Abstract
Adrenal medullary chromaffin cells are a major peripheral output of the sympathetic nervous system. Catecholamine release from these cells is driven by synaptic excitation from the innervating splanchnic nerve. Acetylcholine (ACh) has long been shown to be the primary transmitter at the splanchnic-chromaffin synapse, acting through ionotropic nicotinic ACh receptors to elicit action potential-dependent secretion from the chromaffin cells. This cholinergic stimulation has been shown to desensitize under sustained stimulation, yet catecholamine release persists under this same condition. Recent evidence supports synaptic chromaffin cell stimulation through alternate transmitters. One candidate is pituitary adenylate cyclase activating peptide (PACAP), a peptide transmitter present in the adrenal medulla shown to have an excitatory effect on chromaffin cell secretion. In this study we utilize native neuronal stimulation of adrenal chromaffin cells in situ and amperometric catecholamine detection to demonstrate that PACAP specifically elicits catecholamine release under elevated splanchnic firing. Further data reveal that the immediate PACAP-evoked stimulation involves a phospholipase C (PLC) and protein kinase C (PKC) dependant pathway to facilitate calcium influx through a Ni2+ and mibefradil-sensitive calcium conductance that results in catecholamine release. These data demonstrate that PACAP acts as a primary secretagogue at the sympatho-adrenal synapse under the stress response.
Keywords: adrenal medulla, amperometry, catecholamine, chromaffin cells, PACAP, stress
Introduction
The body responds to stress by increasing sympathetic firing. A major consequence of heightened sympathetic activity is increased catecholamine release from the adrenal medulla into the general circulation. Sympathetic control of the adrenal medulla takes place at cholinergic synapses between innervating splanchnic neurons and neurosecretory chromaffin cells (Carmichael 1986). Acetylcholine released from the presynaptic terminal binds to postsynaptic ionotropic nicotinic acetylcholine receptors (nAChR) on the chromaffin cells. Ligand-gated conductance through the nAChR leads to chromaffin cell depolarization, firing of an action potential and opening of voltage operated calcium channels. Resultant increases in cytosolic Ca2+ lead to fusion of transmitter-containing large dense core secretory granules and exocytosis of their transmitter cargoes into the bloodstream (Aunis 1998, Carmichael 1986). It has been shown, however, that this cholinergic response rapidly desensitizes at multiple stages along the exocytic pathway and that frequent stimulation or exposure to maintained levels of ACh at the sympatho-adrenal synapse results in a decreased efficiency of evoked catecholamine release from chromaffin cells (Aunis 1998, Marley 1988, Ohara-Imaizumi & Kumakura 1986). Physiologically, however, it is known that under the acute stress response, or heightened splanchnic firing, catecholamine release persists and elevated serum catecholamine levels are observed (Habib et al. 2001, Klevans & Gebber 1970, Kumar et al. 2006, McFadden & Koshland 1990, Wakade 1988). Thus, a secondary synaptic excitation mechanism other than ACh must exist.
Pituitary Adenylate Cyclase Activating Peptide (PACAP) is a member of the secretin family and is implicated in regulating the release of many peptide transmitters from different secretory cells, including insulin from pancreatic cells, thyroid stimulating hormone (TSH) in the pituitary, and renin from granular cells in the kidney (Bjorkqvist et al. 2005, Hautmann et al. 2007, Jamen et al. 2002, Kawai et al. 1992, Okada et al. 2007). PACAP is also a known secretagogue for catecholamine release from adrenal chromaffin cells (Chowdhury et al. 1994, Hamelink et al. 2002b, Lamouche et al. 1999, Przywara et al. 1996, Tornoe et al. 2000, Watanabe et al. 1992). Splanchnic nerve terminals in the adrenal medulla have been shown to contain PACAP while chromaffin cells express the high affinity PACAP receptor, PACR-1 (Hamelink et al. 2002b). Previous studies have shown that prolonged (20 minutes) PACAP exposure results in a robust persistent catecholamine release from clonal PC-12 cells (Taupenot et al. 1999) and isolated adrenal chromaffin cells (Tornoe et al. 2000, Wakade 1998, Watanabe et al. 1992). As indicated by the name, PACAP exposure is thought to evoke catecholamine release through activation of adenylate cyclase, subsequent cyclic adenosine monophosphate (cAMP) generation and activation of protein kinase A (PKA) (Hagen et al. 2006, Montero-Hadjadje et al. 2006). However, there are conflicting data describing the complete mechanism of action by which PACAP elicits its secretory response. Work in the PC-12 cell line showed 20 minute exposure to PACAP elicits catecholamine secretion that is sensitive to inhibition of phospholipase C (PLC) and evokes Ca2+ influx through a mechanism that does not include L-type channels (Taupenot et al. 1999). Additional studies performed in primary cultures from rat adrenal medulla have shown PACAP stimulation to directly inhibit L-type channel activation (Jorgensen et al. 2002) while other studies have shown that PACAP causes an increased L-type conductance (Geng et al. 1997). Furthermore, PACAP-evoked Ca2+ influx has been shown to depend upon external Na+ and Na+ influx through a route that is not sensitive to blockers of voltage gated Na+ channels (Mustafa et al. 2007, Tanaka et al. 1996). These data suggest an alternate route of Na+ influx independent of action potentials but that may result in depolarization. Other studies point to PACAP-mediated Ca2+ release from internal stores to evoke secretion (Montero-Hadjadje et al. 2006). Lastly, studies conducted in isolated rat cells or PC-12 cells indicate PACAP acts as an acute secretagogue eliciting exocytosis within seconds through a pathway that requires Ca2+ influx independent of the major high voltage activated Ca2+ channels (Przywara et al. 1996, Taupenot et al. 1999). Thus, conflicting interpretations of the action of PACAP in the adrenal medulla exist in the literature.
We set out to study the physiological response to native PACAP release on adrenal chromaffin cells in situ. We directly stimulated the innervating splanchnic nerve to elicit catecholamine release. The nerve was paced at low frequencies designed to match sympathetic tone and with higher frequency bursts designed to match the sympathetic stress response. We provide data that demonstrate PACAP acts as a native chromaffin cell secretagogue specifically under sympathetic burst mode firing. We further show that PACAP-dependent secretion does not depend on PKA signaling. Rather it evokes a rapid activation of PLC, protein kinase C (PKC) and activation of the Na+/Ca2+ exchanger (NCX) to depolarize the cell membrane. Membrane depolarization evokes Ca2+ influx through a NiCl2 and mibefradil-sensitive conductance to evoke a sustained catecholamine secretion. We place this signaling mechanism in the context of the current literature summarized above and propose a single mechanism for PACAP-evoked secretion. We provide data that potentially reconcile both the PACAP-mediated cAMP and PKC signaling paths described in the literature. Exchange Proteins Activated directly by cAMP (Epac) has been shown to be activated by PACAP (Ster et al. 2007) and has been shown to activate PKC (Hucho et al. 2005). We provide direct data demonstrating that Epac activation elicits secretion from chromaffin cells that is blocked by NiCl2 in same manner that that NiCl2 blocks PACAP-dependent secretion.
Materials and Methods
Slice preparation
Mouse adrenal tissue slices were prepared for experimentation as previously described (Chan & Smith 2003). Adult C57BL/6 mice (8-10 wk old; Jackson Laboratories, Bar Harbor, ME) were used in this study. All anesthesia and euthanasia protocols were reviewed and approved by the institutional animal care and use committee (IACUC) of Case Western Reserve University, an accredited oversight body (Federal animal welfare assurance No. A3145-01). Briefly, animals were deeply anesthetized by isoflurane inhalation (Abbott Laboratories, Abbott Park, IL) and sacrificed by decapitation. Adrenal glands were immediately removed and placed in ice-cold, low calcium bicarbonate buffered saline (BBS) containing in mM: 140 NaCl, 2 KCl, 0.1 CaCl2, 5 MgCl2, 26 NaHCO3, and 10 glucose that was bubbled with 95% O2/ 5% CO2. Osmolarity of the BBS was 320 mOsm. All chemicals were acquired from Fischer Scientific (Hanover Park, IL) unless otherwise noted. The adrenal glands were trimmed for excess fat and connective tissue and embedded in 3% low gelling point agarose (Sigma, St. Louis, MO) that was prepared by melting agar in low calcium BBS at 110° C followed by equilibration to 35° C. The agarose block containing the adrenal glands was trimmed into 3 mm cubes and glued to a tissue stand of a vibratome (WPI, Sarasota, FL). The adrenal glands were sectioned into 200 μm slices in ice-cold bubbled BBS. Sections were cut along the major axis and parallel to the maximum width to preserve native splanchnic innervation for in situ stimulation.
