Abstract
OBJECTIVE
Limited data on patients undergoing Roux-en-Y gastric bypass surgery (RY-GBP) suggest that an improvement in insulin secretion after surgery occurs rapidly and thus may not be wholly accounted for by weight loss. We hypothesized that in obese patients with type 2 diabetes the impaired levels and effect of incretins changed as a consequence of RY-GBP.
RESEARCH DESIGN AND METHODS
Incretin (gastric inhibitory peptide [GIP] and glucagon-like peptide-1 [GLP-1]) levels and their effect on insulin secretion were measured before and 1 month after RY-GBP in eight obese women with type 2 diabetes and in seven obese nondiabetic control subjects. The incretin effect was measured as the difference in insulin secretion (area under the curve [AUC]) in response to an oral glucose tolerance test (OGTT) and to an isoglycemic intravenous glucose test.
RESULTS
Fasting and stimulated levels of GLP-1 and GIP were not different between control subjects and patients with type 2 diabetes before the surgery. One month after RY-GBP, body weight decreased by 9.2 ± 7.0 kg, oral glucose-stimulated GLP-1 (AUC) and GIP peak levels increased significantly by 24.3 ± 7.9 pmol · l−1 · min−1 (P<0.0001) and 131 ± 85 pg/ml (P = 0.007), respectively. The blunted incretin effect markedly increased from 7.6 ± 28.7 to 42.5 ± 11.3 (P=0.005) after RY-GBP, at which it time was not different from that for the control subjects (53.6 ± 23.5%, P = 0.284).
CONCLUSIONS
These data suggest that early after RY-GBP, greater GLP-1 and GIP release could be a potential mediator of improved insulin secretion.
The prevalence of obesity and type 2 diabetes (1) is increasing in the U.S. More and more patients seek bariatric surgery (surgical weight loss) for treatment of their obesity. Up to 30% of patients presenting for bariatric surgery have type 2 diabetes (2,3). Although weight loss surgery generally results in a loss of 50–70% excess body weight and “cures” diabetes in 77% of patients (4), the rapidity in the onset and the magnitude of the benefits of Roux-en-Y gastric bypass surgery (RY-GBP) on diabetes has thus far baffled clinical scientists.
It has been proposed that the incretins could be some of the key mediators of the antidiabetic effects of certain types of bariatric surgery. The incretins are gut peptides secreted in response to meals, which enhance insulin secretion. The two main incretins are gastric inhibitory peptide (GIP), secreted by the K-cells in the proximal small intestine (5), and glucagon-like peptide-1 (GLP-1), secreted by the L-cells of the distal small intestine (6). Both incretins affect meal-related insulin secretion (7,8). GLP-1 also delays gastric emptying (9), decreases appetite (9 –11), inhibits glucagon (12), and may improve insulin sensitivity (13), all effects that are antidiabetogenic. The incretin effect is impaired in patients with type 2 diabetes (14). GLP-1 levels are blunted (15), but the effect of administered GLP-1 on insulin secretion persists (16). Contrary to GLP-1, GIP levels are normal in patients with type 2 diabetes, but the effect of administered GIP on insulin secretion is blunted (17,18). GIP and GLP-1 have additive insulinotropic effects during hyperglycemia. Both GLP-1 and GIP analogs are being developed as antidiabetic agents (19).
Previous data have shown that the significant weight loss observed after various bariatric procedures was accompanied by improvement in diabetes control and increased GLP-1 levels. However, most studies were cross-sectional (20,21), reported fasting (22) rather than postprandial GLP-1 levels, and compared various types of surgery such as jejunoileal bypass (JIB) (23,24) or biliopancreatic diversion (BPD) (24), often leading to inconclusive results. Data on fasting GIP levels after bariatric surgery are inconsistent, with reports of either a decrease (22,25,26) or an increase (20,24).
GLP-1 levels increase after a meal in patients after RY-GBP (27) or with oral glucose after BPD (28). Meal-stimulated GIP levels have been reported to increase after JIB (20) or to decrease after GBP or JIB surgery (23,26,29). None of these studies, however, measured GLP-1 and GIP simultaneously, reported the incretin levels and effect on insulin secretion, or were done in diabetic patients.
Our goal was to investigate the role of incretins as the mechanism for rapid improvement in insulin secretion in obese patients with type 2 diabetes after RY-GBP. Specifically, we wished to measure the changes in GLP-1 and GIP levels in response to oral glucose, as well as the changes in the incretin effect on insulin secretion, in obese patients with type 2 diabetes before and 1 month after RY-GBP.
RESEARCH DESIGN AND METHODS
Obese patients of either sex and all ethnic groups with BMI >35 kg/m2 who were scheduled for RY-GBP surgery and had type 2 diabetes diagnosed for <5 years, were not taking insulin, had A1C <8%, and were aged <60 years were recruited. They were studied before and within 1 month after the surgery to minimize the amount of weight loss at the time of the second study. A group of obese nondiabetic control subjects was studied for incretin levels and effect. Fat mass was measured by anthropometrics (30).
