Abstract
Background:
The vein of Marshall (VOM) is an attractive target during ablation of atrial fibrillation due to its autonomic innervation, its location anterior to the left pulmonary veins and drainage in the coronary sinus.
Methods and results:
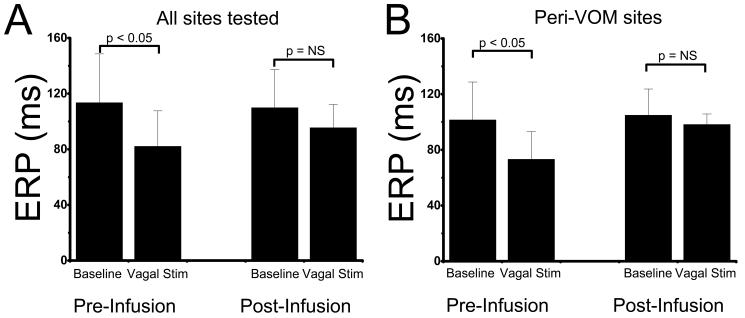
We studied 17 dogs. A coronary sinus venogram showed a VOM in 13, which was successfully cannulated with an angioplasty wire and balloon. In 5 dogs, electroanatomical maps of the left atrium were performed at baseline and after ethanol infusion in the VOM, which demonstrated a new crescent-shaped scar, extending from the annular left atrium towards the posterior wall and left pulmonary veins. In 4 other dogs, effective refractory periods (ERP) were measured at 3 sites in the left atrium, before and after high-frequency bilateral vagal stimulation. The ERP decreased from 113.6±35.0 ms to 82.2±25.4 ms (p<0.05) after vagal stimulation. After VOM ethanol infusion, vagally-mediated ERP decrease was eliminated (from 108.6±24.1 ms to 96.4 ±16.9ms, p=NS). The abolition of vagal effects was limited to sites near the VOM (ERP: 104±14 ms, vs 98.6±12.2 ms post vagal stimulation, p=ns), as opposed to sites remote to VOM (ERP: 107.2±14.9 ms, vs 78.6±14.7ms post vagal stimulation, p<0.05). To test feasibility in humans, 5 patients undergoing pulmonary vein antral isolation had successful VOM cannulation and ethanol infusion: left atrial voltage maps demonstrated new scar involving the infero-posterior left atrial wall extending towards the left pulmonary veins.
Conclusions:
Ethanol infusion in then VOM achieves significant left atrial tissue ablation, abolishes local vagal responses and is feasible in humans.
Keywords: ethanol, ablation, vein of Marshall, atrial fibrillation, vagal
Introduction
Catheter ablation has become an integral part of the management of atrial fibrillation (AF) when a strategy to preserve normal sinus rhythm is required.1 However, critical details of the technique are still controversial, the procedure demands high technical dexterity and carries significant risk of complications. Isolation of the pulmonary veins has been accepted as a requirement for procedural success. However, isolation of the left-sided veins can be difficult due to the presence of a thick pectinate muscle separating the veins from the left atrial appendage (“lateral ridge”).2 Additionally, ablation of the left atrial posterior wall can lead to esophageal damage, which in turn can cause atrio-esophageal fistula, a deadly complication.3, 4
The ligament of Marshall is the embryologic remnant of the left superior vena cava, which, as it becomes atretic during development, may remain open as the so-called vein of Marshall (VOM).5 This vein drains in the coronary sinus and runs posteriorly and superiorly in the epicardial surface of the left atrium, to join the anterior aspect of the left-sided pulmonary veins, as part of the lateral ridge that separates the veins from the appendage.6
The VOM and its neighboring tissue are attractive targets for ablation in AF. It contains autonomic parasympathetic7 and sympathetic6 innervations that have been implicated in the pathogenesis of AF.8 The atrial tissue surrounding the VOM, which connects the mitral annulus (coronary sinus) to the posterior left atrium, as well as the lateral ridge, are routinely targeted during ablation of AF.
The VOM is present in and can be cannulated in ∼70% of patients.5 We tested the hypothesis that infusion of ethanol via VOM could be used to ablate its nerve terminals and neighboring atrial tissues.
Methods
ANIMAL STUDIES
Seventeen normal, adult Mongrel dogs (35-40 kg) were studied. The animal protocol was approved by the animal care and use committee of the Methodist Hospital Research Institute. The protocol adhered to the PHS guidelines for the care and use of laboratory animals. Dogs were premedicated by an intramuscular injection of xylazine (0.75-1.5 mg/kg) and atropine, anesthesized by intravenous injection of propofol (5 mg/kg) and continued on isoflurane inhalation for the duration of the study. The left internal jugular and right femoral veins were cannulated. An 8.5 F sheath was advanced into the coronary sinus under fluorosocopic guidance, and a coronary sinus contrast venogram was obtained by occluding its ostium with a balloon catheter.
