Abstract
Deguelin has shown promising chemopreventive and therapeutic activities in diverse types of cancers. However, the potential side effect of deguelin over a certain dose could be the substantial hurdle in the practical application of the drug. One of the successful strategies for the use of deguelin in clinical trials could be lung-specific delivery of the drug. The present study evaluates the efficacy of liposome-encapsulated deguelin with a dose of 0.4mg/kg, which is 10 times less than the dose (4mg/kg) for preventive and therapeutic activities validated in previous in vivo studies. Liposomal deguelin revealed cytotoxic activity in vitro in premalignant and malignant human bronchial epithelial (HBE) cells and non-small-cell lung cancer (NSCLC) cells through the same mechanistic pathway previously reported for deguelin, i.e., suppression of the HSP90 chaperone function and induction of apoptosis. Delivery of liposomal deguelin at a dose of 0.4mg/kg by intranasal instillation resulted in markedly increased drug partitioning to the lungs compared to that of 4mg/kg deguelin or 0.4mg/kg liposomal deguelin administered by oral gavage. Lung-specific delivery of deguelin (0.4mg/kg) via nasal or intratracheal instillation in a liposomal formulation also showed significant chemopreventive and therapeutic activities in 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone (NNK)/benzo(a)pyrene (BaP)-treated A/J mice and K-rasLAC57Bl6/129/sv F1 mice with no detectable toxicity. Our findings support the potential use of deguelin in a liposomal formulation via lung-specific delivery to improve efficacy and to reduce the potential side effects of the agent.
Keywords: liposome-encapsulated deguelin, chemoprevention, therapy, lung cancer, instillation
Introduction
Lung cancer is the number one cause of cancer death and the second most frequent cancer reported each year in the United States 1. Despite recent advances in therapeutic modalities, the severe mortality of lung cancer has not improved, and the need to develop novel therapeutic approaches for this disease is urgent. In addition to developing effective therapeutic modalities, researchers have made extensive efforts to discover effective drug delivery systems (DDS). Carrier-assisted, localized drug delivery enables drugs to avoid first-pass metabolic degradation in the liver and increases their efficacy and anticancer effects. Liposomes have been proposed and used as a good carrier of chemicals and drugs due to the simplicity of preparation and its high biocompatibility 2, 3. The greatest potential advantage to using a liposome-encapsulated agent to prevent or treat lung cancer is the ability to deliver the agent directly to the lungs through natural or device-assisted inhalation of the aerosol remedy 4, 5.
Given the complexity of the signaling pathways present in most cancers, small-molecule inhibitors, such as deguelin, which can alter multiple aberrant signaling components, may provide better therapeutic outcomes than agents commonly used in cancer prevention and therapy. Deguelin, a natural product isolated from several plant species, including Mundulea sericea (Leguminosae), has emerged as an effective agent for a variety of cancer types, including skin, mammary, colon, prostate, head and neck, and lung cancers 6–12. A potential barrier to the use of deguelin, however, is the possible risk of a Parkinson’s disease-like syndrome (PDS), which has been reported in rats treated with a high dose of deguelin 4. On the basis of the hydrophobicity of the drug, we hypothesized that a selective drug delivery system (DDS) by liposomal delivery of deguelin could increase its bioavailability to desired targets and reduce the effective dose of deguelin, leading to decrease in the risk of the side effect.
In the present study, we sought to determine the chemopreventive and therapeutic activities of deguelin in a liposomal formulation in vitro and in vivo. We found that liposomal deguelin inhibited proliferation of premalignant and malignant HBE and NSCLC cell lines by inducing apoptosis with a greater potency than deguelin did in certain cell lines. We also found that organ-to-plasma distribution of deguelin in mice was better with nasal administration of liposomal deguelin than with oral administration with deguelin. Furthermore, liposomal deguelin in intranasal or intratracheal administration revealed effective antitumor activities in two different mouse models with lung tumors induced either by a tobacco carcinogen or by the oncogenic k-RAS13. Overall, our results suggest that delivery of deguelin via liposomal formulation is an effective strategy for lung cancer chemoprevention and therapy.
Materials and Methods
Preparation of liposomal-deguelin
Liposomal deguelin was formulated as previously reported for liposomal 9-nitrocamptothecin 14. Briefly, stock solutions of dilauroylphosphatidylcholine (DLPC) and deguelin were prepared in t-butanol. Aliquots of deguelin and DLPC (1:10) were mixed and frozen at −70°C. The frozen solution was then lyophilized overnight, stored at −20°C, and freshly reconstituted at room temperature in sterile PBS before use.
Western blot analysis
Whole cell lysates were prepared by incubating cell pellets in lysis buffer (50 mM Tris, pH 7.5, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1mM EDTA, 1 mM Na2VO4, 1 mM NaF with protease inhibitor cocktail [Roche, Heidelberg, Germany]) for 20 min on ice. After centrifugation at 12,000 rpm for 20 min, the supernatant fractions were harvested carefully and the protein concentration was measured using a BCA protein assay kit (Pierce, Rockford, IL). Aliquots of protein (50μg) were electrophoresed through 10% SDS-polyacrylamide gel (Bio-Rad, Hercules, CA), and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). After blocking with tris-buffered saline (TBS) containing 0.05% Tween20 (TBST) and 5% non-fat powdered milk, the membrane was incubated with the primary antibody solution at 4°C overnight. The following antibodies and working dilutions were used for the western blots: anti-phosphorylated-AKT (1:1000), anti-AKT (1:1000), anti-phosphorylated-Gsk3b (1:1000), anti-MEK1 (1:1000), and anti-p85 (1:1000) antibodies (Cell Signaling Technology, Beverly, MA); anti-CDK4 (1:1000), and anti-actin (1:2000) antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); anti-Hif2-alpha (1:1000) antibody (Novus Biologicals, Littleton, CO); and anti-e-NOS (1:1000) antibody (BD Biosciences, San Jose, CA). After overnight incubation with the primary antibody, the membrane was washed with TBST and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 hr at room temperature. The protein-antibody complexes were detected by enhanced chemiluminescence (ECL kit) in accordance with the manufacturer’s directions (Amersham, Arlington Heights, IL).
Cell proliferation assay
NSCLC cell lines (H1299, A549, H460, and 226B) were maintained in RPMI 1640 with 10% Fetal Bovine Serum (FBS) in a humidified environment with 5% CO2. Normal human bronchial epithelial (NHBE) cells were purchased from Cambrex (East Rutherford, NJ) and cultured according to the manufacturer’s recommended protocol. BEAS-2B cells (NHBE immortalized with a hybrid adenovirus/simian virus 40 15 were cultured in KSFM with recommended supplements. One premalignant (1198) and one malignant (1170) cell line derived from BEAS-2B cells by exposure to beeswax pellets containing cigarette smoke condensate 15, 16 were cultured in KSFM with 3% FBS. The cells were plated on 96-well cell culture plates (Griner Bio-One, Monroe, North Carolina) at a density of 4000 cells/well. The next day, cells were treated with 100 nM deguelin or liposomal deguelin in complete medium with control cells receiving DMSO or liposome vehicle. After 96 hr, viable cells in each well were measured using the MTT cell growth assay as described previously 17 and as per manufacturer’s protocol (Sigma, St. Louis, MO).
Anchorage-dependent colony formation assay
NSCLC cell lines were seeded in a 6-well cell culture plate at a density of 5 × 105 cells/well. On the second day, cells were treated with 100 nM deguelin or liposomal deguelin in complete medium. After 3 days, cells were harvested and re-seeded into a 6-well plate at a density of 500 cells/well in complete medium without drugs. Two weeks later, plates were stained with hematoxylin and the colony number was counted.