Immunohistochemistry
Adrenal glands were removed and prepared as previously described. The adrenal glands were immersion fixed by placement in 2% phosphate buffered paraformaldehyde (PFA) solution for 2 hours at 4°C. They were post-fixed and stored overnight in 30% sucrose solution containing 2% PFA. The glands were embedded in OCT (Ted Pella, Inc., Redding, CA), cryosectioned into 16 μm sections and mounted on slides. To probe for PACAP or the PACR-1 receptor, the adrenal sections were washed three times with PBS and blocked with a solution of 10% donkey serum in PBS containing 0.1% Triton X-100 for 20 minutes at room temperature. The sections were incubated with goat-anti PACAP (1:50) and rabbit-anti PACR-1 (1:50) (Santa Cruz Biotechnology, Inc. Santa Cruz, CA) overnight at 4°C. The sections were washed six times each with PBS and incubated with donkey-anti goat-Alexa 498 (1:100) and donkey anti-rabbit-Alexa 594 (1:100; Invitrogen, Carlsbad, CA) for 1 hour at room temperature. Sections were washed six times with PBS and mounted with immuno-mount (Thermo Scientific, Pittsburgh, PA) under a cover slip. Fluorescence signals were visualized on an Olympus IX81 microscope at 100× magnification. Image stacks were captured at pixel-width z steps (square voxels) and deconvolved with a constrained-iterative algorithm built into SlideBook (Intelligent Imaging Innovations, Denver, CO) image processing software.
Electrophysiology
Adrenal chromaffin slices were visualized using an upright microscope (Olympus, Melville, NY) equipped with a 40× water-immersion objective. Slices were held in place by sliver wires placed over the agar margin and constantly superfused (1 ml/minute) with normal Ca2+ BBS containing in mM: 140 NaCl, 2 KCl, 2 MgCl2, 26 NaHCO3, 10 glucose, 3 CaCl2 bubbled with 95% O2 / 5% CO2. Recordings were performed in the perforated patch configuration of the patch-clamp technique. Patch pipettes were pulled from borosilicate glass (4-5 MΩ). They were partially coated with molten dental wax and polished by a microforge (Narashige, Tokyo, Japan). The perforated patch pipette solution used for voltage clamp contained in mM: 135 CsGlutamate, 10 HEPES-H, 9.5 NaCl, 0.5 TEA-Cl, and 0.53 amphotericin B. The pH was adjusted to 7.3 and osmolarity was adjusted with mannitol to 320 mOsm. The solution for current clamp contained in mM: 145 potassium glutamate, 10 HEPES-H, 8 NaCl, 2 MgATP, 1 MgCl2, 0.1 EGTA and 0.53 amphotericin B. The amphotericin was prepared as a 100× stock solution in DMSO (Sigma, St. Louis, MO) and diluted into the pipette solution. The pipette was backfilled without tip-dipping. Cells were allowed to perforate to a series resistance of no more than 30 MΩ and held at -80 mV. For current clamp configuration, series resistance was compensated at 80% and 100 μs. Records were acquired at 20 kHz through an EPC-9 voltage-clamp amplifier (HEKA Elektronik, Lambrecht, Germany) under the control of Pulse software (v 8.40, HEKA Elektronic). In the I-V protocol, cells were held at -100 mV and depolarized to potentials between -80 and 15 mV in 5 mV increments. Each depolarization was 100 ms in duration and separated from the previous by 45 s to allow for complete recovery from inactivation. The peak Ni2+ sensitive Ca2+ conductance was isolated by the subtraction of currents recorded in the above recording BBS containing 50 μM NiCl2 from currents recorded in the absence of NiCl2. All records were corrected for junction potential. Data were analyzed using IGOR Pro (Wavemetrics, Lake Oswego, OR).
Bipolar electrical stimulation
The splanchnic nerve was stimulated by a parallel bipolar stimulator fitted with two platinum electrodes (250 μm spacing) (FHC, Bowdoin, ME) and connected to an ISO-Stim 01-D stimulator (ALA Scientific Instruments, Westbury, NY) and controlled by TTL triggers generated by the EPC-9 through the Pulse software. The adrenal gland slice was placed between the two electrodes and visualized using an upright microscope (Olympus, Melville, NY) equipped with a 40× water-immersion objective. The innervating splanchnic nerve was stimulated to mimic either basal tone firing conditions or stress firing conditions. To compare relative cholinergic and PACAP-evoked secretion, stimulation protocols were balanced to evoke quantitatively identical amounts of catecholamine under cholinergic control (i.e. blocking the PACAP signaling path under stress firing elicits the same nicotinic component as elicited by basal stimulation; see figure 2C). Under basal stimulation the nerve was stimulated for 10 pulses at a frequency of 0.2 Hz. For stress stimulation, 4 bursts of 15 pulses were delivered at 2 Hz frequency with an inter-burst interval of 15 s. All stimuli were delivered at constant voltage (35 V) with 10 μs duration.
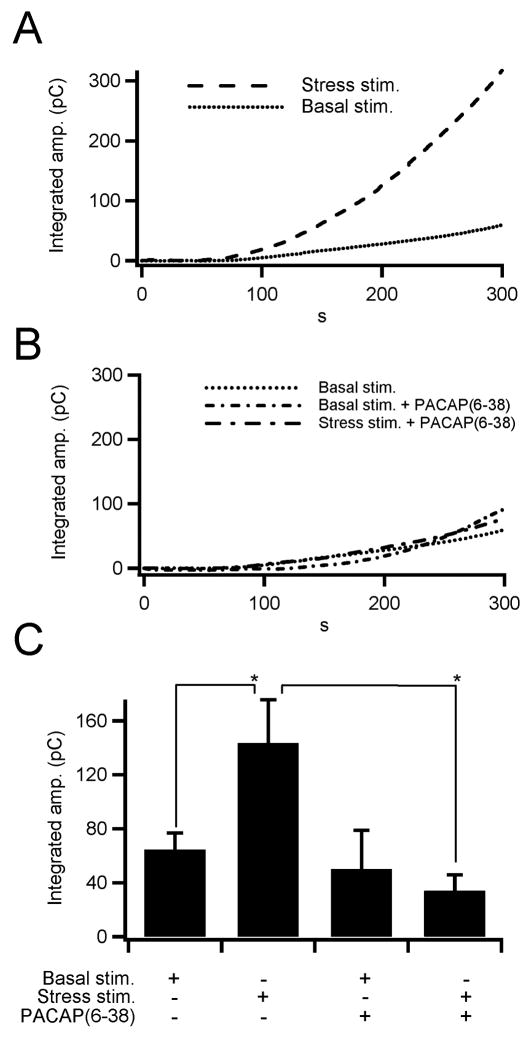
Figure 2. PACAP evokes catecholamine release under elevated sympathetic input.
(A) Bipolar stimulation of the splanchnic nerve evokes catecholamine release from chromaffin cells. Sample traces for total catecholamine release (integrated amperometric current) under firing rates that mimic basal sympathetic firing (dotted line) and bursting stress firing patterns (dashed line). These sample traces indicate that elevated frequency burst mode firing elicits greater catecholamine release than basal firing. (B) Catecholamine release as a function of PACAP signaling was measured under basal and burst mode firing. Inhibition of the PACR1 receptor by bath application of the PACAP antagonist, PACAP(6-38), decreased catecholamine release under burst mode firing but not under basal firing patterns. (C) Total catecholamine release was measured and pooled for each protocol indicated in panels A and B and are plotted (n = 11, 10, 4 and 4, respectively). The data show that the PACAP-mediated catecholamine release occurs under burst mode firing, but not under basal firing conditions (* = p < 0.02; paired Student's t-test).
Amperometry
Carbon fiber electrodes with 5 μm diameter tips were used for amperometric detection of catecholamine release (ALA scientific, Westbury, NY, USA). Fibers were cut fresh daily and again whenever debris accumulated on the tip. A +650 mV potential was placed on the fiber. After placement into the bath, the initial fiber oxidation current was allowed to relax to a steady value. For recordings, the fiber was placed as close to the cell of interest without physically distorting the cell. PACAP was puffed on the slice with a micro-perfusion system (Warner Instruments; Hamden, CT). Oxidative amperometric currents, indicating catecholamine release, were recorded using a dedicated VA-10 amperometry amplifier (ALA Scientific). Experiments were conducted primarily with either a 500 MOhm or 1 GOhm feedback head stage and currents were calibrated prior to pooling. The current was filtered at 1.5 kHz and sampled at 20 kHz. All pharmacological blockers used to isolate the PACAP signaling pathway were added to the BBS and were puffed on the slice for 5 minutes prior to, and during PACAP stimulation. Acute PACAP-evoked catecholamine secretion was determined as the integrated amperometric current 60 s after PACAP exposure. The concentration of pharmacological blockers is as follows: U73122 (10 μM), U73433 (10 μM), Gö6983 (100 nM), Benzamil (10 μM), NiCl2 (50 μM), 8-pCPT-2′-O-Me-cAMP (100 μM). The Ca2+-free BBS was prepared as above with an equimolar substitution of MgCl2 for CaCl2.