Initial studies were used to develop a reliable technique of VOM cannulation. After multiple strategies were attempted, selective cannulation of the VOM was reliably achieved by introducing a left internal mammary artery (LIMA) catheter in the coronary sinus, engaging the VOM ostium. Through the LIMA catheter, an angioplasty wire and balloon catheter (2 mm × 8 mm) were advanced into the VOM. The balloon was inflated at the ostium of the VOM and selective VOM venograms were obtained. Substantial anatomical variations were present in the length and caliber of the VOM. See Figure 1. Electrophysiologic evaluations were performed before and after VOM ethanol infusion, following the protocols described below. The angioplasty balloon was then inflated at the ostium of the VOM and 5 cc of 100% ethanol were delivered over 2 minutes through the balloon lumen.
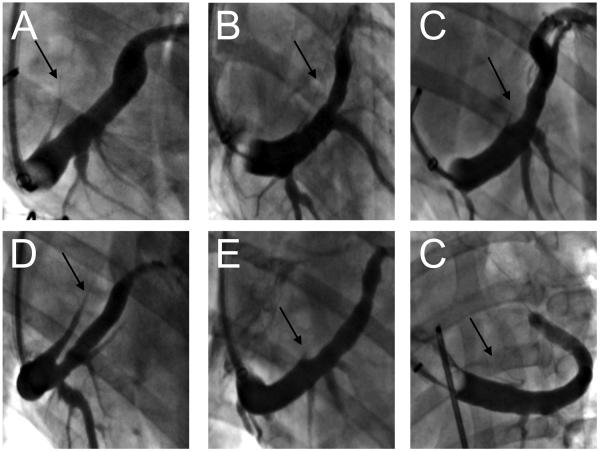
Figure 1.
Angiographic appearance of the VOM in dogs in balloon occlusion venograms. The VOM (black arrows) can be readily identified in the right anterior oblique radiographic projection (A through E) as a posteriorly-directed branch of the coronary sinus. In the left anterior oblique projection (F), it is superiorly directed. Significant variations in caliber and length are obvious.
Protocol 1: Extent of left atrial tissue ablation by retrograde VOM ethanol infusion
After securing cannulation of the VOM with the angioplasty wire and balloon, we performed a trans-septal puncture from the right femoral vein. A sheath was advanced into the left atrium, through which a three-dimensional mapping catheter (CARTO, Biosense-Webster, Diamond Bar, CA) was maneuvered to generate a volume rendition of the left atrium and quantify regional amplitudes of the bipolar voltage signals. After a baseline map was obtained, ethanol was injected in the VOM. Five to 10 minutes after ethanol injection, a repeat three-dimensional voltage map was performed to delineate the extent and distribution of tissue ablated (areas of low voltage post ablation). Complete pre- and post-ethanol infusion maps were obtained in 5 dogs. In three dogs, a third voltage map of the left atrium was performed 30 minutes after completion of the initial map to assess the persistence of the lesions.
Protocol 2: VOM ethanol infusion and parasympathetic control in the left atrium
Because the ligament of Marshall has been implicated as a parasympathetic conduit,7 we evaluated the effects of ethanol injection in the parasympathetic modulation of the left atrium in 4 dogs. Similar to protocol 1, after securing the cannulation of the VOM, we performed a trans-septal puncture form the right femoral vein. A sheath was advanced into the left atrium, through which a multi-electrode basket catheter (Constellation, EP technologies) was inserted and expanded. Bilateral cervical dissections were performed to expose the vagus trunk. Cuff electrodes were positioned in each vagus nerve and were connected to a Grass stimulator. High frequency vagal stimulation was titrated to achieve 30-50% decrease in heart rate. Left atrial effective refractory periods (ERP) were measured at 3 left atrial sites with and without vagal stimulation, and both before and after VOM ethanol infusion. The basket catheter was pushed towards the lateral atrial wall so that contact close to the VOM region was obtained. One pacing site was chosen from such region, and the other two from equidistant splines, as determined by tissue contact and capture.
Induction of AF during vagal stimulation was performed by burst pacing before and after ethanol infusion. Pacing from three different atrial sites was performed, starting at a cycle length of 300 ms and decreasing every ∼5 seconds by 10 ms until loss of 1:1 capture or AF induction. Fiver induction attempts were performed from each site. If AF persisted more than one minute, it was considered sustained, and vagal stimulation was discontinued in order to allow return of normal sinus rhythm. Mean AF cycle length was quantified by averaging beat-to-beat intervals from selected atrial sites.
