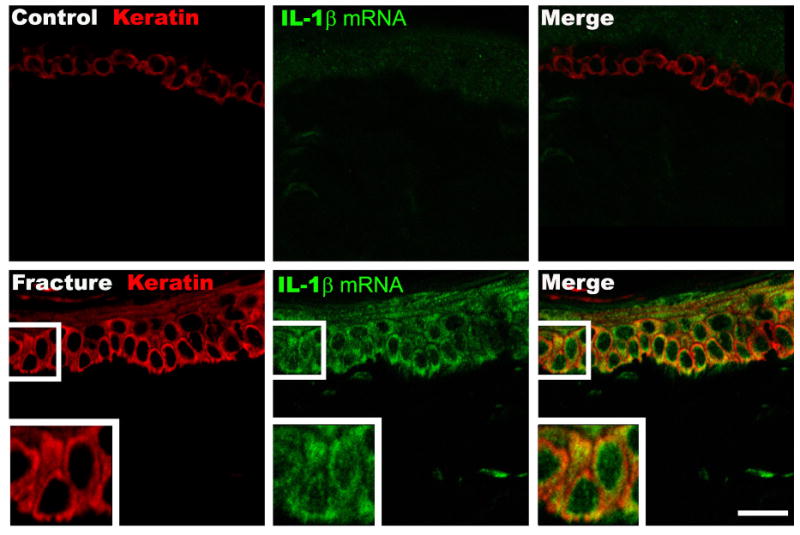
Representative fluorescence photomicrographs of IL-1β mRNA in situ hybridization in keratinocyte and endothelial cells in the dorsal hindpaw skin at 4 weeks post-fracture. Top panels are from a normal control rat, bottom panels are from the fracture hindpaw. Co-immunostaining for keratin, (a keratinocyte marker, red) and IL-1β mRNA (green) in the epidermis demonstrates dramatically increased IL-1β mRNA expression in keratinocytes after fracture with increased epithelial thickness and keratinocyte proliferation. Scale bar = 20 um.
Figure 3.
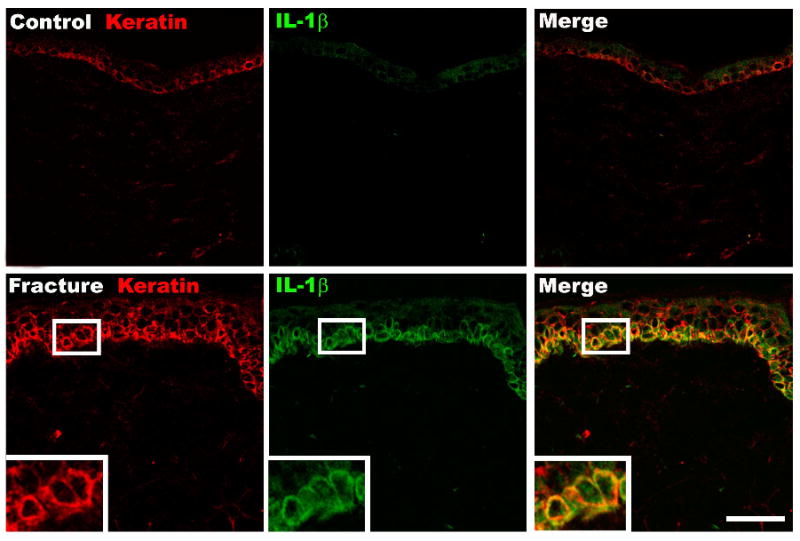
Fluorescence photomicrographs of IL-1β protein in the dorsal hindpaw skin at 4 weeks post-fracture. Top panels are from a normal control rat, bottom panels are from the fracture hindpaw. Co-immunostaining for keratin, (a keratinocyte marker, red) and IL-1β (green) in the dermis demonstrates dramatically increased IL-1β protein expression in keratinocytes after fracture, especially in the basal layer of the epidermis. Scale bar = 50 um.
