Abstract
Several prior studies have been performed to determine the feasibility of tissue decellularization to create a non-immunogenic xenogenic tissue replacement for bladder, vasculature, heart valves, knee meniscus, temporomandibular joint disc, ligament, and tendon. However, limited work has been performed with articular cartilage, and no studies have examined the decellularization of tissue engineered constructs. The objective of this study was to assess the effects of different decellularization treatments on articular cartilage constructs, engineered using a scaffoldless approach, after 4 wks of culture, using a two-phased approach. In the first phase, five different treatments were examined: 1) 1% SDS, 2) 2% SDS, 3) 2% Tributyl phosphate, 4) 2% Triton X-100, and 5) Hypotonic followed by hypertonic solution. These treatments were applied for either 1 h or 8 h, followed by a 2 h wash in PBS. Following this wash, the constructs were assessed histologically, biochemically for cellularity, GAG, and collagen content, and biomechanically for compressive and tensile properties. In phase II, the best treatment from phase I was applied for 1, 2, 4, 6, or 8 h in order to optimize the application time. Treatment with 2% SDS for 1 h or 2 h significantly reduced the DNA content of the tissue, while maintaining the biochemical and biomechanical properties. On the other hand, 2% SDS for 6 h or 8 h resulted in complete histological decellularization, with complete elimination of cell nuclei on histological staining, although GAG content and compressive properties were significantly decreased. Overall, 2% SDS, for 1 or 2 h, appeared to be the most effective agent for cartilage decellularization, as it resulted in decellularization while maintaining the functional properties. The results of this study are exciting as they indicate the feasibility of creating engineered cartilage that may be non-immunogenic as a replacement tissue.
Keywords: Cartilage, decellularization, ECM, mechanical testing
INTRODUCTION
Injuries to articular cartilage, whether traumatic or from degeneration, generally result in the formation of mechanically inferior fibrocartilage, due to the tissue’s poor intrinsic healing response.[1] As such, tissue engineering strategies have focused on developing replacement tissue in vitro for eventual in vivo implantation. One such strategy employs a “self-assembly process”[2] in which chondrocytes can be used to form robust tissue engineered constructs without the use of a scaffold.
Although engineered articular cartilage tissue has recently been created with biochemical and biomechanical properties in the range of native tissue values, [3] there are currently two significant limitations to cartilage tissue engineering. First, human cells are scarce in number and difficult to procure, and passage of these cells leads to dedifferentiation. [4] These issues make the use of autologous cells for cartilage repair difficult. Additionally, the majority of cartilage tissue engineering approaches have employed bovine or other animal cells, and tissues grown from these cells are xenogenic. Thus, their use may result in a severe immune response following implantation, though this has not been fully elucidated.
It is believed that a decellularized xenogenic tissue may be a viable option as a replacement tissue, as the antigenic cellular material will be removed while preserving the relatively non-immunogenic extracellular matrix (ECM), as described in an earlier review. [5] Ideally, this will also preserve the biomechanical properties of the tissue. For instance, an acellular dermal matrix[6] has seen successful use clinically as the FDA approved Alloderm product. Additionally, acellular xenogenic tissues have been created for many musculoskeletal applications, including replacements for the knee meniscus, [7] temporomandibular joint disc, [8] tendon, [9] and ACL, [10] as well as in other tissues including heart valves, [11–17] bladder, [18] artery, [19] and small intestinal submucosa.[20, 21]
However, studies demonstrating the effects of tissue decellularization on cartilage are limited, [22] and there are no studies demonstrating the effects of decellularization on musculoskeletal tissue engineered constructs. In the only other study examining cartilage decellularization, [22] a photo-oxidation approach was employed to decellularize bovine xenografts, after which they were implanted in vivo into a sheep model. It was determined that the photo-oxidation approach, which resulted in nonviable chondrocytes, resulted in a reduced monocyte and plasma cell infiltration in the implant after 6 months. In this study, the primary benefit of using an approach involving tissue engineered cartilage constructs, rather than explants, is the potential to modulate the geometry and functional properties of the construct to match those of a specific patient or joint surface. Additionally, xenograft approaches are appealing, as animal tissue provides a relatively limitless and cost-effective cell source. However, decellularization of the xenograft tissue may be required to eliminate the immunogenicity of the tissue. While the use of decellularized xenograft explants can also be considered, both approaches must be explored before determining which approach should be pursued. As such, we are also pursuing an explant-based approach.[23]
Therefore, the objective of this study was to determine the effects of multiple decellularization treatments on tissue engineered construct cellularity, biochemical, and biomechanical properties. A two-phased approach was used in which an appropriate agent for decellularization was selected in phase I, and an appropriate treatment time was selected in phase II. It was hypothesized that cells could be removed from self-assembled constructs while preserving the biochemical and biomechanical properties. To test this hypothesis, self-assembled articular cartilage constructs were cultured for 4 wks, and then treated with 1% sodium dodecyl sulfate (SDS), 2% SDS, 2% Tributyl Phosphate (TnBP), 2% Triton X-100, or a hypotonic/hypertonic solution, for either 1 or 8 h. These treatments were selected from prior literature, [7–17, 20, 21] as well as from pilot experiments in our lab. Next, the treatment selected in phase I was applied for 1, 2, 4, 6, or 8 h in phase II. The effects of the decellularization treatments were assessed on construct cellularity and functional properties.
