Abstract
There is a need to investigate exactly how memory breaks down in the course of Alzheimer's disease (AD). Examining what aspects of memorial processing remain relatively intact early in the disease process will allow us to develop behavioral interventions and possible drug therapies focused on these intact processes. Several recent studies have worked to understand the processes of recollection and familiarity in patients with mild cognitive impairment (MCI) and very mild AD. Although there is general agreement that these patient groups are relatively unable to use recollection to support veridical recognition decisions, there has been some question as to how well these patients can use familiarity. The current study used receiver operating characteristic (ROC) curves and a levels of processing manipulation to understand the effect of MCI and AD on the estimates of recollection and familiarity. Results showed that patients with MCI and AD were impaired in both recollection and familiarity, regardless of the depth of encoding. These results are discussed in relation to disease pathology and in the context of recent conflicting evidence as to whether familiarity remains intact in patients with MCI. The authors highlight differences in stimuli type and task difficulty as possibly modulating the ability of these patients to successfully use familiarity in support of memorial decisions.
Keywords: Alzheimer's disease, recognition memory, familiarity, recollection, receiver operating characteristics, ROC, dual process
Introduction
Dual-process models of recognition memory theorize that accurate recognition decisions rely on two independent neural processes: recollection and familiarity (Jacoby & Dallas, 1981; Mandler, 1980; Yonelinas, 1994). Recollection refers to the retrieval of specific context-bound information about an item or event, while familiarity is defined as a more general, acontextual sense that an item or event has been previously encountered. These two constructs are often vividly experienced in daily life. For example, the unexpected sight of a particular man on a crowded city street may elicit an immediate feeling of knowing him without being able to produce any specific details about who he is or how he is known. After a moment of thought, these details may come into mind and the man’s identity – say, the waiter at a restaurant you had visited one week earlier – becomes apparent. Familiarity describes the initial feeling of knowing the man without being able to place him, while recollection captures the subsequent remembering of the specific details of his identity.
Several behavioral paradigms have been devised to empirically quantify familiarity and recollection for individual recognition decisions in the laboratory (for review see Yonelinas, 2002). These process-estimation methods include process-dissociation (Jacoby, 1991), remember/know (Tulving, 1985), and confidence-based ROC procedures (Yonelinas, 1994) – the latter being the focus of the current investigation. In a prototypical recognition memory experiment, the participant is exposed to a series of items during a “study” phase. These items are then re-presented along with some number of novel items during a “test” phase. The participant must indicate at test whether each item is “old” (previously studied) or “new” (not previously studied). In the confidence-based ROC paradigm, this binary old/new decision is expanded to reflect how confident the participant is that each test item has or has not been previously encountered. For each test item, the participant provides a response ranging from certainty that the item was previously studied (i.e., “certain the item is old”) to certainty that the item was not previously studied (i.e., “certain the item is new”) with several intermediate options (e.g., “sort of certain the item is old”, “not at all certain the item is old”, “not at all certain the item is new”, “sort of certain the item is new”).
Analysis using ROC curves has been used since the 1950s to describe recognition memory decisions (e.g., Egan, 1958), and Yonelinas (1994) devised a dual-process model of confidence-based ROC data that could estimate the separate contributions of recollection and familiarity. The Yonelinas high threshold model assumes that recognition memory decisions are made based on either recollection or familiarity (Yonelinas, 1994). In recent years, these ROC analyses have been used to estimate recollection and familiarity in healthy older adults (Howard et al., 2006; Prull et al., 2006), individuals with thalamic lesions (Kishiyama et al., 2005), and individuals with selective hippocampal or more diffuse medial temporal lobe lesions (Aggleton et al., 2005; Cipolotti et al., 2006; Wais et al., 2006; Yonelinas et al., 1998; Yonelinas et al., 2002). These investigations, in addition to numerous functional neuroimaging studies, have provided an understanding of the neuroanatomical basis of recognition memory decisions. Though far from settled, research has argued that the hippocampus (Cansino et al., 2002; Dobbins et al., 2003; Eldridge et al., 2000; Yonelinas et al., 2005), prefrontal regions (Burgess & Shallice, 1996; Dobbins et al., 2002; Simons et al., 2005), and parietal regions (Skinner & Fernandes, 2007; Wagner et al., 2005) are critical to recollection, whereas more anterior medial temporal and parahippocampal regions are critical to familiarity (Brown & Xiang, 1998; Cansino et al., 2002; Eichenbaum et al., 2007; Henson et al., 2003).
Understanding the neural and cognitive correlates of recollection and familiarity is critically important in determining the nature of memory impairment in clinical populations (Aggleton et al., 2005; Cipolotti et al., 2006; Wais et al., 2006; Yonelinas et al., 1998; Yonelinas et al., 2002). Along with the understanding of the nature of memory impairment of AD, we hope that the current study can help to elucidate how memory breaks down in the earliest stages of the disease. This understanding may in turn allow new drug therapies and early behavioral interventions to be developed. The processes of recollection and familiarity have only recently begun to be systematically investigated in patients with Mild Cognitive Impairment (MCI) and Alzheimer's disease (AD). Two such studies have examined recollection and familiarity in patients with MCI (Westerberg et al., 2006; Wolk, Signoff, & DeKosky, 2008), but neither study used ROC procedures that have proven particularly informative in other clinical populations. Based largely on the methodology of Yonelinas et al. (1998), the goal of the present study is to use the Yonelinas high threshold model to estimate recollection and familiarity for word stimuli in healthy older adults, patients with MCI-amnestic type (a-MCI), and patients with mild AD.
Evidence in healthy older adults using ROC and other process estimation methods has suggested that compared to young adults, recollection is differentially impaired for certain groups of healthy older adults (Cabeza et al., 2002; Davidson & Glisky, 2002; Duarte et al., 2004) or for certain types of stimuli (Ally et al., 2008), while familiarity generally is spared (Daselaar et al., 2006; Howard et al., 2006; Jacoby, 1999; Jennings & Jacoby, 1993, 1997; Rybash & Hoyer, 1996; Spencer & Raz, 1995; Titov & Knight, 1997; Yonelinas, 2001). It has been suggested that a decline in the attentional resources allocated at encoding and retrieval, perhaps due to frontal lobe changes associated with normal aging, may be responsible for a decrease in recollection in this group (Anderson, Craik, & Naveh-Benjamin, 1998; Buckner, 2004; Park et al., 1989; Salthouse, 1994; Whiting & Smith, 1997).
In addition to the cognitive changes that may occur with normal aging, Alzheimer’s disease damages key brain structures involved in language, executive functioning, and memory. The earliest and most prominent of these cognitive abilities to be affected is episodic memory. Studies have shown that when memory loss is clinically apparent, significant AD pathology is evident in medial temporal structures including perirhinal cortex, entorhinal regions, hippocampus, amygdala, and nucleus basalis (Arriagada et al., 1992; Braak and Braak, 1991; Gomez-Isla et al., 1996; Mesulam, 2000; Van Hoesen et al., 1991). Many researchers and clinicians believe that MCI may be the transitional state between normal aging and mild AD (Bell-McGinty et al., 2005; Petersen et al., 2001), and note that the amnestic variant of MCI has the highest rate of conversion to AD (Petersen, 2004). Patients with a-MCI have significant memory loss for their age, but do not have impaired activities of daily living needed to meet the clinical diagnosis of AD (Petersen, 2004; Petersen et al., 2001). Neuropathology and structural imaging studies lend support to the supposition that MCI may be the earliest stage of AD, showing a significant link between structures affected by the two groups (Grundman et al., 2004; Killiany et al., 2002; McKee et al., 2006; Micthell et al., 2002; Petersen, 2004). By the time memory loss is clinically evident, warranting a diagnosis of MCI, significant AD neurofibrillary pathology is seen in limbic regions, including transentorhinal regions, perirhinal cortex, amygdala, nucleus basalis (Arriagada et al., 1992; Braak & Braak, 1991; Mesulam, 2000; Van Hoesen et al., 1991), and most prominently in hippocampus and entorhinal cortex (Gomex-Isla et al., 1996). These regions continue to be affected as AD progresses (Mesulam, 1999), with pathology spreading to neocortical areas such as temporal, parietal, occipital association, and frontal cortex in clinical AD (Braak & Braak, 1991; Delacourte et al., 1999; Grady et al, 1988; Ibanez et al., 1998, McKee et al. 2006).