3.3. IL-1ra attenuates fracture induced nociceptive behavior
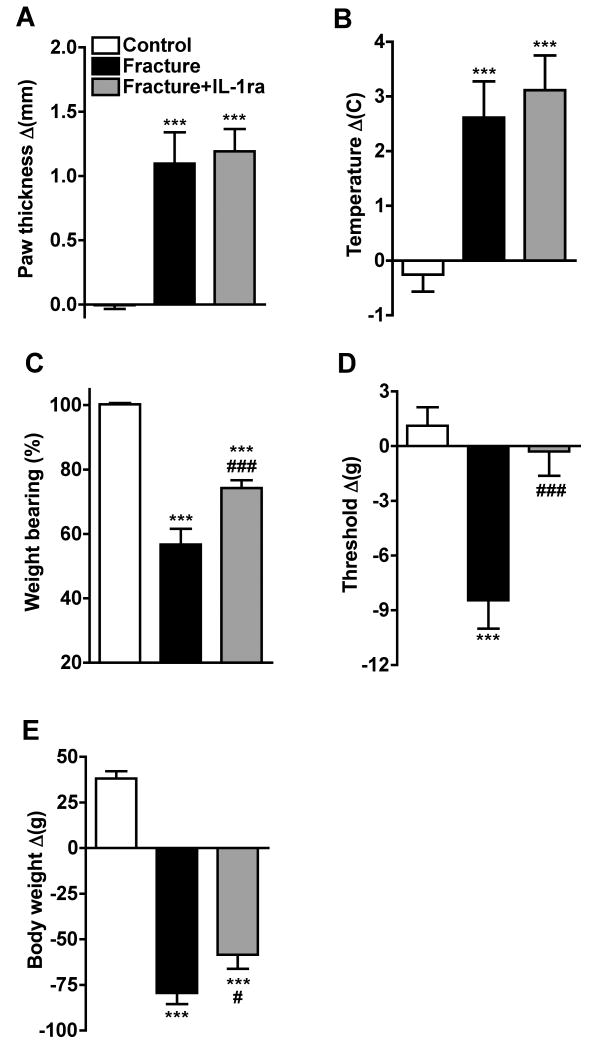
The effects of IL-1ra treatment on fracture induced hindpaw warmth, edema, mechanical sensitivity and weight bearing were evaluated (Fig. 4). There were three treatment groups; 1) control rats (no fracture), 2) control fracture rats that underwent sham surgery for a pump insertion, and 3) fracture rats that were continuously perfused with IL-1ra (10mg/kg/day) via a subcutaneously implanted osmotic pump for 28 days post-fracture. At 4 weeks post-fracture the right hindpaw thickness (Fig. 1A) and temperature (Fig. 1B) were increased in the fracture rats (n = 10) compared to controls (n = 12). anakinra had no effect on hindpaw thickness and warmth in the fracture limb (n = 10, Fig. 4A, B). Figure 4C illustrates that von Frey nociceptive thresholds in the right hindpaw were reduced after fracture, but IL-1ra treatment prevented the development of this mechanical allodynia. Fracture rats unweighted the ipsilateral hindpaw by 44% at 4 weeks post-fracture and IL-1ra treatment partially restored weight bearing in the fracture hindlimb (Fig. 4D). Fracture had no effect on nociceptive thresholds in the contralateral hindpaw vs. control rat thresholds, and IL-1ra treatment had no effect on contralateral nociceptive thresholds after unilateral tibia fracture, when compared to the contralateral thresholds observed in the untreated fracture rats. (data not shown).
Figure 4.
After baseline testing, the right distal tibia was fractured and the hindlimb casted for 4 weeks, then the cast was removed and the animals retested. There were 3 treatment groups, control rats (no fracture, n = 12), fracture rats (sham pump placement surgery at the time of fracture, n = 10), and fracture rats treated with IL-1ra (10mg/kg/day perfusion by osmotic pump for 4 weeks, pump placed at the time of fracture, n = 10). IL-1ra treatment had no effect on the hindpaw edema (A) and warmth (B), but did partially restore the hindlimb weight bearing (C) and completely inhibited the mechanical allodynia (D) that develops after fracture. Measurements for (C) represent weight bearing on the fracture hindlimb as a ratio to 50% of the total bilateral hindlimb loading, thus a percentage lower than 100% represents hindpaw unweighting. Body weight was reduced after fracture and casting, and IL-1ra treatment partially inhibited this weight loss (E). ***P < 0.001 for fracture vs control rat values, #P < 0.05, ###P < 0.001 for IL-1ra treated fracture vs fracture rat values.
3.4. IL-1ra had minimal effect on trabecular and cortical bone loss after fracture
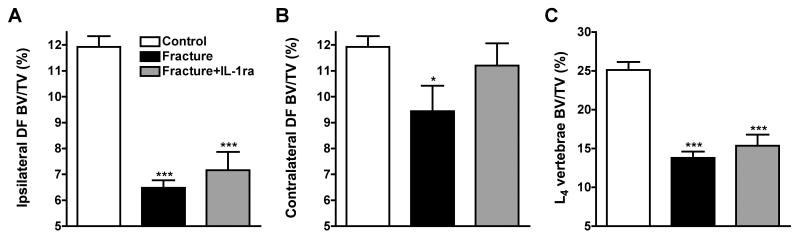
Figure 5 shows quantitative data for the effects of fracture and IL-1ra treatment on trabecular and cortical bone. In the ipsilateral distal femur there was a 45% reduction in trabecular bone volume at 4 weeks post-fracture (Fig. 5A). IL-1ra treated fracture rats lost 39% of their ipsilateral distal femur trabecular bone after fracture. In the contralateral distal femur there was a 20% loss of trabecular bone in the fracture rats and a nonsignificant 5% reduction in bone volume in the IL-1ra treated fracture cohort (Fig. 5B). The L4 vertebral analysis demonstrated a 45% bone loss in the fracture rats and a 39% loss in the IL-1ra treated fracture rats compared to controls (Fig. 5C), an effect which did not reach statistical significance. There were also changes in midfemur cortical bone parameters at 4 weeks after tibia fracture (Table 1). Fracture caused a significant decrease in TAr, BAr, and BPm cortical bone parameters both ipsi- and contralateral to the fracture, but there was no effect on BAr/TAr in either femur. IL-1ra had no effect on any cortical bone parameters either ipsilateral or contralateral to fracture.