MATERIALS AND METHODS
Chondrocyte Isolation and Seeding
Cartilage was harvested from the distal femur of wk-old male calves[24–26] (Research 87, Boston, MA) shortly after slaughter, and chondrocytes were isolated following digestion with collagenase type 2 (Worthington, Lakewood, NJ). To normalize variability among animals, each leg came from a different animal, and cells from all legs were combined together to create a mixture of chondrocytes; a mixture of cells from five legs was used in the study. Cell number was determined on a hemocytometer, and a trypan blue exclusion test indicated that viability remained >90%. Chondrocytes were frozen in culture medium supplemented with 20% FBS (Biowhittaker, Walkersville, MD) and 10% DMSO at −80°C for 1 day prior to use. After thawing, viability was greater than 90%. To construct each agarose well, sterile, molten 2% agarose was added to wells of a 48-well plate, and a stainless steel mold consisting of 5 mm dia. × 10 mm long cylindrical prongs was placed into a row of the plate to construct the cylindrical molds. The agarose solidified at room temperature for 60 min, after which the mold was removed from the agarose. Two changes of culture medium were used to completely saturate the agarose well by the time of cell seeding. The medium was DMEM with 4.5 g/L-glucose and L-glutamine (Biowhittaker), 100 nM dexamethasone (Sigma, St. Louis, MO), 1% Penicillin/Streptomycin/Fungizone (P/S/F) (Biowhittaker), 1% ITS+ (BD Scientific, Franklin Lakes, NJ), 50 μg/mL ascorbate-2-phosphate, 40 μg/mL L-proline, and 100 μg/mL sodium pyruvate (Fisher Scientific, Pittsburgh, PA). To seed each construct, 5.5 × 106 cells were added in 100 μl of culture medium. Constructs formed within 24 h in the agarose wells and were cultured in the same well until t=10 days, after which they were unconfined for the remainder of the study, as described previously;[27] t=0 was defined as 24 h after seeding. Throughout the studies, constructs were cultured in an incubator at 37°C and 10% CO2.
Decellularization Phase I
At t=4 wks, self-assembled constructs (n=6/group) were removed from culture and exposed to one of five decellularization treatments, for either 1 h or 8 h. The decellularization treatments included:
1% SDS
2% SDS
2% TnBP
2% Triton X-100
-
Hypotonic/Hypertonic Solution (half-time of each)
Hypotonic: 10 mM Tris HCl, 5 mM EDTA, 1 μM PMSF
Hypertonic: 50 mM Tris HCl, 1 M NaCl, 10 mM EDTA, 1 μM PMSF
All treatments included 0.5 mg/ml DNase Type I, 50 μg/ml RNase, 0.02% EDTA, and 1% P/S/F, in PBS. Both 1 h control and 8 h control groups were exposed to this same solution without detergent treatments. These treatments were applied at 37°C with agitation. Following the 1 h or 8 h treatment, the constructs were washed for 2 h in PBS at 37°C with agitation. Additionally, an untreated control was assessed immediately following construct removal from culture, without the treatment or wash steps.
Decellularization Phase II
At t=4 wks, self-assembled constructs (n=6/group) were removed from culture and exposed to 2% SDS for 1, 2, 4, 6, or 8 h. As in phase I, all treatments included 0.5 mg/ml DNase Type I, 50 μg/ml RNase, 0.02% EDTA, and 1% P/S/F, in PBS. These treatments were applied at 37°C with agitation. Following the SDS treatment, the constructs were washed for 2 h in PBS at 37°C with agitation. Additionally, an untreated control was assessed immediately following construct removal from culture, without the treatment or wash steps.
Histology
After freezing, samples were sectioned at 14 μm. To determine construct cellularity, a hematoxylin & eosin (H&E) stain was used. A Safranin-O/fast green stain was used to examine GAG distribution, [28, 29] and picrosirius-red was employed for collagen content.
Quantitative Biochemistry
Samples were frozen overnight and lyophilized for 48 h, followed by re-suspension in 0.8 mL of 0.05 M acetic acid with 0.5 M NaCl and 0.1 mL of a 10 mg/mL pepsin solution (Sigma) at 4°C for 72 h. Next, 0.1 mL of 10x TBS was added along with 0.1 mL pancreatic elastase and mixed at 4°C overnight. A Picogreen® Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR) was used to assess total DNA content. GAG content was quantified using the Blyscan Glycosaminoglycan Assay kit (Biocolor), based on 1,9-dimethylmethylene blue binding. [30, 31] After hydrolysis with 2 N NaOH for 20 min at 110°C, total collagen content was determined using a chloramine-T hydroxyproline assay.[32]
Indentation Testing
Samples were assessed with an indentation apparatus, as described previously. [33] A 0.7 g (0.007 N) mass was applied with a 1 mm flat-ended, porous indenter tip, and specimens crept until equilibrium, as described elsewhere. [2] For the constructs treated for 1 h with the hypotonic/hypertonic solution and 8 h with 1% SDS, 2% TnBP, or 2% Triton X-100, a 0.27 g (0.0027 N) mass was applied instead such that all constructs would experience a similar strain during indentation testing. Examined over the entire study, strains from 3–9% were recorded. The thickness was measured using digital calipers with accuracy to 0.01 mm. Preliminary estimations of the aggregate modulus of the samples were obtained using the analytical solution for the axisymmetric Boussinesq problem with Papkovich potential functions. [34, 35] The sample biomechanical properties, including aggregate modulus, Poisson’s ratio, and permeability were then calculated using the linear biphasic theory. [36]
Tensile Testing
A uniaxial materials testing system (Instron Model 5565, Canton, MA) was employed to determine tensile properties with a 50 N load cell, as described previously.[37] Briefly, samples were cut into a dog-bone shape with a 1-mm-long gauge length. Samples were glued to paper tabs with cyanoacrylate glue outside of the gauge length. The 1-mm-long sections were pulled at a 1% constant strain rate. All samples broke within the gauge length. The gauge length, thickness, and initial cross-sectional area were measured using digital calipers. For each construct, a stress-strain curve was created from the load-displacement curve and the Young’s modulus was calculated from each stress-strain curve using the initial cross-sectional area.