HUMAN FEASIBILITY STUDIES
The human protocol was approved by the Institutional Review Board at the Methodist Hospital and all subjects signed an informed consent form describing the experimental nature of the study. Patients subjected to ablation of AF were included in the study. After obtaining routine intravenous access, an 8F sheath was advanced into the coronary sinus under fluoroscopy. A balloon occlusion venogram was performed to delineate the coronary sinus anatomy. The presence of the VOM was established when a posteriorly-directed vein branch was visible in the right anterior oblique projection. An angiographic LIMA catheter was then inserted in the coronary sinus aiming its tip towards the VOM, and an angioplasty wire was advanced over the VOM. An angioplasty balloon was then advanced over the wire and placed in the proximal VOM.
We then proceeded with obtaining double trans-septal access under intracardiac echocardiographic guidance. A baseline three-dimensional map of the left atrial geometry and bipolar voltage amplitudes was constructed using either the CARTO (Biosense-Webster) or the NavX-EnSite (St Jude Medical) systems.
Subsequently, we inflated the angioplasty balloon in the proximal VOM as defined by radiographic contrast injection. In order to ascertain flow in the VOM, one ml of echocardiographic contrast agent (Definity ®) was delivered via the lumen of the angioplasty balloon. Afterwards, we performed 2 infusions of 1 cc each of 100% ethanol, 2 minutes apart. We then repeated a three-dimensional bipolar voltage map of the left atrium, in order to define the tissue areas ablated by ethanol. Finally, conventional radiofrequency ablation of the pulmonary vein antra was performed. Pacing in the coronary sinus was performed to assess for peri-mitral conduction block after ethanol infusion.
Data analysis
Data are presented as mean ± standard deviation. In order to compare ERP before and after vagal stimulation, we used paired T-test. In order to compare ERP before and after vagal stimulation, before and after VOM ethanol infusion, we used repeated measures ANOVA with Bonferroni test for multiple comparisons. A p value less than 0.05 was considered significant.
Results
ANIMAL STUDIES
Vein of Marshall characteristics and cannulation
A visible VOM was present in 13/17 dogs, identified as a posteriorly-directed branch of the coronary sinus in the right anterior oblique fluoroscopic view. Figure 1 shows representative appearances of the VOM.
In 4 dogs, there was no discernable VOM. In the remainder, the length of the vein or Marshall was variable (29.7±10.7 mm), and so was its caliber. In all dogs, the vein communicated with the left atrial lumen, as shown by the presence of contrast in the left atrium via venous collaterals in the left atrial appendage. In one dog, a large VOM communicated with the innominate vein as a persistent left superior vena cava (Figure 1D).
Successful cannulation of the vein with an angioplasty wire was performed in 11 dogs. After several tools and strategies were attempted, the most reliable technique to cannulate the VOM involved advancing an angiographic left internal mammary (LIMA) catheter in the coronary sinus, so that its tip faced upwards, in contact with the roof of the coronary sinus. Two angioplasty wires were used: one was advanced in the lumen of the coronary sinus for fluoroscopic reference, and a second one into the VOM through the LIMA catheter, with a pre-loaded angioplasty balloon (8 mm long, 2 mm diameter). The angioplasty wire (most commonly a Cross-It 0.014″ wire) was then advanced through the LIMA catheter at different points of the roof of the coronary sinus, probing it at different locations until it could be advanced in the VOM. The wire was advanced in the VOM for a length of 25.4±11.7 mm from the vein ostium.
Once enough wire was advanced in the VOM, the angioplasty balloon was advanced over the wire into the proximal VOM. Contrast was injected through the balloon lumen to confirm VOM cannulation. The balloon was then inflated to maintain it in place while we proceeded with the experimental protocol. Direct contrast injection via the angioplasty balloon lumen demonstrated the full extent of the VOM and consistently some degree of myocardial staining. In 2 dogs, the length and caliber of the VOM significantly enlarged after selective contrast injection via the balloon (Figure 2A). Despite balloon inflation to achieve 2 mm diameters, complete occlusion of the VOM was never achieved, and contrast leakage into the coronary sinus lumen was consistently present.
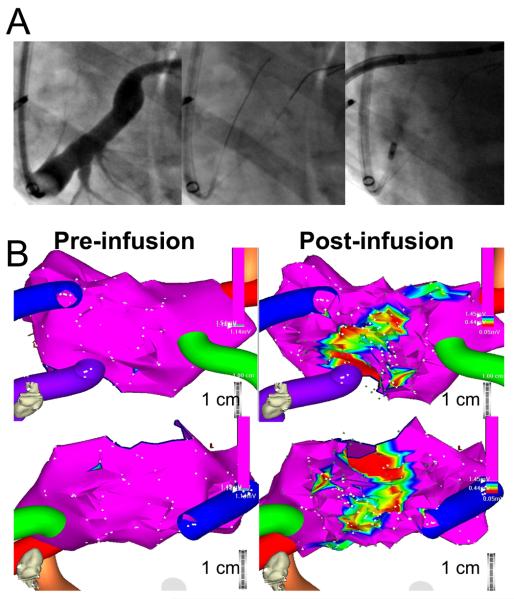
Figure 2.