Numerous studies have reported impaired recollection in patients with AD (Budson et al., 2000; Christensen et al., 1998; Dalla Barba, 1997; Gallo et al., 2004; Knight, 1998; Koivisto et al., 1998; Smith and Knight, 2002). In fact, recollection appears to be severely impaired even in the earliest stages of the disease, resulting in an increased reliance on familiarity-based memory (Balota et al., 2002; Budson et al., 2000; Lekeu et al., 2003; Wolk et al., 2005). Although patients with AD may be more reliant on familiarity (Budson et al., 2000, Smith & Knight, 2002), it remains unclear whether this type of memory is impaired in MCI or mild AD (see Westerberg et al., 2006 and Wolk, Signoff, & DeKosky, 2008). Given the early pathological changes to areas critical to the processes of recognition in patients with MCI and AD, we would expect both groups to be impaired in recollection and familiarity compared to healthy older adults on a standard old/new recognition test.
The goal of the current study is to use a depth of encoding manipulation and ROC procedures similar to Yonelinas et al. (1998) to investigate how MCI and mild AD affect the memorial processes of recollection and familiarity. A possible concern using ROC methodology in patients with MCI or AD may be the ability of these patients to assess confidence for memory decisions. However, research investigating the ability to retrieve and monitor stored general knowledge in patients with AD has shown that these patients can successfully make confidence ratings regarding the certainty of their answers (Bäckman & Lipinska, 1993). Given evidence that AD pathology affects brain structures critical to both recollection and familiarity in MCI and the earliest stages of AD (Csernansky et al., 2004; Gomez-Isla et al., 1996; Jack et al., 2004; Kantarci et al., 2005; Karas et al., 2004), we hypothesized that both patient groups would show impairment in recollection and familiarity compared to healthy older adults.
Methods
Participants
Ten patients with a diagnosis of probable AD as determined by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria (NINCDS-ADRDA; McKhann, Drachman, Folstein, Katzman, & Price, 1984), 11 patients with a diagnosis of probable MCI (Petersen, 2004), and 12 controls were recruited for this experiment. Patients with MCI in the current study reported a subjective memory compliant, showed abnormal memory performance for their age as evidenced by performing greater than 1.5 standard deviations below the healthy adult group on either the recall or the recognition portion of the word list memory test of the CERAD, and they did not display functional impairment according to caregiver report. All MCI subjects met criteria for single or multiple domain amnestic-type MCI (Petersen, 2004). The participants with AD and MCI were recruited from the clinical populations of the Memory Disorders Unit, Brigham and Women’s Hospital, and the Boston University Alzheimer’s Disease Center, both in Boston, Massachusetts. Participants were excluded if they were characterized by having clinically significant depression, alcohol or drug use, cerebrovascular disease, traumatic brain damage, or if English was not their primary language. Patients with AD were also excluded if their Mini Mental State Examination (Folstein, Folstein, & McHugh, 1975) score fell below 21. Healthy older adults were also excluded if they had a first-degree relative with a history of AD, another neurodegenerative disorder, or dementia.
Each participant completed a brief neuropsychological battery in a 45-minute session either directly following the experimental session or on a separate date. This battery included the Mini Mental State Examination, the CERAD word list memory test (Morris et al., 1989), Trail Making Test Part B (Adjutant General’s Office, 1944), Verbal Fluency (Monsch et al., 1992), the 15-item Boston Naming Test (Mack, Freed, Williams, & Henderson, 1992), Symbol Cancellation (Goodglass & Kaplan, 1983), and the Hooper Visual Organization Test (Hooper, 1958). Table 1 presents demographic and neuropsychological data for the three groups. Analyses of variance revealed no significant differences in age or years of education among the AD, MCI, and healthy older adult groups. The human subjects committees of Brigham and Women’s Hospital and the Edith Nourse Rogers Memorial Veterans Hospital approved this study. Written informed consents were obtained from all participants and from their caregivers where appropriate, and participants were modestly compensated for their time.
Table 1.
Demographic and Neuropsychological Data for all Subjects
| Older Adults | aMCI | AD | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Years of age | 77.3 | 5.8 | 76.5 | 7.8 | 77.1 | 4.7 |
| Years of education | 15.8 | 3.5 | 16.0 | 3.1 | 16.6 | 4.1 |
| MMSE | 29.4 | 0.8 | 27.7* | 2.2 | 24.9** | 1.7 |
| CERAD | ||||||
| Immediate recall | 21.2 | 3.9 | 14.3** | 4.1 | 11.1** | 3.4 |
| Delayed recall | 7.5 | 1.5 | 3.1** | 1.9 | 0.6** | 1.1 |
| Recognition | 9.8 | 0.5 | 8.0** | 1.7 | 3.7** | 2.7 |
| Letter fluency | 48.3 | 13.4 | 33.5** | 7.9 | 37.3 | 16.8 |
| Category fluency | 50.0 | 8.4 | 32.5** | 10.5 | 27.3** | 7.7 |
| Trails-B (sec.) | 83.3 | 18.9 | 146.8* | 64.5 | 207.0** | 81.3 |
| Symbol cancellation (sec.) | 109.2 | 35.6 | 139.3 | 45.4 | 212.9** | 102.5 |
| Boston naming test | ||||||
| Immediate | 14.3 | 1.2 | 13.3 | 1.3 | 13.6 | 2.5 |
| Semantic cue | 0.1 | 0.3 | 0.3 | 0.6 | 0.1 | 0.3 |
| Phonemic cue | 0.2 | 0.4 | 1.1* | 1.3 | 0.4 | 0.7 |
| Hooper visual organization test | 24.4 | 3.9 | 21.1 | 3.8 | 17.9** | 7.0 |
Note: Significant differences from the control group are indicated by * < .05, and ** < .01
Stimuli
The stimuli were 640 common words chosen from a larger set of words used in Budson, Wolk, Chong, and Waring (2006) and originally selected from the University of Western Australia MRC Psycholinguistic Database (http://www.psy.uwa.edu.au/MRCDataBase/uwamrc.htm). The stimuli were randomly divided into eight lists of 80 words. The presentation of these lists was counterbalanced across participants in each group such that each list was used equally often in the deep and shallow encoding depths, or as unstudied items.
Design and Procedure
Each participant was tested individually in a single session. Stimuli were presented on a Dell Inspiron 640m laptop computer via E-Prime software (Psychology Software Tools Inc.; www.pstnet.com/eprime). The procedure consisted of two blocks each composed of a study phase and a test phase.
