Abstract
There has been an increasing interest in understanding how the mechanical properties of the microenvironment influence stem cell fate. We describe studies of the proliferation and differentiation of neural stem cells (NSCs) encapsulated within three-dimensional scaffolds – alginate hydrogels – whose elastic moduli were varied over two orders of magnitude. The rate of proliferation of neural stem cells decreased with increase in the modulus of the hydrogels. Moreover, we observed the greatest enhancement in expression of the neuronal marker β-tubulin III within the softest hydrogels, which had an elastic modulus comparable to that of brain tissues. To our knowledge, this work represents the first demonstration of the influence of modulus on NSC differentiation in three-dimensional scaffolds. Three-dimensional scaffolds that control stem cell fate would be broadly useful for applications in regenerative medicine and tissue engineering.
1. Introduction
Considerable recent work has focused on understanding the factors that influence stem cell fate, motivated by both a fundamental interest in stem cell biology as well as by the potential applications of stem cells in regenerative medicine [1-3]. While a majority of such studies have concentrated on the influence of biochemical cues on stem cell behavior, it has become increasingly clear that the mechanical properties of the microenvironment also influence the behavior of a wide variety of cells including stem cells [4-16]. In particular, in a recent seminal study, Engler et al. demonstrated that the elasticity of the matrix influences the differentiation of mesenchymal stem cells (MSCs) into neurons, osteoblasts, and myoblasts [12]. Saha et al. have also studied the influence of the mechanical properties of the substrate on the behavior of neural stem cells (NSCs). NSCs have the ability to differentiate into the three major neural lineages – neurons, oligodendrocytes, and astrocytes [17, 18] – and are thus of interest for a range of neuroregenerative applications [2, 17, 19-22]. Saha et al. found that NSCs optimally differentiated into neurons when on substrates of intermediate stiffness (500 Pa) and that hard surfaces favor astroctytic differentiation, whereas soft surfaces promote neuronal differentiation [15]. These previous studies were carried out by culturing stem cells on hydrogel substrates with controllable stiffness. It would, however, be particularly important to understand the influence of the mechanical properties of the microenvironment on the behavior of stem cells cultured in three-dimensional scaffolds, as cell behavior can differ in 2 vs. 3 dimensions [23]. Such an environment would not only be extremely relevant for clinical applications of stem cells, but would also more closely mimic the stem cell microenvironment in vivo.
Alginate is a particularly promising material for generating three-dimensional cell-encapsulating scaffolds [24, 25]. Alginates are salts of alginic acid, a linear heteropolysaccharide consisting of (1→4) linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) [26, 27]. Alginate has been studied in past as a 3-D scaffold for encapsulating NSCs because of its compatibility with central nervous system tissue [24, 25, 28, 29], In the presence of divalent cations – for example, Ca2+, Sr2+ or Ba2+ – these alginate salts form gels due to the ability of the divalent ions to bridge G residues on different chains [19, 26, 30-32]. In general, the elastic modulus of an alginate gel depends on the number of cross-links and the length and stiffness of the chains between cross-links, and the modulus of the gel can thus be tuned by varying the molecular weight of the alginate used, the G content in the alginate, and the calcium ion concentration [33].
Here, we report the effect of the modulus of alginate hydrogels on the proliferation and differentiation of encapsulated NSCs. By modulating the concentration of alginate and calcium ions, we were able to vary the modulus of the hydrogels by more than two orders of magnitude, which impacted both the proliferation and the differentiation of encapsulated NSCs. In particular, we observed the greatest enhancement in expression of the neuronal marker β-tubulin III within hydrogels having an elastic modulus comparable to that of brain tissues. The ability to influence stem cell behavior by tuning the mechanical properties of three-dimensional scaffolds should be broadly useful for applications in regenerative medicine and tissue engineering.
2. Materials and Methods
2.1 Materials
Sterile high molecular weight (200–300kDa MW, 69% guluronate content) PRONOVA SLG 100 sodium alginate was purchased from FMC BioPolymer (PA, USA). Live-dead and cell proliferation assay kits were purchased from Molecular Probes.