Statistical Analysis
All biomechanical and biochemical assessments were made using n=6. In phase I, the three control groups were compared using a single factor ANOVA. As no difference was noted, only the culture control was used in the final analysis. To compare treatment groups in both phases, a single factor ANOVA was used, and a Tukey HSD post hoc test was used when warranted. Significance was defined as p< 0.05.
RESULTS
Gross Appearance and Histology
In all groups, the construct diameter was approximately 6 mm at 4 wks. In phase I, treatment for 8 h with either 1% SDS or the hypotonic/hypertonic solution resulted in a significant decrease in construct thickness (Table I). Additionally, treatment for 8 h with 1% SDS, 2% SDS, 2% Triton X-100, or the hypotonic/hypertonic solution resulted in a significant decrease in construct wet weight (Table I). In phase II, treatment with 2% SDS for 6 h or 8h resulted in a significant decrease in construct thickness and wet weight (Table II).
Table I.
Phase I. Construct wet weight and thickness values. Treatment with 1% and 2% SDS for 8 h, 2% Triton X-100 for 8 h, and Hypotonic/Hypertonic treatment for 8 h all resulted in a significant decrease in construct wet weight. Only treatment with 1% SDS for 8 h and Hypotonic/Hypertonic treatment for 8h resulted in a significant decrease in construct thickness.
| Treatment Group | Construct Wet Weight (mg) |
Thickness (mm) |
|---|---|---|
| Control | 14.8±1.1 | 0.49±0.03 |
| 1% SDS, 1 h | 14.3±1.0 | 0.50±0.02 |
| 1% SDS, 8 h | 8.8±1.2a | 0.38±0.04a |
| 2% SDS, 1 h | 12.3±1.1 | 0.43±0.05 |
| 2% SDS, 8 h | 9.3±2.6a | 0.47±0.08 |
| 2% TnBP, 1 h | 15.2±1.1 | 0.53±0.06 |
| 2% TnBP, 8 h | 12.2±1.2 | 0.49±0.04 |
| 2% Triton X-100, 1 h | 13.7±1.2 | 0.47±0.05 |
| 2% Triton X-100, 8 h | 11.2±1.7a | 0.47±0.08 |
| Hypo/Hyper 1 h | 15.0±3.0 | 0.40±0.09 |
| Hypo/Hyper 8 h | 7.0±1.3a | 0.35±0.04a |
Significantly lower than control (p<0.05)
Table II.
Phase II. Construct wet weight and thickness values. Treatment with 2% SDS for 6 h or 8 h resulted in a significant decrease in construct wet weight and construct thickness.
| Treatment Group | Construct Wet Weight (mg) |
Thickness (mm) |
|---|---|---|
| Control | 19.9±3.3 | 0.73±0.14 |
| 1 h | 16.0±4.1 | 0.73±0.16 |
| 2 h | 15.8±3.6 | 0.66±0.10 |
| 4 h | 14.8±2.5 | 0.56±0.09 |
| 6 h | 9.3±1.9a | 0.53±0.07a |
| 8 h | 10.7±1.8a | 0.53±0.08a |
Significantly lower than control (p<0.05)
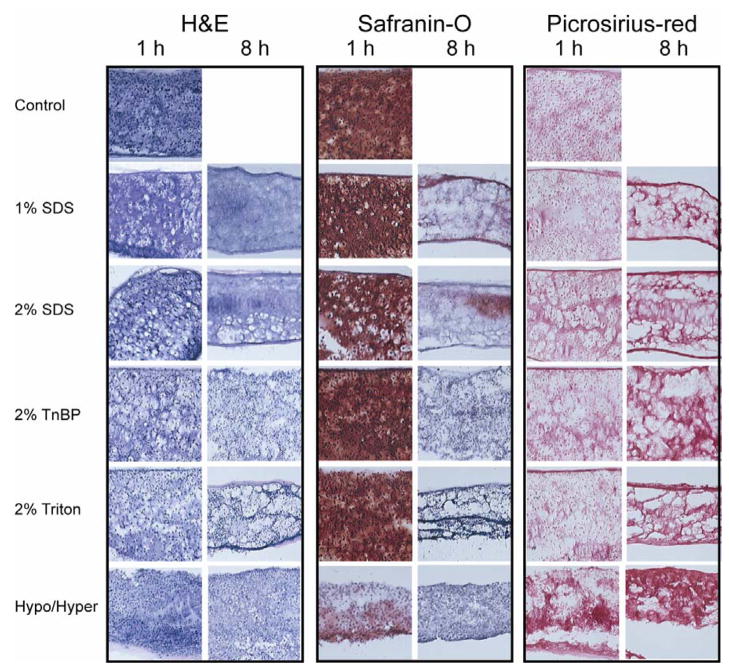
Figure 1 displays the histological results of phase I. Extensive staining for cell nuclei was observed in the H&E staining of the control group. Treatment with 1% SDS for 1 h reduced the number of cell nuclei, while treatment for 8 h eliminated all nuclei from the construct. The 2% SDS treatment had similar results. However, treatment with 2% TnBP or 2% Triton X-100, for either timepoint, had no effect on the number of nuclei. Both hypotonic/hypertonic treatments resulted in a slight reduction in number of cell nuclei. All decellularization treatments for 8 h resulted in a significant reduction or complete elimination of staining for GAGs. Additionally, 1 h treatment with the hypotonic/hypertonic solution reduced the GAG content. However, there were no apparent differences in GAG staining among the 1 h treatments with 1% SDS, 2% SDS, 2% TnBP, 2% Triton X-100, and the control. Finally, all constructs demonstrated extensive staining for collagen.
Fig. 1.
Photomicrographs demonstrating construct cellularity, GAG content, and collagen content for various treatment groups in phase I. 10× original magnification. Treatment with 2% SDS for 1 h decreased cellularity while preserving GAG content, while treatment for 8 h eliminated all nuclei, but also eliminated all GAG.