Assessment of apoptosis in HBE cells and NSCLC cells by Annexin-V/PI double-staining assay
For detection of apoptotic cells, expression of Annexin-V-FITC and exclusion of propidium iodide (PI) were simultaneously detected using two-color flow cytometry. The HBE and NSCLC cells were seeded into wells of a 6-well cell culture plates and allowed to attach overnight. Cells were treated with a 100 nM concentration of liposomal deguelin or deguelin in complete medium. After 36 hr of treatment, cells were harvested by trypsinization. Cell pellets were suspended in 100 μl of 1X binding buffer, to which was then added 3 μl of FITC-conjugated Annexin-V and 5 μl of PI (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After incubation at room temperature for 10 min, 400 μl of 1X binding buffer was added, and cells were immediately analyzed by flow cytometry (Becton Dickinson, San Jose, CA).
Measurement of caspase-3 activity
Caspase-3 activity was determined using the Caspase-3 Colorimetric Assay (R&D Systems, Minneapolis, MN), according to the manufacturer’s protocol. Briefly, the cell lysate was tested for protease activity by the addition of a caspase-specific peptide conjugated to the color reporter molecule, p-nitroanilide(p-NA). Cleavage of the peptide by caspase releases the chromophore p-NA, which is quantitated spectrophotometrically at a wavelength of 405 nm. The caspase-3 activity was compared in fold changes by setting the control reading as 1.
Evaluation of therapeutic effects of liposomal deguelin in the A/J and K-rasLAC57Bl6/129/sv F1 mutant mice
To assess the chemopreventive effects of liposomal deguelin for tobacco-induced tumorigenesis, six week-old female A/J mice (Jackson Laboratory, Bar Harbor, Maine) were treated with 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone (NNK) and benzo(a)pyrene [BaP] (3uM each in 0.1 ml cottonseed oil) once a week for 8 weeks. After the first treatment, mice were randomly divided into four groups. One group of mice received liposomal deguelin (0.4 mg/kg), the second group received 4 mg/kg of deguelin, the third group received vehicle alone, and the fourth group of mice was kept untreated. Deguelin and DMSO control were administered by oral gavage, and liposomal deguelin and empty liposome were administered nasally. Deguelin, liposomal deguelin, and vehicle were administered once a week for 16 weeks. After 20 weeks, all mice were sacrificed, and the lungs were removed and fixed in a 10% formalin PBS solution. Twelve week-old K-rasLAC57Bl6/129/sv F1 mutant mice were used as a spontaneous lung tumor model and treated with either PBS or the liposomal deguelin (0.4 mg/kg) via intratracheal instillation once a week for 8 weeks. At the end of the ninth week, the mice were sacrificed, and the lungs were removed and fixed in a 10% formalin PBS solution. As an indicator of drug efficacy, the total number of tumor nodules in each lung was counted under a dissecting microscope. The tissue was submitted for histological evaluation with hematoxilin and eosin (H&E) staining, and tumor multiplicity, volume, and load were quantitated. The tumor volume was calculated as volume (mm3 = 1/2 × long diameter × short diameter2). The tumor load was calculated as tumor number multiplied by tumor volume.
Deguelin concentration analysis
Fifty ICR out-bred tumor-free mice were randomized into two groups for pharmacokinetic assays. One group received liposomal deguelin at 0.4 mg/kg via intranasal instillation. The other group received deguelin at 4 mg/kg via oral gavage. Mice were sacrificed using isofurane for blood collection and harvesting of organs at the following time points: pre-treatment, 1, 4 and 24 hours after drug administration. Blood samples were collected through the orbital plexus and allowed to clot. Serum was isolated by centrifugation at 5000 × g at 4°C for 5 min. Serum samples were stored at −70°C until analysis. The organs were rapidly excised, weighed, frozen, and stored in liquid nitrogen until analyzed. All samples were processed and analyzed by high performance liquid chromatography with mass spectrophotometry detection as previously described 18.
Immunohistochemistry