VOM cannulation technique and VOM ethanol infusion results. A, Cannulation technique. After balloon occlusion venogram, the VOM was indentified. For reference, an angioplasty wire was inserted in the main lumen of the coronary sinus. An angiographic left internal mammary artery (LIMA) catheter was then inserted to probe the roof of the coronary sinus with an angioplasty wire until the VOM was cannulated. In the case shown, significant expansion of the VOM lumen was present upon selective VOM venogram (rightmost panel). B, Bipolar voltage three-dimensional maps of the left atrial geometry before (left) and after VOM ethanol infusion (right), in the posterior-anterior view (top) and in a left lateral view (bottom). The color tubes indicate the pulmonary veins (left inferior vein not shown). At baseline, the left atrial wall had bipolar voltage signals with amplitudes exceeding 1.5 mV throughout the atrium. After VOM ethanol infusion a new area of low voltage amplitude is apparent in the anatomical distribution of the VOM, which in this case runs superiorly towards the anterior aspect of the left pulmonary veins.
Extent of endocardial tissue ablated
At baseline, three-dimensional maps demonstrated that bipolar voltage signals in the left atrium exceeded 2 mV in all dogs, consistent with normal healthy tissue. Infusion of 5 cc of 100% ethanol in the VOM was performed over 2 minutes and was not associated with hemodynamic or heart rate changes. (In one dog that did not have a discernable VOM, rapid ethanol bolus infusion in the main coronary sinus was associated with ST-segment elevation and cardiac arrest.) Immediately after ethanol infusion, a repeat bipolar voltage map was performed. This consistently demonstrated a new area of low voltage in the region corresponding to the VOM, extending from the mitral annulus, posteriorly and superiorly towards the left pulmonary veins. The low-voltage area measured 4.1 ± 2.7 cm2 using a threshold of less then 1 mV, and 2.2 ± 1.7 cm2 using a threshold of less than 0.5 mV. Figure 2 shows a typical example. In Figure 2A, the angiographic appearance of the VOM is shown, with significant enlargement of the vein after cannulation and angioplasty balloon inflation. Figure 2B shows three-dimensional voltage maps pre- and post-infusion of ethanol, with a clearly demarcated area of low voltage that extends from the mitral annulus (Figure 2B, top) towards the posterior wall of the left atrium, encompassing the area adjacent and anterior to the left pulmonary veins.
The low-voltage surface areas were variable in extent and distribution, depending on the course, size and length of the VOM. Figures 2, 3 and 4 show different examples. In Figure 3, the lesion extends mostly towards the posterior wall of the left atrium in between the left and right pulmonary veins.
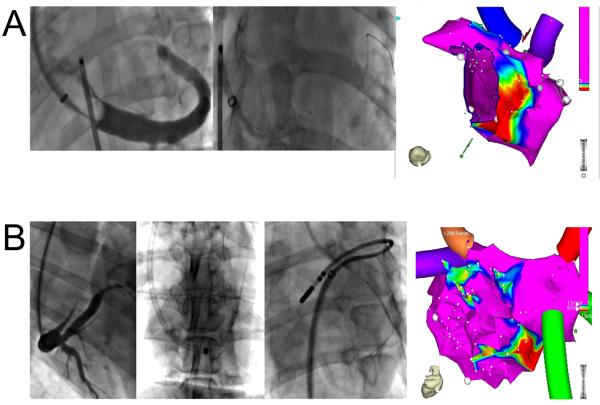
Figure 3.
Posterior left atrial ablation after VOM ethanol infusion. A, Course of the VOM on the coronary sinus venogram (left panel), wire cannulation (mid panel: top wire is in the VOM advanced via a left internal mammary artery angiographic catheter, bottom wire in the main coronary sinus lumen), and selective VOM venogram via an inflated angioplasty balloon. B, Bipolar voltage three-dimensional maps of the left atrial geometry before (left) and after VOM ethanol infusion (right), in the posterior-anterior view (top) and in an inferior view (bottom). The low-voltage area created by VOM ethanol infusion is posteriorly directed, in between left and right pulmonary veins.
Figure 4.
Anatomical factors leading to reduced ablation area. A, Small VOM. On CS venogram (left panel, left anterior oblique projection), the VOM is a small twig arising from the roof of the coronary sinus. Cannulation was successful, but the angioplasty balloon could only be advanced in the very proximal VOM (mid panel). A small annular area of ablation was created (right panel). B, Communication between VOM and left innominate vein superiorly. Despite a large VOM on the coronary sinus venogram (panel Ba, right anterior oblique projection), and successful VOM cannulation with an angioplasty balloon (panel Bb, left anterior oblique projection), multiple ethanol infusions only created small ablation lesions on voltage maps (panel Bd). A communication between the VOM and the superior vena cava was obvious when the VOM was cannulated from above (panel Bc).