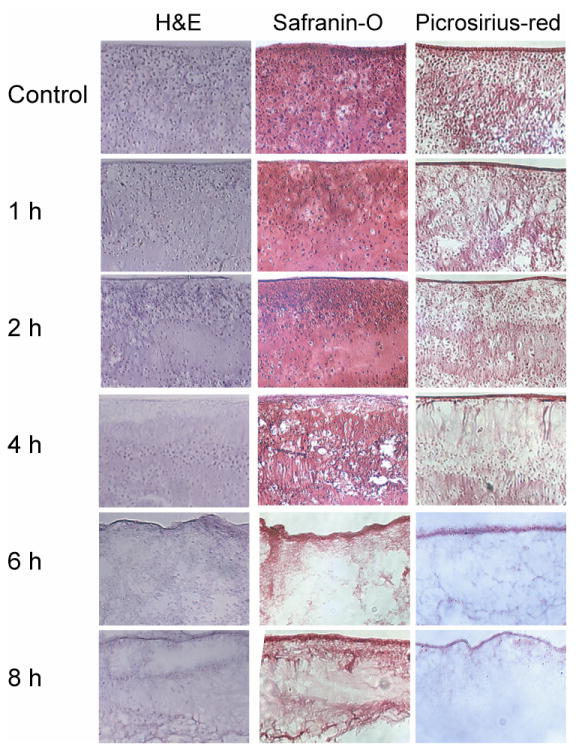
Figure 2 displays the histological results of phase II. Extensive staining for cell nuclei was observed in the H&E staining of the control group. Increasing decellularization was observed with 2% SDS treatment from 1–4 h, while 6 or 8 h application times were required for complete histological decellularization. Treatment for 1 and 2 h resulted in maintenance of GAG and collagen staining, while the 4 h treatment resulted in decreased staining. However, treatment for 6 and 8 h resulted in no GAG staining and poor collagen staining.
Fig. 2.
Photomicrographs demonstrating construct cellularity, GAG content, and collagen content for treatment groups in phase II. 10× original magnification. Treatment with 2% SDS for 1, 2, and 4 h decreased cellularity while preserving GAG and collagen content, while treatment for 6 and 8 h eliminated all nuclei, but also eliminated GAG and reduced collagen.
Quantitative Biochemistry
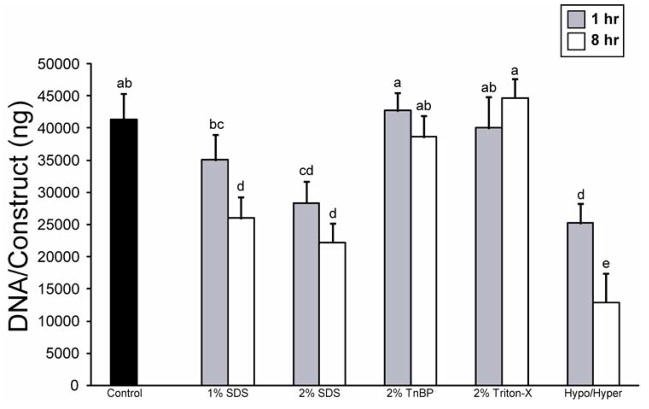
In phase I, several decellularization treatments resulted in a significant reduction in construct DNA (Fig. 3). Treatment for 1 h with 2% SDS or the hypotonic/hypertonic solution, as well as 8 h treatment with 1 or 2% SDS or the hypotonic/hypertonic solution all resulted in a significant reduction of the DNA in the constructs. However, treatment with 2% TnBP or 2% Triton X-100 for either amount of time had no effect on construct DNA. In phase II, all application times resulted in a significant decrease in DNA content, although treatment for 8 h resulted in the greatest decrease (Fig. 4).
Fig. 3.
DNA content of constructs following decellularization treatment in phase I. Treatment with 2% SDS or the hypotonic/hypertonic solutions at either application time significantly decreased construct DNA content. Columns and error bars represent mean values and standard deviations. Groups denoted by different letters are significantly different (p<0.05).
Fig. 4.
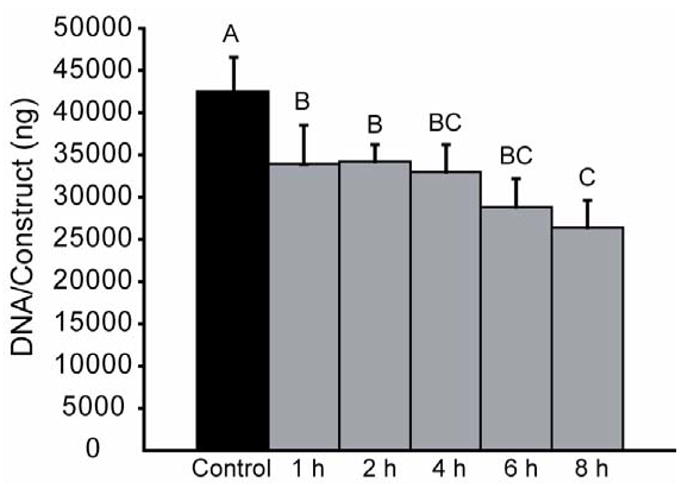
DNA content of constructs following decellularization treatment in phase II. Treatment with 2% SDS at all application times significantly reduced DNA content, while treatment for 8 h resulted in the greatest reduction in DNA content. Columns and error bars represent mean values and standard deviations. Groups denoted by different letters are significantly different (p<0.05).