Prior to the start of the first block, participants were instructed that they would be studying lists of words for a subsequent recognition memory test. In the study phase of the first block, participants studied 80 words under deep-encoding conditions by a like/dislike judgment for each item. Here subjects were asked whether they liked or disliked the real-world exemplar of the study word. The responses were participant-paced and were recorded by the experimenter. Following the first study list, participants studied a second list of 80 words under shallow-encoding conditions. Participants mentally counted the number of syllables in each word and responded “more” if the word contained three or more syllables, or “less” if the word contained one or two syllables. Responses were participant-paced and were recorded by the experimenter. All participants understood and were able to appropriately complete both the deep and shallow encoding tasks.
Prior to the start of the test phase of the first block, participants were instructed that all of the studied words plus an equal number of new words would appear, and that each word would appear only once. Participants received instruction in providing one of six responses to each item: (6) certain that the word is old; (5) sort of certain that the word is old; (4) not at all certain that the word is old; (3) not at all certain that the word is new; (2) sort of the certain that the word is new; (1) certain that the word is new. Participants were instructed to be as accurate as possible in their responses, but also to spread out their answers among all six of the confidence intervals if possible (an instruction deemed necessary by Yonelinas et al. [1998] to prevent bimodal “old-new” responding by impaired participants). Participants were reminded of these guidelines after their first few responses and the six possible choices were listed on every slide during the test phase. In the test phase, participants orally provided one of the six responses for each of 320 randomly presented words: 80 that had been studied in the deep encoding condition, 80 that had been studied in the shallow encoding condition, and 160 that had not been studied. The test phase was participant-paced and the experimenter recorded the responses.
The second block was similar to the first block, except that the order of the two encoding tasks in the study was reversed such that participants studied a list of words using the number of syllables (shallow encoding) task first, then a list of words using the like-dislike (deep encoding) task. None of the words from the first block reappeared in the second block.
Results
It is important to note that the Yonelinas high threshold model is just one of many models to describe ROC curves, and may not provide the best fit for some recognition memory data (for review see Parks & Yonelinas, 2007; Wixted, 2007; Yonelinas & Parks, 2007). Other models, such as unequal variance signal detection theory models (UVSDT; Egan, 1975; Ratcliff, Sheu, & Gronlund, 1992; Wixted, 2007), the sum-difference theory of remembering and knowing (Rotello, Macmillan, & Reeder, 2004), and mixture models (DeCarlo, 2002; DeCarlo, 2003), may be as viable as the Yonelinas high threshold model in terms of explaining item recognition ROCs. However, the Yonelinas model is the only model of ROC data that directly provides estimates of recollection and familiarity. Since we view the current experiment primarily as an opportunity to characterize the impairments in recognition memory in patients with MCI and AD in terms of recollection and familiarity, the Yonelinas model will be the focus of our analyses.
Yonelinas High Threshold Model Analyses
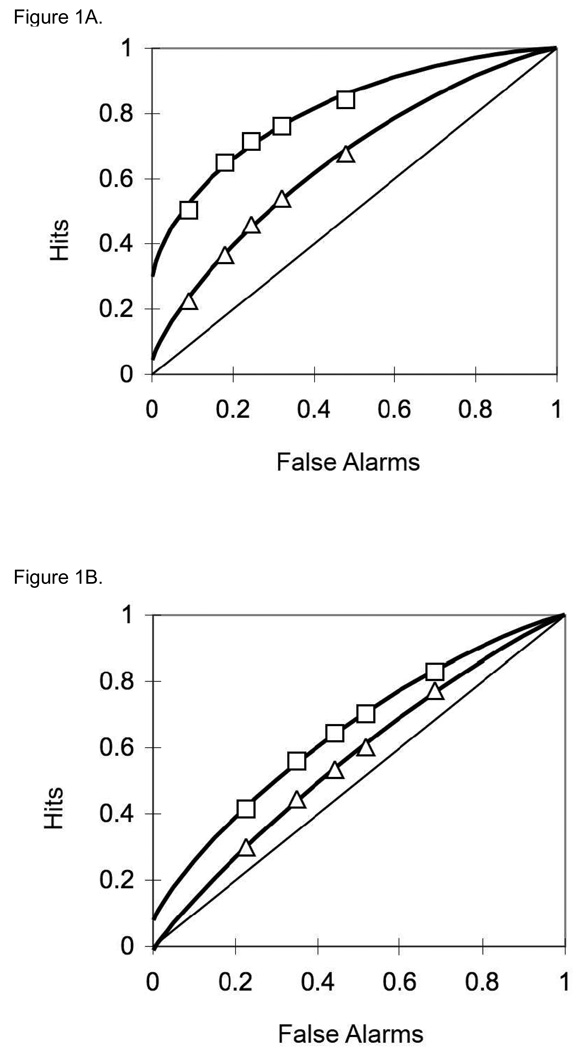
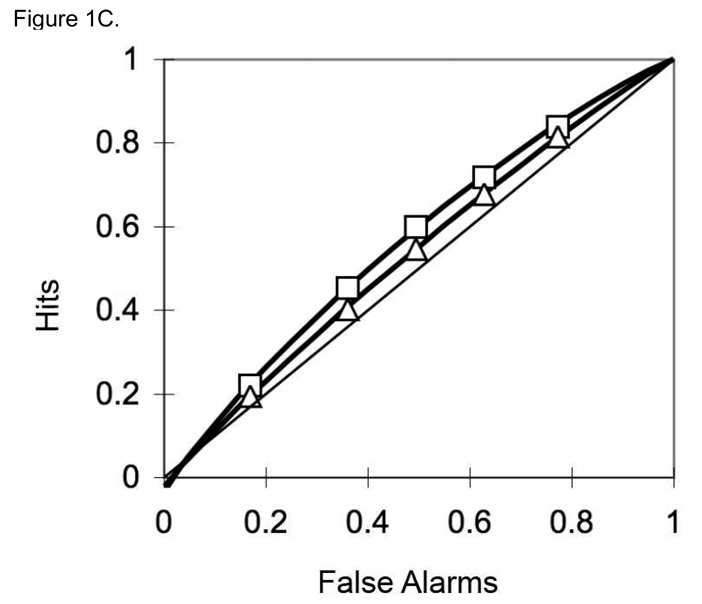
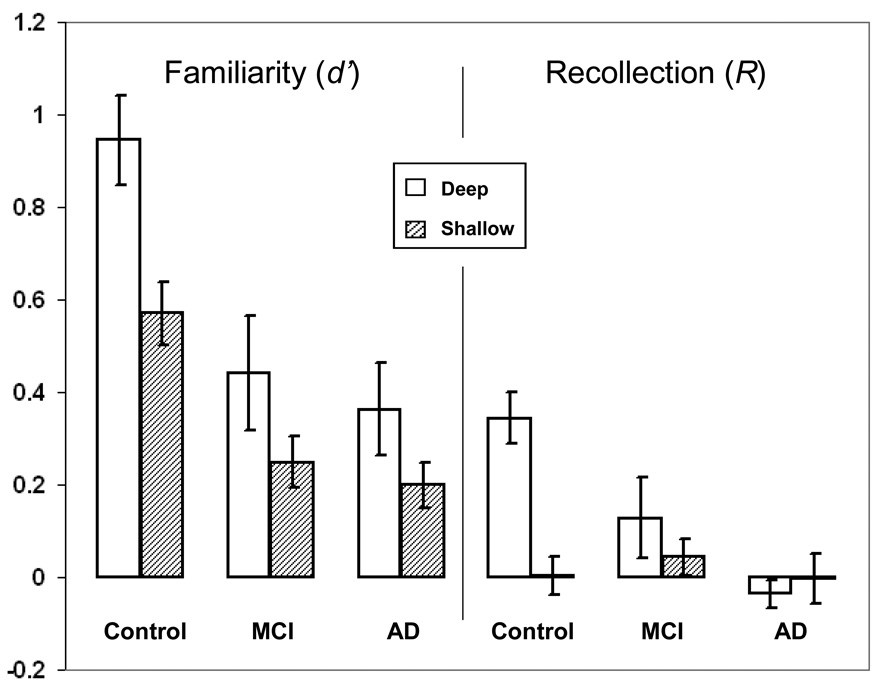
Confidence-based ROC curves were generated for the two encoding conditions for each participant using the standard Yonelinas high threshold methodology (Yonelinas, 1994; Yonelinas et al., 1998). Responses of 6, 5, 4, 3, and 2 to unstudied items were used to calculate false alarm rates. Responses of 6, 5, 4, 3, and 2 to previously studied items were used to form hit rates for deep and shallow encoded items. These false alarm and hit rates were plotted as five (x, y) coordinates in an additive fashion: (false alarm rates for responses of 6, hit rate for responses of 6); (false alarm rate for responses of 6 and 5, hit rate for responses of 6 and 5); (false alarm rate for responses of 6 and 5 and 4, hit rate for responses of 6 and 5 and 4); and so on. A Microsoft Excel solver routine designed by Yonelinas (available at http://psychology.ucdavis.edu/labs/Yonelinas) generated the ROC curves for each participant. Figure 1 shows the aggregate ROC curves for (A) controls, (B) MCI patients, and (C) AD patients. For each participant, the Yonelinas Microsoft Excel solver routine was then used to generate Yonelinas high threshold model-based recollection (R) and familiarity (d’) estimates for deep and shallow encoding conditions. Figure 2 presents the means of the individual R and d’ values by group and encoding depth. Error bars represent the standard error of the mean. It is important to note that both the MCI and mild AD group demonstrated nearly equal responding using all 6 response types. Table 2 shows the proportion of response for each group.