Fura recording
Intracellular Ca2+ dynamics were measured in cells loaded with the calcium indicator Fura-2 penta-acetoxymethyl ester (Fura-2 AM) (Molecular Probes, Eugene, OR). Isolated chromaffin cells were prepared as described in the literature (Fulop et al. 2005) and incubated in 2 μM FURA (50 μg Fura2-AM was dissolved in DMSO and diluted to 1 mM) at 37°C for 15 minutes. After loading, the cells were washed for 5 minutes by superfusion with HEPES-buffered Ringer solution containing in mM: 150 NaCl, 10 HEPES, 10 glucose, 2.8 CaCl2, 2.8 KCl, 2 MgCl2, pH 7.4. Cells were visualized with an inverted microscope (Olympus IX81 microscope; Tokyo Japan) with a UV-optimized 40× water-immersion objective. Ratiometric measurements of intracellular Ca2+ were recorded by FURA dye excitation with light alternating between 360 nm and 390 nm using a fast monochromator-based system (TILL Photonics, Eugene OR). Emission was measured at >510 nm. All fluorescence signals were recorded in the Pulse X-Chart program and analyzed in IGOR.
Results
PACAP has been shown to act as both a both a neuromodulator and a secretagogue for catecholamine secretion from adrenal chromaffin cells. Heterogeneous effects of PACAP exposure may be encoded by the multi-potent HOP cassette of the PACR1 receptor that activates both Gs and Gq-mediated signaling cascades (Hagen et al. 2006, Mustafa et al. 2007, Taupenot et al. 1999). Much attention has been focused on understanding the long term modulatory effects of exogenous PACAP exposure while the mechanism responsible for the direct secretagogic action is not yet resolved. Evidence points to a variety of potential mechanisms for acute PACAP-evoked Ca2+-dependent secretion including influx through high voltage activated (HVA) L-type Ca2+ channels (Geng et al. 1997), influx through non HVA Ca2+ channels (Przywara et al. 1996) and release from intracellular stores (Taupenot et al. 1999). In order to understand which of these mechanisms, if any, are responsible for physiological catecholamine release under the sympathetic stress response, we initiated a series of experiments in an acute tissue slice preparation from mouse adrenal medulla. We introduce a native splanchnic-chromaffin stimulation method to show for the first time that splanchnic-chromaffin signaling under elevated burst-mode sympathetic firing occurs through a largely PACAP-dependent mechanism. We test a variety of signaling paths potentially responsible for the secretion in situ. We present data defining a mechanism that includes activation of PLC, PKC, Na+/Ca2+ exchange and Ca2+ influx through a voltage-dependent, nickel and mibefradil-sensitive conductance to evoke catecholamine release.
PACAP is localized in the splanchnic synaptic terminals
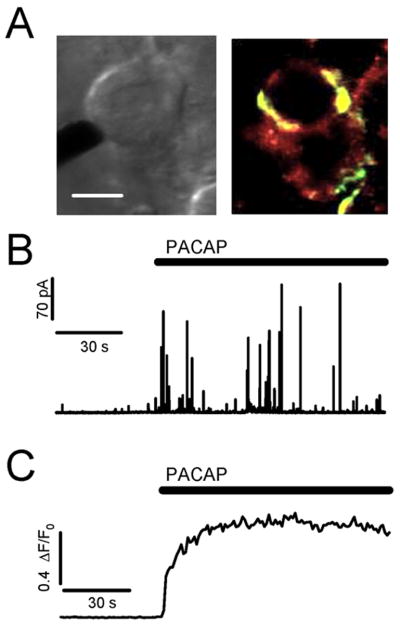
The experimental configuration is shown in Figure 1A. A bright-field image (left) of the in situ slice preparation with a 5 μm amperometric carbon fiber electrode placed near the chromaffin cell to measure catecholamine release. Immuno-histochemistry (right) shows that PACAP, stained green, is located peripheral to the chromaffin cells, but not in the chromaffin cells themselves. These data are consistent with the literature (Hamelink et al. 2002b, Moller & Sundler 1996), showing PACAP expression in the innervating splanchnic terminals. The high affinity PACAP PACR-1 receptor, stained red, is expressed over the surface of the chromaffin cells (Mazzocchi et al. 2002a, Mazzocchi et al. 2002b, Moller & Sundler 1996). Next, we employed single cell carbon fiber amperometry to directly measure catecholamine release under PACAP stimulation. Carbon fiber amperometry has been a valuable tool to measure catecholamine release on the quantal level (Chow et al. 1992, Jankowski et al. 1992). Briefly, a carbon fiber is placed near a cell and held at a constant positive potential (+650 mV). As catecholamine molecules are released from the cell, they are oxidized in the local electric field near the fiber tip, resulting in a current in the fiber. A representative amperometric recording shows that local application of exogenous PACAP (1 μM) results in an immediate and robust signal (Fig. 1B). Each spike represents the fusion of a single catecholamine-containing granule. In order to compare PACAP-evoked secretion to intracellular calcium dynamics, we measured relative intracellular Ca2+ in freshly isolated FURA2-AM loaded cells stimulated with 1 μM exogenous PACAP. Relative Ca2+ levels are reported as ΔF/F0 (see methods for recording and analysis). As demonstrated in the representative recording (Fig. 1C), PACAP evokes a rapid and long-lasting elevation in cytosolic Ca2+. Thus, based on immunostaining, we confirm that PACAP is found peripheral to chromaffin cells and that chromaffin cells express PACR1 receptors. Additionally, chromaffin cells exhibit a rapid secretory response to focal PACAP stimulation in situ.
Figure 1. PACAP stimulation of adrenal chromaffin cells results in an immediate and robust release of catecholamine.
(A) Left image: A bright-field image of the in situ adrenal slice preparation demonstrates the amperometric recording configuration used to measure catecholamine release from chromaffin cells. Right image: Immuno-histochemical staining shows that PACAP (green) is localized peripheral to chromaffin cells. The PACAP specific receptor (PACR-1, red) is expressed in chromaffin cells. (scale = 10 μm) (B) An amperometric trace recorded from a chromaffin cell in situ that has been stimulated by focal perfusion with a Ringer containing 1μM PACAP causes an immediate catecholamine exocytosis. (C) Ratiometric FURA signals from a freshly isolated cell demonstrates an immediate rise in intracellular Ca2+ upon PACAP stimulation.
PACAP specifically evokes catecholamine release under elevated sympathetic input
As demonstrated in figure 1, PACAP is localized peripheral to single chromaffin cells in the medulla, chromaffin cells express the PACR1 receptor. PACAP stimulation causes a rapid elevation in cytosolic Ca2+ and subsequent exocytosis of catecholamine. Yet, no studies to date have demonstrated that endogenous splanchnic PACAP release stimulates chromaffin cells. We developed an intact in situ splanchnic-adrenal preparation to deliver native synaptic stimulation to single chromaffin cells. Briefly, tissue slices were cut and placed on the microscope recording chamber. A platinum bipolar stimulator was placed peripheral to the medulla and vertically spanned across the adrenal cortex. Low frequency pulses were delivered to the bipolar stimulator while a carbon fiber electrode was moved within the proximal adrenal medulla until a responsive chromaffin cell was identified. After establishing functional neuronal-chromaffin cell coupling, the nerve was pulsed at either a low periodic frequency to match sympathetic tone or at a higher frequency bursting pattern to mimic sympathetic activation (see Methods). Cumulative stimulus-evoked catecholamine release from the cell was quantified by integrating the raw amperometric current. Example integral traces for basal and stress firing stimulus patterns are provided in figure 2A. Basal firing elicited a modest catecholamine release compared to burst mode firing. To determine PACAP-dependent secretion under each condition, we bathed slices in the competitive PACAP inhibitor PACAP(6-38) (10 μM), a PACAP receptor antagonist (Ciccarelli et al. 1995). Pretreatment with PACAP(6-38) did not affect the total catecholamine release under basal stimulation but attenuated release under burst mode stimulation to the level observed under basal stimulation (Fig. 2B). These data show that PACAP acts as a transmitter under the bursting firing pattern. Furthermore, the data show that PACAP represents the magnitude difference between catecholamine secretion under conditions designed to match basal sympathetic tone and those designed to match the sympathetic stress response. Combined data from each of the above stimulation protocols were combined and are plotted (Fig. 2C). These data show that PACAP is the predominant synaptic transmitter under burst mode splanchnic firing.