Figure 5.
After the right tibia was fractured and the hindlimb casted for 4 weeks, the rats (n = 10) were sacrificed and the bilateral distal femurs (DF) and L4 vertebrae were collected for ex-vivo uCT scanning. After tibia fracture there was extensive regional trabecular bone loss in the ipsilateral (A) and contralateral (B) distal femur, as well as in the L4 vertebra (compared to control rats, n = 10). There was no effect on midfemur cortical bone (data not shown). A 4-week course of IL-1ra treatment had no effect on trabecular bone loss ipsilateral to the fracture or in the L4 vertebra, but did reduce the bone loss in the contralateral distal femur. *P < 0.05, ***P < 0.001 for fracture vs control rat values.
Table 1.
Effect of fracture on trabecular and cortical bone parameters assessed by ex vivo uCT scanning
| Control | Fracture ipsilateral | Fracture+IL-1ra ipsilateral | Fracture contralateral | Fracture+IL1-ra contralateral | |
|---|---|---|---|---|---|
| Distal femur | |||||
| BV/TV (%) | 11.7 ± 0.4 | 6.49 ± 0.29*** | 7.17 ± 0.71*** | 9.44 ± 0.98* | 11.2 ± 0.9 |
| TbN (mm-1) | 2.34 ± 0.09 | 2.32 ± 0.10 | 2.44 ± 0.15 | 2.70 ± 0.15* | 2.92 ± 0.11** |
| TbTh (μm) | 73.0 ± 1.0 | 60.6 ± 1.3*** | 59.9 ± 1.4*** | 63.3 ± 2.2*** | 66.5 ± 1.4** |
| TbSp (μm) | 463 ± 21 | 444 ± 20 | 430 ± 28 | 387 ± 22* | 352 ± 13* |
| ConnD (1/mm3) | 26.9 ± 1.9 | 12.8 ± 1.0*** | 15.3 ± 2.6** | 23.6 ± 3.6 | 31.2 ± 3.8 |
| L4 vertebra | |||||
| BV/TV (%) | 25.1 ± 1.0 | 13.8 ± 0.8*** | 15.4 ± 1.5*** | ||
| TbN (mm-1) | 4.05 ± 0.10 | 3.97 ± 0.1 | 3.95 ± 0.1 | ||
| TbTh (μm) | 79.1 ± 1.2 | 62.1 ± 1.3*** | 64.5 ± 1.7*** | ||
| TbSp (μm) | 247 ± 6.6 | 252 ± 5.4 | 254 ± 8.9 | ||
| ConnD (1/mm3) | 79.0 ± 3.1 | 48.4 ± 4.4*** | 57.0 ± 8.0** | ||
| Femur midshaft | |||||
| BAr (mm2) | 8.02 ± 0.09 | 7.09 ± 0.16*** | 7.28 ± 0.14*** | 7.03 ± 0.19*** | 7.27 ± 0.14*** |
| TtAr (mm2) | 14.1 ± 0.2 | 12.6 ± 0.3** | 13.0 ± 0.2** | 12.4 ± 0.3*** | 13.0 ± 0.3** |
| MeAr (mm2) | 6.06 ± 0.13 | 5.56 ± 0.18 | 5.70 ± 0.15 | 5.41 ± 0.20* | 5.68 ± 0.17 |
| CtTh (μm) | 725 ± 10 | 711 ± 10 | 722 ± 12 | 712 ± 15 | 713 ± 10 |
| BPm (mm) | 23.9 ± 0.6 | 21.1 ± 0.3* | 21.4 ± 0.3* | 21.4 ± 0.5* | 22.1 ± 0.3 |
| BAr/TtAr (%) | 57.1 ± 0.4 | 56.1 ± 0.7 | 56.1 ± 0.7 | 56.5 ± 0.9 | 56.2 ± 0.7 |
Statistical significance determined from one-way ANOVA followed by post hoc Newman-Keuls testing. All data expressed as means ± SEM.
p < 0.05, vs controls.
p < 0.01 vs controls.
p < 0.001 vs controls.
3.5. Serum chemistry panel
Table 2 presents the serum chemistries for control (n = 9) and fracture cohorts (n = 10 for each cohort) at 28 days post-fracture. Twenty serum chemistry components were measured with a total of 8 showing alterations after fracture. Serum electrolytes were unchanged by fracture. IL-1ra appeared to have a marginally significant effect to slightly reduce serum chloride levels in the fracture plus IL-1ra group, a finding of uncertain significance.
Table 2.