2.2 NSC culture
Adult NSCs isolated from the hippocampi of 6-week-old female Fisher 344 rats were cultured as described elsewhere [18]. Briefly, cells were grown on poly-ornithine/laminin-coated dishes in DMEM/F12 medium with N2 supplement (Invitrogen) and 20 ng/mL FGF-2 (Promega). The medium was changed every other day, and upon reaching ∼80% confluency, cells were subcultured on poly-ornithine/laminin-coated plates using Accutase (Phoenix Flow Systems).
2.3 NSC encapsulation inside alginate hydrogel
Neural stem cells were grown in alginate hydrogels at a density of 4000 cells per well in a 96-well plate. Briefly, sterile sodium alginate was dissolved in DMEM/F12 medium and mixed with 4000 cells resuspended in DMEM/F12 medium to the final volume of 40 μL, yielding final alginate concentrations of 0.25% or 1% wt/vol. 40 μL of this solution was added to each well of a 96-well plate, followed by the addition of 100 μL of a chilled CaCl2 solution (10 mM, 50 mM, or 100 mM). The plate was kept on ice for 10 min, 50 μL of liquid were then removed from each well, and 150 μL of DMEM/F12 media containing 20 ng/mL FGF-2, 1% penicillin streptomycin, and 5 μg/mL laminin were added. In some wells, NSCs were allowed to grow without any alginate hydrogel (negative control). We performed a 50% media change every other day for 7 days. We cultured NSCs within hydrogels having different values of modulus, prepared by varying the concentrations of alginate and CaCl2. Specifically, the following combinations were tested: 1% alginate /100 mM CaCl2 (1-100), 0.25% alginate/100 mM CaCl2 (0.25-100), 0.25% alginate/50 mM CaCl2 (0.25-50) and 0.25% alginate/10 mM CaCl2 (0.25-10).
2.4 Testing the viability of NSCs
A live dead assay (Molecular Probes) was used as per the manufacturer's instructions. Briefly, the medium from the wells was removed and cells in the hydrogel were washed once with phosphate buffer saline (PBS) followed by incubation with the dye. Live cells stained with Calcein AM and dead cells marked with Ethidium homodimer-1 (EthD-1) were visualized using a Zeiss LSM 510 Meta Confocal microscope.
2.5 Measuring the proliferation of NSCs
After 7 days of growth inside the hydrogel, with an initial seeding density of 4000 cells per well, medium was removed, and NSCs were washed once with PBS. To release encapsulated cells, the alginate hydrogel was incubated with 100 μL of a solution containing 15 mM sodium citrate and 150 mM NaCl for an hour [17, 30]. Using a pipette, the contents of each well were mixed until a homogenous solution was obtained. The 96-well plates were stored overnight at -80 °C. Cell quantities in all hydrogels were assayed in parallel using the Molecular Probes CyQuant™ Assay.
2.6 Rheological characterization of alginate hydrogels
Rheological measurements were carried out using a TA instrument AR-G2 stress-controlled rheometer with a parallel-plate geometry. Stress sweeps at a constant frequency of 1 Hz were first performed to obtain the linear viscoelastic region for collecting subsequent data. Frequency sweeps were performed in the linear viscoelastic regime to determine values of the elastic (G′) and viscous (G″) modulus. Since the 0.25 wt% gels were very fragile, they were cast directly on the rheometer. In a typical method of sample preparation, 0.25 wt% alginate solutions were poured in a mold placed on top of the bottom plate, quickly followed by the addition of a CaCl2 solution of the desired concentration. After approximately 5 minutes, the mold was removed, and the sample was cut in the shape of a disc with a diameter of 40 mm. 1 wt% gels were prepared in a similar fashion, though the gels were first cast in a Petri dish prior to loading onto the instrument. A solvent trap was used, and water evaporation was not significant for the temperature and timescales investigated. Gels were gently pressed with tissue paper to remove surface water before lowering the top plate. Samples were allowed to equilibrate for 10-15 minutes before starting the measurements, which were taken under ambient conditions at 25 °C unless otherwise specified.
