Abstract
Many antipsychotic drugs disrupt active components of maternal behavior such as pup approach, pup retrieval and nest building at clinically relevant doses in postpartum female rats. However, the neurochemical mechanisms underlying such a disruptive effect remain to be determined. This study examined the neurochemical mechanisms that mediate the disruptive effects of haloperidol (a typical antipsychotic) and clozapine (an atypical antipsychotic) on rat maternal behavior. Postpartum rats were administered with haloperidol (0.2 mg/kg, sc) or clozapine (10.0 mg/kg, sc) together with either vehicle (saline or water), quinpirole (a selective dopamine D2/D3 agonist, 0.5 or 1.0 mg/kg, sc), or 2,5-dimethoxy-4-iodo-amphetamine (DOI, a selective 5-HT2A/2C agonist, 1.0 or 2.5 mg/kg, sc), and their maternal behaviors were tested at different time points before and after drug administration. Haloperidol and clozapine treatment disrupted pup approach, pup retrieval, pup licking and nest building. Pretreatment of quinpirole, but not DOI, dose-dependently reversed the haloperidol-induced disruptions. In contrast, pretreatment of DOI, but not quinpirole, dose-dependently reversed the clozapine-induced disruptions. Quinpirole pretreatment even exacerbated the clozapine-induced disruption of pup retrieval and nest building. These findings suggest a double dissociation mechanism underlying the disruption of haloperidol and clozapine on rat maternal behavior. Specifically, haloperidol disrupts maternal behavior primarily by blocking dopamine D2 receptors, whereas clozapine exerts its disruptive effect primarily by blocking the 5-HT2A/2C receptors. Our findings also suggest that 5-HT receptors are involved in the mediation of rat maternal behavior.
Keywords: haloperidol, clozapine, dopamine D2/D3 receptor, quinpirole, 5-HT2A/2C receptor, DOI, rat maternal behavior
Introduction
Maternal behavior in rats is a complex behavior that shares many features with human mothering behavior (Fleming and Corter, 1988). It is naturally expressed for the first time with the birth of the first litter. Within hours of parturition, the mother rat reconstructs the nest, retrieves the displaced pups, gathers them together in the nest site and adopts a nursing posture over the pups to permit suckling (Dollinger et al., 1980; Rosenblatt and Lehrman, 1963). The mother rat (dam) continues to exhibit maternal behavior (pup licking, pup retrieval, nest building and nursing) over the subsequent 3-week period, although as the pups mature, the intensity and quality of her behavior changes (Galef, 1981; Rosenblatt and Lehrman, 1963).
In recent years, we have used the rat maternal behavior as an ecologically relevant model to investigate the behavioral mechanisms of action of antipsychotic drugs and attempted to capture the possible side effects of antipsychotic medications on human parental behaviors. We and others have shown that clinically comparable doses of haloperidol (HAL), clozapine (CLZ), risperidone and quetiapine (~50%–70% dopamine D2 occupancy) disrupt active components of rat maternal behavior, such as pup retrieval, pup licking and nest building (Li et al., 2004; Stern and Taylor, 1991; Stern and Keer, 1999; Zhao and Li, 2009). HAL causes a prolonged disruption (up to 6 hrs), whereas CLZ and other atypical antipsychotics induce an early onset but transient disruption (less than 6 hrs). Novel antipsychotics such as amisulpride and aripiprazole also exhibit a certain degree of inhibition on active maternal responses in a dose-dependent fashion (Li et al., 2005a). Chronic treatment with HAL or olanzapine via mini-pumps or repeated daily injections significantly inhibits rat active maternal responses as well (Li et al., 2005b). It seems that antipsychotic-induced inhibition on pup retrieval, pup licking and nest building may be an inherent feature of all currently available antipsychotics (Li et al., 2004, 2005a; Silva et al., 2001; Stern and Taylor, 1991; Stern and Keer, 1999; Zhao and Li, 2009). Our recent work further identifies that sedation and suppression of maternal motivation are two important behavioral mechanisms underlying the disruptive effects of antipsychotic treatment on maternal behavior (Zhao and Li, 2009).