Serum chemistry in control, fracture, and IL-1ra treated fracture rats.
| Control | Fracture | Fracture+IL-1ra | |
|---|---|---|---|
| calcium (mg/dL) | 9.57 ± 0.09 | 9.34 ± 0.08 | 9.59 ± 0.11 |
| phosphorus (mg/dL) | 5.43 ± 0.28 | 5.40 ± 0.10 | 5.30 ± 0.12 |
| sodium (mmol/L) | 141.6 ± 0.38 | 141.2 ± 0.20 | 140.5 ± 0.34 |
| potassium (mmol/L) | 5.86 ±0.13 | 5.41 ±0.17 | 5.53 ± 0.14 |
| chloride (mmol/L) | 100.8 ±0.66 | 100.9 ± 0.38 | 98.5 ± 0.45**/## |
| glucose (mg/dL) | 172.9 ±8.07 | 173.8 ±6.18 | 184.0 ±8.52 |
| blood urea nitrogen (mg/dL) | 24.1 ±3.98 | 20.1 ±1.32 | 20.0 ±1.71 |
| creatinine (mg/dL) | 0.52 ± 0.04 | 0.35 ± 0.02*** | 0.32 ± 0.01*** |
| albumin (g/dL) | 1.21 ± 0.04 | 0.81 ± 0.02*** | 0.83 ± 0.03*** |
| total protein (g/dL) | 5.62 ± 0.09 | 5.20 ± 0.06*** | 5.25 ± 0.06** |
| cholesterol (mg/dL) | 133.1 ± 13.7 | 95.6 ± 5.74 | 123.8 ± 12.3 |
| triglycerides (mg/dL) | 139.9 ± 17.2 | 63.0 ± 6.27*** | 62.2 ± 8.64*** |
| HDL cholesterol (mg/dL) | 108.4 ± 9.76 | 75.7 ± 4.18* | 92.6 ± 8.16 |
| alkaline phosphatase (IU/L) | 131.1 ± 12.1 | 173.5 ± 5.70* | 160.7 ± 13.9 |
| alanine transaminase (IU/L) | 72.0 ± 10.5 | 74.4 ± 2.80 | 67.8 ± 3.56 |
| aspartate transaminase (IU/L) | 137.6 ± 13.0 | 89.2 ± 4.25*** | 85.7 ± 6.67*** |
| γ-glutamyl transpeptidase (IU/L) | 3.86 ± 0.70 | 2.78 ± 0.43 | 3.50 ± 0.73 |
| total bilirubin (mg/dL) | 0.50 ± 0.07 | 0.37 ± 0.03 | 0.40 ± 0.02 |
| creatinine phosphokinase (IU/L) | 434.9 ± 82.5 | 156.9 ± 9.40*** | 186.6 ± 21.5*** |
Significantly different values for fracture cohorts (n = 10 for each cohort) compared with controls (n = 9) depicted in bold font. Differences were determined by one-way ANOVA followed by Newman-Keuls post hoc testing. All data expressed as means ± SEM.
p < 0.05, vs controls,
p < 0.01, vs controls,
p < 0.001 vs controls,
p < 0.01 for fracture+IL-1ra vs fracture cohorts.
Serum protein and albumin were lower in both the fracture and fracture plus IL-1ra group. In addition, the muscle related metabolites creatinine and creatinine phosphokinase were strongly reduced in the fracture group, and this effect not reversed by IL-1ra treatment. Muscle mass has been previously reported to fall after hind limb immobilization in rats and to recover slowly [23]. IL-1ra did modestly reverse the weight loss seen in rats after fracture (Fig. 4E), though this effect was not reflected in these serum chemistry values.
Alkaline phosphatase levels were increased in the fracture rats consistent with the bone resorption observed in these animals by uCT. The levels of this enzyme were not different from control for the fracture plus IL-1ra group, though the alkaline phosphatase values did not appear to return to baseline by virtue of drug treatment.
Overall, while fracture and immobilization altered several of the serum chemistries in largely expected ways, anakinra was relatively ineffective in normalizing these values. It does not appear that anakinra works to reduce nociceptive sensitization in tibial fracture rats by reducing any of the aberrations in the serum components measured in this panel.
3.6. Intraplantar IL-1β injection stimulated NGF protein expression in hindpaw keratinocytes
Based on reports that complete Freund's adjuvant can induced mechanical hyperalgesia in rats due to a sequential increase in cutaneous levels of the inflammatory mediators TNF, IL-1β, and NGF [32, 40], our previous results showing up-regulated TNF, IL-1β, and NGF expression in the hindpaw skin after fracture [30, 31, 38], and our previous report that anti-NGF administration reduced nociceptive sensitization in this model [38], we postulated that the post-fracture IL-1β expression observed in the hindpaw keratinocytes contributes to an inflammatory signaling cascade leading to up-regulated cutaneous NGF expression and NGF mediated nociceptive sensitization. Plantar hindpaw skin samples were collected 3 hours after intraplantar injection of IL-1β (1000 pg/50ul of PBS) or PBS (50ul) in normal control rats and the sections were immunostained with an antibody directed against the rat keratin Pan-Ab1 antigen and co-stained for NGF-β protein. Figure 6 illustrates that NGF protein was strongly up-regulated in keratinocytes at 3 hours after IL-1β injection.