2.7 NSC staining and confocal microscopy imaging
After 7 days of culture within alginate hydrogels, the NSCs were washed three times with PBS containing 5% donkey serum and 0.3% Triton X-100 (PBS-DT) and pre-blocked with PBS-DT for an hour at 37°C. NSCs were then stained overnight at 4°C with primary antibodies for nestin and β-Tubulin III at a 1:500 dilution in PBS-DT. NSCs were then washed three times with PBS-DT and incubated with secondary antibodies (Alexa fluor labeled), diluted 1:250 fold in PBS-DT in the dark. Cells were then washed three times with PBS-DT. The second to last wash was used to stain nuclei with DAPI. Images were taken on Zeiss LSM 510 Meta Confocal microscope with 40× magnification at constant settings.
2.8 Quantitative gene expression measurements
2.8.1 RNA extraction
Total RNA of the NSCs growing inside the alginate hydrogel was extracted using Trizol Reagent (Invitrogen, USA), according to the single step acid–phenol guanidinium extraction method described by Chomczynski and Sacchi [34]. In brief, after growing NSCs within hydrogels in 96-well plates for seven days, the medium from each well was replaced by Trizol reagent. After 6 hours, Trizol reagent and hydrogel from the wells were collected, vortexed, and treated with chloroform. Total RNA was precipitated using chilled ethanol. The acquired RNA samples were treated with RNase-free DNase I, and RNA integrity was tested on a 1.7% agarose gel containing 0.0025% (v/v) ethidium bromide. The RNA concentration was quantified spectrometrically at 260 and 280 nm, and purity was determined at 230 and 260 nm (NanoDrop ND-1000, USA).
2.8.2 Preparation of complementary DNA by reverse transcription
One hundred ng of total RNA from each sample was reverse transcribed into cDNA using ImProm-II™ Reverse Transcription System (Promega, USA) according to the manufacturer's protocol. At the end of the procedure, cDNA was diluted to a concentration of 10 ng/μl.
2.8.3 Quantitative real-time polymerase chain reaction assay (QRT-PCR)
Gene-specific sense and antisense primer for β-Tubulin III and 18S (house keeping gene) were purchased from IDT (USA), and TaqMan probes were synthesized by Biosearch, USA. The nucleotide sequences and values of the melting temperature (Tm) of the primers and probes are shown in Table 1. QRT-PCR was performed in a LightCycler 489 (Roche, Basel, Switzerland) in a 96-well plate. A 25 μl reaction volume with 12.5 μl Brilliant QRT-PCR Master Mix (Stratagene, USA), 10 ng cDNA as template, and 0.2 mM of the primer-pairs and TaqMan probe were used for the reaction. Target genes were run in triplicate on a single plate, which included different samples as well as no-template controls. PCR conditions were as follows: denaturation (95 °C for 10 min) followed by 70 amplification cycles (95 °C for 15 s, 60 °C for 1 min). The QRT-PCR analysis was repeated using RNA isolated from three independent experiments.
Table 1.
Primers and probes for quantitative RT- PCR. β-Tubulin III: neuronal marker. 18S (18S ribosomal subunit): internal control. FAM490: FAM490 fluorophore. CAL610: CAL610 fluorophore. BHQ: Black Hole Quencher®. Tm: Melt temperature.
| Gene | Sense and Antisense primers/ TaqMan Probe | Tm (°C) |
|---|---|---|
| β-Tubulin III | S: 5′-GCATGGATGAGATGGAGTTCACC-3′ | 65.2 |
| A: 5′-CGACTCCTCGTCGTCATCTTCATAC-3′ | 65.4 | |
| P:5′-FAM490-AGGACAGTCAGCAGTGCCTGCA-BHQ-3′ | 67.8 | |
| 18S | S: 5′-GTAACCCGTTGAACCCCATTC-3′ | 62.6 |
| A: 5′-CCATCCAATCGGTAGTAGCGA-3′ | 62.2 | |
| P: 5′-CAL610-AAGTGCGGGTCATAAGCTTGCG-BHQ-3′ | 67.6 | |
3. Results and Discussion
3.1 Alginate hydrogel characterization
We wished to test the influence of the mechanical properties of a three-dimensional scaffold – an alginate hydrogel – on the proliferation and differentiation of encapsulated NSCs. To that end, we first prepared and characterized alginate hydrogels having different elastic modulus values, which can be tuned by varying the concentrations of alginate and calcium chloride [35-37]. Previous studies of cell encapsulation in alginate hydrogels utilized 1-2% sodium alginate solutions [17, 24, 30]; however, we prepared hydrogels using four different combinations of alginate and CaCl2 concentrations to test the behavior of NSCs in softer scaffolds: 1% alginate /100 mM CaCl2 (1-100), 0.25% alginate/100 mM CaCl2 (0.25-100), 0.25% alginate/50 mM CaCl2 (0.25-50) and 0.25% alginate/10 mM CaCl2 (0.25-10).