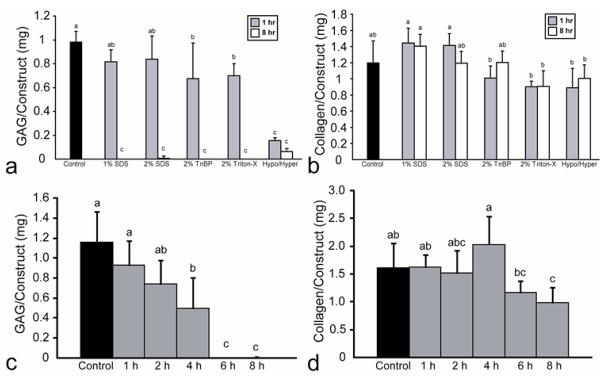
For phase I, the effects of the decellularization agents on construct GAG content are found in Fig. 5a. Treatment with 1% or 2% SDS for 1 h had no effect on GAG content, while all other treatments significantly reduced the GAG content of the constructs. Additionally, all 8 h treatments resulted in complete or nearly complete removal of GAG from the constructs. Finally, there were no significant changes in total collagen content following treatment with the decellularization agents (Fig. 5b). For phase II, the effects of the decellularization agents on construct GAG content are found in Fig. 5c. Treatment with 2% SDS for 1 or 2 h maintained GAG content, while 4 h treatment resulted in a significant decrease in GAG content. However, treatment for 6 or 8 h resulted in complete elimination of GAG. Treatment for 1, 2, 4, or 6 h did not significantly alter the collagen content, while treatment for 8 h resulted in a slight decrease in collagen content, as shown in Fig. 5d.
Fig. 5.
Construct biochemical properties following decellularization in phases I and II. (a) In phase I, all 8 h treatments resulted in nearly complete GAG removal, while both 1% and 2% SDS for 1 h maintained GAG content. (b) In phase I, treatment with SDS or TnBP maintained collagen content, while treatment with Triton X-100 or the hypotonic/hypertonic combination significantly reduced total collagen content. (c) In phase II, treatment for 1 or 2 h maintained GAG content, while treatment for 6 or 8 h resulted in near complete GAG removal. (d) In phase II, treatment for 1, 2, 4, or 6 h maintained collagen content, while treatment for 8 h resulted in a reduction in collagen content. Columns and error bars represent mean values and standard deviations. Groups denoted by different letters are significantly different (p<0.05).
Biomechanical Evaluation
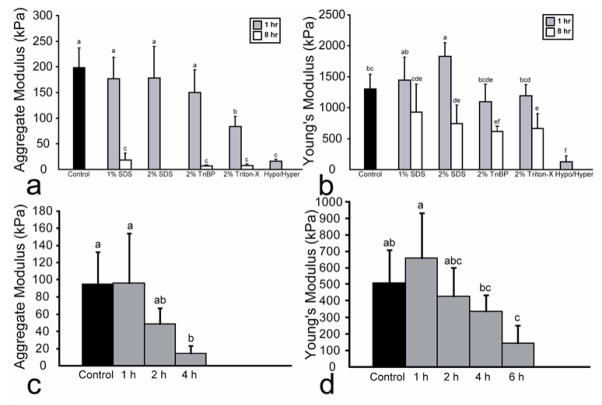
For phase I, the effects of the various decellularization treatments on construct aggregate modulus are displayed in Fig. 6a. Treatment for 1 h with 1% or 2% SDS as well as with 2% TnBP maintained the aggregate modulus. However, treatment for 8 h with 1% SDS, 2% TnBP, and 2% Triton X-100 significantly reduced the aggregate modulus. The groups treated for 8 h with either 2% SDS or the hypotonic/hypertonic solutions were too weak to be mechanically tested with creep indentation. Additionally, the effects of the various decellularization treatments on Poisson’s ratio and permeability are displayed in Table III. A significant decrease in Poisson’s ratio was noted for the groups treated for 8 h with 1% SDS, 2% TnBP, and 2% Triton X-100. Finally, only treatment for 8 h with 1% SDS resulted in a significantly decreased permeability. Figure 6b indicates the tensile properties of the constructs treated with the various agents in phase I. Treatment for 1 h with 1% SDS, 2% TnBP, or 2% Triton X-100 maintained the Young’s modulus. A 1 h treatment with 2% SDS actually increased the Young’s modulus. However, 8 h treatments with 2% SDS, 2% TnBP, and 2% Triton X-100 significantly decreased the Young’s modulus.
Fig. 6.
Construct biomechanical properties following decellularization in phases I and II. (a) In phase I, all 8 h treatments either significantly reduced aggregate modulus, or were untestable. Treatment for 1 h with 1% or 2% SDS, or 2% TnBP maintained aggregate modulus. (b) In phase I, treatment with 1% SDS for 1 h maintained Young’s modulus, while treatment with 2% SDS for 1 h increased Young’s modulus. (c) In phase II, 2% SDS treatment for 1 or 2 h maintained compressive properties, while treatment for 6 or 8 h resulted in constructs that were untestable in compression. (d) In phase II, treatment for 1, 2, or 4 h maintained Young’s modulus, while 6 and 8 h treatments significantly reduced Young’s modulus. Columns and error bars represent mean values and standard deviations. Groups denoted by different letters are significantly different (p<0.05).
Table III.
Phase I. Values of Poisson ratio and permeability following decellularization. Treatment with 1% SDS for 8 h, 2% TnBP for 8 h, and 2% Triton X-100 for 8 h resulted in a significant reduction in Poisson ratio. Only treatment with 1% SDS for 8 h resulted in a significant decrease in permeability.