PACAP stimulation acts through a PLC and PKC dependent process
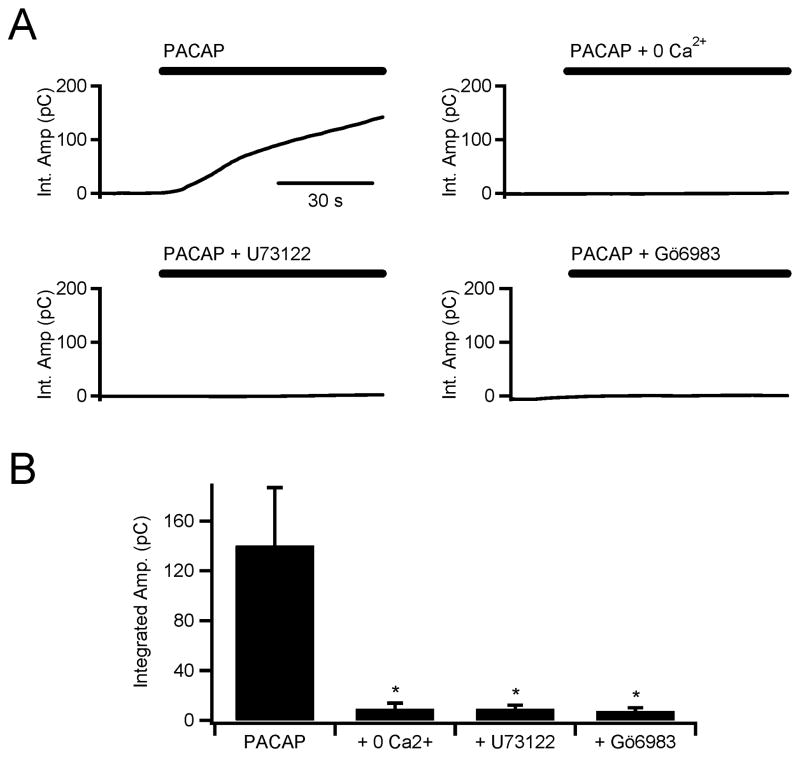
The mechanism by which PACAP acutely elicits catecholamine release is unclear. Our effort to define the mechanism responsible for catecholamine release in situ initially focused on whether PACAP-evoked Ca2+ signals originate from release from internal stores or through influx from the extracellular space. Examples collected under diverse stimulation protocols for each route exist in the literature (Morita et al. 2002, Payet et al. 2003). In this set of experiments we chose to stimulate cells through focal superfusion with exogenous PACAP (1 μM) to isolate its specific mechanism independent from other presynaptic transmitters (i.e. acetylcholine, adenosine and Ca2+). To test for the requirement of external Ca2+, we bathed tissue slices in a Ca2+-deficient Ringer solution and stimulated with PACAP. As in figure 1, catecholamine secretion was measured by carbon fiber amperometry and quantified by integrating the amperometric current. The top left panel of figure 3A shows a rapid and robust catecholamine release in response to stimulation under control conditions. The top right plot shows that Ca2+-free Ringer did not support PACAP-evoked catecholamine release. In paired experiments, PACAP-evoked cytosolic Ca2+ increases were also shown to be dependent on extracellular Ca2+ (supplemental figure S1). Thus, acute PACAP stimulation requires extracellular Ca2+ for catecholamine exocytosis.
Figure 3. PACAP-dependent secretion is dependent on extracellular Ca2+ and blocked by PLC and PKC inhibition.
(A) Carbon fiber amperometry was used to measure total catecholamine release (integral of the amperometric current) from single chromaffin cells in situ. Cells were stimulated by focal perfusion with PACAP (1 μM, as indicated by the bar above each sample recording). Sample single recordings for PACAP stimulation alone (top left) and PACAP in the presence of a Ca2+-free Ringer (top right), PACAP in the presence of the PLC inhibitor U73122 (10 μM, bottom left) and PACAP in the presence of the PKC inhibitor Gö6983 (100 nM, bottom right). (B) Pooled data from 10 experiments for each condition shown in panel A demonstrate that extracellular Ca2+, PLC, and PKC, are all necessary components of the immediate PACAP secretory mechanism in adrenal chromaffin cells (* = p < 0.02; paired Student's t-test).
Next, we considered the route of PACAP-evoked Ca2+ entry. Previous studies provide a variety of signaling cascades initiated by PACAP excitation, as would be expected through the multi-potent HOP cassette of the PACR1 receptor. First, we considered PKA-mediated modulation of L-type HVA calcium conductance described in clonal PC-12 cells (Taupenot et al. 1999). We challenged cells with pharmacological inhibitors that target this pathway. We pre-incubated slices in H-89 (1 μM), a PKA inhibitor, and in nifedipine (1 μM), an L-type channel blocker. Neither of these reagents had any effect on acute PACAP-dependent catecholamine release (data not shown). We then considered the prospect of a phospholipase C-dependent PACR1-mediated pathway being responsible for secretion (Jorgensen et al. 2002, Taupenot et al. 1999). Cells were pre-treated with the pharmacological PLC blocker, U73122 (10 μM). Pre-treatment with the active U73122 PLC-blocker completely inhibited catecholamine release in situ upon PACAP stimulation (Fig. 3A, lower left plot). The inactive U73122 analog, U73433 (10 μM), had no significant effect on PACAP secretion compared to PACAP stimulated untreated control cells (97.8 ± 20.7 pC [n=9] for U73433 + PACAP, 140.3 ± 46.5 pC [n=10] for PACAP alone and 9.1 ± 3.1 pC for U73122 + PACAP [n=10]). Again, in paired experiments, Ca2+ transients measured in freshly isolated cells showed the same behavior, U73122 pretreatment blocked PACAP-evoked Ca2+ signals (supplemental figure S1). Thus, acute stimulation with PACAP in situ elicits catecholamine secretion through a PLC-dependent pathway.
PLC activity results in generation of two second messenger molecules, inositol triphosphate (IP3) to release intracellular Ca2+ stores and diacyl glycerol (DAG) to activate protein kinase C (PKC). PACAP-evoked secretion requires influx of external Ca2+, thus IP3-mediated release of internal stores does not significantly contribute to the immediate catecholamine secretion. Moreover, previous studies have shown that PACAP-evoked Ca2+ mobilization is not due to IP3 signaling, but is dependent on PKC signaling (Tanaka et al. 1996). For this reason we tested DAG-mediated activation of PKC as an effector for secretion. We pretreated tissue slices with Gö6983 (100 nM), a blocker of both classical and novel PKC isoforms, and then stimulated by focal PACAP perfusion. PACAP-dependent catecholamine release was blocked by Gö6983 pretreatment (lower right plot, Fig 3A). As above, paired experiments showed that Gö6983 treatment had a similar effect of blocking evoked Ca2+ signals in FURA2-loaded cells (supplemental data, Fig. S1). Combined data from each of the above protocols were pooled and are presented in figure 3B and show that the immediate PACAP-dependent catecholamine secretion in situ is blocked by PLC and PKC inhibition and requires extracellular Ca2+. In order to confirm that native synaptic PACAP signaling also included a PKC-mediated signaling mechanism, we repeated the basal and burst mode firing patterns delivered by direct bipolar stimulation presented in figure 2 in the presence of Gö6983. Data from these experiments confirmed that elevated catecholamine release under burst mode firing is sensitive to PKC inhibition (143.4 ± 40.1 pC [n=10] versus 46.3 ± 10.0 pC [n=4], untreated cells versus Gö6983-treated cells respectively).
PACAP stimulation depolarizes the cell but does not fire an action potential
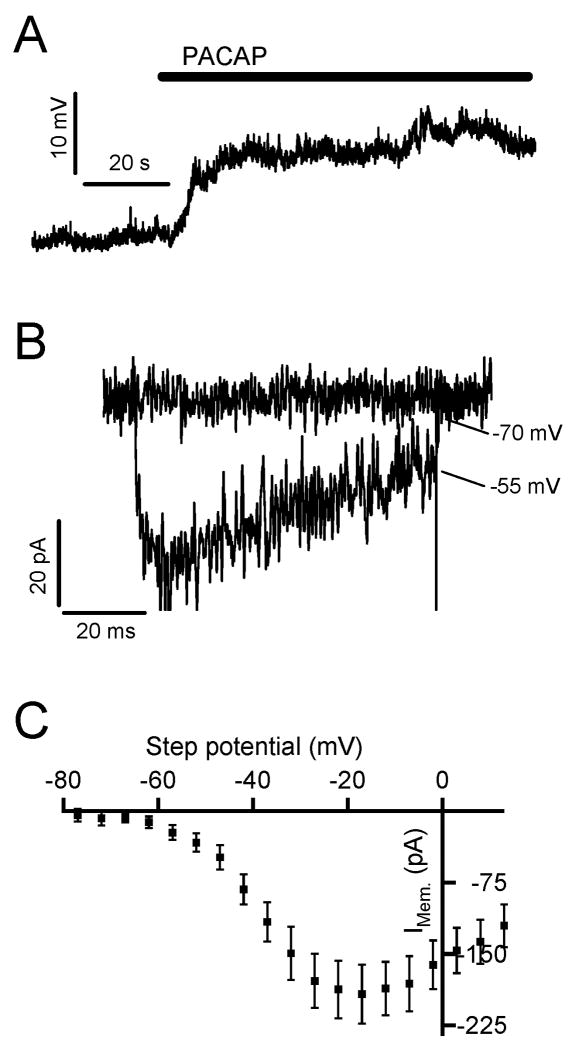
PACAP exposure has been shown to cause a sub-threshold membrane depolarization that is dependent upon external Na+ (Tanaka et al. 1996). We tested if PACAP stimulation of chromaffin cells in situ expressed the same behavior. We held chromaffin cells in perforated patch current clamp and focally perfused 1 μM PACAP. A representative recording is provided in figure 4A and demonstrates a sub-threshold depolarization upon PACAP exposure (+12.4 ± 2.8 mV from a resting potential of -69.6 ± 1.9 mV; n= 7). Next, we tested the possibility that this sub-threshold depolarization may evoke the influx of Ca2+ through activation of low voltage activated (LVA) Ca2+ channels.