We characterized the mechanical properties of the hydrogels by carrying out oscillatory shear measurements in a rheometer, and Figure 1 shows the results of the dynamic frequency sweep tests. The elastic modulus of the hydrogels was weakly dependent on frequency over the entire range studied (Fig. 1a) and significantly larger than the loss modulus (Fig. 1b). Figure 2, which compares the elastic modulus for hydrogels of different composition, shows that the elastic modulus of the three hydrogels formed using 0.25 wt% alginate decreased with decrease in CaCl2 concentration. The lowest modulus (∼ 180 Pa) was observed for the “0.25-10” hydrogel and lies in the range reported for the modulus of brain tissues (100-1000 Pa) [38]. In contrast, the hydrogel formed using the highest concentrations of alginate and CaCl2 (1-100) had the largest elastic modulus (∼20,000 Pa). This dramatic change in the mechanical behavior can be attributed to a higher crosslink density in these gels.
Fig 1.
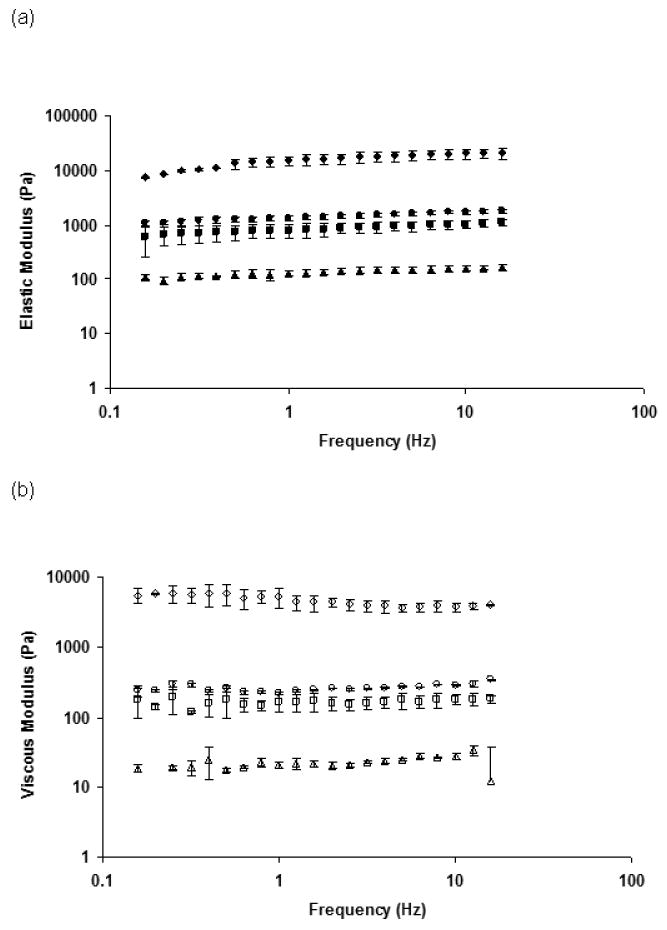
Characterization of modulus of alginate hydrogels by rheological measurements: a) Elastic modulus of different alginate hydrogels during frequency sweep analysis - 0.25-10 (▲); 0.25-50 (■); 0.25-100 (●); 1-100 (◆); b): Viscous modulus of different alginate hydrogels during frequency sweep analysis - 0.25-10 (Δ); 0.25-50 (□); 0.25-100(○); 1-100(◊).