In some cases, the extension of the ablation lesions was small. In Figure 4, two examples are shown where small lesions were created: Figure 4A shows an example of a very small VOM in which cannulation with an angioplasty balloon was only possible in its very proximal portion; and Figure 4B shows a case where a communication between VOM and the innominate vein was present (persistent left superior vena cava), and the lesion size was also small due to ethanol shunting to the central venous circulation.
Lesions were persistent over time. In 3 dogs, repeat voltage maps were performed 30 minutes after completion of the initial map and no significant differences were found. Figure 5 shows the endocardial and epicardial appearance of the lesions after extraction of the heart.
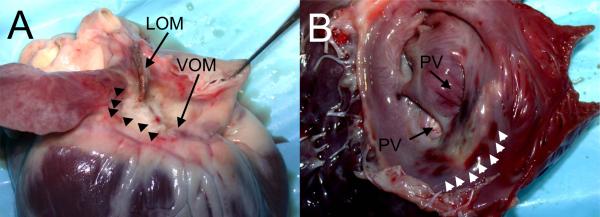
Figure 5.
Ethanol ablation lesions. A, Epicardial aspect, showing the VOM and ligament of Marshall (LOM) areas, with pale discoloration of the ablated areas (arrowheads). B, Endocardial aspect after incision in the left atrial appendage. A pale area of discoloration is shown anterior to the left pulmonary veins (PV), surrounded by small areas of tissue hemorrhage (arrowheads).
Vagal denervation by VOM ethanol infusion: effects on AF induction
Effective refractory periods were measured at three atrial sites via basket electrodes before and after vagal stimulation. A decrease in ERP was demonstrable after vagal stimulation. After ethanol infusion, a significant blunting of vagally-mediated decrease in ERP was found (Figure 6). However, this effect was not uniform across the three left atrial sites tested: only sites in the neighborhood of the ablated tissue exhibited blunting of the vagal effect (Figure 6B).
Figure 6.
VOM ethanol infusion abolishes vagally-mediated decreases in effective refractory periods (ERP). A, ERPs measured in three left atrial sites before and after VOM ethanol infusion, showed blunting for vagal decreases in ERP after VOM ethanol infusion. This was particularly obvious at sites fluoroscopically adjacent to the VOM (B).
Sustained AF during vagal stimulation was uniformly inducible (n=4 dogs) after burst pacing in 3 atrial sites (37/45 attempts). In one dog, AF organized into atrial flutter prior to spontaneous return of sinus rhythm. After VOM ethanol infusion, AF was induced in 19/45 attempts (p<0.001). Mean cycle length of AF after VOM ethanol infusion was slightly longer after VOM ethanol infusion (118.8± 22.3 ms vs 111.3 ± 19.2 ms at baseline, p<0.05). Organization into atrial flutter was present in 2/4 dogs after VOM ethanol infusion.
Left atrial propagation patterns and perimitral conduction after VOM ethanol infusion
It has been suggested that the ligament of Marshall participates in the electrical communication between the right and left atria, as the terminal component of the interatrial communication via the coronary sinus.9 We evaluated left atrial activation maps before and after VOM ethanol infusion and were unable to show appreciable differences in left atrial activation patterns. Similarly, there were no changes in perimitral conduction patterns after VOM ethanol infusion.
HUMAN FEASIBILITY STUDIES
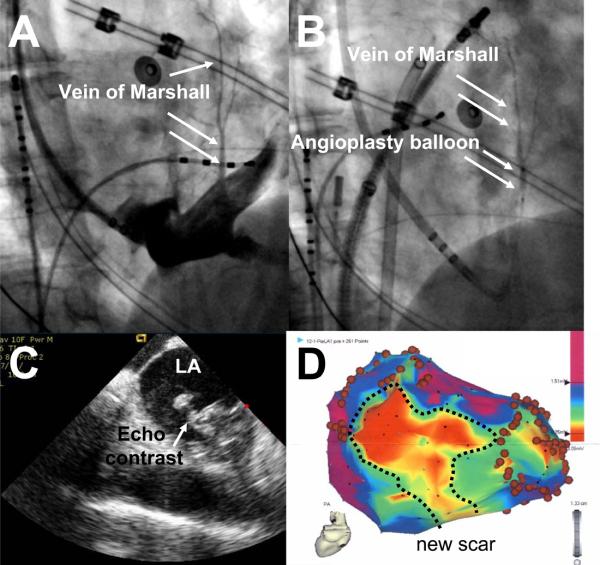
Nine patients were consented for the study. In two patients there was no visible VOM in the coronary sinus venogram. In one patient, a small coronary sinus dissection was created during attempts to cannulate the VOM with an angioplasty wire, and the procedure was aborted without clinical complications. In the remaining 6 patients, successful cannulation of the VOM was performed. Injection of echocardiographic contrast in the VOM demonstrated earliest contrast appearance in the left atrial lumen, adjacent to the left-sided pulmonary veins (Figure 7 and movie 1 online). There were no acute hemodynamic or clinical changes during or after ethanol infusion.