Figure 1.
Aggregate ROC curves for (1A) controls, (1B) MCI patients, and (1C) AD patients. The curves with rectangular points represent the ROCs for deeply encoded items while the lines with triangular points represent the ROCs for shallowly encoded items.
Figure 2.
YHT model-generated estimates of familiarity (d’) and recollection (R) for deeply and shallowly encoded items for controls, MCI patients, and AD patients. Error bars represent the standard error of the mean.
Table 2.
Proportions of response types for the deep and shallow encoding conditions.
| Response | Deep | Shallow | |||||
|---|---|---|---|---|---|---|---|
| Older Adults | aMCI | AD | Older Adults | aMCI | AD | ||
| 1 | 15 | 17 | 16 | 33 | 23 | 18 | |
| 2 | 8 | 13 | 12 | 14 | 17 | 14 | |
| 3 | 5 | 6 | 12 | 8 | 7 | 13 | |
| 4 | 7 | 8 | 15 | 9 | 9 | 14 | |
| 5 | 15 | 14 | 23 | 14 | 15 | 21 | |
| 6 | 50 | 42 | 22 | 22 | 30 | 20 | |
Note: Response types are as follows: (1) certain that the word is new; (2) sort of the certain that the word is new; (3) not at all certain that the word is new; (4) not at all certain that the word is old; (5) sort of certain that the word is old; (6) certain that the word is old.
To analyze the recollection parameter (R), a Group (control, MCI, AD) by encoding depth (deep, shallow) repeated measures ANOVA was performed. The ANOVA revealed a significant effect of group [F(2, 30) = 5.33, p = .010], which demonstrated a greater estimate of recollection for the control group compared to the MCI group [t(21) = 2.139, p = .044] and the AD group [t(20) = 5.721, p < .001]. However, the MCI and AD comparison revealed no significant differences between the two groups [t(19) = 1.710, p = .103]. The ANOVA also revealed a significant effect of encoding depth [F(1, 30) = 10.18, p = .003], and a significant interaction of group and encoding depth [F(2, 30) = 7.34, p = .003]. Post-hoc t-tests revealed that items in the deep encoding condition resulted in greater estimates of recollection than items in the shallow encoding condition for the healthy older adult group [t(11) = 4.834, p = .001], but not for the MCI group [t(10) = 1.149, p = .277] or the AD group [t(9) > 1].
To analyze the familiarity parameter (d’), a Group (control, MCI, AD) by encoding depth (deep, shallow) repeated measures ANOVA was performed. The ANOVA revealed a significant effect of group [F(2, 30) = 14.61, p < .001], which demonstrated a greater estimate of familiarity for the older adult control group compared to the MCI group [t(21) = 4.033, p = .001] and the AD group [t(20) = 5.347, p < .001]. However, the MCI and AD comparison revealed no significant differences between the two groups [t(19) = .645, p = .527]. The ANOVA also revealed a significant effect of encoding depth [F(1, 30) = 15.40, p < .001], but no interaction of group and encoding depth [F(2, 30) = 1.18, p = .323]. Follow-up t-tests revealed that the estimate of familiarity was greater in the deep condition than in the shallow condition for the healthy older adult group [t(11) = 3.612, p = .004], but not for the MCI [t(10) = 1.743, p = .112] or AD group [t(9) = 1.528, p = .161].
Using the recollection and familiarity parameters, we then set out to determine the degree of impairment in familiarity compared with recollection between the groups. In a method similar to Wolk, Signoff, and DeKosky (2008), z-scores were calculated for the MCI and AD groups referenced to the control mean and standard deviation (Nunally & Bernstein, 1994; Yonelinas, 2002). Control-references z-scores for the MCI group are presented in Table 3. A repeated-measures ANOVA with the factors of group (OC, MCI, AD), parameter (recollection, familiarity), and encoding depth (deep, shallow) revealed main effects of encoding depth [F(1, 30) = 32.75, p < .001] (attributable to overall memory being stronger in the deep versus shallow condition) and parameter [F(1, 30) = 5.15, p = .031] (attributable to overall memory being stronger with the recollection versus familiarity parameter). The ANOVA also revealed a significant interactions of encoding depth and group [F(2, 30) = 8.99, p = .001] encoding depth and parameter [F(1, 30) = 6.83, p = .014]. These interactions can best be explained by follow-up paired sample t-tests that revealed that familiarity was more impaired than recollection in the shallow encoding condition for the MCI [t(10) = 3.47, p = .002] and AD [t(9) = 2.71, p = .024] groups, but not in the deep encoding condition: MCI [t(10) = 0.52, p = .626], AD [t(9) = .60, p = .559].
Table 3.
Control-referenced z-scores for the MCI and AD groups. d’ represents the YHT model of familiarity and R represents the YHT model of recollection.
| MCI | AD | |
|---|---|---|
| Shallow d’ | −1.37 | −1.58 |
| Deep d’ | −1.51 | −1.74 |
| Total d’ | −1.81 | −2.10 |
| Shallow R | 0.28 | −0.05 |
| Deep R | −1.13 | −1.99 |
| Total R | −0.77 | −1.69 |
Accuracy
In addition to using the Yonelinas high threshold model to generate recollection and familiarity parameters, we analyzed accuracy between all groups. To perform this analysis, response types were dichotomized such that responses 4, 5, and 6 were classified as “old” responses and responses 1, 2, and 3 were classified as new responses. Group hit and false alarm data can be seen in Table 4.
Table 4.