Fig 2.
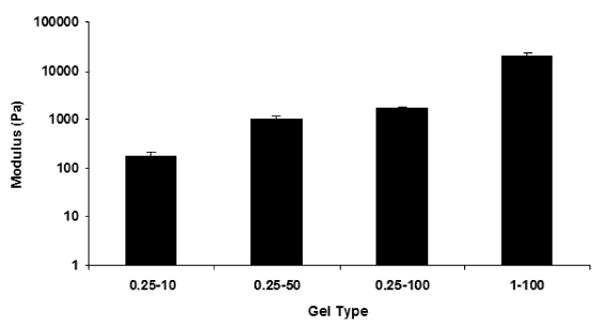
Influence of composition of alginate hydrogels on the elastic modulus (G′) at 8 Hz. Error bars represent the standard deviation.
3.2 NSC proliferation inside hydrogels
Having demonstrated the ability to control the mechanical properties of alginate hydrogels, we next proceeded to study the effect of hydrogel stiffness on the behavior of encapsulated NSCs. We found that the proliferation of NSCs in the hydrogels increased significantly with a decrease in the modulus of the hydrogels (Fig. 3). After 7 days of culture, the NSC number had increased 14-fold in the lowest modulus hydrogel (0.25-10), but only approximately two fold for the highest modulus hydrogel (1-100). We note that based on previous reports in the literature, we do not anticipate the diffusion of medium components to be a significant limitation for the hydrogels used in this study [39, 40], and we found that NSC viability was greater than 80% inside the hydrogels (data not shown). The data in Fig. 3 are consistent with proliferation rate results we had previously obtained for NSCs cultured inside 1% alginate hydrogels [29]. Consistent with prior reports, we also found a greater increase in NSC number (approximately fiftyfold in seven days) in two-dimensional monolayer culture without alginate.
Fig 3.
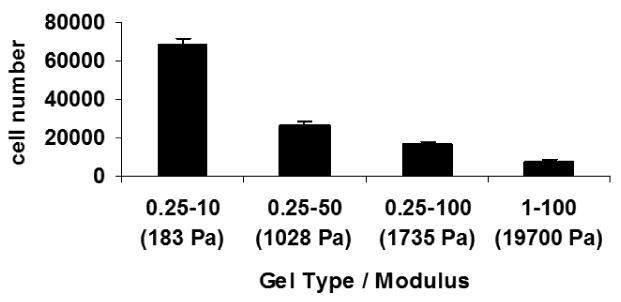
Proliferation of NSCs after 7 days in alginate hydrogels having different values of the modulus. Error bars represent the standard deviation; p<0.01 based on Student's t-test.
3.3 Effect of hydrogel modulus on neuronal differentiation of NSCs
Next, we tested whether the mechanical properties of the three-dimensional scaffolds influence the differentiation of NSCs. NSCs were encapsulated in alginate hydrogels and cultured in DMEM/F12 media containing FGF-2 (see methods for details). After 7 days, to test the extent of neuronal differentiation, we first monitored levels of β-tubulin III (a marker for neuronal differentiation) using immunofluorescence analysis (Fig. 4). We also monitored levels of nestin (an immature neural marker); transient co-expression of nestin and NSC lineage markers are seen during the process of differentiation [41]. As seen in the confocal micrographs (Fig. 4), the maximum intensity of β-tubulin III staining was observed in cells grown in the lowest modulus hydrogel (0.25-10), whereas the intensity was considerably lower for cells cultured in the other hydrogels. These results clearly indicate that culturing NSCs in three-dimensional alginate hydrogels having a modulus of ∼180 Pa – a value in the range reported for the modulus of brain tissues – promotes neuronal differentiation.
Fig 4.