RY-GBP protocol
All patients underwent a laparoscopic RY-GBP with a 30-ml gastric pouch, 40-cm afferent limb, 150-cm Roux limb, and 12-mm gastrojejunostomy. The post–RY-GBP diet recommendations included a daily intake of 600–800 kcal, 70 g protein, and 64 oz fluid. This was achieved, on an individual basis, with multiple small meals and snacks with various commercial protein supplements. The diet after RY-GBP was monitored by food records but not directly supervised. The diet in the few days preceding the testing in patients before surgery or in control subjects was not controlled.
Measurement of incretin effect
Insulin secretion after oral and isoglycemic intravenous glucose load
Oral glucose tolerance tests (OGTTs) and isoglycemic intravenous glucose tests (IsoG IVGTs) were administered in the morning after a 12-h overnight fast, on two different days, separated by <5 days.
3-h OGTT
All patients underwent first a 3-h OGTT with 50 g glucose (in a total volume of 300 ml). After insertion of an intravenous (IV) catheter, at 8:00 a.m., subjects received 50 g glucose orally. Blood samples, collected in chilled EDTA tubes with added aprotinin (500 kallikrein inhibitory units/ml blood) and dipeptidyl-peptidase IV (DPPIV) inhibitor (Linco, St. Charles, MO) (10 µl/ml blood), were centrifuged at 4°C before storage at −70°C.
IsoG IVGT
The goal of the IsoG IVGT was to expose the pancreas to blood glucose levels matched to the ones obtained during the OGTT in the same subject. Glucose (sterile 20% dextrose solution in water) was infused intravenously over 3 h using a Gemini pump (Gemini). A sample of blood was collected every 5 min using a contralateral antecubital IV catheter and then was transferred in a picofuge tube without any additive and centrifuged immediately for measurement of glucose levels at the bedside. The glucose infusion rate was adjusted to match the glucose concentrations obtained for the same patient during the OGTT at each time point for 3 h. During the OGTT and the IsoG IVGT, the arm used for blood sampling was kept warm with a heating pad.
Incretin effect
The difference in β-cell responses (insulin total area under the curve [INS AUC 0–180 min]) to the oral and the isoglycemic IV glucose stimuli represents the incretin effect (INC), the action of the incretin factor expressed as the percentage of the physiological response to oral glucose, which is taken as the denominator (100%) (14). The formula is
Assays
Total GLP-1, an indicator of secretion, was measured by radioimmunoassay (Phoenix Pharmaceutical, Belmont, CA) after plasma ethanol extraction. The intra-assay and interassay coefficients of variation (CVs) were 3– 6.5 and 4.7– 8.8%, respectively. This assay has 100% specificity for GLP-1(7–36) and GLP-1(9 –36), 60% specificity for GLP-1(7–37) and does not cross-react with glucagon (0.2%), GLP-2 (<0.001%), or exendin (<0.01%). Active GLP-1, an indicator of potential action, was measured by ELISA (Linco). The intra-assay and interassay CVs were 3–7 and 7–8%, respectively. The assay is 100% specific for GLP-1(7–36) and GLP-1(7–37) and does not react with GLP-1(9 –36), glucagon, or GLP-2. Total GIP was measured by ELISA (Linco). The assay is 100% specific for GIP 1–42 and GIP 3–42 and does not cross-react with GLP-1, GLP-2, oxyntomodulin, or glucagon. The intra-assay and interassay CVs were 3.0–8.8 and 1.8–6.1%, respectively. Plasma insulin and C-peptide concentrations were measured by radioimmunoassay (Linco) with, intra-assay CVs, respectively, of 5–8 and 3–6% and interassay CVs of 7.2 and 5.2–7.7%. The glucose concentration was measured at the bedside by the glucose oxidase method (glucose analyzer; Beckman, Fullerton, CA). All hormonal and metabolites assays were performed at the Hormone and Metabolite Core Laboratory of the New York Obesity Research Center.
Statistical analysis
Outcome variables were serum glucose and plasma insulin, C-peptide, GLP-1, and GIP concentrations. Total AUC 0–180 min for outcome variables were calculated using the trapezoidal method. ANOVA with repeated measures was used to detect hormonal changes over time during the OGTT within each condition and for comparison before and after RY-GBP or between control subjects and patients with type 2 diabetes. Paired t tests were used to compare data from before and after RY-GBP. Statistical significance was set at P < 0.05. Statistical analyses were performed with SPSS 13.0 (SPSS, Chicago, IL) (31).
RESULTS
Subject characteristics are shown in Table 1. Eight obese women with type 2 diabetes of 20.1 ± 12.9 months’ duration, A1C of 6.9 ± 0.7%, and liver enzymes, thyroid function tests, and blood pressure within normal limits were studied before and 1 month (31 ± 14 days) after RY-GBP. Type 2 diabetes was diagnosed in one patient at the time of screening for surgery. Patients’ diabetes medications, sulfonylureas, and/or metformin were discontinued 3 days before they were studied before the surgery. None of the patients were taking insulin, thiazolinediones, or β-blockers before RY-GBP or any diabetes medication after RY-GBP. Seven obese nondiabetic women were studied as control subjects, while consuming their regular diet at stable weight and taking no medications. Control subjects did not differ from patients in terms of age, body weight, or BMI (Table 1).
Table 1.