Hit and false alarm rates.
| Group | ||||||
|---|---|---|---|---|---|---|
| Condition | Older Adults | aMCI | AD | |||
| Hits | FA | Hits | FA | Hits | FA | |
| Shallow | .46 | .24 | .53 | .44 | .50 | .44 |
| Deep | .72 | .24 | .64 | .44 | .56 | .44 |
A repeated measures ANOVA was performed to analyze hit rates with the factors of Group (older adults, MCI, AD) and Condition (deep, shallow). The ANOVA revealed an effect of Condition [F(1, 30) = 52.28, p < .001], and an interaction of Group and Condition [F(2, 30) = 9.99, p < .001]. Post-hoc t-tests revealed a greater number of hits for the deep encoding condition versus the shallow for the healthy older adults [t(11) = 7.59, p < .001] and the patients with MCI [t(10) = 3.46, p = .006], but not for the patients with AD [t(9) = 1.61, p = .154]. ANOVA was then used to analyze false alarm rates using the factor of Group (older adults, MCI, AD). (Note: Given that there was only one test phase for each subject, false alarm rates were the same for the deep and shallow encoding conditions.) The ANOVA revealed an effect of Group on false alarm rate [F(2, 30) = 3.72, p = .036]. Post-hoc t-tests showed that the older adult group had fewer false alarms that the MCI group [t(21) = 2.56, p = .019] and the AD group [t(20) = 2.63, p = .016, but the two patient groups did not differ in false alarm rates [t(19) = 0.01, p = .993].
A univariate ANOVA was then performed on the accuracy measure Pr (% hits - % false alarms, Snodgrass & Corwin, 1988) to compare groups. An effect of Group [F(2, 30) = 47.74, p < .001] revealed that the healthy older adults performed better than the MCI [t(21) = 7.15, p < .001] and AD [t(20) = 9.74, p < .001] groups, and that there was a trend towards the MCI group performing better than the AD group [t(19) = 1.98, p = .063].
Discussion
The current investigation set out to understand the effect of MCI and mild AD on the memorial processes of recollection and familiarity using a depth of processing manipulation and confidence-based ROC analysis. Because AD pathology affects brain structures thought to be critical to both recollection and familiarity, even in the earliest stages of the disease, we hypothesized that compared to older adults, patients with MCI and AD would demonstrate impairment in both recollection and familiarity. The results of the current study confirmed this hypothesis; patients with MCI and mild AD demonstrated significantly diminished estimates of both processes compared to healthy older adults. Interestingly, we also found that recollection and familiarity appear to be modulated by depth of encoding. This is particularly evident in the patient populations, where familiarity appeared to be more impaired when items were encoded in the shallow condition compared to when items were encoded in the deep condition.
Our data revealed that the healthy older adult group showed moderate evidence of recollection for items in the deep encoding condition. The recollection parameter (R) for the deep encoding condition was just over 0.3 and the ROC curve was asymmetric. Under the assumptions of the Yonelinas model, any recollection-based responses are assumed to support decisions made with high confidence, which distort the shape of the ROC curve (making it asymmetrical). By contrast, the older adults showed no evidence of recollection in the shallow encoding condition. In this condition, the recollection parameter was near 0, and the ROC curve was rather symmetrical. It should be emphasized here that the basis of the current investigation was to characterize estimates of recollection and familiarity in patients with MCI and mild AD compared to healthy age-matched peers. Understanding the effect of aging was not the goal of the current study, and no young adult controls were used as comparison for the healthy older adult group. Therefore, we are notably cautious in characterizing estimates of recollection and familiarity as impaired or intact in healthy older adults. Nonetheless, these results are generally consistent with previous literature. Studies focusing on healthy aging suggest that while familiarity remains intact, recollection can vary based on individual differences or by stimulus type (Ally et al., 2008; Cabeza et al., 2002; Davidson & Glisky, 2002; Duarte et al., 2004). Although it is unclear whether the older adults in the current study would have shown impaired recollection compared to younger adults, perhaps the deep encoding task allowed the older adults to use recollection in some cases to support their memorial decisions.
In contrast, the MCI group showed no evidence of recollection for either encoding condition. The ROC curves were very symmetrical for both conditions. The recollection parameter (R) was significantly impaired for the deep condition compared to the healthy older adults. There was no difference in these two groups for the shallow encoding task--neither healthy older adults nor patients with MCI showed evidence of recollection in the shallow encoding task. These results were not surprising given previous findings showing significantly impaired episodic memory performance in patients with MCI (Perri et al., 2005; Petersen et al, 1999; Westerberg et al., 2006). However, despite being generally accepted that recollection is impaired in MCI, there has been some recent debate as to whether familiarity remains intact for this group (Bayley et al., 2008; Westerberg et al., 2006; Wolk et al., 2008). The results of the current study suggest that familiarity is also impaired in this patient group. The familiarity parameter (d’) was significantly diminished compared to the healthy older adults. These results are generally consistent with neuroanatomical and neuroimaging data. Research has shown that AD pathology is evident very early in the course of MCI in anterior medial temporal cortex, which is thought to be critical to familiarity, (Gomez-Isla et al., 1996; Guillozet et al., 2003; Mesulam, 2000; Mitchell et al., 2002).
The results of the present investigation are also consistent with a recent study by Wolk, Signoff, and DeKosky (2008). In a well controlled study of patients with amnestic-type MCI, Wolk et al. used three process-estimation techniques to show that familiarity was at least as impaired as recollection in this patient group. Because previous research has shown recollection tends to be impaired in healthy older adults, but familiarity remains relatively spared, Wolk et al. proposed that impairment in familiarity may reflect early tangle pathology in the perirhinal and entorhinal regions, thereby being a specific marker for early pathological changes of AD leading to amnestic-type MCI. The findings of the current study lend some support to this hypothesis that impaired familiarity separates patients with MCI from healthy older adults. We, like Wolk et al. (2008), found that familiarity was at least as impaired as recollection in patients with MCI. These differences between familiarity and recollection were highly notable in the shallow encoding condition where familiarity appeared to be more impaired than recollection. These results suggest that the relative balance of recollection and familiarity may be modulated by factors such as depth of encoding or task difficulty. Viewed another way, our data show that deeper encoding actually enhances familiarity more so than recollection in patients with MCI. Although our data and statistics support this supposition, we must remain cautious, as the older adults and MCI curves show a restricted range near zero for recollection. Further, we acknowledge that recollection and familiarity are measured in different ways and may not be directly comparable. However, the general premise of this hypothesis is consistent with Wolk et al. (2008), who found that familiarity was enhanced in patients with MCI when items were repeated three times compared to when items were seen only once.
The results of the current study are somewhat divergent from an earlier study by Westerberg et al. (2006), who suggested that familiarity remains intact in patients with MCI. Westerberg et al. (2006) administered two separate recognition memory tests to groups of healthy older adults, patients with MCI, and patients with mild AD. The first test required subjects to make standard old/new recognition memory decisions, while the second test required subjects to make forced-choice recognition decisions in which the target was grouped with highly related foils. Based on earlier evidence (Bastin & Van der Linden, 2003; Gardiner, Java, & Richardson-Klavehn, 1998), it has been suggested that standard recognition memory tests rely more on the process of recollection, whereas forced-choice tests rely more on familiarity. Results of the Westerberg et al. (2006) showed that patients with MCI and mild AD performed significantly worse on the standard old/new test compared to the healthy older adults, but performance on the forced-choice test was indistinguishable for the MCI group and the healthy older adults. The authors concluded that familiarity remains intact for these patients, and that it could successfully be used in even difficult tasks such as recognizing targets from highly similar foils.