After serial de-paraffination and rehydration steps, antigen was retrieved in citrate buffer(10mM, pH 8.0) by boiling in a microwave for 10 minutes and by typsin-digestion (1μg/ml in PBS) for 20 minutes at 37°C. After incubation in 3% H2O2 for 5 minutes, the tissue sections were blocked with 10% FBS for overnight at 4°C. Then, tissues were incubated at 25°C with the primary rabbit p-Akt(Thr308) antibodies(Santa Cruz Biotechnology, Inc.) at 1:100 dilution in the antibody diluent (Invitrogen Co., Carlsbad, California). After washing three times for 5 minutes, the tissues were incubated using Vectorstain ABC Elite kit (Vector Laboratories Inc., Burlingame, CA) and stained using DAB Substrate Kit, 3,3′-diaminobenzidine (Vector Laboratories Inc.) as the manufacturer’s manuals. Positive nuclear staining was counted for each node in lung tissue sections, and the total counted nuclei were divided by the number of total nodes for each group.
Statistical analysis
Colony formation number, lung tumor multiplicity, and positive nuclear staining for p-Akt were compared among treatment groups using unpaired, two-tailed Student’s t test, and p<0.05 was considered statistically significant for all tests.
Results
Inhibition of in vitro growth and colony formation of premalignant, malignant, and NSCLC cell lines by liposomal deguelin
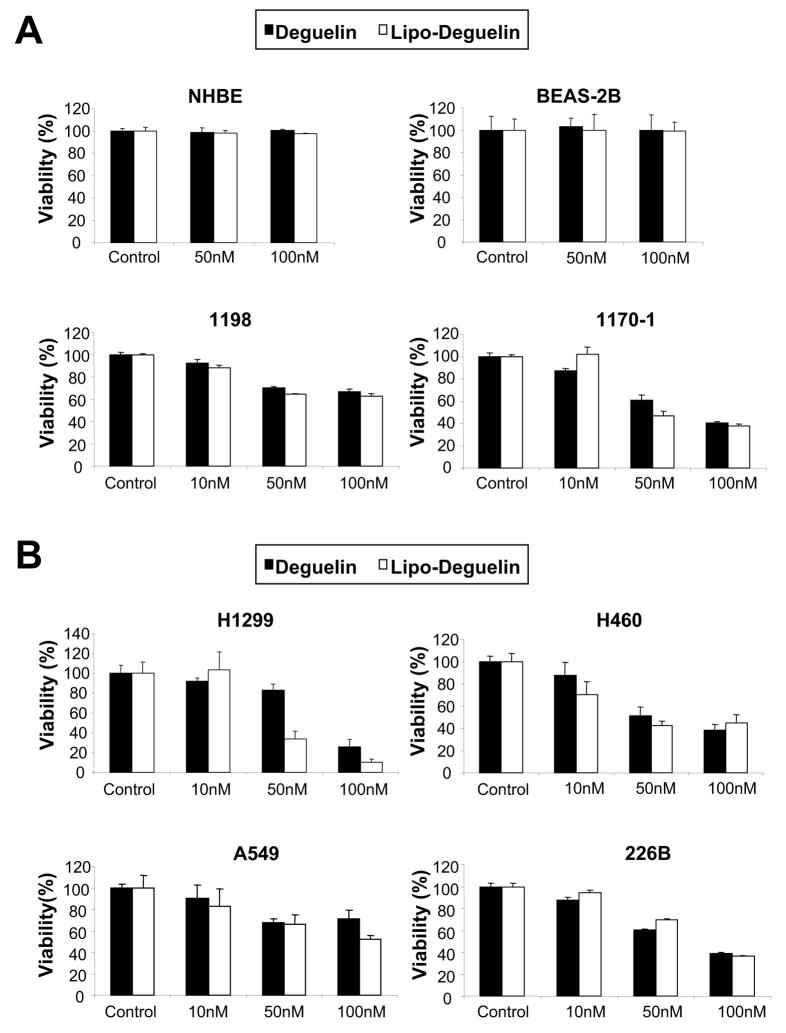
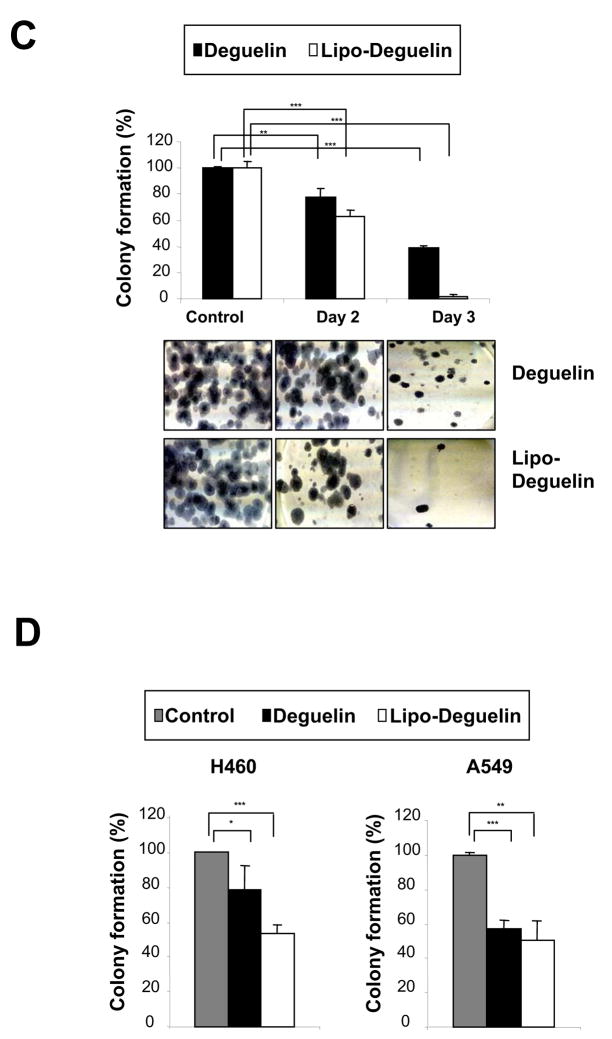
The effect of liposomal deguelin on cell proliferation was assessed in primary cultured normal (NHBE), immortalized (BEAS-2B), premalignant (1198), and malignant (1170-1) HBE cells and a subset of NSCLC cell lines (H1299, H460, 226B and A549) (Figure 1). Liposomal and parental deguelin showed similar levels of dose-dependent antiproliferative activities in 1198, 1170-1, H1299, A549 and H460 cells. However, neither compound showed any significant cytotoxicity on NHBE and BEAS-2B cells at doses of 50 nM and 100 nM (Fig.1A). We next examined the effect of the drugs on NSCLC cell survival. Liposomal deguelin significantly inhibited the anchorage-dependent colony forming abilities of H1299, H460 and A549 cells with a greater potency than parental deguelin (Fig. 1C). MTT and colony formation assays demonstrated a ~80% growth inhibition in H1299 cell lines after treatment with 100 nM liposomal deguelin (Figures 1B and 1C).
Fig.1. Effect of liposomal deguelin on inhibition of cell proliferation in normal, precancerous, and cancerous human bronchial epithelial cells and in non-small cell lung cancer (NSCLC) cells.
Effect of liposomal deguelin on cell proliferation in (A) NHBE, BAES-2B, 1198, and 1170-1 cell lines and (B) NSCLC cell lines. Cells were treated with indicated concentrations of deguelin and liposomal-deguelin. After 96 h incubation, cell viability was measured using the MTT assay as described in Materials & Methods. Results are expressed as percent viability relative to the viability of vehicle treated cells (Control). Each bar represents the mean value of 4 individual wells. (C) Anchorage-dependent colony formation assay of NSCLC treated with deguelin and liposomal deguelin. Results from H1299 cells are shown as a representative case. The graph indicates the number of colonies relative to the number of colonies formed by vehicle control. (D) Colony formation assay graphs for A549 and H460 cells. * p<0.05, ** p<0.01, *** p<0.001
Induction of apoptosis in precancerous, cancerous, and NSCLC cells by liposomal deguelin
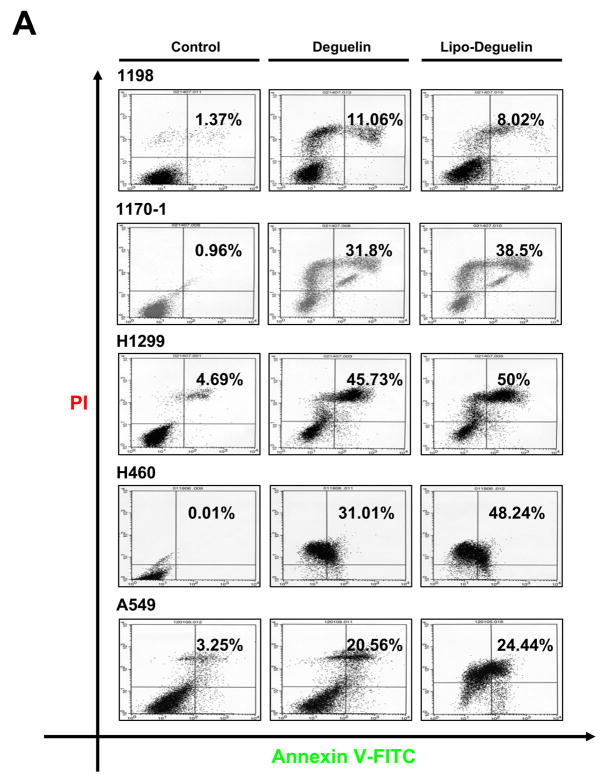
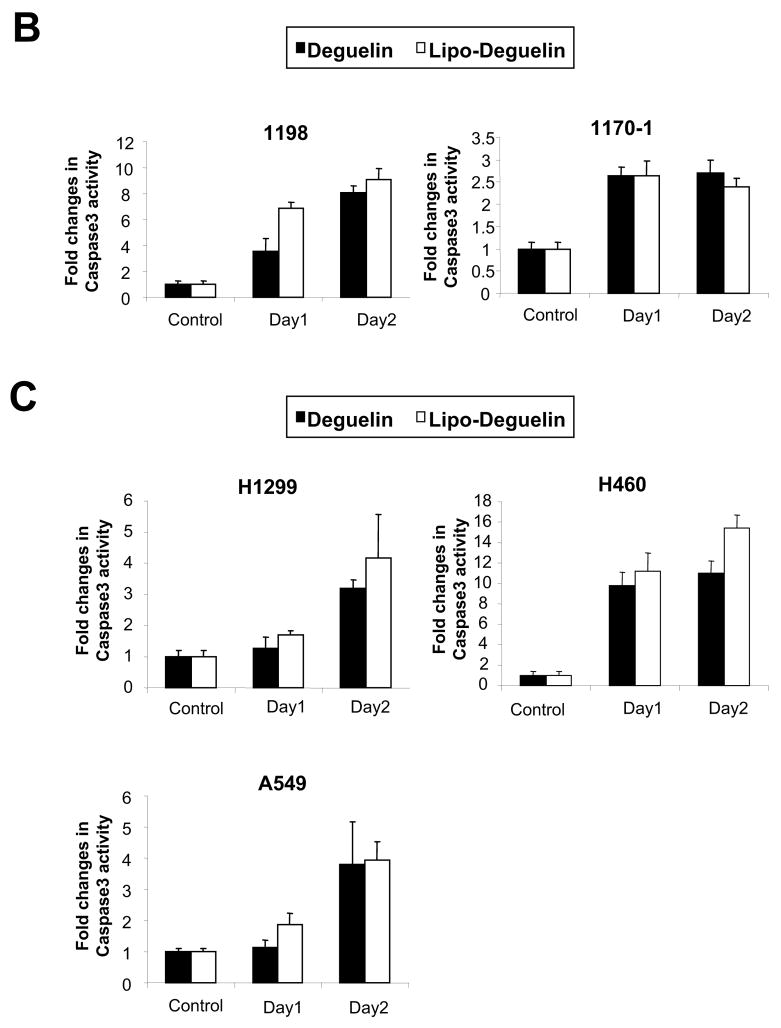
Because apoptosis is one of the main biological events caused by deguelin, we investigated whether liposomal deguelin would also induce apoptosis in 1198, 1170-1, H1299, H460, and A549 cells. Figure 2A shows the density plots of PI fluorescence versus annexin V-FITC fluorescence obtained from control (untreated), deguelin- or liposomal deguelin-treated cells. Apoptotic cell death was obviously increased after the treatment with deguelin or liposomal deguelin; approximately 11.1%, 31.8%, 45.7%, 31.0%, and 20.6% of deguelin-treated vs. 8.0%, 38.5%, 50.0%, 48.2%, and 24.4% of liposomal deguelin-treated 1198, 1170-1, H1299, H460, and A549 cells, respectively, underwent apoptosis (Figure 2A). Apoptotic cell death was further confirmed by detecting increased caspase-3 activities following treatment with either parental or liposomal deguelin in the cell lines (Figures 2B and 2C). These results show that liposomal deguelin and parental deguelin have apoptotic activities in premalignant and malignant HBE and NSCLC cells.