Subject characteristics
| Control subjects |
Patients before RY-GBP |
P value (vs. control subjects) |
Patients after RY-GBP |
Δ | P value (Δ) | |
|---|---|---|---|---|---|---|
| Age (years) | 38.8 ± 7.8 | 44.8 ± 10.2 | 0.210 | |||
| Weight (kg) | 100.4 ± 29.9 | 113.9 ± 16.4 | 0.267 | 104.8 ± 17.1 | 9.2 ± 7.0 | 0.008 |
| BMI (kg/m2) | 37.1 ± 11.6 | 43.6 ± 6.8 | 0.189 | 40.1 ± 7.0 | 3.5 ± 3.6 | 0.029 |
| Fat mass (%) | 41.1 ± 8.3 | 47.1 ± 1.3 | 0.06 | 45.3 ± 3.1 | 1.8 ± 1.8 | 0.192 |
| Fasting glucose (mmol/1) | 5.4 ± 0.4 | 8.05 ± 1.82 | 0.001 | 6.45 ± 0.84 | 1.60 ± 1.45 | 0.017 |
| Fasting insulin (pmol/l) | 123 ± 93 | 171 ± 74 | 0.303 | 128 ± 54 | 43 ± 53 | 0.057 |
| 120-min glucose (mmol/l) | 6.04 ± 1.48 | 11.2 ± 1.72 | <0.0001 | 7.10 ± 1.72 | 4.10 ± 1.62 | <0.0001 |
| Peak insulin (pmol/l) | 749 ± 532 | 507 ± 304 | 0.301 | 723 ± 327 | 216 ± 245 | 0.042 |
| AUC glucose (mmol · l−1 · min−1) |
1,113 ± 191 | 2,018 ± 343 | <0.0001 | 1,500 ± 212 | 517 ± 187 | <0.0001 |
| AUC insulin (pmol · l−1 · min−1) |
252 ± 147 | 356 ± 199 | 0.294 | 323 ± 137 | 32 ± 134 | 0.514 |
| AUC total GLP-1 (pmol · l−1 · min−1) |
5.26 ± 3.06 | 7.54 ± 2.79 | 0.155 | 31.82 ± 8.10 | 24.3 ± 7.9 | <0.0001 |
| AUC GIP (pmol · l−1 · min−1) |
37.12 ± 20.97 | 50.96 ± 9.62 | 0.103 | 52.66 ± 18.93 | 1.7 ± 24.3 | 0.849 |
| Peak GIP (pmol/l) | 151 ± 77 | 213 ± 52 | 0.088 | 324 ± 130 | 111 ± 98 | 0.015 |
| Incretin effect on insulin (%) | 53.6 ± 23.5 | 7.6 ± 28.7 | 0.020 | 42.5 ± 11.3 | 34.8 ± 24.2 | 0.005 |
| Incretin effect on C-peptide (%) |
49.9 ± 19.4 | −17.8 ± 52.8 | 0.079 | 26.6 ± 18.1 | 44.4 ± 43.6 | 0.024 |
Data are means ± SD. Control subjects are obese individuals without type 2 diabetes (n = 7), and patients are obese individuals with type 2 diabetes (n = 8) before and after RY-GBP. Fasting values are the average of two baseline values. Peak (120-min value) and AUC (total area under the curve, 180 min) values were obtained during the OGTT. Δ, difference between pre– and post–RY-GBP.
Side effects
Although a 50-g glucose drink was used rather than 75 g to minimize the risk of dumping syndrome after RY-GBP, three patients experienced stomach cramping and discomfort, nausea, sweating, flushing, and palpitations 5–20 min into the OGTT. No severe adverse effects were observed.
Glucose and insulin levels during the OGTT
Changes in outcome variables after RY-GBP are shown in Table 1. After RY-GBP, body weights and BMIs decreased significantly. Fasting and 120-min glucose levels decreased to the nondiabetic range while peak insulin levels increased significantly.
GLP-1 and GIP levels during the OGTT
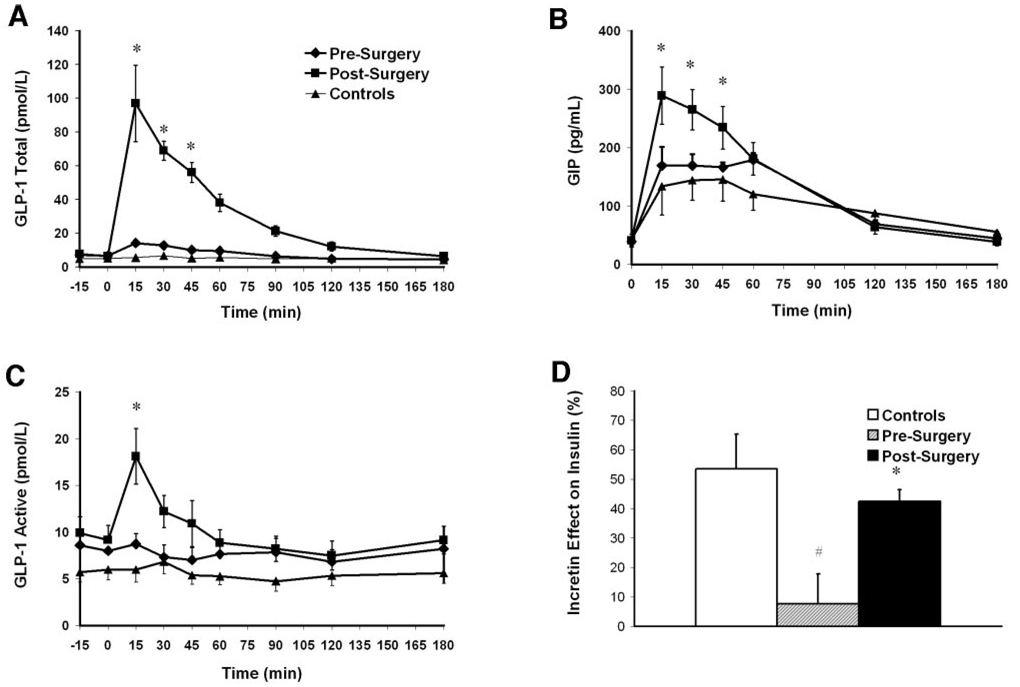
Fasting and stimulated GLP-1 and GIP levels were not different between control subjects and patients before RY-GBP (Table 1 and Fig. 1A–C). GLP-1 AUC and peak GIP increased significantly after surgery by factors of 4.2 and 1.6, respectively (Table 1 and Fig. 1A and B). There was no change in GLP-1 or GIP levels during the IsoG IVGT (data not shown). The blunted active GLP-1 levels increased significantly at 15 min during the OGTT after GBP (Fig. 1C). To indirectly assess the activity of the enzyme DPPIV, the ratio of active GLP-1 to total GLP-1 (using OGTT peak values) was calculated. The ratio, which was not statistically different between control subjects and patients before surgery (P = 0.132), decreased significantly from 0.70 ± 0.12 to 0.18 ± 0.06 (P < 0.0001) after RY-GBP.