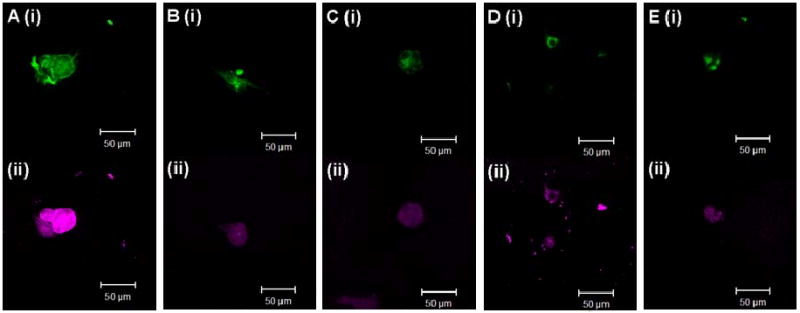
Confocal images showing nestin (i) and β-tubulin III (ii) staining of NSCs grown inside alginate hydrogels having different values of the modulus (A: 0.25-10 (183 Pa); B: 0.25-50 (1028 Pa); C: 0.25-100 (1735 Pa); D: 1-100 (19700 Pa); E: Control) in medium containing 20 ng/mL FGF.
The imaging results were complemented by an analysis of β-tubulin III expression using quantitative real time polymerase chain reaction (QRT-PCR). Expression of 18S was monitored as an internal control, and Fig. 5 shows the ratio of β-tubulin III and 18S expression levels (normalized relative to the ratio in cells cultured in the highest modulus hydrogel (1-100)). Consistent with the immunofluorescence analysis, the relative expression of β-tubulin III is ∼20-fold greater in cells cultured in alginate hydrogels having the lowest value of modulus and decreases with increasing hydrogel modulus.
Fig 5.
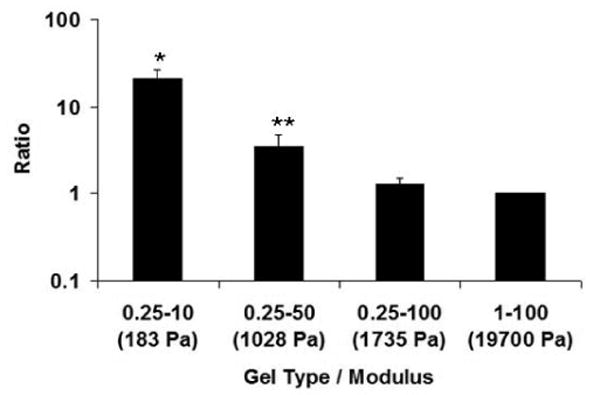
QRT-PCR results for β-tubulin III after 7 days of growth in alginate hydrogels having different values of the elastic modulus. The data reported represent the ratio of the expression of β-tubulin III and 18S ribosomal RNA normalized relative to the ratio in cells cultured in highest modulus hydrogel (1-100). Error bars represent the standard deviation; *p<0.0005, **p<0.05 compared with highest modulus hydrogel (1-100) based on Student's t-test.
We note our observed increase in neuronal differentiation of NSCs within soft hydrogels is consistent with other reports on the promotion of neuronal differentiation of stem cells cultured on soft substrates. For instance, Discher and collegues [12] found a ∼ 6 fold increase in the ratio of β-tubulin III and actin expression levels when MSCs were grown on hydrogels with a modulus of 100 Pa relative to the ratio in naive MSCs. Saha et al. have recently shown enhanced neuronal differentiation of NSCs cultured on interpenetrating polymer networks having a modulus of 500 Pa; the ratio of β-tubulin III and 18S expression levels was >3-fold greater on these hydrogels than for cells on stiffer substrates [15]. To our knowledge, however, we have shown for the first time the effect of modulus on NSC differentiation within a 3-D microenvironment. Moreover, we observed a ∼20 fold increase in relative β-tubulin III expression for NSCs cultured within the lowest modulus hydrogels as compared to control cells, significantly greater than that reported in previous studies. Thus we see a significantly greater influence of modulus on neuronal marker expression on going from a 2-D to 3-D microenvironment. Understanding the mechanisms by which the mechanical properties of the hydrogel influence stem cell fate remains a fundamental challenge in stem cell biology. Discher and colleagues have shown that cytoskeletal motors may be involved in the matrix-elasticity sensing that drives lineage specification in mesenchymal stem cells grown on hydrogels [12]. Probing a possible role for cytoskeletal motors in influencing the function of neural stem cells in three-dimensional environments will be an area of interest for future work.