Fig.2. Effect of liposomal deguelin on apoptosis in precancerous and cancerous human bronchial epithelium cells and in NSCLC cells.
(A) Results of fluorescence-activated cell sorter analysis using premalignant (1198) and malignant (1170-1) HBE cell lines, and the H1299, H460, and A549 NSCLC cell lines. Each cell line was treated with 100 nM liposomal deguelin or deguelin for 36 hours. Left lower quadrant, unstained cells; left upper quadrant, PI-stained cells (necrotic); right upper quadrant, annexin V and PI-stained cells (late apoptotic cells and necrotic cells); right lower quadrant, annexin V stained cells (early apoptotic cells). (B) Caspase-3 activity was measured at the time points indicated in control and treated cells.
Effect of liposomal deguelin on the PI3K/AKT pathway and HSP90 client proteins
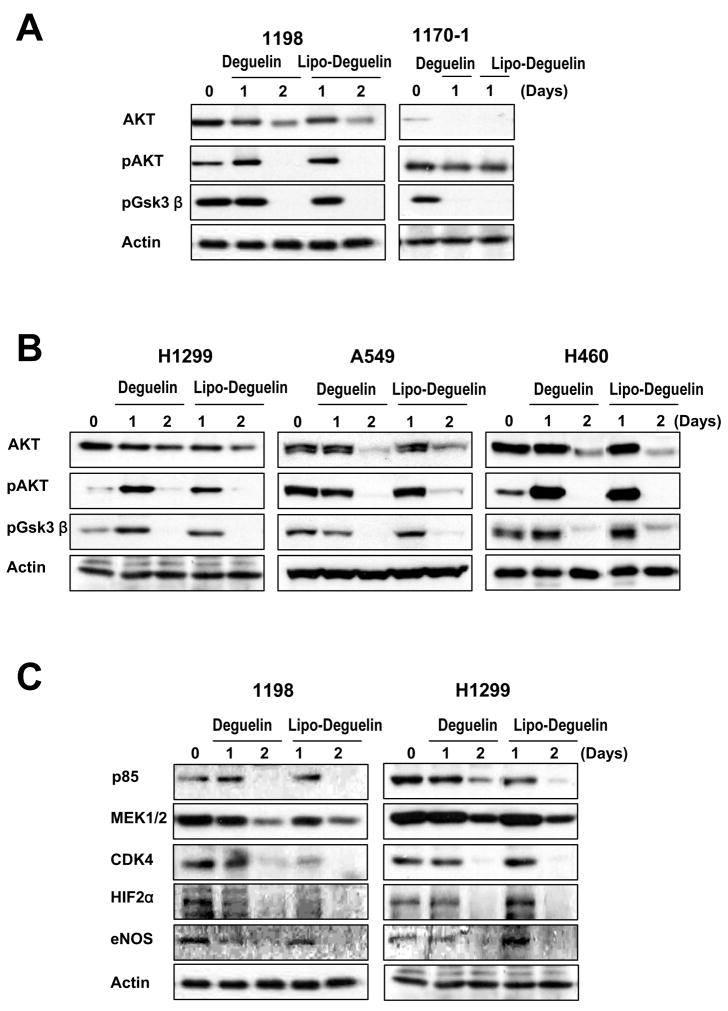
The PI3K/AKT pathway is important in regulating cell survival and proliferation 19–21. We previously demonstrated that deguelin downregulates HSP90 client proteins, including AKT, HIF-1α, HIF-2α, CDK4, and eNOS in vitro and in vivo in premalignant and malignant HBE cells 22, 23 and/or NSCLC cells 10, 24. Consistent with previous results, we observed down-regulation of total and phosphorylated AKT as well as pGSK3β, a direct downstream target of the PI3K/AKT pathway, by liposomal deguelin in 1198, 1170-1, H1299, A549, and H460 cell lines to a degree comparable to that observed with the parental drug (Figs. 3A and B). Furthermore, we observed decreases in the expression of client proteins of HSP90, including CDK4, MEK1/2, HIF-2α, and eNos, in 1198 and H1299 cell lines after treatment with 100 nM liposomal deguelin or deguelin (Fig. 3C). All protein levels were down-regulated to a similar degree by liposomal deguelin and parental deguelin (Figs. 3A and B). As a whole, the results of the tissue culture studies suggest that liposome-encapsulated deguelin has the same efficacy as the parental compound on cell proliferation, apoptosis, and downregulation of the HSP90 function.
Fig.3. Effect of liposomal deguelin on the phosphatidylinositol 3-kinase pathway (PI3K)/AKT and HSP90 client proteins in premalignant and malignant human bronchial epithelium cell lines and in NSCLC cell lines.
Western blot analysis showing the level of (A, B) AKT, phosphorylated AKT, and phosphorylated glycogen synthase kinase-3beta protein, and (C) HSP90 client proteins in cell lines treated with 100 nM deguelin or liposomal deguelin.