| Treatment Group | Poisson Ratio | Permeability (×10−14 m4/Ns) |
|---|---|---|
| Control | 0.30±0.07 | 14.3±3.9 |
| 1% SDS, 1 h | 0.26±0.04 | 15.6±8.0 |
| 1% SDS, 8 h | 0.07±0.09a | 2.0±1.6a |
| 2% SDS, 1 h | 0.26±0.10 | 12.6±6.3 |
| 2% SDS, 8 h | Not testable | Not testable |
| 2% TnBP, 1 h | 0.24±0.13 | 5.5±3.1 |
| 2% TnBP, 8 h | 0.04±0.03a | 7.3±7.5 |
| 2% Triton X-100, 1 h | 0.16±0.11 | 4.3±2.6 |
| 2% Triton X-100, 8 h | 0.04±0.04a | 5.1±4.7 |
| Hypo/Hyper 1 h | 0.14±0.14 | 14.9±6.6 |
| Hypo/Hyper 8 h | Not testable | Not testable |
Significantly lower than control (p<0.05)
For phase II, the effects of the various application times on construct aggregate modulus are displayed in Fig. 6c. There was no significant difference in aggregate modulus with treatment for 1 and 2 h, while the 4 h treatment significantly reduced the stiffness. Additionally, the 6 and 8 h treatment resulted in constructs that were untestable in compression. As shown in Table IV, the 1, 2, and 4 h treatments did not result in significant changes in permeability and Poisson’s ratio. Figure 6d displays the tensile properties of the constructs treated in phase II. Treatment with 2% SDS for 1 h resulted in a slight increase in tensile properties, although this was not significant. Treatment for 2 and 4 h maintained the Young’s modulus while treatment for 6 h resulted in a reduced Young’s modulus. Constructs treated for 8 h were untestable in tension.
Table IV.
Phase II. Values of Poisson ratio and permeability following decellularization. No treatments resulted in significant reductions in either Poisson ratio or permeability.
| Treatment Group | Poisson Ratio | Permeability (×10−14 m4/Ns) |
|---|---|---|
| Control | 0.13±0.07 | 32.0±18.2 |
| 1 h | 0.09±0.08 | 27.0±15.2 |
| 2 h | 0.08±0.08 | 15.5±4.4 |
| 4 h | 0.09±0.09 | 66.3±77.3 |
| 6 h | Not testable | Not testable |
| 8 h | Not testable | Not testable |
Significantly lower than control (p<0.05)
DISCUSSION
The objective of this study was to assess the effectiveness of multiple decellularization protocols on self-assembled articular cartilage constructs, and to determine an appropriate application time for the treatment. A two-phased approach was used. In phase I, a two-factor approach was employed, in which five different treatments were examined at two application times each. In phase II, the effects of multiple treatment times were examined.
The results of this study indicated that SDS, at concentrations of either 1% or 2%, is an effective treatment for tissue decellularization, thus confirming our hypothesis that the number of cells could be significantly reduced from engineered constructs. An ionic detergent, SDS typically is able to solubilize the nuclear and cytoplasmic cell membranes. Although all SDS treatments led to cell removal, treatment with 2% SDS appeared the most promising, although application time also had significant effects. For instance, treatment with 2% SDS for 1 h resulted in a 33% decrease in DNA content, while maintaining both GAG and collagen content, as well as maintaining the aggregate modulus. This treatment even resulted in an increase in Young’s modulus; a similar increase in tensile properties was observed in a study of ACL decellularization.[10] On the other hand, treatment with 2% SDS for 8 h led to complete histological decellularization, with complete elimination of all cell nuclei on histological staining, as well as a 46% decrease in DNA content. However, this treatment also resulted in loss of all GAG and significant reduction in aggregate modulus, as well as a decrease in Young’s modulus. Treatment with 2% SDS for 8 h also resulted in a significant decrease in construct wet weight, presumably as a result of the GAG loss, which would also decrease the tissue hydration. As 2% SDS for 8 h resulted in the greatest decrease in DNA content, and treatment for 1 h maintained or increased biomechanical and biochemical properties, 2% SDS was selected for use in phase II.
The assessed histological, biochemical and biomechanical properties of the untreated tissue engineered constructs are in the range of the starting immature bovine cartilage, although the tensile properties are only about 10–15% of native tissue. For instance, the aggregate modulus of immature bovine cartilage is 252±31, the Young’s modulus is 7.2±4.6 MPa, the GAG/WW is 0.04±0.03 mg/mg, and the collagen/WW is 0.13±0.01 mg/mg.[23] Additionally, the constructs treated for 1 h with 1% SDS, 2% SDS, and 2% TnBP had an aggregate modulus, GAG/WW, and collagen/WW in the range of native tissue. However, the tensile properties of the tissue are lacking compared to those of native tissue. Therefore, 2% SDS treatment for 1 h, with a significant increase in Young’s modulus, results in a value closer to that of native tissue. Additionally, it is important to note that ‘control’ constructs from our prior tissue engineering studies were used as the starting point in this study, due to ease of use; however, with the use of growth factor application and mechanical stimulation such as hydrostatic pressure, we have achieved an aggregate modulus, GAG/WW, and collagen/WW matching those of native tissue, and a Young’s modulus approaching 50% of that of native tissue.[3, 38, 39] It is believed that the aggregate modulus and Young’s modulus likely will be the most important properties to match to native tissue in future tissue engineering approaches. Similar biomechanical properties between the implanted construct and the surrounding native tissue will prevent added stress at the interface site. The Poisson ratio, a measure of the tissue’s apparent compressibility, and the permeability, a measure of the resistance to fluid flow, should also approach native tissue values in order to achieve similar deformations and fluid movement under joint loading.
Treatment with 2% SDS for 1 h resulted in tissue decellularization while maintaining construct functional properties. Although SDS at all application times led to decellularization, 6 or 8 h was required for complete histological decellularization. However, these timepoints resulted in complete removal of GAG as well as an extremely poor aggregate modulus. However, the reduction in collagen content and tensile properties was less pronounced. On the other hand, as in phase I, treatment for 1 h resulted in a significant reduction in DNA content, while maintaining all biochemical and biomechanical properties, and even increasing Young’s modulus. The observed increase in Young’s modulus with a 1 h application of SDS suggests an effect of the detergent on collagen fibers within the engineered construct. SDS is known to have a propensity to disrupt non-covalent bonds in proteins and confer negative charges on proteins that have been denatured. The application of SDS for 1 h followed by a wash step may have had a transient effect on collagen architecture, wherein collagen fibers unfold as described previously, [40] and then return to their native conformations, reforming non-covalent bonds and strengthening interactions in the process. The putative mechanism may have led to the observed increased Young’s modulus at 1 h. With greater time in SDS, the effect is not observed, suggesting that any recovery undergone by collagen is counterbalanced by the detergent’s aggregate effect on the rest of the tissue architecture.