Figure 4. Ni2+-sensitive Ca2+ currents in adrenal tissue slices.
(A) A sample recording from a chromaffin cell held in the current clamp mode of the perforated-patch configuration in situ. Focal perfusion with 1 μM PACAP (bar) elicits a 12 mV depolarization. (B) To test for a Ni-sensitive component in adrenal chromaffin cells, a chromaffin cell was voltage clamped in situ in the perforated patch configuration. The cell was depolarized from -80 mV to 15 mV in normal Ringer and Ringer containing 50 μM Ni2+. The nickel-sensitive component was determined by subtraction of currents recorded in these two Ringer solutions. Sample currents recorded at relevant membrane potentials are shown; -70 mV (the resting potential of chromaffin cells in situ) and -55 mV (the mean potential recorded after PACAP stimulation). (C) The peak Ni2+-sensitive current was measured in response to depolarization as above from 5 recordings and are plotted as an I-V relationship.
Zinc selectively inhibits currents through LVA T-type Ca2+ channels relative to currents through HVA channels (Perez-Reyes et al. 1998, Traboulsie et al. 2007). One study noted a Zinc sensitivity for PACAP-evoked Ca2+ signaling, with Zn2+ being more effective than other Ca2+ channel blockers at inhibiting PACAP excitation (Przywara et al. 1996). Other groups have shown that isolated adrenal chromaffin cells recruit the LVA T-type Ca2+ channels to regulate a rapid and long lasting catecholamine release in response to acute stress (Carabelli et al. 2007, Carbone et al. 2006). We set out to determine if there is also a pronounced T-type Ca2+ current in adrenal slices and to determine its potential role in PACAP signaling. NiCl2 has been shown to be a potent and more selective T-type blocker at low concentrations than Zn2+ (Perez-Reyes et al. 1998). We tested if there was a Ni2+-sensitive current component in adrenal chromaffin cells in situ. We depolarized chromaffin cells to potentials that match the measured PACAP-evoked depolarization and are expected to evoke low voltage activated current. Sample records are provided in figure 4B and show the activation of a Ni2+-sensitive influx at -55 mV, a potential and pharmacological profile indicative of LVA T-type currents. Furthermore, an analysis of Ni2+-sensitive currents provides an I-V relationship matching that for T-type currents (Fig. 4C). The mechanistic role for Ni2+-sensitive currents as a component of PACAP-evoked secretion will be considered in the context of a signaling cascade below.
PACAP-evoked stimulation involves Na+/Ca2+ exchange
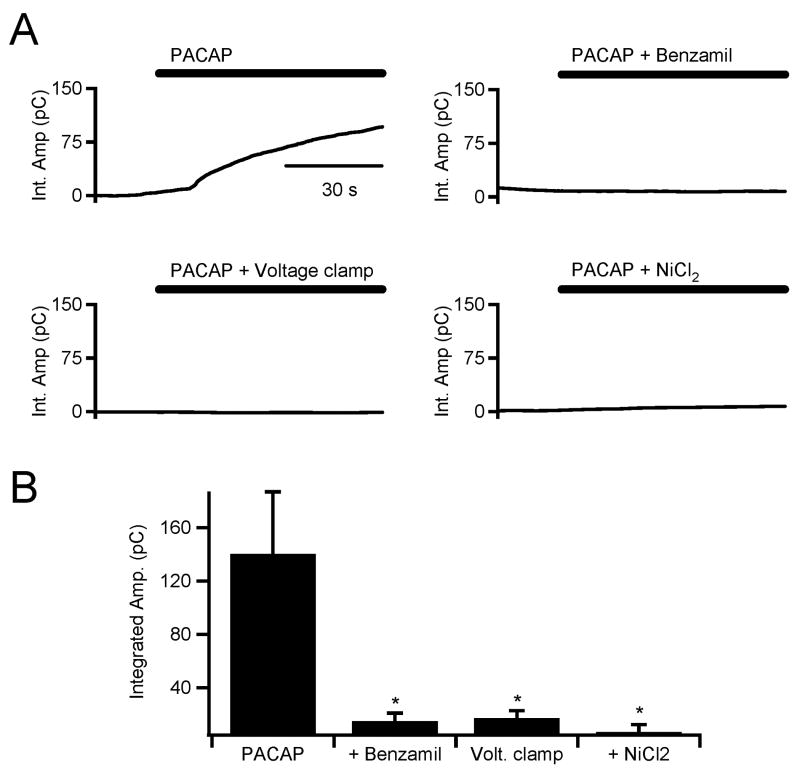
We tested a potential signaling event that could link the requirement for external Na+ and PKC activation (Fig. 3) in PACAP-evoked catecholamine secretion. It has been previously shown that the electrogenic Na+/Ca2+ exchanger (NCX) activity is upregulated by PKC (Houchi et al. 1994, Houchi et al. 1995, Iwamoto et al. 1999, Pan et al. 1998). Increased NCX activity would therefore provide an inward Na+ flux to depolarize the cell membrane and increase T-type conductance. We tested for the involvement of the NCX in the PACAP-evoked secretory response. Adrenal chromaffin slices were bathed in a NCX blocker, benzamil (10 μM), prior to the application of PACAP. A sample recording is provided in figure 5 (top right plot) and demonstrates that PACAP-evoked catecholamine release is sensitive to NCX inhibition. We conducted parallel experiments in FURA2-loaded cells and showed that benzamil also blocked PACAP-evoked increases in cytosolic Ca2+ (see supplemental figure S2). We further tested if membrane depolarization is required for rapid PACAP-evoked catecholamine release. Chromaffin cells were held in the perforated patch voltage clamp configuration and the membrane potential was clamped at -80 mV to block activation of any voltage-sensitive processes. Cells that were clamped at -80 mV prior to PACAP stimulation did not show PACAP-dependent catecholamine secretion (Fig. 5A, lower left plot). Again, parallel experiments showed that voltage clamp at -80 mV also blocked PACAP-evoked cytosolic Ca2+ increases (supplemental Fig. S2). In order to confirm that PACAP-mediated membrane depolarization is dependent on NCX activity, we bathed cells in benzamil as above and measured membrane potential in response to PACAP stimulation. We found that benzamil significantly inhibited the depolarization observed under PACAP stimulation (3.4 ± 0.5 [n=4] in PACAP + benzamil versus 12.4 ± 2.8 mV [n=7] in PACAP alone). Lastly, to test for involvement of Ni2+-sensitive T-type calcium channels in the immediate PACAP response, adrenal slices were bathed in 50 μM NiCl2 prior to PACAP stimulation. This again blocked catecholamine secretion (Fig. 5A, lower right plot) and Ca2+ signals (supplemental figure S2). Pooled data for each of these data sets is provided in figure 5B and paired pooled data demonstrating similar results for PACAP -dependent cytosolic Ca2+ signals is provided in supplemental data (Fig. S2B). We bathed cells in 10 μM mibefradil, another blocker specific to T-type channels at this concentration (Randall & Tsien 1997). We found mibefradil to also block catecholamine release and Ca2+ elevations in a manner statistically identical to Ni2+ (data not shown).
Figure 5. PACAP-dependent catecholamine secretion is blocked by benzamil, voltage clamp and extracellular Ni2+.
(A) Carbon fiber amperometry was used to measured total catecholamine release under PACAP stimulation as indicated in figure 3. Sample single recordings for PACAP stimulation alone (top left) and PACAP in the presence of a benzamil (10 μM, top right), PACAP stimulation under perforated patch voltage clamp at -80 mV (bottom left) and PACAP stimulation in the presence of NiCl2 (50 μM, bottom right). (B) Pooled data from 10 experiments for each condition shown in panel A show that Na+/Ca2+ exchange and membrane depolarization are required for PACAP-dependent catecholamine release. They also show that that PACAP-secretory response is sensitive to extracellular Ni2+ (* = p < 0.02; paired Student's t-test).