Figure 6.
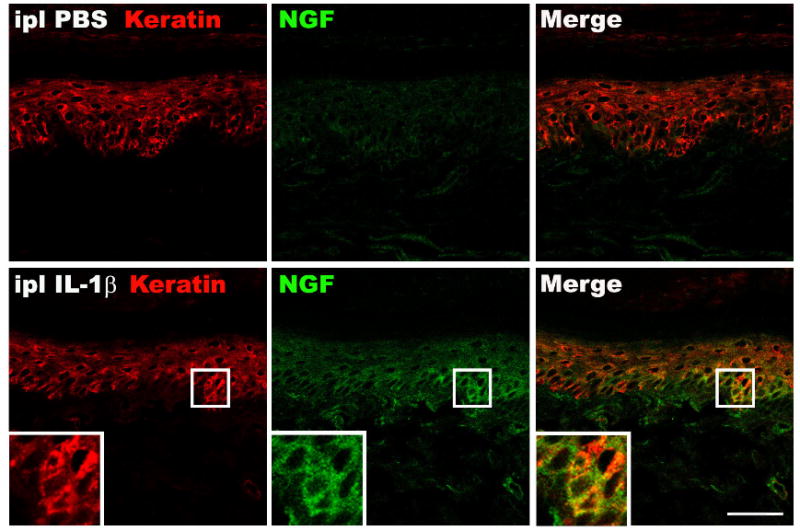
Fluorescence photomicrographs of NGF protein immunostaining in the hindpaw plantar skin collected 3 hour after IL-1β (1000 pg/50ul PBS) intraplantar injection. Top panels are from a vehicle injected (PBS 50 ul) hindpaw, bottom panels are from the IL-1β injected hindpaw. Co-immunostaining for keratin (red) and NGF-β (green) in the dermis demonstrates dramatically increased NGF protein expression in keratinocytes after IL-1β injection. Scale bar = 50 um.
3.7. Intraplantar NGF dose-dependently induced mechanical allodynia in intact rats
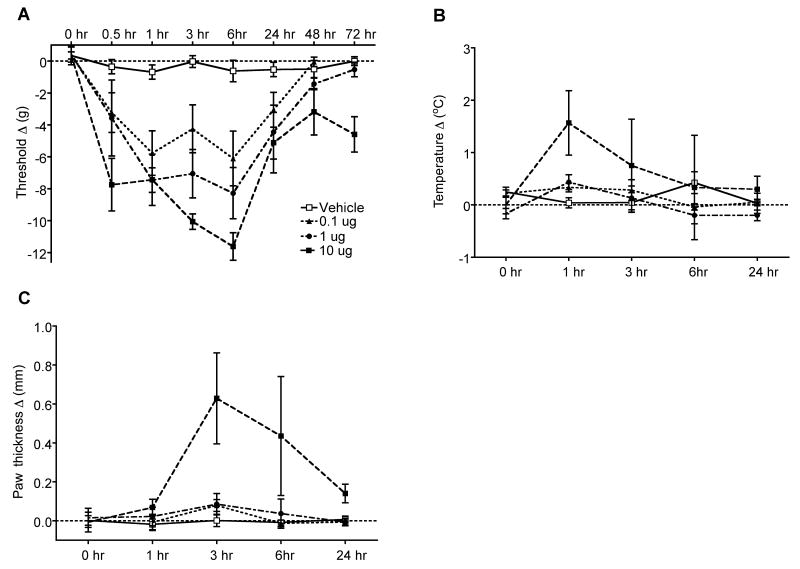
The preceding experiments suggest NGF could be a mediatior downstream of IL-1β responsible for some signs of CRPS in the fracture model. Therefore, the nociceptive and vascular effects of nerve growth factor (NGF) injection were examined in normal intact rats. Intraplantar NGF injection dose-dependently induced prolonged mechanical allodynia lasting between 0.5 up to 6 hours after a dose of 0.1 ug and between 0.5 up to 24 hours after a dose 10ug (Fig. 7A). There was a trend towards elevation of hindpaw temperature at 1 hour post-injection with the highest dose of NGF (10ug, Fig. 7B). In addition, there was a significant increase in hindpaw thickness between 3 and 6 hours post-injection with the highest dose of NGF (10ug, Fig. 7C).
Figure 7.
Changes in hindpaw von Frey thresholds, temperature, and thickness induced by intraplantar injection of various doses of NGF (0.1, 1, 10 ug) in normal control rats (n = 6 per injection cohort). (A) Intraplantar NGF injection dose-dependently induced prolonged mechanical allodynia lasting between 0.5 up to 6 hours after a dose of 0.1 ug and between 0.5 up to 24 hours after a dose 10ug. (B) There was also an insignificant elevation of hindpaw temperature at 1 hour post-injection with the highest dose of NGF (10ug). (C) There was a significant increase in hindpaw thickness between 3 and 6 hours post-injection with the highest dose of NGF (10ug).