The present study investigated the neurochemical basis of antipsychotic-induced disruptive effects on rat maternal behavior. For typical antipsychotics, it is generally assumed that they disrupt maternal behavior by blocking dopamine D2 receptors because they are primarily dopamine D2 antagonists (Dragunow et al., 1990; Seeman et al., 1976), and because apomorphine, a dopamine receptor agonist, can reverse the inhibitory effects of haloperidol (Giordano et al., 1990). However, solid evidence demonstrating that typical antipsychotics do act through this specific D2 mechanism is still lacking. For atypical antipsychotics (e.g., clozapine, olanzapine), because they generally have multiple-receptor binding profiles (Meltzer, 1989; Miyamoto et al., 2005), it is hard to pinpoint their exact neurochemical mechanisms. Most atypical antipsychotics possess a much more potent antagonism on the 5-HT2 receptor in addition to relatively weak antagonism on D2 receptor (Meltzer et al., 2003). It is thus possible that the disruptive effects of atypical antipsychotics on maternal behavior could be attributed to their action on D2 receptor alone (Kapur and Seeman, 2001) or to its dual action on both 5-HT2 and D2 receptor (Meltzer et al., 1989a, 1989b) or on other receptors.
In the present study, we administered quinpirole, a selective D2/D3 dopaminergic receptor agonist or 2,5-dimethoxy-4-iodo-amphetamine (DOI), a selective 5-HT2A/2C serotonergic receptor agonist together with HAL (0.2 mg/kg) or CLZ (10.0 mg/kg) to postpartum rats. We examined which of these two agonists was able to reverse the disruptive effects induced by HAL or CLZ. We hypothesized that quinpirole, but not DOI, would be able to reverse the HAL-induced disruption on maternal behavior and may also be effective in alleviating the CLZ-induced disruption to some extent. We had no prior conviction regarding the effect of DOI due to lack of sufficient evidence in the literature on the role of serotonin in maternal behavior (Numan and Insel, 2003).
Materials and Methods
Subjects
Animals were naive pregnant female Sprague-Dawley rats (9–12 weeks old at the start of pregnancy) purchased from Charles River Inc. (Portage, MI). Upon arrival to our animal facility, the rats were between gestational days 13–15. Each subject was housed individually in 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cage lined with aspen shavings in a colony on a 12-h light/dark cycle (lights on at 6:30 AM). All behavioral tests were performed in the light phase between 9:00 AM and 4:00 PM. The colony was maintained under standard vivarium conditions with an ambient temperature of 23°C, relative humidity of 55–60%. Standard laboratory rat chow and water were available ad libitum. All animal procedures were approved by the University of Nebraska Institutional Animal Care and Use Committee.
Drugs and choices of doses
HAL (5.0 mg/ml ampoules, Sicor Pharmaceuticals, Inc, Irvine, CA) was diluted with sterile water. CLZ (a gift from NIMH drug supply program) was dissolved in 1.0% glacial acetic acid in distilled water. Quinpirole (QUI) and DOI (RBI-Sigma, Natick, MA) were dissolved in 0.9% saline. HAL (0.2 mg/kg), CLZ (10.0 mg/kg), QUI (0.5 or 1.0 mg/kg), DOI (1.0 or 2.5 mg/kg) and vehicle (water or saline) were all administered subcutaneously in a volume of 1.0 ml/kg body weight. Choices of drug doses for HAL and CLZ were based on our previous studies showing that, at these doses, HAL and CLZ effectively disrupt active maternal behaviors in rats (Li et al., 2004; Zhao and Li, 2009). The doses of QUI were chosen based on our preliminary study showing that 1.0 mg/kg of QUI improved the HAL’s disruption on pup retrieval. DOI doses were chosen on the basis of reports showing that DOI at these doses potentiates the effects of amphetamine on dopamine release in the striatum, medial prefrontal cortex and nucleus accumbens (Ichikawa and Meltzer, 1995; Kuroki et al., 2003) and blocks the CLZ-induced dopamine release in the medial prefrontal cortex (Ichikawa et al., 2001).