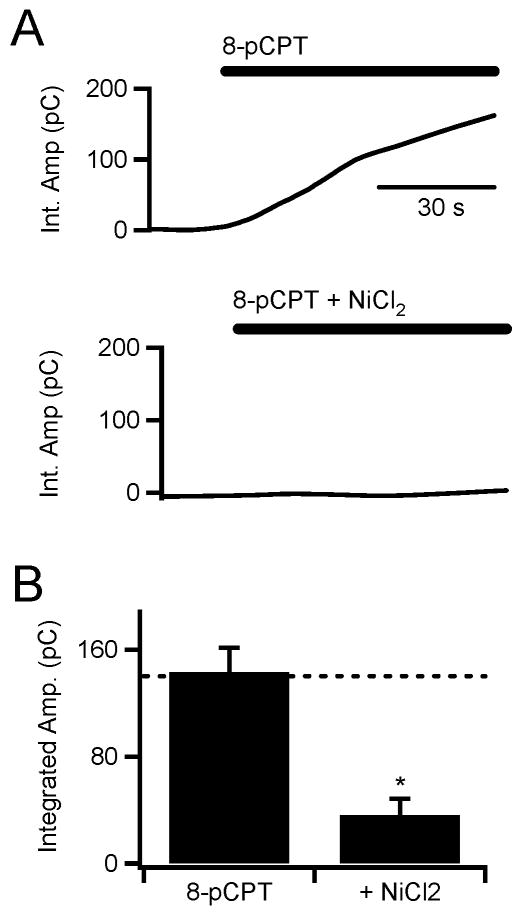
As described above, previous studies have reported divergent potential signaling paths for PACAP-evoked catecholamine secretion, including cAMP-mediated (Przywara et al. 1996) and PKC-mediated (Tanaka et al. 1996, Turquier et al. 2001) pathways. These divergent data have been interpreted as due to a multi-potent HOP cassette of the PACR1 receptor eliciting multiple signaling cascades. However Epac, a signaling molecule indicated to play a modulatory role in secretion from a variety of cell types (Geng et al. 1997, Hatakeyama et al. 2007, Kang et al. 2003), may provide a simplification of these data. Epac acts to stimulate PLC through a non-canonical cAMP-dependent pathway (Hucho et al. 2005). In a last set of experiments, we tested a potential that Epac is involved in PACAP signaling. No specific Epac inhibitors are yet available, rather we turned to the Epac activator 8-pCPT-2′-O-Me-cAMP (8-pCPT) (Kang et al. 2003, Holz et al. 2006). As demonstrated by the raw data in figure 6A and the pooled data in 6B, acute exposure to 8-pCPT elicits a statistically identical catecholamine release as PACAP stimulation. Also reminiscent of PACAP stimulation, 8-pCPT stimulation is sensitive to block by NiCl2.
Figure 6. Epac stimulation elicits nickel-sensitive catecholamine secretion.
(A) Carbon fiber amperometry was used to measure total catecholamine release under 8-pCPT exposure in (100 μM, top) and 8-pCPT + NiCl2 exposure (100 μM and 50 μM, respectively, bottom trace). (B) Pooled data show that 8-pCPT elicits catecholamine release identical in magnitude to PACAP stimulation (dotted line) and that this secretion is sensitive to block by NiCl2 (* = p < 0.02; paired Student's t-test).
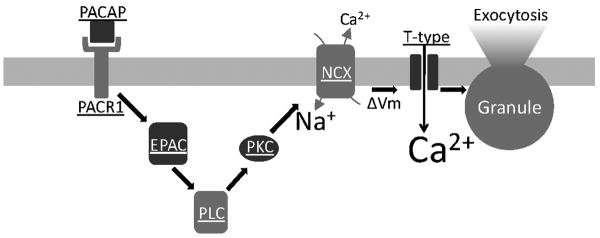
Taken together, we demonstrate that PACAP stimulation in situ is a potent and rapidly-acting secretagogue. We show that PACAP is natively released from the innervating splanchnic nerve under elevated burst stimulation that mimics the sympathetic stress response. As summarized in figure 7, we show that this immediate PACAP response mechanistically involves PLC and PKC activation and that the electrogenic Na+/Ca2+ exchange depolarizes the cell allowing LVA T-type Ca2+ channels to evoke catecholamine release.
Figure 7. Summary diagram for the proposed PACAP signaling pathway.
Key components of the proposed PACAP-mediated signaling path as described in the text are shown.
Discussion
Prolonged release of ACh from the splanchnic terminals results in a rapid desensitization of the cholinergic response (Bevington & Radda 1985). Yet, elevated levels of catecholamine are maintained in the blood stream under acute sympathetic stress. This phenomenon suggests that excitation of the adrenal medulla must occur by another mechanism under sympathetic stress. Here we demonstrate that pacing native neuronal input elicits an activity-dependent PACAP-evoked catecholamine release from adrenal medullary chromaffin cells. We show that this signaling path is selectively active only under burst mode firing and does not appreciably contribute to medullary stimulation under low frequency splanchnic firing. PACAP stimulation could potentially be through activation of three different receptors. PACAP has a relatively lower binding affinity to vasointestinal peptide receptors 1 and 2 (VPAC1 and 2) and a higher affinity for PACR1. This is a significant difference in that the VPACR1 signals through a Gs-coupled path, leading to adenylate cyclase activation and ultimately cAMP-mediated signaling. VPACR2 signals through a Gq-coupled path leading to PLC activity and PKC signaling while the PACR1 receptor, with the HOP cassette signals through both pathways (Mustafa et al. 2007). Thus, identification of the relevant receptor for PACAP signaling in the medulla could help define the signaling path for evoked catecholamine secretion. Previous work has shown that all three potential receptors are expressed in adrenal chromaffin cells (Mazzocchi et al. 2002a, Mazzocchi et al. 2002b, Moller & Sundler 1996). The data contained in this study are consistent with either signaling through both VPAC receptors or through the single PACR1 receptor. Work in human adrenal chromaffin cells showed that block of the VPAC receptors does not block PACAP-mediated catecholamine release (Mazzocchi et al. 2002b) indicating that the PACR1 receptor is likely the relevant receptor for PACAP-evoked secretion. Further studies will be necessary to confirm this conclusion in the mouse model.
Closer examination of the data set provides for mechanistic description of PACAP-evoked excitation in situ. We show that sequentially, PKC activation is required for elevated cytosolic Ca2+, placing PKC activation temporally prior to the Ca2+ response. In combination with the absolute requirement for external Ca2+, this point implies that PKC is not being activated in a Ca2+-dependent manner (as might be expected from conventional PKC isoforms). It also implies that acute PACAP-evoked secretion does not include Ca2+ mobilization from internal stores (as might be expected by PLC activation and subsequent IP3 production). The lack of release from internal stores may be due to the fact that the majority of the IP3 receptors in chromaffin cells are located on the nuclear envelope, rather than the endoplasmic reticulum (Huh et al. 2005). This location distal to the site of granule fusion may attenuate its efficacy. Rather, the nuclear location of IP3 receptors may facilitate the role of long-term PACAP exposure in gene regulation.
Initially, the data presented here seemingly remain inconsistent with elements of the existing literature. However, closer consideration provides a potential common interpretation. As outlined above, different studies have provided a complex and sometimes conflicting mechanism for PACAP-signaling in adrenal chromaffin cells. One possibility is due to the multi-potent HOP cassette of the PACR1 receptor (Mustafa et al. 2007) that initiates PKA and PKC activation. It is likely that these paths represent the divergent physiological effects of PACAP stimulation, ranging from immediate transmitter exocytosis (Przywara et al. 1996) to long term regulation of gene transcription (Vaudry et al. 2005, Hamelink et al. 2002a). Another intriguing possibility is that the immediate PACAP-evoked secretion may represent a non-canonical cAMP/PLC signaling mechanism. Specifically, one study stimulated cells with PACAP and showed an immediate secretory effect that was dependent on influx of extracellular Ca2+, but this Ca2+ influx was not through HVA Ca2+ channels. Influx was also shown to be blocked by LVA Ca2+ channel blockers (Przywara et al. 1996). Furthermore Przywara et al. showed that secretion was blocked by rp-cAMPs, a blocker of cAMP signaling. Based on this result the authors interpreted that the secretion was elicited through a PKA-mediated mechanism, but did not directly test this interpretation by blocking PKA specifically. We did not see any effect of direct PKA inhibition on PACAP-evoked secretion in situ. Moreover, exchange proteins activated directly by cyclic AMP (Epac) have recently been recognized as a non-canonical signaling effecter for cAMP (Gerdin & Eiden 2007). Studies have shown a signaling path through cAMP-dependent Epac activation that then stimulates PLC activity. Indeed, when challenged with the Epac activator, 8-pCPT, we found an evoked catecholamine release identical in magnitude and nickel sensitivity to that measured under acute PACAP stimulation. This represents a potential convergence point where cAMP signaling may initiate PKC-dependent processes (Holz et al. 2006, Hucho et al. 2005). The presence of Epac activation by cAMP and its ability to initiate a PLC signaling suggests that while cAMP formation is required for the PACAP response, the cAMP may in fact act through PLC and PKC activation and subsequent influx of Ca2+ through a LVA to elicit the robust catecholamine release in situ (Fig. 7).