4. Discussion
CRPS type I is a frequent sequelae of distal tibia [33] and radius fractures [3]. Recently we described a distal tibia fracture model in rats that exhibits chronic unilateral hindlimb warmth, edema, facilitated spontaneous protein extravasation, allodynia, unweighting, and periarticular osteoporosis [13]. This constellation of post-fracture changes closely resembles the clinical scenario in CRPS patients.
We recently demonstrated elevated levels of IL-1β, IL-6, and TNF in the hindpaw skin of the fractured limb in the rat CRPS model [31, 38]. Because of previously established links between IL-1β and pain in various clinical scenarios and rodent pain models, we examined whether IL-1β signaling contributes to the development of nociceptive sensitization, vascular changes and bone loss in our rat model of CRPS type I. In the normal control rats doses of IL-1β as low as 0.1ng directly sensitized skin after cutaneous injection, and this sensitization began within 30 minutes of injection and persisted for up to 24 hours (Fig. 1). IL-1β mRNA was barely detected in the hindpaw skin from normal control animals, whereas tibia fracture chronically up-regulated IL-1β mRNA expression in the epidermal keratinocytes 28 days after injury (Fig 3). Using immunohistochemistry to examine IL-1β protein expression, we found that little or no IL-1β signal was expressed in the control rat hindpaw skin, while IL-1β protein was markedly expressed at 4 weeks post-fracture. Furthermore, there was a dramatic increase in epidermal keratinocye proliferation after fracture, indicative of keratinocyte activation. We had previously examined the hindpaw skin after tibia fracture in an attempt to identify the infiltrating immune cells that are the primary sources of cytokines in most inflammatory processes. However, none of the major classes of immunocytes were observed in skin samples collected 4 weeks after fracture [38]. Collectively, these data indicate that keratinocytes are activated and express high levels of IL-1β after fracture. Several reports utilizing the measurement of fluid from skin suction blisters in humans with CRPS also demonstrate elevated levels of inflammatory cytokines, perhaps due to enhanced production in resident skin cells [12, 17, 18, 25].
An additional important finding from the current study is that chronic nociceptive sensitization was markedly suppressed by 28 day course of IL-1ra (anakinra) perfusion, started on the day of fracture (Fig. 4). It is unlikely that systemically administered IL-1ra can penetrate the blood-brain barrier due to its high molecular weight (17.3 kD) and we postulated that the antinociceptive effects of IL-1ra were mediated in the skin of the injured limb, where increases in IL-1β expression were observed. Furthermore, there were few systemic effects of IL-1ra on serum chemistry values (Table 2). This drug had mild positive effects on reduction in weight loss after fracture (Fig. 4E). These data are consistent with many previously published reports that pharmacological blocking of IL-1β signaling can reverse or mitigate nociceptive sequelae in different acute, neuropathic and inflammatory pain models in rodents [2, 4, 7, 15, 16, 21, 22, 24, 26, 35, 36, 39, 42-45]. The IL-1ra agent used in these studies is a clinically available drug and might therefore be of use in treating clinical CRPS.
While clear effects on nociceptive sensitization were observed, IL-1ra treatment did not reverse all aspects of CRPS in the rat tibia fracture model (Fig. 4). For example, hindpaw swelling and warmth were not altered, suggesting that these features do not rely exclusively on IL-1β signaling. Consistent with the IL-1ra infusion results in the fracture model, the intraplantar injection of IL-1β in normal control rats did not lead to hindpaw edema or warmth, though nociceptive sensitization was seen (Fig. 1). Indeed, based on substance P NK1 antagonist studies in the fracture model we have previously established that swelling and warmth might have a neurogenic etiology [13, 14]. Therefore we postulate that IL-1β cytokine signaling might be more important to the development of nociceptive sensitization than vascular abnormalities after fracture; no single mediator identified to date appears to mediate all components of the CRPS syndrome in this model.
We were able to show that IL-1β directly sensitized skin after cutaneous injection, and that this sensitization was long lived (Fig. 1). These results are consistent with the reports of others that the direct injection of IL-1β into skin causes nociceptive sensitization, especially to mechanical stimuli [8, 10, 32]. Though the specific mechanism has not been fully described, it is notable that intraneural injection of IL-1β causes pain [41], and that IL-1β stimulates discharges in primary afferent neurons [28]. Importantly, the acute peripheral effects of IL-1β following intraplantar injections could be mediated directly by excitation of nociceptive small-diameter Aδ and C fibers as suggested by Fukuoka et al., who demonstrated activation of somatosensory fibers within 1 min after intraplantar IL-1β injection [11]. Thus it is reasonable to suppose that the elevated levels IL-1β in the skin of tibia fracture rats could directly cause nociceptive activation and sensitization.