4. Conclusions
There has been an increasing emphasis in recent times on elucidating the influence of the mechanical properties of the microenvironment on stem cell fate. This work provides insights into the influence of the mechanical properties of three-dimensional alginate hydrogel scaffolds on the proliferation and differentiation of NSCs, where varying the concentrations of alginate and calcium chloride provided facile control over the elastic modulus of the hydrogels. We demonstrated that the properties of the three-dimensional scaffolds significantly impacted both the proliferation and the neuronal differentiation of encapsulated NSCs. In addition, we observed the greatest enhancement in expression of the neuronal marker β-tubulin III within hydrogels having an elastic modulus comparable to that of brain tissues. We note that the optimal value of the elastic modulus may depend on the stem cell type and the lineage to which differentiation is being directed. Such three-dimensional platforms will undoubtedly be useful for fundamental studies of cell mechanobiology. Moreover, the ability to control stem cell fate – possibly without the use of chemical inducers – would also be broadly useful for applications in regenerative medicine and tissue engineering.
Acknowledgments
We acknowledge support from the NIH (EB007295), a New York State Spinal Cord Injury Research Board CART grant and from the NSF-sponsored ICE IGERT grant (DGE-0654128).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wulffraat NM, Kuis W. Autologous stem cell transplantation: a possible treatment for refractory juvenile chronic arthritis? Rheumatology (Oxford, England) 1999;38(8):764–6. doi: 10.1093/rheumatology/38.8.764. [DOI] [PubMed] [Google Scholar]
- 2.Pfeifer K, Vroemen M, Caioni M, Aigner L, Bogdahn U, Weidner N. Autologous adult rodent neural progenitor cell transplantation represents a feasible strategy to promote structural repair in the chronically injured spinal cord. Regenerative medicine. 2006;1(2):255–66. doi: 10.2217/17460751.1.2.255. [DOI] [PubMed] [Google Scholar]
- 3.Brehm M, Strauer BE. Stem cell therapy in postinfarction chronic coronary heart disease. Nature clinical practice. 2006;3 1:S101–4. doi: 10.1038/ncpcardio0431. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. The Journal of cell biology. 2003;163(3):583–95. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas TW, DiMilla PA. Spreading and motility of human glioblastoma cells on sheets of silicone rubber depend on substratum compliance. Medical & biological engineering & computing. 2000;38(3):360–70. doi: 10.1007/BF02347059. [DOI] [PubMed] [Google Scholar]
- 6.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. The Journal of cell biology. 2004;166(6):877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28(4):671–9. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. American journal of physiology. 2000;279(5):C1345–50. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 9.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deroanne CF, Lapiere CM, Nusgens BV. In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovascular research. 2001;49(3):647–58. doi: 10.1016/s0008-6363(00)00233-9. [DOI] [PubMed] [Google Scholar]
- 11.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophysical journal. 2004;86(1 Pt 1):617–28. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Hsiong SX, Carampin P, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. Journal of biomedical materials research. 2008;85(1):145–56. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- 14.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 2004;6(4):483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 15.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, et al. Substrate modulus directs neural stem cell behavior. Biophysical journal. 2008;95(9):4426–38. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Park Y, Tae G, Lee KB, Hwang CM, Hwang SJ, et al. Characterization of low-molecular-weight hyaluronic acid-based hydrogel and differential stem cell responses in the hydrogel microenvironments. Journal of biomedical materials research. 2009;88(4):967–75. doi: 10.1002/jbm.a.31947. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Liu T, Song K, Yao L, Ge D, Bao C, et al. Culture of neural stem cells in calcium alginate beads. Biotechnology progress. 2006;22(6):1683–9. doi: 10.1021/bp060185z. [DOI] [PubMed] [Google Scholar]