Figure 1.
Total GLP-1 (A), GIP (B), and active GLP-1 (C) levels during OGTTs in patients before (♦) and after (■) RY-GBP (A) and in control subjects (▲) and incretin effect on insulin secretion (D) in control subjects (□), patients before RY-GBP ( ), and patients after RY-GBP (■). The incretin effect was calculated by comparing the insulin response to oral and matched IV glucose loads. Data are means±SEM. *P<0.05 compared with patients before RY-GBP. #P < 0.05 compared with control subjects.
), and patients after RY-GBP (■). The incretin effect was calculated by comparing the insulin response to oral and matched IV glucose loads. Data are means±SEM. *P<0.05 compared with patients before RY-GBP. #P < 0.05 compared with control subjects.
Incretin effect
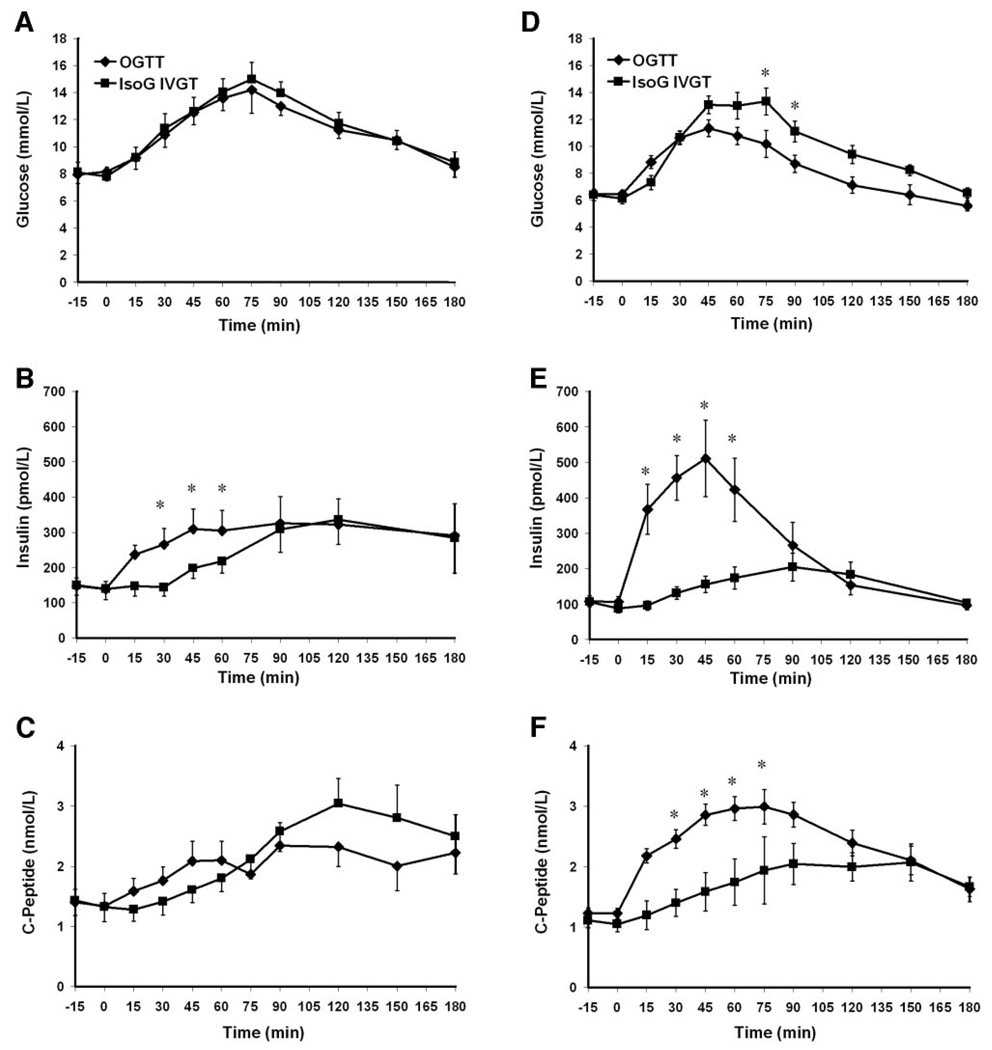
In control subjects, the glucose concentrations were well matched between the IsoG IVGT and the OGTT (mean levels 5.87 ± 0.76 vs. 5.92 ±0.82 mmol/l, P _ 0.639). As expected, insulin levels were greater during the OGTT (AUC insulin 252 ± 147 pmol · l−1 · min−1) than during the IsoG IVGT (132 ± 101 pmol · l−1 · min−1, P = 0.045), with a calculated incretin effect on insulin of 53.6 ± 23.5%. In patients before RY-GBP, the glucose levels were well matched between the IsoG IVGT and the OGTT (11.06 ± 1.93 vs. 10.76 ± 1.97 mmol/l, P=0.058) (Fig. 2A). Contrary to results from the control subjects, as expected, the insulin response was not greater after oral glucose (AUC insulin 356 ± 199 pmol · l−1 · min−1) than after IV glucose (AUC insulin 305 ± 161 pmol · l−1 · min−1, P = 0.195) (Fig. 2B), with a resulting blunted incretin effect of 7.6 ± 28.7% (Fig. 1C). After RY-GBP, glucose levels were higher during the IsoG IVGT (mean levels 9.39 ± 1.00 mmol/l) compared with the OGTT (8.42 ± 1.04 mmol/l, P = 0.046) (Fig. 2D). However, the insulin response was greater during the oral test (323 ± 137 pmol · l−1 · min−1) than during the IV test (184 ± 80 pmol · l−1 · min−1, P = 0.001) (Fig. 2E), and the incretin effect was markedly increased by a factor of 5 to 42.5 ± 11.3% (mean increase 34.8 ± 24.2%, P = 0.007) (Fig. 1C), a level at which it was not significantly different from that for the control subjects (53.6 ± 23.5%, P=0.284) (Fig. 1D). The incretin effect on C-peptide increased significantly after RY-GBP, to a level not statistically different (P = 0.157) from that for the control subjects (Table 1). There was a correlation between GIP AUC and the insulin incretin effect in patients before surgery (r = 0.737, P = 0.037) but not in patients after RY-GBP nor in control subjects. GLP-1 AUC was positively correlated to the C-peptide incretin effect in control subjects and patients after RY-GBP (r = 0.915, P = 0.046).