One possible explanation for the difference in results observed between Westerberg et al. (2006) and the current study may be due to stimuli type. Westerberg and colleagues used black and white pictures of objects, whereas the current study used words. Ally and Budson (2007) demonstrated differences in the neural correlates of recognition memory for pictures versus words, and Ally et al. (2008) recently reported that in older adults, pictures enhanced the neural correlate of both recollection and familiarity relative to words. Thus, it may be that familiarity is differentially affected for pictures versus words in patients with MCI. Ally, Gold, and Budson (revision submitted) demonstrated that the picture superiority effect remained intact in patients with MCI and mild AD, and that despite possible changes in visual cognition to these patients, the relative benefit of studying pictures versus words is similar to healthy controls. If recollection is impaired for pictures (Westerberg et al., 2006) and words (Wolk et al., 2008) in patients with MCI, but the picture superiority effect remains intact, we suspect that patients are likely relying on successful use of familiarity for the recognition of pictures. Perhaps future investigations using event-related potentials (ERPs) or functional magnetic resonance imaging (fMRI) can help to determine dissociations of the neural correlates of picture versus word recognition in patients with MCI.
Another explanation for the differences between our results and those of Westerberg et al. (2006) may be related to how familiarity is used in different recognition memory decisions. As pointed out by Westerberg and colleagues, familiarity can lead to a correct response in the forced-choice format because an individual is able to directly measure familiarity strength for the target and simultaneously presented lures. In this situation, presumably a subject with impaired recollection endorses the item that engenders the greatest sense of familiarity. However, on a standard old/new recognition test, subjects are presented with targets and lures separately, and there may be overlap of target and lure perceptual characteristics. Therefore, lures with high perceptual overlap may produce a strong familiarity signal, possibly outweighing a target stimulus with low familiarity (Westerberg et al., 2006). With impaired recollection and no other items to measure a sense of familiarity against, patients are forced to respond old or new based on familiarity of a single item on a standard recognition test.
Electrophysiological studies have provided evidence that responding based on familiarity requires increased post-retrieval processing (Ally & Budson, 2007; Wolk et al., 2004; Wolk et al., 2005). This post-retrieval processing often involves the executive monitoring and evaluation of the product of a retrieval attempt by the frontal lobes (Allan et al., 1998; Goldmann et al., 2003; Wilding & Rugg, 1996). Indeed, the frontal lobes have been implicated in response inhibition (Shimamura, 1995), which is critical to the suppression of responding based on familiarity alone (Budson et al., 2002). Numerous investigations have also found that the frontal lobes are involved in distinguishing between identical versus highly similar and familiar items, and thus are important in avoiding false recognition (Budson et al., 2002; Delbecq-Derouesne, Beauvois, & Shallice, 1990; Goldmann et al., 2003; Henson, Shallice, & Dolan, 1999; Melo, Winocur, & Moscovitch, 1999; Parkin et al., 1996; Parkin, Ward et al., 1999; Rapcsak et al., 1998; Rapcsak et al., 2001; Schacter et al., 1996). MacPherson et al. (2008) reported that estimates of familiarity, but not recollection, are impaired in patients with frontal lobe lesions, and argued that a reduction in familiarity estimates in this group may reflect difficulty distinguishing between target and distractor items when they have a high degree of similarity. It is possible that the MCI patients in the current study, some of whom demonstrated executive difficulties as well as memory impairment, demonstrated decreased familiarity estimates due to frontal lobe pathology. Given the findings of Westerberg et al. (2006), it is possible that the basic process of familiarity remains intact, but patients with impaired executive retrieval monitoring strategies cannot appropriately use or asses the strength of familiarity.
Similar to the MCI group, the mild AD group in the present study showed no evidence of recollection in either the shallow or deep encoding conditions. The ROC curves were symmetrical and almost flat, and the Yonelinas high threshold recollection parameters were at 0 for both conditions, and significantly diminished compared to the healthy older adults in the deep encoding condition. Estimates of familiarity were similar for the mild AD and MCI groups in both the deep and shallow conditions. Impaired recollection in patients with AD has been well documented using a wide range of behavioral tasks (Budson et al., 2000; Dalla Barba, 1997; Gallo et al., 2004; Knight, 1998; Petersen, 2004; Smith & Knight, 2002). Additional research focusing on memorial processing has shown that patients with AD become reliant on familiarity in the face of impaired recollection; in fact, patients with AD are often overly dependent on this type of memory (Budson et al., 2000; Gallo et al., 2006; Gold et al., 2007; Wolk et al., 2005). The results of the current study showed that in patients with mild AD, the process of familiarity was also impaired. The familiarity parameter (d’) generated by the Yonelinas high threshold model was significantly diminished for the AD group compared to the healthy older adults in both encoding conditions. It should be noted however, as discussed in Yonelinas and Parks (2007), that differences in response criterion can cause distortions in ROCs. Because patients with AD are known to show a more liberal response bias compared to healthy older adults (Budson et al., 2006), the results of this study should be compared with those using other methodologies to assure validity.
Given the widespread neuropathological changes in the medial temporal lobes, and frontal and parietal cortices in AD, impairment in recollection was not surprising in this group. Indeed, previous studies have reported impaired recollection in patients with AD (Budson et al., 2000; Christensen et al., 1998; Dalla Barba, 1997; Gallo et al., 2004; Knight, 1998; Koivisto et al., 1998; Smith and Knight, 2002). In addition to the regions listed above, perirhinal cortex, entorhinal regions, hippocampus, amygdala, and nucleus basalis appear to be ravaged with Alzheimer pathology in the earliest stages of the disease (Arriagada et al., 1992; Braak and Braak, 1991; Gomez-Isla et al., 1996; Mesulam, 2000; Van Hoesen et al., 1991). Based on these pathology studies, we predicted that familiarity would also be impaired in patients with AD. Consistent with this hypothesis, the current results showed that familiarity is impaired in this group. These data are supported by previous studies showing impaired gist memory in patients with AD (Budson, Todman, & Schacter, 2006; Pierce et al., 2005), and Westerberg et al. (2006) also reported that performance on the multiple-choice format was just as impaired as on the standard old/new format in patients with AD.
In summary, the results of the current study showed that recollection and familiarity are impaired very early in the AD process. These results are consistent with neuropathological studies showing that the hippocampus (Jack et al., 2004; Karas et al., 2004) and anterior medial temporal regions (Csernansky et al., 2004; Gomez-Isla et al., 1996; Kantarci et al., 2005) appear to be the earliest affected by neurofibrillary tangle pathology in patients with MCI. The hippocampus is thought to be critical to recollection, whereas perirhinal and possibly entorhinal regions are thought to be critical to familiarity (Brown & Aggleton, 2001; Eichenbaum et al., 2007). Further, the results of the current study are consistent with recent work by Wolk et al. (2008) who reported that on process dissociation tasks, familiarity was at least at impaired as recollection in patients with MCI. It is possible that impaired familiarity may help to identify patients in the earliest stages of AD, as previous work has demonstrated that familiarity is generally spared in healthy aging. The data of the current study also suggest that perhaps the relative balance of recollection and familiarity is modulated by task difficulty. It was notable that patients with MCI demonstrated a similar level of impairment in recollection and familiarity when items were deeply encoded, but impairment in familiarity appeared to be greater than recollection when items were encoded in the shallow condition. Given these results and the divergent results of Westerberg et al. (2006) using pictures, future studies should be aimed at examining differences in recollection and familiarity based on stimuli type and experimental task. Perhaps focusing interventions on using deeply encoded picture stimuli can help patients with MCI and mild AD better use the process of familiarity to support memorial decisions, and help them remain in the home longer.
Acknowledgments
This research was supported by National Institute on Aging grants F32 AG027632 (BAA), R01 AG025815 (AEB), and P30 AG13846 (AEB), and by a Boston University Alzheimer’s Disease Center Pilot Award (BAA). This material is also the result of work supported with resources and the use of facilities at the Edith Nourse Rogers Memorial Veterans Hospital in Bedford, MA. The authors would like to thank Andrew Yonelinas for providing the algorithm needed to construct the ROC curves and for his guidance throughout this project. We also would like to thank John Wixted and Joel Quamme for their advice on ROC analyses, and David Wolk for his invaluable input on this manuscript.