Our results regarding the roles of IL-1β in our model of CRPS I may pertain most strongly to the relatively acute superficial or cutaneous aspects of the disease. The changes in expression of IL-1β both at the mRNA and protein levels revealed that those changes were most easily observed in the keratinocytes. In addition, the von Frey assay where anakinra had its largest effects stimulates cutaneous nociceptors most directly. When a more evenly distributed pressure was applied during hind paw weighting, conceivably activating deeper nerves, the effects of anakinra were more limited. Furthermore, anakinra had no effect on bone mineralization. It should be noted that while cutaneous mechanical sensitization is commonly observed in CRPS, many patients with chronic CRPS perceive their pain as having a deep tissue origin [34]. In fact, recent studies using the infusion of acidified saline provide strong evidence for the sensitization of muscle in the affected limbs [6]. Other investigators have concluded that deep somatic autonomic innervation may be more important than superficial innervation in supporting CRPS [34]. In addition to the question of superficial versus deep tissue, it is possible that the mechanisms supporting CRPS change over time. In a recent survey of 145 patients with CRPS, manifestations of CRPS like edema and elevated temperature lessened with time [5]. At this point, the rat tibia fracture model of CRPS has been characterized primarily for changes appearing within 4 weeks of fracture. Whether longer-term changes in rats resemble those in humans has not been determined.
In addition to directly activating sensory neurons, IL-1β also plays an important role as an intermediate mediator in peripheral tissue inflammatory signaling [36]. IL-1β up-regulates NGF expression in fibroblasts and glial cells in vitro [1, 37] and we observed a dramatic increase in keratinocyte NGF expression in the hindpaw skin after IL-1β intraplantar injection (Fig. 6), corresponding with the development of nociceptive sensitization after IL-1β injection (Fig. 7). Interestingly, anti-NGF antibodies prevent the development of hindpaw hyperalgesia after intraplantar injection of IL-1β [32]. NGF is an inflammatory mediator that contributes to the development of hyperalgesia in many animal pain models, including chronic inflammation [8, 32, 36, 40]. Our own observations demonstrate that NGF levels in skin are chronically increased after fracture in rats, a process that appears linked to the development of post-traumatic allodynia [31]. Similar to our IL-1ra results, anti-NGF therapy affected nociceptive sensitization with very limited effects on the vascular and bone changes characteristic of the rat fracture CRPS model [31]. Collectively, these data support the hypothesis that IL-1β acts as an intermediate inflammatory mediator in the skin by stimulating keratinocyte expression of NGF, which subsequently induces nociceptive sensitization.
Taken together, these results indicate that activated rat keratinocytes are a major source of cutaneous IL-1β production in the rat fracture model of CRPS. While it appears unlikely that IL-1β signaling can cause all of the of changes characteristic of CRPS, it does appear to play a role in the development of nociceptive sensitization, either by direct excitation of nociceptive fibers or indirectly by inducing keratinocytes to express the pro-nociceptive inflammatory mediator NGF. The aspects of CRPS involving vascular changes and bone loss may be less amenable to anti-IL-1β agents. Therefore, therapy with IL-1ra might represent a viable option for the treatment of patients affected by CRPS and future investigations might be directed at evaluating this hypothesis in the clinic.
Acknowledgments
None of the authors has a conflict of interest to disclose. This work was funded by Department of Veteran Affairs, Veterans Health Administration, Rehabilitation Research and Development Service grant F4516I and NIH NIGMS grant GM079126. We would like to thank Amgen (Thousand Oaks, CA) for generously providing the IL-1ra used in these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe Y, Akeda K, An HS, Aoki Y, Pichika R, Muehleman C, Kimura T, Masuda K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–42. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 2.Ahn DK, Kim KH, Jung CY, Choi HS, Lim EJ, Youn DH, Bae YC. Role of peripheral group I and II metabotropic glutamate receptors in IL-1beta-induced mechanical allodynia in the orofacial area of conscious rats. Pain. 2005;118:53–60. doi: 10.1016/j.pain.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Atkins RM, Duckworth T, Kanis JA. Features of algodystrophy after colles' fracture. J Bone Joint Surg. 1990;72 B:105–110. doi: 10.1302/0301-620X.72B1.2298766. [DOI] [PubMed] [Google Scholar]
- 4.Baamonde A, Curto-Reyes V, Juarez L, Meana A, Hidalgo A, Menendez L. Antihyperalgesic effects induced by the IL-1 receptor antagonist anakinra and increased IL-1beta levels in inflamed and osteosarcoma-bearing mice. Life Sci. 2007;81:673–82. doi: 10.1016/j.lfs.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Birklein F, Riedl B, Sieweke N, Weber M, Neundorfer B. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol Scand. 2000;101:262–9. doi: 10.1034/j.1600-0404.2000.101004262x./. [DOI] [PubMed] [Google Scholar]
- 6.Birklein F, Weber M, Ernst M, Riedl B, Neundorfer B, Handwerker HO. Experimental tissue acidosis leads to increased pain in complex regional pain syndrome (CRPS) Pain. 2000;87:227–34. doi: 10.1016/S0304-3959(00)00286-4. [DOI] [PubMed] [Google Scholar]