Figure 7.
Representative example of VOM ethanol infusion in humans. A, Large VOM shown by contrast injection through a LIMA catheter pointing towards the VOM ostium. B, Selective VOM venogram via angioplasty balloon. C, Echocardiographic contrast injection shows earliest contrast appearance in the left atrial lumen close to the left pulmonary veins. D, Bipolar voltage map after ethanol infusion showing a large are of lower voltage (<0.5 mV, new scar).
Tissue ablation by VOM ethanol infusion
Ethanol infusion in the VOM lead to the generation of a new, low voltage region that appeared posterior and superior to the coronary sinus, encompassing variable extents of the posterior left atrial wall and the anterior aspect of the left inferior pulmonary vein. Figure 7 shows a typical example. The area of scar (bipolar voltage amplitude less than 0.5 mV) measured 10.2 ± 5.7 cm2 (range 3.3 to 15.3 cm2), depending on the size of the VOM as shown in the selective venogram.
Procedural impact
The total added procedure time (including coronary sinus cannulation and venogram, VOM cannulation, balloon occlusion and ethanol infusion, and repeat three-dimensional voltage map) was 52.6 ± 12.8 min (range 31 to 66 min) and the added fluoroscopy time was 8.2 ± 2.8 min (range 4.1 to 10 min). There was no evidence of mitral annular conduction block after VOM ethanol infusion. In all six patients, ethanol levels measured at the end of the procedure in venous blood from the femoral vein were undetectable. In 4/6 patients, the left inferior PV became electrically disconnected solely with ethanol infusion (in 1/6 patients, the left superior PV as well). The total radiofrequency ablation times required to achieve left inferior PV isolation in the two patients that required it were 0.8 and 4.6 minutes. On follow-up, one patient was taken back for atrial flutter caused by macro-reentry in the right PV antrum, remote to areas affected by VOM ethanol infusion and likely unrelated to the VOM procedure. This was successfully ablated by radiofrequency application in the septal aspect of the right superior PV, and remains in normal sinus rhythm. All other patients remain in normal rhythm as well.
Discussion
The major findings of our study are: 1) the VOM is a viable route to deliver therapeutic agents into left atrial tissues; 2) retrograde VOM ethanol infusion leads to tissue ablation in areas of the left atrium routinely targeted during catheter ablation of AF; 3) regional parasympathetic ablation is achieved by retrograde VOM ethanol infusion; and 4) retrograde VOM ethanol infusion is feasible in humans.
VOM as a therapeutic target in atrial fibrillation
The ligament of Marshall has been solidly implicated in arrhythmogenesis. Scherlag et al10 demonstrated an inducible ectopic rhythm arising from the ligament area upon left cardiac sympathetic nerve stimulation. Doshi et al went on to specifically demonstrate the role of the ligament of Marshall in such adrenergic atrial tachycardia.11 A wealth of clinical data supports the VOM as a source of ectopic foci driving the genesis and maintenance of AF.12-17 Mechanistically, there are electrical connections between left superior pulmonary vein, left atrium, and ligament of Marshall.18 The ligament of Marshall contains parasympathetic7 and sympathetic6 innervation19 that mediate autonomic modulation of the left atrial tissue and have been implicated in the pathogenesis of AF.8 Ulphani et al7 have shown that ablation of the ligament of Marshall blunts the vagally–mediated decreases in left atrial ERP. Here we show that similar effects are achieved via retrograde VOM ethanol infusion. Additionally, focal ectopy arising in the VOM triggering AF has been demonstrated clinically5, 16 and in experimental models of AF.20
VOM ethanol infusion for left atrial ablation
The aforementioned data support the VOM as a target of ablation in its own right from the mechanistic standpoint. Two additional facts support targeting the VOM:1) the thick pectinate muscle between the left atrial appendage and left-sided pulmonary veins can be difficult to ablate using current techniques and it is precisely the course of the VOM on the epicardial side;2 and 2) the posterior wall of the left atrium in the vicinity of the VOM can also be close to the esophagus, and current ablation techniques entail risk of esophageal damage and atrio-esophageal fistula which can be deadly.