Figure 2.
Glucose (A and D), insulin (B and E), and C-peptide (C and F) concentrations during OGTTs (♦) and IsoG IVGTs (■) before (A–C) and after (D–F) RY-GBP. Data are means ± SEM. *P < 0.05, significant difference between OGTT and IsoG IVGT values within each condition (before or after RY-GBP).
CONCLUSIONS
We investigated the changes in incretin levels and effect after RY-GBP in patients with morbid obesity and type 2 diabetes. Our main findings are that the release of incretin after oral glucose is of greater magnitude and the incretin effect on insulin secretion is markedly improved 1 month after RY-GBP.
It has long been hypothesized that the incretins could play a role in the marked immediate improvements of diabetes control observed after bariatric surgery (32). Limited data (23–25,27,33) suggested that the improvement in insulin secretion after bariatric surgery occurs rapidly. Thus, it may not be wholly accounted for by weight loss but could be a consequence of changes of the enteroinsular axis, particularly in the incretins. However, most of the studies to date have been cross-sectional (20,23,24) or measured only fasting levels of incretins (22,25).
In our study, fasting and glucose-stimulated levels of GLP-1 and GIP were not different between patients before the surgery and control subjects. This is contrary to findings by others showing lower fasting levels and impaired stimulated release of incretins in patients with type 2 diabetes (7,14,17). This discrepancy could be due to different patient populations. Our patients were relatively young (aged 45 years) with recently diagnosed type 2 diabetes (<2 years).
One month after RY-GBP, our data demonstrate a clear and significant increase of GLP-1 (total and active) and GIP release in response to oral glucose in patients with type 2 diabetes. An increase in levels of circulating incretins was previously reported in nondiabetic patients after RY-GBP (27) or BPD (28). Some cross-sectional and longitudinal studies have shown an increase in the stimulated levels of incretins, after a meal or oral glucose, after JIB (24,34) or after RY-GBP (27,35). Our data show that the changes in stimulated incretin levels occur as early as 1 month after RY-GBP, similarly to changes in enteroglucagon obtained after JIB (34). Future studies will address the long-term changes of incretin and help clarify the controversy about β-cell hypertrophy after RY-GBP (36,37).