Basic experimental procedure
Starting 2 or 3 days prior to the first possible expected parturition date, the subjects were monitored every morning for signs of parturition. Once the dam was found with pups in the morning (that day was designated as Day 1 postpartum), the mother was transferred into a clean cage with wood shavings for bedding. Two shredded paper towels were also provided for nesting material. The litter was culled to 8 pups (4 males and 4 females with the most visible milk bands). Maternal behavior tests were conducted on one day between Days 6 and 8 postpartum.
Maternal behavior test
The basic procedure was similar to what has been described in Zhao and Li (2009). Using a laptop computer with an event recording program (JWatcher, http://www.jwatcher.ucla.edu/), we recorded various components of maternal behavior in an 8-min testing session at different time points before and after drug administration. Each test started by removing the 8 pups from the dam and destroying the nest. Ten sec later, the pups were returned to the corner of the cage diagonal to the nest site or dam sleeping corner. When the subject picked up a pup in her mouth and carried it back to the nest site, it was referred to as a successful pup retrieval. The total number of pups retrieved was recorded. Approach latency was defined as the time taken for mother rats to approach and sniff the pups from the reunion. First and last pup retrieval latency was defined as the time elapsed from the first pup approach to the retrieval of the first and eighth pup into the nest, respectively. A score of 480 sec was assigned to non-responders who did not approach or retrieve the testing pups at all. The occurrence of other behaviors was also recorded, including pup nursing behavior (a rat positioning herself over the pups with legs splayed to accommodate the pups, including hover, high and low crouching over postures), pup licking (a female rat placing its tongue on the anogenital area and the rest of a pup’s body), nest building (a rat picking up nesting material in her mouth and transporting it back to the nest site or pushing the material with her forepaws toward the nest site). At the conclusion of the test, any unretrieved pups were returned to the nest site.
Experiment 1. Effects of pretreatment with quinpirole or DOI on haloperidol-induced maternal behavior deficits in rats
Forty-four postpartum rats were randomly assigned to one of the following six groups: VEH+VEH (n = 4, sterile water or saline), VEH+HAL (n = 8), QUI-0.5+HAL (n = 8), QUI-1.0+HAL (n = 8), DOI-1.0+HAL (n = 8), and DOI-2.5+HAL (n = 8). On the drug test day (one day on either Postpartum Day 6, 7, or 8), all subjects were tested 5 times at different time points before and after drug administrations, with the first maternal behavior test starting at 30 min prior to the HAL or vehicle injection (i.e., baseline) and the other tests occurring at 30, 60, 120 and 240 min after HAL or vehicle injection. QUI, DOI or vehicle was injected s.c. twice with the first injection at 10 min before and the second at 50 min after the HAL or vehicle injection. We adopted such an injection strategy because our preliminary data showed that injection of QUI at 10 min prior to the administration of HAL could optimally reverse its disruption on pup retrieval. Also, agonists were injected twice separately on the basis of their relatively short duration of action and rapid peak plasma concentrations (Deutch and Duman, 1996; Whitaker and Lindstrom, 1987).
Experiment 2. Effects of pretreatment with quinpirole or DOI on clozapine-induced maternal behavior deficits in rats
The basic procedure was identical to that of Experiment 1 with the exception that CLZ was tested in this experiment instead of haloperidol. Forty-four postpartum rats were randomly assigned to one of the following six groups and their maternal behaviors were tested: VEH+VEH (n = 4, sterile water or saline), VEH+CLZ (n = 8), QUI-0.5+CLZ (n = 8), QUI-1.0+CLZ (n = 8), DOI-1.0+CLZ (n = 8), and DOI-2.5+CLZ (n = 8).