Comparison of biodistribution and availability of deguelin and liposomal deguelin
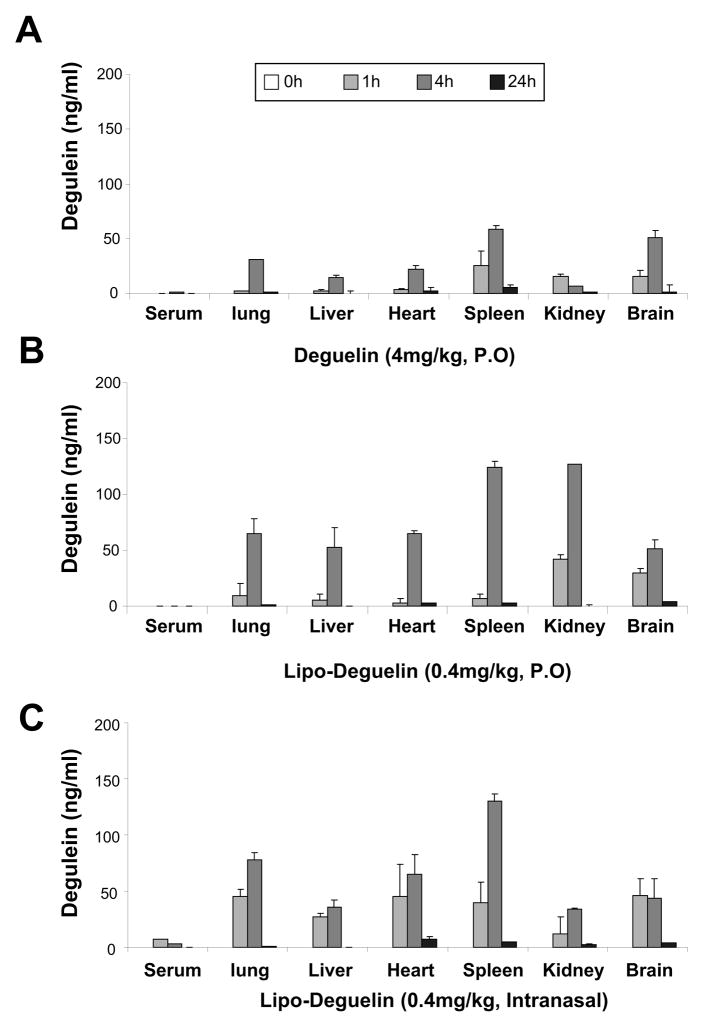
We attempted to examine the chemopreventive and therapeutic activities of deguelin and liposomal deguelin in vivo. We first evaluated the serum and tissue distribution of deguelin in ICR mice treated with deguelin or liposomal deguelin orally or intranasally. Fig. 4A shows the concentration-time curve of deguelin in serum and various organs after oral gavage or intranasal single dose administration of 0.4 mg/kg or 4 mg/kg deguelin. A peak concentration was detected from 1 to 4 hr after the administration with 4 mg/kg deguelin, and deguelin was distributed more to the lung, spleen, and brain (Fig. 4A). In contrast, mice treated with liposomal deguelin showed different patterns of organ biodistribution. Oral-gavage administration of 0.4 mg/kg deguelin resulted in an increased peak concentration of deguelin at 4 hr in most organs, especially kidney and spleen (Fig. 4B). Similarly, the intranasal administration of liposomal deguelin also showed increased accumulation of deguelin in most organs, but the distribution was more confined to lung, heart and spleen. Interestingly, the liposomal formulation showed twofold increase in the accumulation of deguelin at 4 hours in the lung compared to deguelin while the level of accumulation in the brain seemed to be changed. As indicated by Figure 4C, the intranasal administration of liposomal deguelin shows a rapid increase in distribution in the lungs at 1 hour suggesting the effectiveness of intranasal administration of liposomal deguelin for delivery to the lungs.
Fig.4. Pharmacokinetics of liposomal deguelin and deguelin.
(A) Deguelin was administered using oral gavage, and liposomal deguelin were administered using either oral gavage (B) and nasal instillation (C) on ICR out-bred, tumor free mice and sacrificed at 0, 1, 4, and 24 hours later. Sera and organs were collected as indicated in the Materials and Methods and drug concentrations were measured by HPLC.
Chemopreventive effects of liposomal deguelin in the A/J mouse/NNK/B[a]P model
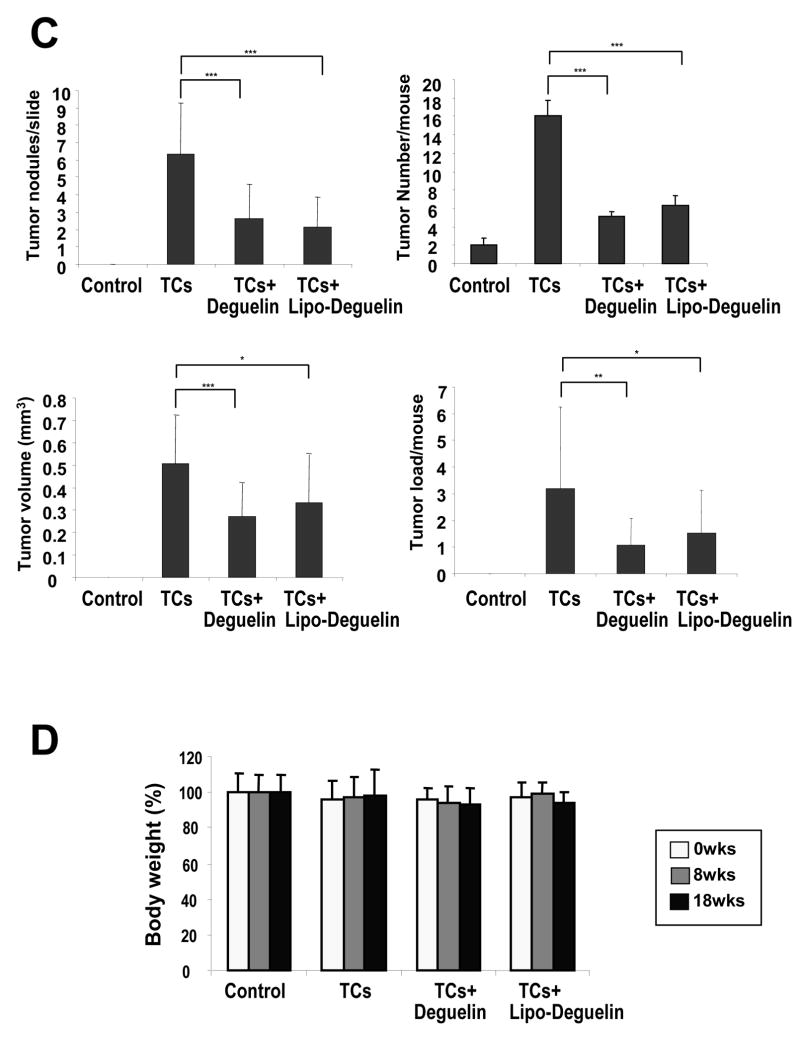
To assess the chemoprevention activity of liposomal deguelin, we used the A/J mouse and NNK/BaP system as described in Materials and Methods and Fig. 5A because NNK and B[a]P have been shown to effectively induce lung tumor formation in laboratory animals, including mice, rats, and hamsters, regardless of the route of administration 25. The animals receiving NNK/BaP (n=13) showed significantly higher tumor incidence and tumor multiplicity compared to the control animals (Fig. 5B). However, liposomal deguelin (n=13) or parental deguelin (n=13) significantly reduced tumor multiplicity, volume, and load compared to NNK/BaP (Fig. 5B, C). The tumor multiplicity was reduced to 34±27% at dose at a dose of 0.4 mg/kg of liposomal deguelin administered by nasal instillation and to 41±30% at a dose 4.0 mg/kg of deguelin administered by oral gavage when compared to that of the NNK/BaP treated control. The tumor volume was significantly deceased by both liposomal deguelin (66±42%) and parental deguelin (54±28%) (Fig. 5C). The tumor load was also notably deceased by liposomal deguelin and parental deguelin to 48±52% and 34±32% respectively in comparison to the NNK/BaP control (Fig. 5C). We measured body weight three times over the period of this experiment, and found no significant change. Other signs of toxicity were also absent in all groups of mice.
Figure 5. Evaluation of the chemoprevention effect of liposomal deguelin in a tobacco-induced tumor model.