Cannulating the VOM allows delivery of therapeutic agents in left atrial tissue. The anatomical distribution of tissues reached includes not only the vein and associated innervation, but also areas routinely targeted during catheter ablation of AF. Ethanol infusion leads to effective tissue ablation in these areas, as limited by the size and extent of the VOM. Ethanol is routinely used to ablate tissue in hypertrophic cardiomyopathy,21 and occasionally ventricular tachycardias that do not respond to conventional RF ablation.22 In these instances, ethanol is delivered arterially. Besides direct toxic effects, ethanol-induced arterial sclerosis is thought to mediate tissue damage through ischemia, a mechanism absent when retrogradely delivered. Nevertheless, we found adequate tissue ablation from the electrical standpoint by retrograde venous infusion. Transmyocardial communication between the epicardial VOM and the left atrial endocardium must have occurred in order for the toxic effects of ethanol to take place. Contrast leakage into the left atrial lumen was consistenly demonstrable during selective VOM venograms. This was confirmed by the human data showing echocardiographic contrast in the left atrium when selectively injected in the VOM. Therefore, transmyocardial transit occurs. It should be emphasized that electroanatomical voltage maps showing tissue ablation by voltage criteria were obtained endocardially, whereas the VOM is an epicardial structure.
Ethanol infusion in the VOM is attractive in ablation of AF because local toxicity of ethanol would be limited to tissues in direct contact with it, and would spare neighboring structures such as the esophagus, potentially increasing safety of the procedure. Second, VOM ethanol infusion would directly address mechanistic sources of AF located in the VOM such as autonomic nerves and ectopic foci triggering AF. Third, it could help in achieving adequate ablation of the lateral ridge from the epicardial side. Fourth, a large area of tissue could be ablated at once with a simple infusion, potentially saving procedural time. Furthermore, if performed in isolation, it would be a purely right-sided procedure, obviating the need for a trans-septal puncture and anticoagulation, which are significant sources of procedural complications. Although several surgical reports have reported some efficacy of similarly limited left atrial interventions.23, 24 most included pulmonary vein isolation as well, and we do not anticipate isolated ethanol VOM infusion as a stand alone procedure, even if it achieved significant vagal denervation.25
Conclusions
Retrograde ethanol infusion in the VOM leads to left atrial tissue and vagal ablation, and is feasible in humans. Future studies are needed to evaluate its potential role in ablation of AF.
Movie Online. Echocardiographic contrast injection (Definity ®) in the VOM. An intracardiac echocardiographic view of the left atrium with the left-sided pulmonary veins is shown. Upon contrast injection, the earliest site of contrast appearance is between the left superior and inferior pulmonary vein, to then reach the let atrial lumen. This supports transmyocardial flow between the epicardial VOM and the left atrial lumen.
Acknowledgements
The authors thank April Gilbert, BS, and Daryl Schulz, RTR for technical assistance. This study was supported by an award from the Houston Texans (MV and DSK), and by research grants RO1HL068768 (DSK) and R21HL085215 (MV) from the National Institutes of Health.
Footnotes
Disclosures
All authors have read the manuscript and take responsibility for its contents. There are no conflicts of interest.
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr., Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera JA, Ho SY, Climent V, Sanchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–362. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 3.Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso F, Torracca L, Benussi S, Alfieri O, Hong R, Lau W, Hirata K, Shikuma N, Hall B, Morady F. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 4.Cummings JE, Schweikert RA, Saliba WI, Burkhardt JD, Kilikaslan F, Saad E, Natale A. Brief communication: atrial-esophageal fistulas after radiofrequency ablation. Ann Intern Med. 2006;144:572–574. doi: 10.7326/0003-4819-144-8-200604180-00007. [DOI] [PubMed] [Google Scholar]
- 5.Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–1505. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 6.Kim DT, Lai AC, Hwang C, Fan LT, Karagueuzian HS, Chen PS, Fishbein MC. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36:1324–1327. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- 7.Ulphani JS, Arora R, Cain JH, Villuendas R, Shen S, Gordon D, Inderyas F, Harvey LA, Morris A, Goldberger JJ, Kadish AH. The ligament of Marshall as a parasympathetic conduit. Am J Physiol Heart Circ Physiol. 2007;293:H1629–1635. doi: 10.1152/ajpheart.00139.2007. [DOI] [PubMed] [Google Scholar]