The mechanisms by which incretin levels increase after surgery are not fully understood. After RY-GBP, as a consequence of the bypass of the upper gut, the lower gut is exposed sooner to the ingested nutrients, thus changing the timing of the physiological release of gut incretins. The time of the peak release of GLP-1 and GIP after oral glucose was at 15 min, although GIP is released by the K-cells of the proximal small intestine and GLP-1 by the more distal L-cells of the small intestine (5,6). More frequent blood sampling could possibly identify different release times for each incretin, according to the anatomical distribution of the secretory cells. Our stimulus was a solution of glucose. Future studies should investigate whether other stimuli, such as amino acids, lipids, or a change in pH, stimulate secretion of the incretins with the same magnitude as a solution of oral glucose. Elegant studies in a rat model of diabetes suggest that the exclusion of the upper gut, rather than weight loss, benefits glucose tolerance (38). Rats after gastrojejunal bypass have better glucose tolerance than sham-operated pair-fed control animals with equivalent body weight (38). Similarly, ileal transposition results in an early improvement in glucose tolerance with an increase of GLP-1 levels in a nonobese type 2 diabetes rat model, compared with sham-operated animals (39). The improvement in glucose tolerance was shown to be independent of weight and food intake (40). These rodent studies underline potential mechanisms by which diabetes improves after bariatric surgery and support a role for the gut incretins in glucose tolerance after RY-GBP.
Additionally, the change in circulating incretin levels could result from changes in the level and/or activity of the enzyme DPPIV. Both GLP-1 and GIP are highly susceptible to enzymatic degradation in vivo. The cleavage by DPPIV is an important determinant of incretin action, as it occurs rapidly and generates noninsulinotropic metabolites. DPPIV inhibitors are used to modulate incretin levels for the treatment of type 2 diabetes (41). We found that the release of active GLP-1 is also increased after RY-GBP, although the increase is short-lived. The ratio of circulating active GLP-1 levels to total GLP-1 levels decreased after RY-GBP. It is difficult to speculate on the significance of these findings, which could represent a change in levels and/or activity of the enzyme DPPIV. To our knowledge, there are no available data on in vivo DPPIV activity and/or levels in type 2 diabetes or on the effect of diabetes control and/or weight loss on the activity or levels of the enzyme.
As shown by others (14,42), we found that our patients with type 2 diabetes had an impaired incretin effect. After RY-GBP, in addition to increases in the levels of stimulated circulating GLP-1 and GIP levels, the severely impaired incretin effect on insulin and/or C-peptide improved significantly, reaching a magnitude similar to that of the nondiabetic control subjects. To our knowledge, this is the first report of simultaneous increases in stimulated incretin levels and the incretin effect (by comparing the insulin response to the oral and matched IV glucose load) in patients with type 2 diabetes after RY-GBP. Although the incretin effect of the patients normalized to the levels of control subjects after RY-GBP, the magnitude of the increase of stimulated GIP and GLP-1 levels was far greater than that for control subjects, with a fivefold increase for GLP-1. Interestingly, a similar discrepancy between incretin levels and effect occurred in patients before RY-GBP. Stimulated GLP-1 and GIP levels of patients were not different from those of control subjects, yet the incretin effect, normal for the control subjects, was severely impaired for the patients with type 2 diabetes. Other have shown discrepancies between circulating levels of incretins in response to the ingestion of glucose and the incretin effect (43), underlining the importance of looking at both the incretin effect and plasma levels.
The changes in incretin levels and effect, albeit very significant, are probably not the only factor responsible for the improvement in insulin secretion early after RY-GBP. We did find a significant correlation between incretin output during the OGTT and the incretin effect. However, the small sample size does not allow pertinent comments on these findings. Other determinants of impaired insulin secretion in type 2 diabetes, such as glucose toxicity (44,45) and lipotoxicity (46,47), probably improve after weight loss. At 1 month after RY-GBP, the daily calorie intake was minimal (range of 500–700 kcal by 24-h diet recall, data not shown) and the participants had lost about 10 kg. It is known that both caloric restriction and weight loss improve diabetes control (48 –51). Diet-induced weight loss also improves the release of incretins in obese nondiabetic individuals (52,53). However, as all of these events occur simultaneously, it is hard to isolate one factor from the others. Future researchers will have to separate the weight loss effect from the effect of the surgical bypass.
Insulin secretion in diabetes is extremely variable (54,55), depending on, among other factors, the age of the patient (56), the duration of the disease (impossible to measure accurately), the degree of diabetes control (45,46), and the degree of insulin resistance (57). The effects of weight loss will depend upon the prediet β-cell capacity (58). In this study, the mean age of our patients was 45 years, the duration of diagnosed diabetes was <2 years, and the A1C at baseline was <7%. After RY-GBP, the patients discontinued their antidiabetes medications, and fasting and postprandial glucose levels decreased significantly to nondiabetic levels. The changes in insulin and C-peptide (data not shown) levels after the RY-GBP were variable, and changes were not statistically significant. However, the relative amount of insulin secreted in response to glucose (insulin-to-glucose ratio) was greater after the surgery, a marker of improved insulin secretion.
Our study has some weaknesses. Our sample was of small size and ethnically diverse (data not shown) with only women, and, therefore, we could not assess sex or ethnic differences. We did not control for diet before the OGTT testing. Indeed, after the RY-GBP, patients were calorie restricted, and their amount of carbohydrate intake was probably much lower than that before RY-GBP. It has been shown that caloric and carbohydrate restriction could affect the result of the OGTT (59). It is possible that this caloric and carbohydrate restriction could have affected incretin release during the OGTT. Future diet-controlled studies will address this issue. Finally, the matching of the glucose levels was not perfect between OGTTs and IsoG IVGTs in patients after RY-GBP, and the levels of glucose were higher during the IsoG IVGT. However, this does not affect the interpretation of the results. If anything, it strengthens our findings. Although glycemia is greater during the IsoG IVGT, patients still released significantly more insulin during the OGTT than during the IsoG IVGT.