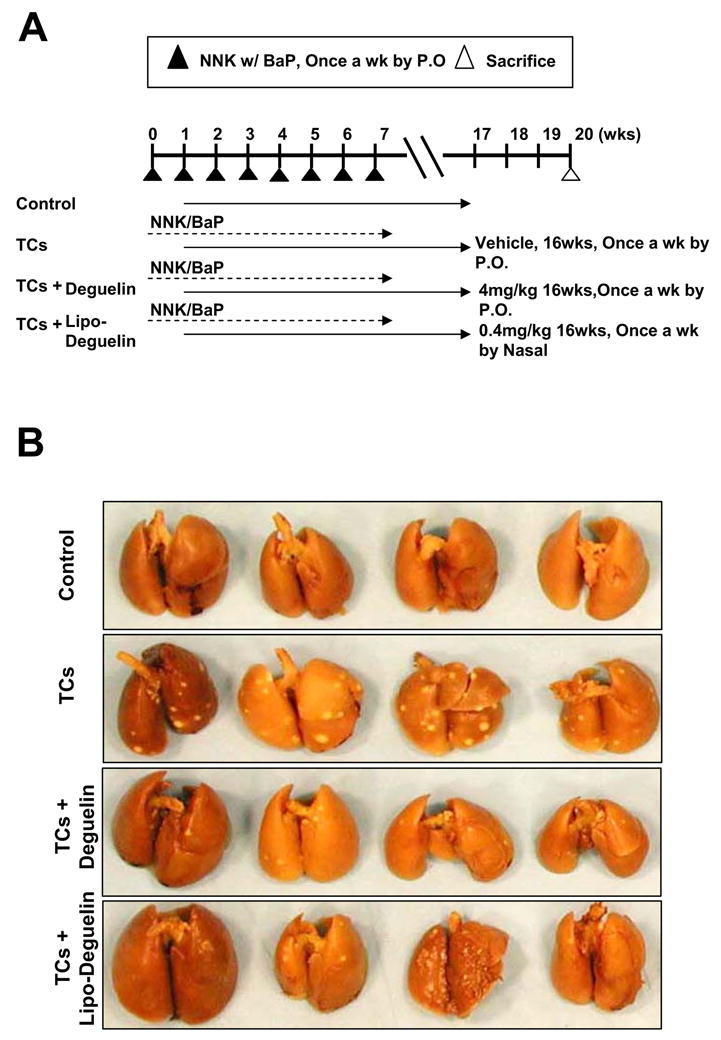
(A) Diagram showing treatment protocol for deguelin or liposomal deguelin and NNK/BaP. The NNK/BaP was administered orally once each week. Animals also received liposomal deguelin (0.4 mg/kg through nasal), deguelin (4 mg/kg through oral gavages), or vehicle (DMSO through oral gavages) once each week. After 20 weeks all mice were sacrificed and lungs were removed and examined. (B) Photographs of lungs removed from A/J mice treated with NNK/BaP and liposomal deguelin, deguelin, or vehicle control. (C) Effects of liposomal deguelin and deguelin on lung tumor induction by NNK/BaP in A/J mice. Graphs show: tumor number per slide, tumor number per animal, average tumor volume (mm3 = 1/2 × long diameter × short diameter2), and tumor load (tumor load = tumor number × tumor volume). (D) Body weight of NNK/BaP-treated A/J mice inoculated each drug. * p<0.05, ** p<0.01, *** p<0.001
Therapeutic efficacy of liposomal deguelin in the K-rasLAC57Bl6/129/sv F1 mutant mouse model
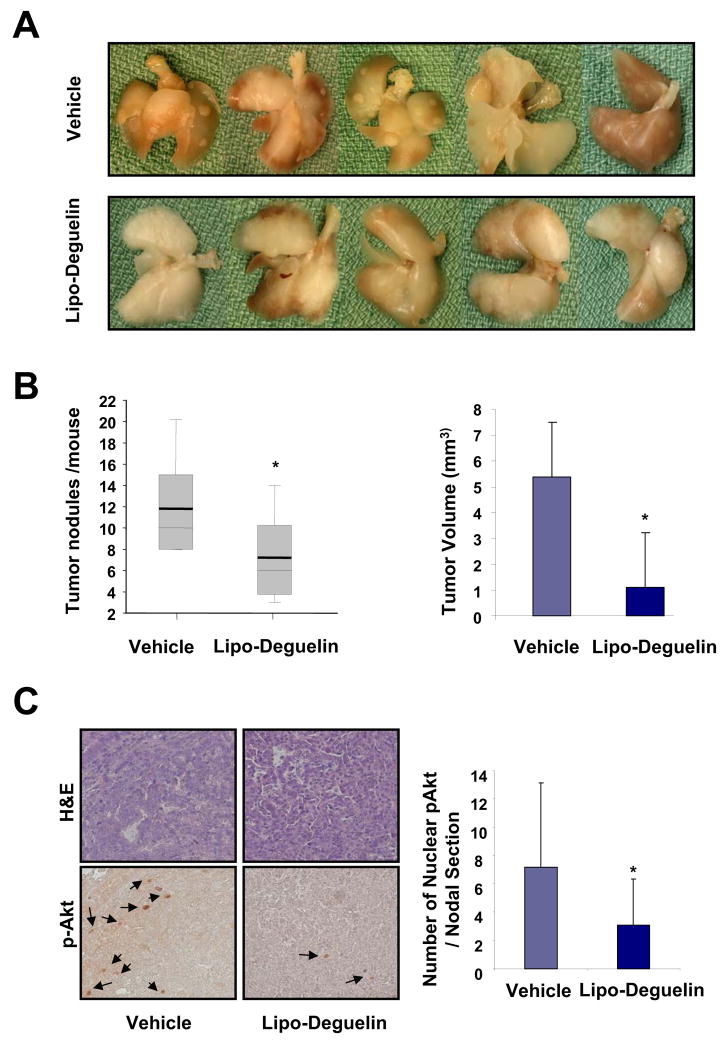
The K-rasLAC57Bl6/129/sv F1 mutant mice develop lung tumors spontaneously with 100% incidence rate 13. In this current study, we used this model to assess the therapeutic efficacy of liposomal deguelin. We began treating the animals with the liposomal deguelin when they reached 3 months of age. The drug was administered once a week for 8 weeks by the intratracheal instillation (0.4 mg/kg in 100 μl of PBS). Liposomal deguelin showed remarkably high efficacy in reducing tumor burden in the mouse lung (Fig. 6). There was a 40% reduction in the number of tumor nodules in the mice group treated with liposomal deguelin in comparison to the control. (p<0.05; Figs. 6A and 6B). Also, total tumor volume was dramatically reduced by 80% in the liposomal deguelin-treated animals compared to the control group (p<0.05, Fig. 6B). Furthermore, the liposomal deguelin reduced p-Akt(Thr308) levels by half compared to the vehicle-treated tumors (p<0.05, Fig. 6C). In spite of the significant reduction of tumor burden by liposomal deguelin, there was no significant weight loss or behavioral abnormality observed.
Figure 6. Therapeutic effects of liposomal deguelin on lung tumorigenesis in K-rasLAC57Bl6/129/sv F1 mutant mice.
Liposomal deguelin (0.4 mg/kg) or vehicle was administered via tracheal instillation once a week for 8 weeks. The mice were sacrificed at the end of the ninth week and lungs were excised for tumor evaluation. (A) Photographs of lungs from either vehicle-treated or liposomal deguelin-treated mice. (B) Number of tumor nodes on the lungs as counted under a dissecting microscope. For H/E staining and histological evaluation, the lungs were fixed and paraffin-blocked. Each block was sectioned at 5 μm intervals, and the first, 21st and 41st sections were mounted on a glass slide. Tumors were measured on sections carrying the highest number of tumors as seen under a dissecting microscope and the volumes were calculated as described in the text. (C) The lung tissue sections were immunostained against p-Akt(Thr308) as described in the materials and methods and the repreentitive results are shown. The arrows indicate the positive nuclear staining. The positive nuclear stainings were counted for each tumor node and compared by the average number of positive nuclei per node. * p<0.05
Discussion
In this study, we have demonstrated that a liposomal formulation of deguelin, a well-studied chemopreventive and therapeutic agent, leads to an improved pharmacokinetic activity, resulting in significant antitumor activities similar to the levels induced by a 10 times higher dose of the parent drug. These results are important because there is a concern regarding potential side effects of deguelin 26. We and others have shown important preclinical evidence of the cancer chemopreventive and therapeutic activities of deguelin in a variety of cancer types 10, 11, 23, 27 implicating deguelin as an effective lung cancer chemopreventive and therapeutic agent.