It must be noted that although treatment with 2% SDS for 6 or 8 h resulted in complete histological decellularization, it did not result in complete elimination of DNA, which would be defined as ‘complete decellularization.’ It appeared that SDS treatment was effective at achieving complete lysis of cell membranes and nuclear membranes, as H&E staining did not reveal any indication of the presence of cell nuclei, while the DNase treatment was not completely effective in degrading the DNA following membrane lysis. It is possible that a higher DNase concentration is required to achieve more complete elimination of DNA. Additionally, as nucleases were only added during detergent treatment, it is possible that adding a nuclease during the wash step would enable the nucleases to more effectively destroy the remaining DNA.
However, the exact level of tissue decellularization requisite to eliminate an immune response, as well as the proper assessment of decellularization, is currently unclear. For instance, a recent study by Gilbert et al.[41] demonstrated that several commercially available ECM scaffold materials contained measurable amounts of DNA; some even demonstrated histological staining for nuclear material. Most of these products have been used successfully clinically, so it is possible that having some remnant DNA and nuclear material in engineered cartilage constructs may result in a limited host response, though of course this needs to be demonstrated in in vivo studies. Additionally, as it is believed that the joint space is relatively immune privileged, as reviewed previously, [42] it is possible that complete decellularization of the tissue is not required. Furthermore, it is unclear if decellularization should be assessed histologically merely as elimination of cell nuclei, or if a more complete assessment involves quantifying the tissue’s DNA content, as prior studies have utilized differing approaches. For example, Lumpkins et al.[8] found that 1% SDS treatment for 24 h resulted in complete removal of cell nuclei, although they did not assess the DNA content of the tissue. On the other hand, Dahl et al.[19] examined the effects of a hypotonic/hypertonic treatment and found that there was complete removal of cell nuclei, but no decrease in DNA content. To study this issue further, in vivo studies are warranted to determine if there is threshold of decellularization at which an immune response is eliminated.
A drawback of using a decellularized xenograft is that it lacks chondrocytes, which are essential for the homeostasis of cartilage tissue. Eliminating the cells from the tissue leaves the ECM, which is responsible for the biomechanical properties of the tissue. Additionally, it has previously been demonstrated that decellularized bovine cartilage remained intact when implanted in a sheep for up to 6 months, and that there was cell infiltration, possibly from surrounding bone marrow MSCs.[22] Therefore, it is possible that bone marrow infiltration of the decellularized constructs after implantation will allow for long term viability.
Although it was less effective than the 2% concentration, 1% SDS displayed similar effects. For example, treatment for 1 h resulted in a 15% decrease in DNA content, while maintaining GAG and collagen content, as well as maintaining biomechanical properties. Additionally, treatment for 8 h resulted in a 37% decrease in DNA content, loss of all GAG and aggregate modulus, as well as a decrease in Young’s modulus.
On the other hand, treatment with Triton X-100 and TnBP did not appear promising, as they had a minimal effect on tissue decellularization, and resulted in a slight decrease in GAG content. Several prior studies have indicated the ineffectiveness of Triton X-100, although it was used in this study as it is believed to have minimal effects on protein-protein interactions.[5] For example, Dahl et al.[19] examined the effects of 1% Triton X-100 on porcine carotid arteries, and found that this treatment resulted in similar cellularity to control and no decrease in DNA content. In another study on tendon decellularization, Cartmell and Dunn[9] examined the effect of 1% Triton X-100 for 24 h, and found that cell density remained similar to control. Contrary to our results, this study also demonstrated complete histological decellularization with 1% TnBP, although a 48 h treatment was required. Therefore, it is possible that TnBP treatment may result in decellularization of self-assembled constructs at longer application times, although the GAG loss after as little as 8 h prevents the use of longer application times.
Finally, although a hypotonic/hypertonic treatment has been an effective decellularization agent in this study as well as prior studies, [10, 19] it did not appear to be a viable treatment for self-assembled cartilage constructs, as it had severely detrimental effects on construct functional properties. For instance, treatment for as little as 1 h resulted in nearly complete loss of compressive and Young’s modulus, while constructs treated for 8 h were untestable mechanically. Additionally, treatment at both application times resulted in nearly complete elimination of GAG content.
Based on these results, treatment with 2% SDS appears to be the most promising and should be examined further in future studies. Once complete SDS removal has been ensured, in vivo studies should be performed to determine the immune response to the decellularized tissue. Additionally, the effects of different decellularization treatments on complement activation should be addressed in future in vitro studies. Furthermore, as this study aimed to reduce the cellularity, additional studies are needed to investigate the elimination of the Galα1,3 epitope, which plays a significant role in hyperacute rejection of xenografts.[43]
CONCLUSIONS
Treatment with 2% SDS appears to be the most promising method for decellularization. Treatment for 1 or 2 h maintained biomechanical and biochemical properties, while simultaneously significantly reducing the DNA content of the tissue. Additionally, treatment for 6 or 8 h resulted in complete histological decellularization, but also resulted in elimination of GAG and decreased aggregate modulus. Although the results of this study did not result in a completely decellularized construct with maintenance of biochemical and biomechanical properties, the results are promising and indicate the potential of a decellularized articular cartilage construct that could be used to treat damaged cartilage tissue without eliciting an immune response.
Acknowledgments
The authors would like to acknowledge funding from NIH NIAMS AR 053286.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Benjamin D. Elder, Department of Bioengineering, Rice University.