- 18.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nature neuroscience. 2003;6(1):21–7. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 19.Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27(19):3560–9. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 20.Vroemen M, Aigner L, Winkler J, Weidner N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. The European journal of neuroscience. 2003;18(4):743–51. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- 21.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nature reviews. 2006;7(8):628–43. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 22.Pfeifer K, Vroemen M, Blesch A, Weidner N. Adult neural progenitor cells provide a permissive guiding substrate for corticospinal axon growth following spinal cord injury. The European journal of neuroscience. 2004;20(7):1695–704. doi: 10.1111/j.1460-9568.2004.03657.x. [DOI] [PubMed] [Google Scholar]
- 23.Green JA, Yamada KM. Three-dimensional microenvironments modulate fibroblast signaling responses. Advanced drug delivery reviews. 2007;59(13):1293–8. doi: 10.1016/j.addr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Read TA, Stensvaag V, Vindenes H, Ulvestad E, Bjerkvig R, Thorsen F. Cells encapsulated in alginate: a potential system for delivery of recombinant proteins to malignant brain tumours. Int J Dev Neurosci. 1999;17(56):653–63. doi: 10.1016/s0736-5748(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 25.Read TA, Sorensen DR, Mahesparan R, Enger PO, Timpl R, Olsen BR, et al. Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nature biotechnology. 2001;19(1):29–34. doi: 10.1038/83471. [DOI] [PubMed] [Google Scholar]
- 26.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 27.Remminghorst U, Rehm BH. Bacterial alginates: from biosynthesis to applications. Biotechnology letters. 2006;28(21):1701–12. doi: 10.1007/s10529-006-9156-x. [DOI] [PubMed] [Google Scholar]
- 28.Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai H, et al. Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue engineering. 2004;10(34):493–504. doi: 10.1089/107632704323061852. [DOI] [PubMed] [Google Scholar]
- 29.Ashton RS, Banerjee A, Punyani S, Schaffer DV, Kane RS. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials. 2007;28(36):5518–25. doi: 10.1016/j.biomaterials.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 30.Markusen JF, Mason C, Hull DA, Town MA, Tabor AB, Clements M, et al. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue engineering. 2006;12(4):821–30. doi: 10.1089/ten.2006.12.821. [DOI] [PubMed] [Google Scholar]
- 31.Morris ER, Rees DA, Thom D, Boyd J. Chiroptical and stoichiometric evidence of a specific, primary dimerization process in alginate gelation. Carbohydr Res. 1978;66:145–54. [Google Scholar]
- 32.Grant GT, Morris ER, Rees DA, Smith PJC, Thom D. Biological interactions between polysaccharides and divalent cations - egg-box model. FEBS Lett. 1973;32(1):195–8. [Google Scholar]
- 33.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22(6):511–21. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 34.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical biochemistry. 1987;162(1):156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 35.Stevens MM, Qanadilo HF, Langer R, Prasad Shastri V. A rapid-curing alginate gel system: utility in periosteum-derived cartilage tissue engineering. Biomaterials. 2004;25(5):887–94. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Simpson NE, Stabler CL, Simpson CP, Sambanis A, Constantinidis I. The role of the CaCl2-guluronic acid interaction on alginate encapsulated betaTC3 cells. Biomaterials. 2004;25(13):2603–10. doi: 10.1016/j.biomaterials.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 37.Martinsen A, Skjak-Braek G, Smidsrod O. Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnology and bioengineering. 1989;33(1):79–89. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 38.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13(18):2411–5. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edelman ER, Nugent MA, Karnovsky MJ. Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(4):1513–7. doi: 10.1073/pnas.90.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo CK, Ma PX. Controlling Diffusion of Solutes through Ionically Crosslinked Alginate Hydrogels Designed for Tissue Engineering. Material Research Society Symposium Proceedings. 2001;662:LL1.5.1–5.6. [Google Scholar]
- 41.Abranches E, O'Neill A, Robertson MJ, Schaffer DV, Cabral JM. Development of quantitative PCR methods to analyse neural progenitor cell culture state. Biotechnology and applied biochemistry. 2006;44(Pt 1):1–8. doi: 10.1042/BA20050218. [DOI] [PubMed] [Google Scholar]