- 8.Kamanu S, Tan AY, Peter CT, Hwang C, Chen PS. Vein of Marshall activity during sustained atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:839–846. doi: 10.1111/j.1540-8167.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 9.Omichi C, Chou CC, Lee MH, Chang CM, Lai AC, Hayashi H, Zhou S, Miyauchi Y, Okuyama Y, Hamabe A, Hwang C, Fishbein MC, Lin SF, Karagueuzian HS, Chen PS. Demonstration of electrical and anatomic connections between Marshall bundles and left atrium in dogs: implications on the generation of P waves on surface electrocardiogram. J Cardiovasc Electrophysiol. 2002;13:1283–1291. doi: 10.1046/j.1540-8167.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 10.Scherlag BJ, Yeh BK, Robinson MJ. Inferior interatrial pathway in the dog. Circ Res. 1972;31:18–35. doi: 10.1161/01.res.31.1.18. [DOI] [PubMed] [Google Scholar]
- 11.Doshi RN, Wu TJ, Yashima M, Kim YH, Ong JJ, Cao JM, Hwang C, Yashar P, Fishbein MC, Karagueuzian HS, Chen PS. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–883. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 12.Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: possible roles of the ligament of Marshall. J Cardiovasc Electrophysiol. 1999;10:636–648. doi: 10.1111/j.1540-8167.1999.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 13.Katritsis D, Ioannidis JP, Anagnostopoulos CE, Sarris GE, Giazitzoglou E, Korovesis S, Camm AJ. Identification and catheter ablation of extracardiac and intracardiac components of ligament of Marshall tissue for treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:750–758. doi: 10.1046/j.1540-8167.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 14.Polymeropoulos KP, Rodriguez LM, Timmermans C, Wellens HJ. Images in cardiovascular medicine. Radiofrequency ablation of a focal atrial tachycardia originating from the Marshall ligament as a trigger for atrial fibrillation. Circulation. 2002;105:2112–2113. doi: 10.1161/01.cir.0000016168.49833.ce. [DOI] [PubMed] [Google Scholar]
- 15.Katritsis D, Giazitzoglou E, Korovesis S, Paxinos G, Anagnostopoulos CE, Camm AJ. Epicardial foci of atrial arrhythmias apparently originating in the left pulmonary veins. J Cardiovasc Electrophysiol. 2002;13:319–323. doi: 10.1046/j.1540-8167.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, Chang MS, Chen SA. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–3183. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 17.Kurotobi T, Ito H, Inoue K, Iwakura K, Kawano S, Okamura A, Date M, Fujii K. Marshall vein as arrhythmogenic source in patients with atrial fibrillation: correlation between its anatomy and electrophysiological findings. J Cardiovasc Electrophysiol. 2006;17:1062–1067. doi: 10.1111/j.1540-8167.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 18.Tan AY, Chou CC, Zhou S, Nihei M, Hwang C, Peter CT, Fishbein MC, Chen PS. Electrical connections between left superior pulmonary vein, left atrium, and ligament of Marshall: implications for mechanisms of atrial fibrillation. Am J Physiol Heart Circ Physiol. 2006;290:H312–322. doi: 10.1152/ajpheart.00369.2005. [DOI] [PubMed] [Google Scholar]
- 19.Makino M, Inoue S, Matsuyama TA, Ogawa G, Sakai T, Kobayashi Y, Katagiri T, Ota H. Diverse myocardial extension and autonomic innervation on ligament of Marshall in humans. J Cardiovasc Electrophysiol. 2006;17:594–599. doi: 10.1111/j.1540-8167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu TJ, Ong JJ, Chang CM, Doshi RN, Yashima M, Huang HL, Fishbein MC, Ting CT, Karagueuzian HS, Chen PS. Pulmonary veins and ligament of marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation. 2001;103:1157–1163. doi: 10.1161/01.cir.103.8.1157. [DOI] [PubMed] [Google Scholar]
- 21.Knight C, Kurbaan AS, Seggewiss H, Henein M, Gunning M, Harrington D, Fassbender D, Gleichmann U, Sigwart U. Nonsurgical septal reduction for hypertrophic obstructive cardiomyopathy: outcome in the first series of patients. Circulation. 1997;95:2075–2081. doi: 10.1161/01.cir.95.8.2075. [DOI] [PubMed] [Google Scholar]
- 22.Sacher F, Sobieszczyk P, Tedrow U, Eisenhauer AC, Field ME, Selwyn A, Raymond JM, Koplan B, Epstein LM, Stevenson WG. Transcoronary ethanol ventricular tachycardia ablation in the modern electrophysiology era. Heart Rhythm. 2008;5:62–68. doi: 10.1016/j.hrthm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Imai K, Sueda T, Orihashi K, Watari M, Matsuura Y. Clinical analysis of results of a simple left atrial procedure for chronic atrial fibrillation. Ann Thorac Surg. 2001;71:577–581. doi: 10.1016/s0003-4975(00)02254-2. [DOI] [PubMed] [Google Scholar]
- 24.Sueda T, Imai K, Orihashi K, Shikata H, Wada S, Hirai S, Morita S, Matsuura Y. Efficacy of a left atrial procedure for treatment of chronic atrial fibrillation in elderly patients. Ann Thorac Cardiovasc Surg. 1998;4:201–204. [PubMed] [Google Scholar]
- 25.Katritsis D, Giazitzoglou E, Sougiannis D, Goumas N, Paxinos G, Camm AJ. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol. 2008;102:330–334. doi: 10.1016/j.amjcard.2008.03.062. [DOI] [PubMed] [Google Scholar]