Sriram V. Eleswarapu, Department of Bioengineering, Rice University.
Kyriacos A. Athanasiou, Department of Bioengineering, Rice University.
References
- 1.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 2.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12(4):969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 3.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3(6):e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23(2):425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Chen RN, Ho HO, Tsai YT, Sheu MT. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25(13):2679–2686. doi: 10.1016/j.biomaterials.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton TW, Ingram J, Katta J, Knight R, Korossis S, Fisher J, et al. Development and characterization of an acellular porcine medial meniscus for use in tissue engineering. Tissue Eng Part A. 2008;14(4):505–518. doi: 10.1089/tea.2007.0233. [DOI] [PubMed] [Google Scholar]
- 8.Lumpkins SB, Pierre N, McFetridge PS. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008;4(4):808–816. doi: 10.1016/j.actbio.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res. 2000;49(1):134–140. doi: 10.1002/(sici)1097-4636(200001)49:1<134::aid-jbm17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26(35):7339–7349. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 11.Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29(8):1065–1074. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasimir MT, Rieder E, Seebacher G, Silberhumer G, Wolner E, Weigel G, et al. Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs. 2003;26(5):421–427. doi: 10.1177/039139880302600508. [DOI] [PubMed] [Google Scholar]
- 13.Seebacher G, Grasl C, Stoiber M, Rieder E, Kasimir MT, Dunkler D, et al. Biomechanical properties of decellularized porcine pulmonary valve conduits. Artif Organs. 2008;32(1):28–35. doi: 10.1111/j.1525-1594.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 14.Tudorache I, Cebotari S, Sturz G, Kirsch L, Hurschler C, Hilfiker A, et al. Tissue engineering of heart valves: biomechanical and morphological properties of decellularized heart valves. J Heart Valve Dis. 2007;16(5):567–573. discussion 574. [PubMed] [Google Scholar]
- 15.Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger-de Groot AC, DeRuiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27(4):566–571. doi: 10.1016/j.ejcts.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 16.Meyer SR, Chiu B, Churchill TA, Zhu L, Lakey JR, Ross DB. Comparison of aortic valve allograft decellularization techniques in the rat. J Biomed Mater Res A. 2006;79(2):254–262. doi: 10.1002/jbm.a.30777. [DOI] [PubMed] [Google Scholar]
- 17.Meyer SR, Nagendran J, Desai LS, Rayat GR, Churchill TA, Anderson CC, et al. Decellularization reduces the immune response to aortic valve allografts in the rat. J Thorac Cardiovasc Surg. 2005;130(2):469–476. doi: 10.1016/j.jtcvs.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Rosario DJ, Reilly GC, Ali Salah E, Glover M, Bullock AJ, Macneil S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen Med. 2008;3(2):145–156. doi: 10.2217/17460751.3.2.145. [DOI] [PubMed] [Google Scholar]
- 19.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12(6):659–666. [PubMed] [Google Scholar]
- 20.Hodde J, Hiles M. Virus safety of a porcine-derived medical device: evaluation of a viral inactivation method. Biotechnol Bioeng. 2002;79(2):211–216. doi: 10.1002/bit.10281. [DOI] [PubMed] [Google Scholar]
- 21.Hodde J, Janis A, Ernst D, Zopf D, Sherman D, Johnson C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med. 2007;18(4):537–543. doi: 10.1007/s10856-007-2300-x. [DOI] [PubMed] [Google Scholar]
- 22.von Rechenberg B, Akens MK, Nadler D, Bittmann P, Zlinszky K, Kutter A, et al. Changes in subchondral bone in cartilage resurfacing--an experimental study in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage. 2003;11(4):265–277. doi: 10.1016/s1063-4584(03)00006-2. [DOI] [PubMed] [Google Scholar]
- 23.Elder BD, Kim DH, Athanasiou KA. Developing an Articular Cartilage Decellularization Process Towards Facet Joint Cartilage Replacement. Neurosurgery. 2009 doi: 10.1227/01.NEU.0000367616.49291.9F. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25(3):293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 25.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9(4):597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 26.Saini S, Wick TM. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 2004;10(5–6):825–832. doi: 10.1089/1076327041348545. [DOI] [PubMed] [Google Scholar]
- 27.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu M, Minakuchi K, Kaji S, Koga J. Chondrocyte migration to fibronectin, type I collagen, and type II collagen. Cell Struct Funct. 1997;22(3):309–315. doi: 10.1247/csf.22.309. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- 30.Brown AN, Kim BS, Alsberg E, Mooney DJ. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000;6(4):297–305. doi: 10.1089/107632700418029. [DOI] [PubMed] [Google Scholar]
- 31.Pietila K, Kantomaa T, Pirttiniemi P, Poikela A. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164(1):30–36. doi: 10.1159/000016640. [DOI] [PubMed] [Google Scholar]
- 32.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 33.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12(3):340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 34.Sneddon I. The relaxation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- 35.Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5(5):541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 36.Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop Relat Res. 1995;(316):254–266. [PubMed] [Google Scholar]
- 37.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13(9):2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 38.Elder BD, Athanasiou KA. Effects of Temporal Hydrostatic Pressure on Tissue-Engineered Bovine Articular Cartilage Constructs. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17(1):114–123. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otzen DE. Protein unfolding in detergents: effect of micelle structure, ionic strength, pH, and temperature. Biophys J. 2002;83(4):2219–2230. doi: 10.1016/S0006-3495(02)73982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilbert TW, Freundb SJ, Badylak SF. Quantification of DNA in Biologic Scaffold Materials. J Surg Res. 2008 doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revell CM, Athanasiou KA. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng Part B. 2009 doi: 10.1089/ten.teb.2008.0189. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platt JL, Fischel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991;52(2):214–220. doi: 10.1097/00007890-199108000-00006. [DOI] [PubMed] [Google Scholar]