However, the possibility that deguelin inhibits NADH:ubiquinone oxidoreductasess and thus induces cardiotoxicity, respiratory depression, and nerve conduction blockade at high doses (a dose that is lethal to 50% of those exposed = 10–100 g in humans) 26 could be a concern for its use in cancer chemoprevention and treatment. Caboni and coworkers observed a Parkinson’s disease-like syndrome in the rat brain after treatment with 6 mg/kg deguelin, but not after treatment with 3 mg/kg 4. We have not observee behavioral neurotoxicity in our previous and current studies with 4mg/kg. However, our data concern an animal model of lung cancer development, and as such, are similar but do not mirror human lung cancer, implying the limitations regarding translation of an animal model to practical use in human lung cancer prevention. The possible side effects from the drug treatment emphasize the need to develop a better delivery system for the drug.
Given the fact that liposomal delivery could provide increased accumulation of the encapsulated drug in the target organ, we hypothesized that delivery of deguelin in a liposomal formulation through lung-targeted administration would achieve effective chemopreventive and therapeutic outcomes with a reduced dose and minimize potential side effects. Hence, we attempted to test our hypothesis in vitro in normal, immortalized, premalignant and malignant HBE cells and a subset of NSCLC cells and in vivo in 2 mouse models in which lung tumors were established by treatment with NNK/BaP or by mutant k-ras (G12D).
When tested in vitro in premalignant (1198) and malignant (1170-1) HBE cells and NSCLC cell lines (H1299, H460 and A549), liposomal deguelin decreased viability of these cells and induced apoptotic activities with no detectable cytotoxic effects on NHBE (primary culture) or BAES-2B HBE (immortalized) cells. Moreover, we also confirmed that liposomal deguelin was also able to inhibit the functions of HSP90 in the similar manner and degree as deguelin did. Of note, liposomal deguelin tended to have greater apoptotic activities in certain cell lines than did deguelin. This cell-specific sensitivity may be associated with differences in a cellular chemical metabolism context 4 or the delivery of the encapsulated drug.
Given these promising in vitro findings and data supporting the benefits of liposomal DDS 2, 3, we anticipated that liposomal encapsulation would increase delivery and distribution of deguelin in vivo. We have shown administration of 4mg/kg of deguelin by oral gavage exerts effective lung cancer chemopreventive and therapeutic activities in the A/J and xenograft mouse models 10, 11, 23, 27 In the current study, we show that intranasal administration of deguelin in liposomal formulation at 10 folds lower dose (0.4 mg/kg) resulted in more effective delivery of the drug to the lungs. Our results suggest that liposomal encapsulation could protect deguelin from metabolic degradation, allowing sustained pulmonary release and causing more efficient intracellular delivery. Although the mechanism by which the concentration of deguelin is increased remains to be explored, we hypothesized that the improved pharmacokinetics of deguelin could allow lowering the therapeutic dose of deguelin. Aerosol delivery of liposomal cancer drugs to patients has been successfully done 28. In this instance, liposomal deguelin resuspended in water will be nebulized using a commercially available nebulizer such as the Aerotech II flowing 10 L of air/min and delivered through a facemask or mouthpiece. The patient, breathing normally, will receive a dose of deguelin proportional to his/her minute volume/kg body weight.
Led by this speculation, we sought to determine the antitumor activities of low dose of liposomal deguelin in two animal models, including the NNK/BaP-treated A/J A/J and K-rasLAC57Bl6/129/sv F1 mutant mice. Indeed, we were able to confirm that treatment with 0.4 mg/kg of liposomal deguelin once a week effectively reduced lung tumor burdens in these two mouse models. These results support the use of liposome for drug delivery into the pulmonary tissues as discussed in the preceding report 14. DLPC liposome tended to remain in the lung for a prolonged period which extended drug efficacy. Therefore, it is plausible to suggest that deguelin treatment in liposomal formulation permits a sharp reduction in therapeutic dose, leading to reduce the risk of potential side effects of the drug treatment while maintaining the high drug efficacy.
We recently demonstrated that the antitumor activities of deguelin involve its binding to the ATP-binding pocket of Hsp90, which suppresses Hsp90 function leading to degradation of a variety of HSP90 client proteins carrying important roles in cancer cell homeostasis 29–31 and survival under stress conditions 21, 22, 32–36. In the current study, we showed the ability of liposomal deguelin to regulate the expression of HSP90 client proteins as effectively as parental deguelin. These findings confirm that liposomal encapsulation enhances the therapeutic potency of deguelin without affecting the biochemical activity of the drug. Therefore, the direct administration of deguelin in liposomal formulation would be a preferred method for controlling human lung cancer.
Taken together, our preclinical results present the possibility of using deguelin at a reduced dose and frequency through a direct route of administration for lung cancer chemoprevention and therapy. These findings provide a strong rationale for testing the toxicity and efficacy of liposomal deguelin in clinical trials of lung cancer chemoprevention and therapy. Finally, our results suggest that a smart delivery system targeting tumors specifically may enhance the efficacy of deguelin even in smaller amount and reduce the putative side effects associated with deguelin.
Acknowledgments
This work was supported by National Institutes of Health grants R01 CA100816-01 and R01 CA109520-01 (to Ho-Young Lee), and American Cancer Society grant RSG-04-082-01-TBE 01 (to Ho-Young Lee). W. K. H. is an American Cancer Society clinical research professor.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bethune CR, Geyer RJ, Spence AM, Ho RJY. Lipid Association Improves the Therapeutic Index of Lomustine [1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea] to Suppress 36B-10 Tumor Growth in Rats. Cancer Res. 2001;61:3669–74. [PubMed] [Google Scholar]
- 3.Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4:297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- 4.Caboni P, Sherer TB, Zhang N, Taylor G, Na HM, Greenamyre JT, Casida JE. Rotenone, deguelin, their metabolites, and the rat model of Parkinson's disease. Chem Res Toxicol. 2004;17:1540–8. doi: 10.1021/tx049867r. [DOI] [PubMed] [Google Scholar]
- 5.Koshkina NV, Kleinerman ES, Waldrep C, Jia S-F, Worth LL, Gilbert BE, Knight V. 9-Nitrocamptothecin Liposome Aerosol Treatment of Melanoma and Osteosarcoma Lung Metastases in Mice. Clin Cancer Res. 2000;6:2876–80. [PubMed] [Google Scholar]
- 6.Gills JJ, Kosmeder J, 2nd, Moon RC, Lantvit DD, Pezzuto JM. Effect of deguelin on UVB-induced skin carcinogenesis. J Chemother. 2005;17:297–301. doi: 10.1179/joc.2005.17.3.297. [DOI] [PubMed] [Google Scholar]