Data analysis
With the exception of latency data, various measurements of maternal behavior were expressed as mean ± SEM. To increase statistical power, we combined the two vehicle control groups from Experiments 1 and 2 into a single group since they did not differ significantly on any measure (Independent-Samples T test or Mann-Whitney U test, all ps > 0.10). Data were analyzed using a factorial repeated measures analysis of variance (ANOVA) with the between-subjects factor being the treatment groups (e.g. saline, HAL) and the within-subjects factor being test time points (baseline, 30, 60, 120 and 240 min post-injection). Group differences at different time points were further analyzed using simple main effect test (one-way ANOVA) followed by Turkey’s HSD post hoc tests where appropriate.
For the latency data, because they were not normally distributed (e.g. the cut-off time assigned 480 sec for the latency data), these data were expressed as median ± interquartile range. Nonparametric Kruskal-Wallis test was used for analyzing the differences among the treatment groups. Once the overall significant effects were determined, two-group comparisons were performed using Mann-Whitney U test. A conventional two-tailed level of significance at the 0.05 level was required.
Results
Experiment 1. Effects of pretreatment with QUI or DOI on haloperidol-induced maternal behavior deficits
Haloperidol treatment disrupted various components of maternal behavior
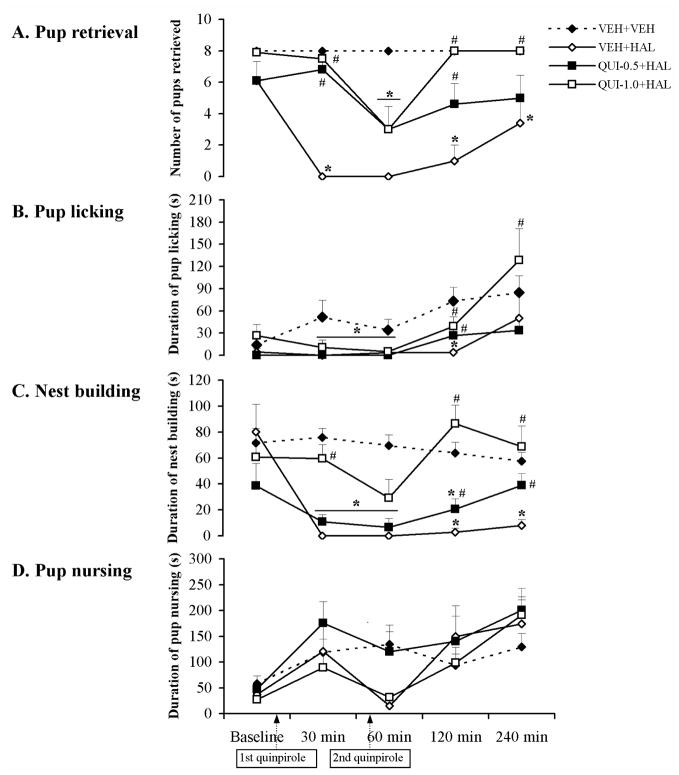
Consistent with our previous findings (Li et al., 2004; Zhao and Li, 2009), a single injection of HAL significantly disrupted various active components of maternal behavior (pup retrieval, pup licking, nest building, but not pup nursing). Rats treated with HAL took a much longer time to initiate contact with pups and retrieve their pups into the nest sites (Table 1) and retrieved fewer pups at the 30, 60, 120 and 240 min test points (Figs. 1,2A) (0.001 < all ps < 0.05) compared to the vehicle-treated rats. They also spent less time licking their pups at the 30, 60 and 120 min test points and rebuilding the nest at the 30, 60, 120 and 240 min test points (Figs. 1,2B,C) (0.001 < all ps < 0.05). However, HAL treatment had no significant effect on nursing activity (Figs. 1,2D).
Table 1.