These data clarify the incretin effect on insulin in the early period after RY-GBP. Our main finding is that incretin levels and the incretin effect are markedly increased 1 month after RY-GBP, in parallel with a significant improvement of diabetes control. The magnitude of the incretin release may be specific to the anatomical changes of the gut resulting from RY-GBP surgery. Further experiments will have to be conducted to separate the effect of the weight loss from the effect of RY-GBP. These results may lead investigators to study other therapeutic maneuvers to alter incretins and develop new treatments for the growing diabetic and prediabetic population. As more obese patients with diabetes undergo RY-GBP, a clear understanding of the mechanism underlying short- and long-term improvements in type 2 diabetes is increasingly important.
Acknowledgment
This work was funded in part by grants from the American Diabetes Association (7-05-CR-18), the National Institutes of Health (R01-DK67561, General Clinical Research Center RR00645, and ORC DK-26687), and Diabetes and Endocrinology Research Center (DK-63068-05).
We thank bariatric surgeons Drs. Christine Ren and Howard Beaton for kindly referring patients for this study, our volunteer participants, and Hao Tran for his help with the figures.
Abbreviations
- AUC
area under the curve
- BPD
biliopancreatic diversion
- DPPIV
dipeptidyl-peptidase IV
- GIP
gastric inhibitory peptide
- GLP-1
glucagon-like peptide-1
- IsoG IVGT
isoglycemic intravenous glucose test
- IV
intravenous
- JIB
jejunoileal bypass
- OGTT
oral glucose tolerance test
- RY-GBP
Roux-en-Y gastric bypass surgery
Footnotes
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. Diabetes trends in the U.S.:1990–1998. Diabetes Care. 2000;23:1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 2.Pories WJ, MacDonald KG, Jr, Morgan EJ, Sinha MK, Dohm GL, Swanson MS, Barakat HA, Khazanie PG, Leggett-Frazier N, Long SD. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55:582S–585S. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- 3.Residori L, Garcia-Lorda P, Flancbaum L, Pi-Sunyer FX, Laferrère B. Prevalence of co-morbidities in obese patients before bariatric surgery: effect of race. Obes Surg. 2003;13:333–340. doi: 10.1381/096089203765887615. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 5.Bloom SR, Polak JM. Gut hormones. Adv Clin Chem. 1980;21:177–244. doi: 10.1016/s0065-2423(08)60089-x. [DOI] [PubMed] [Google Scholar]
- 6.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 7.Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest. 1967;46:1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest. 2004;113:635–645. doi: 10.1172/JCI20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- 10.Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541–R1544. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 11.Naslund E, Bogefors J, Skogar S, Gryback P, Jacobsson H, Holst JJ, Hellstrom PM. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910–R916. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531–544. doi: 10.1053/gast.2002.31068. [DOI] [PubMed] [Google Scholar]
- 13.D’Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest. 1994;93:2263–2266. doi: 10.1172/JCI117225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 15.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 16.Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22:1137–1143. doi: 10.2337/diacare.22.7.1137. [DOI] [PubMed] [Google Scholar]
- 17.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, Habener JF, Andersen DK. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–37) in normal and diabetic subjects. Regul Pept. 1994;51:63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 19.Holst JJ. The Claude Bernard Lecture, 2005: Glucagon-like peptide-1: from extract to agent. Diabetologia. 2006;49:253–260. doi: 10.1007/s00125-005-0107-1. [DOI] [PubMed] [Google Scholar]
- 20.Naslund E, Backman L, Holst JJ, Theodorsson E, Hellstrom PM. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg. 1998;8:253–260. doi: 10.1381/096089298765554449. [DOI] [PubMed] [Google Scholar]
- 21.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. [PubMed] [Google Scholar]
- 23.Lauritsen KB, Christensen KC, Stokholm KH. Gastric inhibitory polypeptide (GIP) release and incretin effect after oral glucose in obesity and after jejunoileal bypass. Scand J Gastroenterol. 1980;15:489–495. doi: 10.3109/00365528009181506. [DOI] [PubMed] [Google Scholar]
- 24.Sarson DL, Scopinaro N, Bloom SR. Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes. 1981;5:471–480. [PubMed] [Google Scholar]
- 25.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirinek KR, O’Dorisio TM, Hill D, McFee AS. Hyperinsulinism, glucose-dependent insulinotropic polypeptide, and the enteroinsular axis in morbidly obese patients before and after gastric bypass. Surgery. 1986;100:781–787. [PubMed] [Google Scholar]
- 27.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Luis MJ, Delgado S, Casamitjana R, Vidal J. GLP-1, PYY, hunger and satiety following gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 28.Valverde I, Puente J, Martin-Duce A, Molina L, Lozano O, Sancho V, Malaisse WJ, Villanueva-Penacarrillo ML. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg. 2005;15:387–397. doi: 10.1381/0960892053576613. [DOI] [PubMed] [Google Scholar]