References
- Adjutant General’s Office. Army Individual Test Battery: Manual of Directions and Scoring. Washington, D.C: War Department; 1944. [Google Scholar]
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43:1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Allan K, Wilding EL, Rugg MD. Electrophysiological evidence for dissociable processes contributing to recollection. Acta Psychol. 1998;98:231–252. doi: 10.1016/s0001-6918(97)00044-9. [DOI] [PubMed] [Google Scholar]
- Ally BA, Budson AE. The worth of pictures: Using high density event-related potentials to understand the memorial power of pictures and the dynamics of recognition memory. NeuroImage. 2007;35:378–395. doi: 10.1016/j.neuroimage.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. The picture superiority effect in patients with Alzheimer's disease and Mild Cognitive Impairment. doi: 10.1016/j.neuropsychologia.2008.10.010. (revision submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Waring JD, Beth EH, McKeever JD, Milberg WP, Budson AE. Aging memory for pictures: Using high-density event-related potentials to understand the effects of age on the dynamics of recognition memory and the picture superiority effect. Neuropsychologia. 2008;46:287–297. doi: 10.1016/j.neuropsychologia.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Craik FI, Naveh-Benjamin M. The attentional demands of encoding and retrieval in younger and older adults: 1. Evidence from divided attention costs. Psychol Aging. 1998;13:405–423. doi: 10.1037//0882-7974.13.3.405. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Backman L, Lipinska B. Monitoring of general knowledge: evidence for preservation in early Alzheimer's disease. Neuropsychologia. 1993;31:335–345. doi: 10.1016/0028-3932(93)90157-u. [DOI] [PubMed] [Google Scholar]
- Balota DA, Burgess GC, Cortese MJ, et al. The word-frequency mirror effect in young, old, and early-stage Alzheimer's disease: Evidence for two processes in episodic recognition performance. Journal of Memory and Language. 2002;46:199–226. [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: A study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Bayley PJ, Wixted JT, Hopkins RO, Squire LR. Yes/No Recognition, Forced-choice Recognition, and the Human Hippocampus. Journal of Cognitive Neuroscience. 2008;20:505–512. doi: 10.1162/jocn.2008.20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-McGinty S, Lopez OL, Meltzer CC, Scanlon JM, Whyte EM, Dekosky ST, Becker JT. Differential cortical atrophy in subgroups of mildcognitive impairment. Arch Neurol. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brown CM, Aggleton JP. Recognition memory: What are the roles of perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown CM, Xiang JZ. Recognition memory: neuronal substrates of the judgment of prior occurance. Progress in Neurobiology. 1998;55:149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sullivan AL, Mayer E, Daffner KR, Black PM, Schacter DL. Suppression of false recognition in Alzheimer's disease and in patients with frontal lobe lesions. Brain. 2002;125:2750–2765. doi: 10.1093/brain/awf277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Todman RW, Schacter DL. Gist memory in Alzheimer’s disease: Evidence from categorized pictures. Neuropsychology. 2006;20:113–122. doi: 10.1037/0894-4105.20.1.113. [DOI] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer's disease: Separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4:359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Christensen H, Kopelman D, Stanhope N, Lorentz L, Owen P. Rates of forgetting in Alzheimer dementia. Neuropsychologia. 1998;36:547–557. doi: 10.1016/s0028-3932(97)00116-4. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Bird C, Good T, Macmanus D, Rudge P, Shallice T. Recollection and familiarity in dense hippocampal amnesia: A case study. Neuropsychologia. 2006;44:489–506. doi: 10.1016/j.neuropsychologia.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Hamstra J, Wang L, McKeel D, Price JL, Gado M, Morris JC. Correlations between antemortem hippocampal volume and postmortem neuropathology in AD subjects. Alzheimer Disease and Associated Disorders. 2004;18:190–195. [PubMed] [Google Scholar]
- Dalla Barba G. Recognition memory and recollective experience in Alzheimer's disease. Memory. 1997;5:657–672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- DeCarlo LT. Signal detection theory with finite mixture distributions: Theoretical developments with applications to recognition memory. Psychological Review. 2002;109:710–721. doi: 10.1037/0033-295x.109.4.710. [DOI] [PubMed] [Google Scholar]
- DeCarlo LT. An application of signal detection theory with finite mixture distributions to source discrimination. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:767–778. doi: 10.1037/0278-7393.29.5.767. [DOI] [PubMed] [Google Scholar]
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- Delbecq-Derouesne J, Beauvois MF, Shallice T. Preserved recall versus impaired recognition. A case study. Brain. 1990;113:1045–1074. doi: 10.1093/brain/113.4.1045. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cognitive Brain Research. 2004;18:255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JP. Recognition memoryand the operating characteristic. Bloomington, IN: Indiana University Hearing and Communication Laboratory; 1958. [Google Scholar]
- Egan JP. Signal detection theory and ROC analysis. New York: Academic Press; 1975. [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ″Mini-mental state″. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Shahid KR, Olson MA, Solomon TM, Schacter DL, Budson AE. Overdependence on degraded gist memory in Alzheimer's disease. Neuropsychology. 2006;20:625–632. doi: 10.1037/0894-4105.20.6.625. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Sullivan AL, Daffner KR, Schacter DL, Budson AE. Associative recognition in Alzheimer's disease: Evidence for impaired recall-to-reject. Neuropsychology. 2004;18:556–563. doi: 10.1037/0894-4105.18.3.556. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RL, Richardson-Klavehn A. Experiences of remembering, knowing, and guessing. Consciousness and Cognition. 1998;7:1–26. doi: 10.1006/ccog.1997.0321. [DOI] [PubMed] [Google Scholar]
- Gold CA, Marchant NL, Koutstaal W, Schacter DL, Budson AE. Conceptual fluency increases false recognition of ambiguous images in Alzheimer's disease. Neuropsychologia. 2007;45:2791–2801. doi: 10.1016/j.neuropsychologia.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann RE, Sullivan AL, Droller DB, Rugg MD, Curran T, Holcomb PJ, Schacter DL, Daffner KR, Budson AE. Late frontal brain potentials distinguish true and false recognition. Neuroreport. 2003;14:1717–1720. doi: 10.1097/00001756-200309150-00012. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The Boston Diagnostic Aphasia Examination. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. Journal of Neuroscience. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Haxby JV, Horwitz B, Sundaram M, Berg G, Schapiro M, Friedland RP, Rapoport SI. Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1988;10:576–596. doi: 10.1080/01688638808402796. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives of Neurology. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Archives of Neurology. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;3:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. J Neurosci. 1999a;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: A functional MRI test of the monitoring hypothesis. Brain. 1999;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Hooper HE. The Hooper Visual Organization Test. Manual. Beverly Hills, CA: Western Psychological Services; 1958. [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: Evidence from modeling and receiver operating characteristic curves. Psychol Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, Rapoport SI, Schapiro MB, Horwitz B. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer's disease. Neurology. 1998;50:1585–1593. doi: 10.1212/wnl.50.6.1585. [DOI] [PubMed] [Google Scholar]