- 7.Murillo G, Salti GI, Kosmeder JW, 2nd, Pezzuto JM, Mehta RG. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur J Cancer. 2002;38:2446–54. doi: 10.1016/s0959-8049(02)00192-2. [DOI] [PubMed] [Google Scholar]
- 8.Peng XH, Karna P, O'Regan RM, Liu X, Naithani R, Moriarty RM, Wood WC, Lee HY, Yang L. Down-regulation of inhibitor of apoptosis proteins by deguelin selectively induces apoptosis in breast cancer cells. Mol Pharmacol. 2007;71:101–11. doi: 10.1124/mol.106.027367. [DOI] [PubMed] [Google Scholar]
- 9.Yan Y, Wang Y, Tan Q, Lubet RA, You M. Efficacy of deguelin and silibinin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice. Neoplasia. 2005;7:1053–7. doi: 10.1593/neo.05532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H-Y, Oh S-H, Woo JK, Kim W-Y, Van Pelt CS, Price RE, Cody D, Tran H, Pezzuto JM, Moriarty RM, Hong WK. Chemopreventive Effects of Deguelin, a Novel Akt Inhibitor, on Tobacco-Induced Lung Tumorigenesis. J Natl Cancer Inst. 2005;97:1695–9. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 11.Lee H-Y, Suh Y-A, Kosmeder JW, Pezzuto JM, Hong WK, Kurie JM. Deguelin-Induced Inhibition of Cyclooxygenase-2 Expression in Human Bronchial Epithelial Cells. Clin Cancer Res. 2004;10:1074–9. doi: 10.1158/1078-0432.ccr-0833-3. [DOI] [PubMed] [Google Scholar]
- 12.Oh SH, Woo JK, Jin Q, Kang HJ, Jeong JW, Kim KW, Hong WK, Lee HY. Identification of novel antiangiogenic anticancer activities of deguelin targeting hypoxia-inducible factor-1 alpha. Int J Cancer. 2008;122:5–14. doi: 10.1002/ijc.23075. [DOI] [PubMed] [Google Scholar]
- 13.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–6. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 14.Knight V, Koshkina NV, Waldrep JC, Giovanella BC, Gilbert BE. Anticancer effect of 9-nitrocamptothecin liposome aerosol on human cancer xenografts in nude mice. Cancer Chemother Pharmacol. 1999;44:177–86. doi: 10.1007/s002800050965. [DOI] [PubMed] [Google Scholar]
- 15.Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–9. [PubMed] [Google Scholar]
- 16.Klein-Szanto AJP, Iizasa T, Momiki S, Garcia-Palazzo I, Caamano J, Metcalf R, Welsh J, Harris CC. A Tobacco-Specific N-Nitrosamine or Cigarette Smoke Condensate Causes Neoplastic Transformation of Xenotransplanted Human Bronchial Epithelial Cells. PNAS. 1992;89:6693–7. doi: 10.1073/pnas.89.15.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HY, Chun KH, Liu B, Wiehle SA, Cristiano RJ, Hong WK, Cohen P, Kurie JM. Insulin-like growth factor binding protein-3 inhibits the growth of non-small cell lung cancer. Cancer Res. 2002;62:3530–7. [PubMed] [Google Scholar]
- 18.Papadimitrakopoulou V, Agelaki S, Tran HT, Kies M, Gagel R, Zinner R, Kim E, Ayers G, Wright J, Khuri F. Phase I Study of the Farnesyltransferase Inhibitor BMS-214662 Given Weekly in Patients with Solid Tumors. Clin Cancer Res. 2005;11:4151–9. doi: 10.1158/1078-0432.CCR-04-1659. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–67. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 20.Forgacs E, Biesterveld EJ, Sekido Y, Fong K, Muneer S, Wistuba II, Milchgrub S, Brezinschek R, Virmani A, Gazdar AF, Minna JD. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene. 1998;17:1557–65. doi: 10.1038/sj.onc.1202070. [DOI] [PubMed] [Google Scholar]
- 21.Lee HY, Oh SH, Suh YA, Baek JH, Papadimitrakopoulou V, Huang S, Hong WK. Response of non-small cell lung cancer cells to the inhibitors of phosphatidylinositol 3-kinase/Akt- and MAPK kinase 4/c-Jun NH2-terminal kinase pathways: an effective therapeutic strategy for lung cancer. Clin Cancer Res. 2005;11:6065–74. doi: 10.1158/1078-0432.CCR-05-0009. [DOI] [PubMed] [Google Scholar]
- 22.Lee HY, Srinivas H, Xia D, Lu Y, Superty R, LaPushin R, Gomez-Manzano C, Gal AM, Walsh GL, Force T, Ueki K, Mills GB, et al. Evidence that phosphatidylinositol 3-kinase- and mitogen-activated protein kinase kinase-4/c-Jun NH2-terminal kinase-dependent Pathways cooperate to maintain lung cancer cell survival. J Biol Chem. 2003;278:23630–8. doi: 10.1074/jbc.M300997200. [DOI] [PubMed] [Google Scholar]
- 23.Chun K-H, Kosmeder JW, II, Sun S, Pezzuto JM, Lotan R, Hong WK, Lee HY. Effects of Deguelin on the Phosphatidylinositol 3-Kinase/Akt Pathway and Apoptosis in Premalignant Human Bronchial Epithelial Cells. J Natl Cancer Inst. 2003;95:291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- 24.Oh SH, Woo JK, Yazici YD, Myers JN, Kim WY, Jin Q, Hong SS, Park HJ, Suh YG, Kim KW, Hong WK, Lee HY. Structural basis for depletion of heat shock protein 90 client proteins by deguelin. J Natl Cancer Inst. 2007;99:949–61. doi: 10.1093/jnci/djm007. [DOI] [PubMed] [Google Scholar]
- 25.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 26.Fang N, Casida JE. Anticancer action of cube insecticide: correlation for rotenoid constituents between inhibition of NADH:ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc Natl Acad Sci U S A. 1998;95:3380–4. doi: 10.1073/pnas.95.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerhauser C, Lee SK, Kosmeder JW, Moriarty RM, Hamel E, Mehta RG, Moon RC, Pezzuto JM. Regulation of ornithine decarboxylase induction by deguelin, a natural product cancer chemopreventive agent. Cancer Res. 1997;57:3429–35. [PubMed] [Google Scholar]
- 28.Verschraegen CF, Gilbert BE, Loyer E, Huaringa A, Walsh G, Newman RA, Knight V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 2004;10:2319–26. doi: 10.1158/1078-0432.ccr-0929-3. [DOI] [PubMed] [Google Scholar]
- 29.Muller L, Schaupp A, Walerych D, Wegele H, Buchner J. Hsp90 regulates the activity of wild type p53 under physiological and elevated temperatures. J Biol Chem. 2004;279:48846–54. doi: 10.1074/jbc.M407687200. [DOI] [PubMed] [Google Scholar]
- 30.Gabai VL, Mosina VA, Budagova KR, Kabakov AE. Spontaneous overexpression of heat-shock proteins in Ehrlich ascites carcinoma cells during in vivo growth. Biochem Mol Biol Int. 1995;35:95–102. [PubMed] [Google Scholar]
- 31.Muller P, Ceskova P, Vojtesek B. Hsp90 is essential for restoring cellular functions of temperature-sensitive p53 mutant protein but not for stabilization and activation of wild-type p53: implications for cancer therapy. J Biol Chem. 2005;280:6682–91. doi: 10.1074/jbc.M412767200. [DOI] [PubMed] [Google Scholar]
- 32.Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, Sessa WC, Altieri DC. Regulation of survivin function by Hsp90. PNAS. 2003;100:13791–6. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harashima K, Akimoto T, Nonaka T, Tsuzuki K, Mitsuhashi N, Nakano T. Heat shock protein 90 (Hsp90) chaperone complex inhibitor, radicicol, potentiated radiation-induced cell killing in a hormone-sensitive prostate cancer cell line through degradation of the androgen receptor. Int J Radiat Biol. 2005;81:63–76. doi: 10.1080/09553000400029460. [DOI] [PubMed] [Google Scholar]
- 34.Munster PN, Basso A, Solit D, Norton L, Rosen N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. See: E. A. Sausville, Combining cytotoxics and 17-allylamino, 17-demethoxygeldanamycin: sequence and tumor biology matters, Clin. Cancer Res., 7: 2155–2158, 2001. Clin Cancer Res. 2001;7:2228–36. [PubMed] [Google Scholar]
- 35.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 36.Carlisle DL, Liu X, Hopkins TM, Swick MC, Dhir R, Siegfried JM. Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells. Pulm Pharmacol Ther. 2006 doi: 10.1016/j.pupt.2006.07.001. [DOI] [PubMed] [Google Scholar]