- 29.Jorde R, Burhol PG, Johnson JA. The effect of jejunoileal bypass on postprandial release of plasma gastric inhibitory polypeptide (GIP) Scand J Gastroenterol. 1981;16:313–319. doi: 10.3109/00365528109181974. [DOI] [PubMed] [Google Scholar]
- 30.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 31.SPSS/PC Statistical Program User’s Guide. Release 6. 1 Edition. Chicago: SPSS; 1993. [Google Scholar]
- 32.Fetner R, Pi-Sunyer FX, Russell CD, Laferrère B. Incretins, diabetes and bariatric surgery: a review. Tenn Med. 2005;6:589–597. doi: 10.1016/j.soard.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Service FJ, Rizza RA, Westland RE, Hall LD, Gerich JE, Go VL. Gastric inhibitory polypeptide in obesity and diabetes mellitus. J Clin Endocrinol Metab. 1984;58:1133–1140. doi: 10.1210/jcem-58-6-1133. [DOI] [PubMed] [Google Scholar]
- 34.Barry RE, Barisch J, Bray GA, Sperling MA, Morin RJ, Benfield J. Intestinal adaptation after jejunoileal bypass in man. Am J Clin Nutr. 1977;30:32–42. doi: 10.1093/ajcn/30.1.32. [DOI] [PubMed] [Google Scholar]
- 35.Morinigo R, Casamitjana R, Moize V, Gomis R, Vidal J. Determinants and relevance of GLP-1 secretion changes following gastric bypass in morbidly obese patients (Abstract) Int J Obes. 2003;31:S87. [Google Scholar]
- 36.Carpenter T, Trautmann ED, Baron AD. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric bypass surgery. N Engl J Med. 2005;353:2192–2194. doi: 10.1056/NEJM200511173532017. [DOI] [PubMed] [Google Scholar]
- 37.Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet cell hyperplasia or increased β-cell turnover. Diabetes Care. 2006;29:1554–1559. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 38.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patriti A, Facchiano E, Annetti C, Aisa MC, Galli F, Fanelli C, Donini A. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg. 2005;15:1258–1264. doi: 10.1381/096089205774512573. [DOI] [PubMed] [Google Scholar]
- 40.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 41.Deacon CF. Therapeutic strategies based on GLP-1. Diabetes. 2004;53:2181–2189. doi: 10.2337/diabetes.53.9.2181. [DOI] [PubMed] [Google Scholar]
- 42.Ebert R, Creutzfeldt W. Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev. 1987;3:1–26. doi: 10.1002/dmr.5610030101. [DOI] [PubMed] [Google Scholar]
- 43.Henchoz E, D’Alessio DA, Gillet M, Halkic N, Matzinger O, Goy JJ, Chiolero R, Tappy L, Schneiter P. Impaired insulin response after oral but not intravenous glucose in heart- and liver-transplant recipients. Transplantation. 2003;76:923–929. doi: 10.1097/01.TP.0000079833.86120.85. [DOI] [PubMed] [Google Scholar]
- 44.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 45.Leahy JL, Bonner-Weir S, Weir GC. β-Cell dysfunction induced by chronic hyperglycemia: current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care. 1992;15:442–455. doi: 10.2337/diacare.15.3.442. [DOI] [PubMed] [Google Scholar]
- 46.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 47.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 48.Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes. 1986;35:155–164. doi: 10.2337/diab.35.2.155. [DOI] [PubMed] [Google Scholar]
- 49.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1985;61:917–925. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- 50.Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med. 1991;151:1334–1340. [PubMed] [Google Scholar]
- 51.Gumbiner B, Polonsky KS, Beltz WF, Griver K, Wallace P, Brechtel G, Henry RR. Effects of weight loss and reduced hyperglycemia on the kinetics of insulin secretion in obese non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1990;70:1594–1602. doi: 10.1210/jcem-70-6-1594. [DOI] [PubMed] [Google Scholar]
- 52.Verdich C, Toubro S, Buemann B, Lysgard MJ, Holst JJ, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 53.Rask E, Olsson T, Soderberg S, Johnson O, Seckl J, Holst JJ, Ahren B. Impaired incretin release after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24:1640–1645. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- 54.DeFronzo RA. Lilly Lecture 1987: The triumvirate:β-cell, muscle, liver: a collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 55.Porte D., Jr Banting Lecture 1990. β-Cells in type II diabetes mellitus. Diabetes. 1991;40:166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- 56.Kahn SE, Larson VG, Schwartz RS, Beard JC, Cain KC, Fellingham GW, Stratton JR, Cerqueira MD, Abrass IB. Exercise training delineates the importance of B-cell dysfunction to the glucose intolerance of human aging. J Clin Endocrinol Metab. 1992;74:1336–1342. doi: 10.1210/jcem.74.6.1592879. [DOI] [PubMed] [Google Scholar]
- 57.Bogardus C. Insulin resistance in the pathogenesis of NIDDM in Pima Indians. Diabetes Care. 1993;16:228–231. doi: 10.2337/diacare.16.1.228. [DOI] [PubMed] [Google Scholar]
- 58.Pi-Sunyer FX. Weight and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1996;63:426S–429S. doi: 10.1093/ajcn/63.3.426. [DOI] [PubMed] [Google Scholar]
- 59.Conn JW. Am J Med Sci. 1940;199:555–564. [Google Scholar]