In a further link between Epac and PACAP signaling, work conducted in isolated rat chromaffin cells showed that increased cAMP levels elicit Epac-mediated increases in low-voltage activated T-type Ca2+ current (Novara et al. 2004). We have shown that the low voltage activated Ca2+ channels play an integral role in the immediate PACAP response. Influx through these channels is necessary to generate the Ca2+ dependent exocytosis of catecholamine. As shown by the Carbone group, hypoxic stress induces the increased expression of α1H T-type Ca2+ channels (Carabelli et al. 2007). Together, these findings further support momentum that T-type channels are involved in catecholamine release under stress (Marcantoni et al. 2008). This emerging hypothesis matches well with the idea that PACAP acts as a stress transmitter in the adrenal medulla. Further studies will need to be conducted on the systems, cell and molecular level to determine the activity-dependent roles of Epac and PLC/PKC/NCX on PACAP-evoked catecholamine release.
Supplementary Material
Acknowledgments
We would like to thank Mr. Bryan Doreian for helpful comments and support in preparing this manuscript. The research is supported by grants from National Institutes of Health (NS-052123 to CS) and the National Science Foundation (IBN-0344768 to CS). BK was supported by the NIH (TS HL07653).
Abbreviations
- ACh
Acetylcholine
- DAG
diacylglycerol
- Epac
exchange proteins activated directly by cyclic AMP
- IP3
inositol triphosphate
- nAChR
nicotinic acetylcholine receptor
- NCX
Na+/Ca2+ exchanger
- PACAP
pituitary adenylate cyclase activating peptide
- PACR1
PACAP receptor 1
- PLC
phospholipase C
- PKA
protein kinase A
- PKC
protein kinase C
- VPAC
vasointestinal peptide receptor
References
- Aunis D. Exocytosis in chromaffin cells of the adrenal medulla. Int Rev Cytol. 1998;181:213–320. doi: 10.1016/s0074-7696(08)60419-2. [DOI] [PubMed] [Google Scholar]
- Bevington A, Radda GK. Declining catecholamine secretion in adrenal medulla on prolonged stimulation with acetylcholine. Biochem Pharmacol. 1985;34:1497–1500. doi: 10.1016/0006-2952(85)90690-2. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, Bernsand M, Eliasson L, Hakanson R, Lindstrom E. Somatostatin, misoprostol and galanin inhibit gastrin- and PACAP-stimulated secretion of histamine and pancreastatin from ECL cells by blocking specific Ca2+ channels. Regul Pept. 2005;130:81–90. doi: 10.1016/j.regpep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Carabelli V, Marcantoni A, Comunanza V, de Luca A, Diaz J, Borges R, Carbone E. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol. 2007;584:149–165. doi: 10.1113/jphysiol.2007.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Marcantoni A, Giancippoli A, Guido D, Carabelli V. T-type channels-secretion coupling: evidence for a fast low-threshold exocytosis. Pflugers Arch. 2006;453:373–383. doi: 10.1007/s00424-006-0100-7. [DOI] [PubMed] [Google Scholar]
- Carmichael SW. Morphology and innervation of the adrenal medulla. In: Rosenheck K, Lelkes P, editors. Stimulus-Secretion Coupling, Vol 1. 1-29. CRC; Boca Raton, Florida: 1986. pp. 40–49. [Google Scholar]
- Chan SA, Smith C. Low frequency stimulation of mouse adrenal slices reveals a clathrin-independent, protein kinase C-mediated endocytic mechanism. J Physiology (London) 2003;553(3):707–717. doi: 10.1113/jphysiol.2003.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Chowdhury PS, Guo X, Wakade TD, Przywara DA, Wakade AR. Exocytosis from a single rat chromaffin cell by cholinergic and peptidergic neurotransmitters. Neuroscience. 1994;59:1–5. doi: 10.1016/0306-4522(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Ciccarelli E, Svoboda M, De Neef P, Di Paolo E, Bollen A, Dubeaux C, Vilardaga JP, Waelbroeck M, Robberecht P. Pharmacological properties of two recombinant splice variants of the PACAP type I receptor, transfected and stably expressed in CHO cells. Eur J Pharmacol. 1995;288:259–267. doi: 10.1016/0922-4106(95)90037-3. [DOI] [PubMed] [Google Scholar]
- Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosci. 2005;25:7324–7332. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng G, Gaspo R, Trabelsi F, Yamaguchi N. Role of L-type Ca2+ channel in PACAP-induced adrenal catecholamine release in vivo. Am J Physiol. 1997;273:R1339–1345. doi: 10.1152/ajpregu.1997.273.4.R1339. [DOI] [PubMed] [Google Scholar]
- Gerdin MJ, Eiden LE. Regulation of PC12 Cell Differentiation by cAMP Signaling to ERK Independent of PKA: Do All the Connections Add Up? Sci STKE. 2007;2007:pe15-. doi: 10.1126/stke.3822007pe15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. vii–viii. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Hagen BM, Bayguinov O, Sanders KM. VIP and PACAP regulate localized Ca2+ transients via cAMP-dependent mechanism. Am J Physiol Cell Physiol. 2006;291:C375–385. doi: 10.1152/ajpcell.00495.2005. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Lee HW, Chen Y, Grimaldi M, Eiden LE. Coincident elevation of cAMP and calcium influx by PACAP-27 synergistically regulates vasoactive intestinal polypeptide gene transcription through a novel PKA-independent signaling pathway. J Neurosci. 2002a;22:5310–5320. doi: 10.1523/JNEUROSCI.22-13-05310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002b;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Takahashi N, Kishimoto T, Nemoto T, Kasai H. Two cAMP-dependent pathways differentially regulate exocytosis of large dense-core and small vesicles in mouse beta-cells. J Physiol. 2007;582:1087–1098. doi: 10.1113/jphysiol.2007.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautmann M, Friis UG, Desch M, Todorov V, Castrop H, Segerer F, Otto C, Schutz G, Schweda F. Pituitary adenylate cyclase-activating polypeptide stimulates renin secretion via activation of PAC1 receptors. J Am Soc Nephrol. 2007;18:1150–1156. doi: 10.1681/ASN.2006060633. [DOI] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H, Kitamura K, Minakuchi K, Ishimura Y, Okuno M, Ohuchi T, Oka M. Mechanism of histamine-induced calcium efflux from cultured bovine adrenal chromaffin cells: possible involvement of an Na+/Ca2+ exchange mechanism. Neurosci Lett. 1994;180:281–284. doi: 10.1016/0304-3940(94)90539-8. [DOI] [PubMed] [Google Scholar]
- Houchi H, Okuno M, Kitamura K, Minakuchi K, Ishimura Y, Ohuchi T, Oka M. Calcium efflux from cultured bovine adrenal chromaffin cells induced by pituitary adenylate cyclase-activating polypeptide (PACAP): possible involvement of an Na+/Ca2+ exchange mechanism. Life Sci. 1995;56:1825–1834. doi: 10.1016/0024-3205(95)00154-x. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac Mediates a cAMP-to-PKC Signaling in Inflammatory Pain: An Isolectin B4(+) Neuron-Specific Mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh YH, Yoo JA, Bahk SJ, Yoo SH. Distribution profile of inositol 1,4,5-trisphosphate receptor isoforms in adrenal chromaffin cells. FEBS Lett. 2005;579:2597–2603. doi: 10.1016/j.febslet.2005.03.076. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Uehara A, Nakamura TY, Imanaga I, Shigekawa M. Chimeric analysis of Na(+)/Ca(2+) exchangers NCX1 and NCX3 reveals structural domains important for differential sensitivity to external Ni(2+) or Li(+) J Biol Chem. 1999;274:23094–23102. doi: 10.1074/jbc.274.33.23094. [DOI] [PubMed] [Google Scholar]
- Jamen F, Puech R, Bockaert J, Brabet P, Bertrand G. Pituitary adenylate cyclase-activating polypeptide receptors mediating insulin secretion in rodent pancreatic islets are coupled to adenylate cyclase but not to PLC. Endocrinology. 2002;143:1253–1259. doi: 10.1210/endo.143.4.8739. [DOI] [PubMed] [Google Scholar]
- Jankowski JA, Schroeder TJ, Holz RW, Wightman RM. Quantal secretion of catecholamines measured from individual bovine adrenal medullary cells permeabilized with digitonin. J Biol Chem. 1992;267:18329–18335. doi: 10.21236/ada251716. [DOI] [PubMed] [Google Scholar]