- 7.Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000;130:1418–24. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755–60. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 10.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 11.Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res. 1994;657:133–40. doi: 10.1016/0006-8993(94)90960-1. [DOI] [PubMed] [Google Scholar]
- 12.Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC musculoskeletal disorders. 2006;7:91. doi: 10.1186/1471-2474-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006;121:158–67. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–18. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, Iwakura Y, Decker MW, Faltynek C, Sullivan J, Jarvis MF. Interleukin-1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res. 2006;167:355–64. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijistra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm. 2002;11:47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunol Lett. 2004;91:147–54. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Kimble RB, Vannice JL, Bloedow DC, Thompson RC, Hopfer W, Kung VT, Brownfield C, Pacifici R. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J Clin Invest. 1994;93:1959–67. doi: 10.1172/JCI117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 2003;104:75–84. doi: 10.1016/s0304-3959(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 21.Kleibeuker W, Gabay E, Kavelaars A, Zijlstra J, Wolf G, Ziv N, Yirmiya R, Shavit Y, Tal M, Heijnen CJ. IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav Immun. 2008;22:200–8. doi: 10.1016/j.bbi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Li A, Lao L, Wang Y, Zhang H, Ren K, Berman BM, Zhang R. Interleukin-1ra inhibits Fos expression and hyperalgesia in rats. Neuroreport. 2007;18:495–8. doi: 10.1097/WNR.0b013e3280586839. [DOI] [PubMed] [Google Scholar]
- 23.Maeda H, Kimmel DB, Raab DM, Lane NE. Musculoskeletal recovery following hindlimb immobilization in adult female rats. Bone. 1993;14:153–9. doi: 10.1016/8756-3282(93)90242-3. [DOI] [PubMed] [Google Scholar]
- 24.Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–40. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munnikes RJ, Muis C, Boersma M, Heijmans-Antonissen C, Zijlstra FJ, Huygen FJ. Intermediate stage complex regional pain syndrome type 1 is unrelated to proinflammatory cytokines. Mediators Inflamm. 2005;2005:366–72. doi: 10.1155/MI.2005.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narita M, Shimamura M, Imai S, Kubota C, Yajima Y, Takagi T, Shiokawa M, Inoue T, Suzuki M, Suzuki T. Role of interleukin-1beta and tumor necrosis factor-alpha-dependent expression of cyclooxygenase-2 mRNA in thermal hyperalgesia induced by chronic inflammation in mice. Neuroscience. 2008;152:477–86. doi: 10.1016/j.neuroscience.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Opp MR, Krueger JM. Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. Am J Physiol. 1991;260:R453–7. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- 28.Ozaktay AC, Kallakuri S, Takebayashi T, Cavanaugh JM, Asik I, DeLeo JA, Weinstein JN. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15:1529–37. doi: 10.1007/s00586-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 29.Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–51. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- 30.Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008;137:507–19. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138:47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115:1265–75. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarangi PP, Ward AJ, Smith EJ, Staddon GE, Atkins RM. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br. 1993;75:450–2. doi: 10.1302/0301-620X.75B3.8496220. [DOI] [PubMed] [Google Scholar]
- 34.Schattschneider J, Binder A, Siebrecht D, Wasner G, Baron R. Complex regional pain syndromes: the influence of cutaneous and deep somatic sympathetic innervation on pain. The Clin J Pain. 2006;22:240–4. doi: 10.1097/01.ajp.0000169672.49438.67. [DOI] [PubMed] [Google Scholar]
- 35.Sommer C, Petrausch S, Lindenlaub T, Toyka KV. Neutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathy. Neurosci Lett. 1999;270:25–8. doi: 10.1016/s0304-3940(99)00450-4. [DOI] [PubMed] [Google Scholar]
- 36.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112:116–38. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 37.von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G, Kirsch J. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J Neuroendocrinol. 2006;18:820–5. doi: 10.1111/j.1365-2826.2006.01478.x. [DOI] [PubMed] [Google Scholar]
- 38.Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. Eur J Pain. 2009;13:253–62. doi: 10.1016/j.ejpain.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf G, Livshits D, Beilin B, Yirmiya R, Shavit Y. Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: Genetic and pharmacological studies in mice. Brain Behav Immun. 2008;22:1072–1077. doi: 10.1016/j.bbi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–24. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116:257–63. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Zhang RX, Li A, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Electroacupuncture attenuates bone cancer pain and inhibits spinal interleukin-1 beta expression in a rat model. Anesth Analg. 2007;105:1482–8. doi: 10.1213/01.ane.0000284705.34629.c5. [DOI] [PubMed] [Google Scholar]
- 43.Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–9. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. 2008;154:1533–8. doi: 10.1016/j.neuroscience.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–36. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]