- Jack CR, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;64:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL. A process-dissociation framework: separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Jacoby LL. Ironic effects of repetition: Measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jacoby JJ, Dallas M. On the relationship between autobiographical memory and perceptual learning. J Exp Psychol Gen. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychology and Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: Telling effects of repetition. Psychology and Aging. 1997;12:352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Petersen RC, Boeve BF, Knopman DS, Weigand SD, O’Brien PC, Shiung MM, Smith GE, Ivnik RJ, Tangalos EG, Jack CR., Jr DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2005;64:902–904. doi: 10.1212/01.WNL.0000153076.46126.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas GB, Scheltens P, Rombouts SA, Visser PJ, van Schijndel RA, Fox NC, Barkhof F. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Kishiyama MM, Yonelinas AP, Kroll NE, Lazzara MM, Nolan EC, Jones EG, Jagust WJ. Bilateral thalamic lesions affect recollection-and familiarity-based recognition memory judgments. Cortex. 2005;41:778–788. doi: 10.1016/s0010-9452(08)70296-x. [DOI] [PubMed] [Google Scholar]
- Knight RG. Controlled and automatic memory process in Alzheimer's disease. Cortex. 1998;34:427–435. doi: 10.1016/s0010-9452(08)70765-2. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Portin R, Seinela A, Rinne J. Automatic influences of memory in Alzheimer's disease. Cortex. 1998;34:209–219. doi: 10.1016/s0010-9452(08)70748-2. [DOI] [PubMed] [Google Scholar]
- Lekeu F, Van der Linden M, Chicherio C, Collette F, Degueldre C, Franck G, Moonen G, Salmon E. Brain correlates of performance in a free/cued recall task with semantic encoding in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2003;17:35–45. doi: 10.1097/00002093-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: Shortened versions for use in alzheimer's disease. J Gerontol. 1992;47:P154–P158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgement of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, Drake J, Wolf PA. Visual association pathology in preclinical Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2006;65:621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:285–297. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Melo B, Winocur G, Moscovitch M. False recall and false recognition: An examination of the effects of selective and combined lesions to the medial temporal lobe/diencephalon and frontal lobe structures. Cognitive Neuropsychology. 1999;16:343–359. [Google Scholar]
- Mesulam MM. Neuroplasticity failure in Alzheimer's disease: Bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Principles of behavioral and cognitive neurology. New York: Oxford University Press; 2000. [Google Scholar]
- MacPherson SE, Bozzali M, Cipolotti L, Dolan RJ, Rees JH, Shallice T. Effect of frontal lobe lesions on the recollection and familiarity components of recognition memory. Neuropsychologia. 2008;46:3124–3132. doi: 10.1016/j.neuropsychologia.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, Han LY, Bienias JL, Lee VM, Trojanowski JQ, Bennett DA, Arnold SE. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early alzheimer's disease. Annals of Neurology. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nunally JC, Bernstein IH. Psychometric Theory. New York: McGraw-Hill; 1994. [Google Scholar]
- Park DC, Smith AD, Dudley WN, Lafronza VN. Effects of age and a divided attention task presented during encoding and retrieval on memory. J Exp Psychol Learn Mem Cogn. 1989;15:1185–1191. doi: 10.1037//0278-7393.15.6.1185. [DOI] [PubMed] [Google Scholar]
- Parks CM, Yonelinas AP. Moving beyond pure signal0detection models” Comment on Wixted (2007) Psychological Review. 2007;114:188–201. doi: 10.1037/0033-295X.114.1.188. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Bindschaedler C, Harsent L, Metzler C. Pathological false alarm rates following damage to the left frontal cortex. Brain Cogn. 1996;32:14–27. doi: 10.1006/brcg.1996.0055. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Ward J, Bindschaedler C, Squires EJ, Powell G. False recognition following frontal lobe damage: The role of encoding factors. Cognitive Neuropsychology. 1999;16:243–265. [Google Scholar]
- the Early Diagnosis Group of the Italian Interdisciplinary Network on Alzheimer's Disease. Perri R, Carlesimo GA, Serra L, Caltagirone C. Characterisation of memory profile in subjects with mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2005;27:1033–1055. doi: 10.1080/13803390490919317. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment. Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Pierce BH, Sullivan AL, Schacter DL, Budson AE. Comparing source-based and gist-based false recognition in aging and Alzheimer’s disease. Neuropsychology. 2005;19:411–419. doi: 10.1037/0894-4105.19.4.411. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, 3rd, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: Adult age differences and neuropsychological test correlates. Psychol Aging. 2006;21:107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Kaszniak AW, Reminger SL, Glisky ML, Glisky EL, Comer JF. Dissociation between verbal and autonomic measures of memory following frontal lobe damage. Neurology. 1998;50:1259–1265. doi: 10.1212/wnl.50.5.1259. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Nielsen L, Littrell LD, Glisky EL, Kaszniak AW, Laguna JF. Face memory impairments in patients with frontal lobe damage. Neurology. 2001;57:1168–1175. doi: 10.1212/wnl.57.7.1168. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Sheu CF, Gronlund SD. Testing global memory models using ROC curves. Psychological Review. 1992;99:518–535. doi: 10.1037/0033-295x.99.3.518. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Mcmillam NA, Reeder JA. Sum-Difference Theory of Remembering and Knowing: A Two-Dimensional Signal-Detection Model. Psychological Review. 2004;111:588–616. doi: 10.1037/0033-295X.111.3.588. [DOI] [PubMed] [Google Scholar]
- Rybash JM, Hoyer WJ. Process dissociation procedure reveals age differences in unconscious influences on memory for possible and impossible objects. Aging, Neuropsychology, & Cognition. 1996;3:251–263. [Google Scholar]
- Salthouse TA. Aging associations: Influence of speed on adult age differences in associative learning. J Exp Psychol Learn Mem Cogn. 1994;20:1486–1503. doi: 10.1037//0278-7393.20.6.1486. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Curran T, Galluccio L, Milberg WP, Bates JF. False recognition and the right frontal lobe: A case study. Neuropsychologia. 1996;34:793–808. doi: 10.1016/0028-3932(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Memory and the prefrontal cortex. Ann N Y Acad Sci. 1995;769:151–159. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- Simons JS, Owen AM, Fletcher PC, Burgess PW. Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia. 2005;43:1774–1783. doi: 10.1016/j.neuropsychologia.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia. 2007;45:2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Smith JA, Knight RG. Memory processing in Alzheimer's disease. Neuropsychologia. 2002;46:666–682. doi: 10.1016/s0028-3932(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: A meta-analysis. Psychol Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Titov N, Knight RG. Adult age differences in controlled and automatic memory processing. Psychol Aging. 1997;12:565–573. doi: 10.1037//0882-7974.12.4.565. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Van Hoesen GW, Hyman BT, Damasio AR. Entorhinal cortex pathology in Alzheimer's disease. Hippocampus. 1991;1:1–8. doi: 10.1002/hipo.450010102. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49:459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and Alzheimer’s disease. Neuropsychology. 2006;20:193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Whiting WL, 4th, Smith AD. Differential age-related processing limitations in recall and recognition tasks. Psychol Aging. 1997;12:216–224. doi: 10.1037//0882-7974.12.2.216. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-Process Theory and Signal-Detection Theory of Recognition Memory. Psychological Review. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. An electrophysiological investigation of the relationship between conceptual fluency and familiarity. Neuroscience Letters. 2004;369:150–155. doi: 10.1016/j.neulet.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. Patients with Alzheimer's Disease attribute conceptual fluency to prior experience. Neuropsychologia. 2005;43:1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, DeKosky ST. Recollection and familiarity in Amnestic Mild Cognitive Impairment: A global decline in recognition memory. Neuropsychologia. 2008;4:1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. J Exp Psychol Learn Mem Cog. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: The contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: Convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauvé MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychological Bulletin. 2007;133:800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]