- Jorgensen MS, Liu J, Adams JM, Titlow WB, Jackson BA. Inhibition of voltage-gated Ca(2+) current by PACAP in rat adrenal chromaffin cells. Regul Pept. 2002;103:59–65. doi: 10.1016/s0167-0115(01)00327-5. [DOI] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Ohse C, Watanabe Y, Suzuki S, Yamashita K, Ohashi S. Pituitary adenylate cyclase activating polypeptide stimulates insulin release from the isolated perfused rat pancreas. Life Sci. 1992;50:257–261. doi: 10.1016/0024-3205(92)90332-j. [DOI] [PubMed] [Google Scholar]
- Klevans LR, Gebber GL. Comparison of differential secretion of adrenal catecholamines by splanchnic nerve stimulation and cholinergic agents. J Pharmacol Exp Ther. 1970;172:69–76. [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouche S, Martineau D, Yamaguchi N. Modulation of adrenal catecholamine release by PACAP in vivo. Am J Physiol. 1999;276:R162–170. doi: 10.1152/ajpregu.1999.276.1.R162. [DOI] [PubMed] [Google Scholar]
- Marcantoni A, Carabelli V, Comunanza V, Hoddah H, Carbone E. Calcium channels in chromaffin cells: focus on L and T types. Acta Physiol (Oxf) 2008;192:233–246. doi: 10.1111/j.1748-1716.2007.01815.x. [DOI] [PubMed] [Google Scholar]
- Marley PD. Desensitization of the nicotinic secretory response of adrenal chromaffin cells. Trends Pharmacol Sci. 1988;9:102–107. doi: 10.1016/0165-6147(88)90177-0. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Malendowicz LK, Neri G, Andreis PG, Ziolkowska A, Gottardo L, Nowak KW, Nussdorfer GG. Pituitary adenylate cyclase-activating polypeptide and PACAP receptor expression and function in the rat adrenal gland. Int J Mol Med. 2002a;9:233–243. [PubMed] [Google Scholar]
- Mazzocchi G, Malendowicz LK, Rebuffat P, Gottardo L, Nussdorfer GG. Expression and function of vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide, and their receptors in the human adrenal gland. J Clin Endocrinol Metab. 2002b;87:2575–2580. doi: 10.1210/jcem.87.6.8571. [DOI] [PubMed] [Google Scholar]
- McFadden PN, Koshland DE., Jr Habituation in the single cell: diminished secretion of norepinephrine with repetitive depolarization of PC12 cells. Proc Natl Acad Sci U S A. 1990;87:2031–2035. doi: 10.1073/pnas.87.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller K, Sundler F. Expression of pituitary adenylate cyclase activating peptide (PACAP) and PACAP type I receptors in the rat adrenal medulla. Regul Pept. 1996;63:129–139. doi: 10.1016/0167-0115(96)00033-x. [DOI] [PubMed] [Google Scholar]
- Montero-Hadjadje M, Delarue C, Fournier A, Vaudry H, Yon L. Involvement of the adenylyl cyclase/protein kinase A signaling pathway in the stimulatory effect of PACAP on frog adrenocortical cells. Ann N Y Acad Sci. 2006;1070:431–435. doi: 10.1196/annals.1317.057. [DOI] [PubMed] [Google Scholar]
- Morita K, Sakakibara A, Kitayama S, Kumagai K, Tanne K, Dohi T. Pituitary adenylate cyclase-activating polypeptide induces a sustained increase in intracellular free Ca(2+) concentration and catecholamine release by activating Ca(2+) influx via receptor-stimulated Ca(2+) entry, independent of store-operated Ca(2+) channels, and voltage-dependent Ca(2+) channels in bovine adrenal medullary chromaffin cells. J Pharmacol Exp Ther. 2002;302:972–982. doi: 10.1124/jpet.102.033456. [DOI] [PubMed] [Google Scholar]
- Mustafa T, Grimaldi M, Eiden LE. The hop cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J Biol Chem. 2007;282:8079–8091. doi: 10.1074/jbc.M609638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novara M, Baldelli P, Cavallari D, Carabelli V, Giancippoli A, Carbone E. Exposure to cAMP and beta-adrenergic stimulation recruits Ca(V)3 T-type channels in rat chromaffin cells through Epac cAMP-receptor proteins. J Physiol. 2004;558:433–449. doi: 10.1113/jphysiol.2004.061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Kumakura K. Receptor desensitization and the transient nature of the secretory response of adrenal chromaffin cells. Neurosci Lett. 1986;70:250–254. doi: 10.1016/0304-3940(86)90472-6. [DOI] [PubMed] [Google Scholar]
- Okada R, Yamamoto K, Ito Y, Mochida H, Tonon MC, Fournier A, Leprince J, Vaudry H, Kikuyama S. VIP and PACAP stimulate TSH release from the bullfrog pituitary. Peptides. 2007;28:1784–1789. doi: 10.1016/j.peptides.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Pan CY, Chu YS, Kao LS. Molecular study of the Na+/Ca2+ exchanger in bovine adrenal chromaffin cells. Biochem J. 1998;336(Pt 2):305–310. doi: 10.1042/bj3360305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payet MD, Bilodeau L, Breault L, Fournier A, Yon L, Vaudry H, Gallo-Payet N. PAC1 receptor activation by PACAP-38 mediates Ca2+ release from a cAMP-dependent pool in human fetal adrenal gland chromaffin cells. J Biol Chem. 2003;278:1663–1670. doi: 10.1074/jbc.M206470200. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Przywara DA, Guo X, Angelilli ML, Wakade TD, Wakade AR. A non-cholinergic transmitter, pituitary adenylate cyclase-activating polypeptide, utilizes a novel mechanism to evoke catecholamine secretion in rat adrenal chromaffin cells. J Biol Chem. 1996;271:10545–10550. doi: 10.1074/jbc.271.18.10545. [DOI] [PubMed] [Google Scholar]
- Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- Ster J, De Bock Fdr, Guérineau NC, Janossy A, Barrère-Lemaire SP, Bos JL, Bockaert JI, Fagni L. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proceedings of the National Academy of Sciences. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Shibuya I, Nagamoto T, Yamashita H, Kanno T. Pituitary adenylate cyclase-activating polypeptide causes rapid Ca2+ release from intracellular stores and long lasting Ca2+ influx mediated by Na+ influx-dependent membrane depolarization in bovine adrenal chromaffin cells. Endocrinology. 1996;137:956–966. doi: 10.1210/endo.137.3.8603609. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Mahata M, Mahata SK, O'Connor DT. Time-Dependent Effects of the Neuropeptide PACAP on Catecholamine Secretion : Stimulation and Desensitization. Hypertension. 1999;34:1152–1162. doi: 10.1161/01.hyp.34.5.1152. [DOI] [PubMed] [Google Scholar]
- Tornoe K, Hannibal J, Jensen TB, Georg B, Rickelt LF, Andreasen MB, Fahrenkrug J, Holst JJ. PACAP-(1-38) as neurotransmitter in the porcine adrenal glands. Am J Physiol Endocrinol Metab. 2000;279:E1413–1425. doi: 10.1152/ajpendo.2000.279.6.E1413. [DOI] [PubMed] [Google Scholar]
- Traboulsie A, Chemin J, Chevalier M, Quignard JF, Nargeot J, Lory P. Subunit-specific modulation of T-type calcium channels by zinc. J Physiol. 2007;578:159–171. doi: 10.1113/jphysiol.2006.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turquier V, Yon L, Grumolato L, Alexandre D, Fournier A, Vaudry H, Anouar Y. Pituitary adenylate cyclase-activating polypeptide stimulates secretoneurin release and secretogranin II gene transcription in bovine adrenochromaffin cells through multiple signaling pathways and increased binding of pre-existing activator protein-1-like transcription factors. Mol Pharmacol. 2001;60:42–52. doi: 10.1124/mol.60.1.42. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Hamelink C, Damadzic R, Eskay RL, Gonzalez B, Eiden LE. Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides. 2005;26:2518–2524. doi: 10.1016/j.peptides.2005.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade AR. Noncholinergic transmitter(s) maintains secretion of catecholamines from rat adrenal medulla for several hours of continuous stimulation of splanchnic neurons. J Neurochem. 1988;50:1302–1308. doi: 10.1111/j.1471-4159.1988.tb10608.x. [DOI] [PubMed] [Google Scholar]
- Wakade AR. Multiple transmitter control of catecholamine secretion in rat adrenal medulla. Adv Pharmacol. 1998;42:595–598. doi: 10.1016/s1054-3589(08)60821-2. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Masuo Y, Matsumoto H, Suzuki N, Ohtaki T, Masuda Y, Kitada C, Tsuda M, Fujino M. Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline. Biochem Biophys Res Commun. 1992;182:403–411. doi: 10.1016/s0006-291x(05)80159-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.