Effects of pretreatment with quinpirole or DOI on pup approach latency and pup retrieval latency in postpartum female rats treated with haloperidol or vehicle
| Gro ups |
Approach latency (s) | First pup retrieval latency (s) | Last pup retrieval latency (s) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aseline | 0 min | 0 min | 20 min | 40 min | aseline | 0 min | 0 min | 20 min | 40 min | aseline | 0 min | 0 min | 20 min | 40 min | |
| VEH+VEH | .1 | .2 | .5 | .5 | .9 | .8 | .0 | .6 | .1 | .9 | 8.3 | 2.6 | 3.9 | 1.7 | 4.0 |
| 2.0) | 0.7) | 1.2) | 0.8) | 4.6) | 87.0) | 0.9) | 1.4) | 0.7) | 0.5) | 104.0) | 9.7) | 7.7) | 9.8) | 14.4) | |
| VEH+HAL | .8 | 5.6a | 7.5a | 5.4a | 3.2a | 1.7 | 80.0a | 80.0a | 80.0a | 45.6a | 12.4 | 80.0a | 80.0a | 80.0a | 80.0a |
| 0.9) | 106.9) | 311.7) | 224.6) | 135.1) | 100.8) | 0.0) | 0.0) | 0.0) | 476.1) | 382.9) | 0.0) | 0.0) | 0.0) | 272.7) | |
| QUI-0.5+HAL | .4 | .2b | .4b | .6b | .8b | 7.4 | .0b | 80.0a | .3b | .3b | 41.0 | 5.9a,b | 80.0a | 85.4a | 4.0a,b |
| 0.8) | 15.1) | 26.9) | 5.6) | 41.6) | 89.6) | 35.7) | 357.8) | 358.2) | 358.4) | 289.1) | 55.9) | 241.2) | 443.6) | 352.6) | |
| QUI-1.0+HAL | 8 | .8b | 2.5b | 7 | 6b | 0.7 | 3b | 47.1a | 1b | .4b | 52.3 | 05.7a,b | 80.0a | 45.9a,b | 7.1a,b |
| 4.1) | 7.4) | 78.1) | 24.5) | 14.2) | 84.1) | 12.0) | 474.9) | 2.0) | 0.7) | 260.0) | 417.8) | 284.9) | 104.0) | 45.5) | |
| DOI-1.0+HAL | .3 | 3.1a | 15.7a | 3.6a | 0.6a | .8 | 93.6a | 80.0a | 80.0a | 5.2a | 79.7 | 80.0a | 80.0a | 80.0a | 80.0a |
| 0.7) | 29.5) | 122.6) | 52.2) | 361.4) | 189.40 | 441.1) | 0.0) | 0.0) | 476.8) | 450.6) | 0.0) | 0.0) | 0.0) | 356.1) | |
| DOI-2.5+HAL | .0 | 9.3a | 80.0a,b | 78.6a | 1.9a | .1 | 80.0a | 80.0a | 80.0a | 80.0a | 9.6 | 80.0a | 80.0a | 80.0a | 80.0a |
| 5.9) | 107.8) | 169.4) | 446.2) | 216.1) | 15.3) | 0.0) | 0.0) | 0.0) | 0.0) | 59.2) | 0.0) | 0.0) | 0.0) | 0.0) | |
Data are represented as median ± interquartile range.
p < 0.05 relative to the VEH+VEH group.
p < 0.05 relative to the VEH+HAL group.
Fig. 1.
The time course of effects of the dopamine D2/D3 receptor agonist quinpirole on various HAL-induced maternal behavior deficits in postpartum female rats. Pup retrieval (A), pup licking (B), nest building (C) and pup nursing (D) were tested at baseline, 30, 60, 120 and 240 min after injection of HAL or vehicle. HAL disrupted all the active maternal behaviors tested (A–C), but had no significant effect on pup nursing (D). Both 0.5 and 1.0 mg/kg of quinpirole improved the maternal behavior deficits induced by HAL in a dose-dependent fashion (A–C). Data were represented as mean + SEM. * p < 0.05 versus VEH+VEH control; # p < 0.05 versus VEH+HAL group.
Fig. 2.
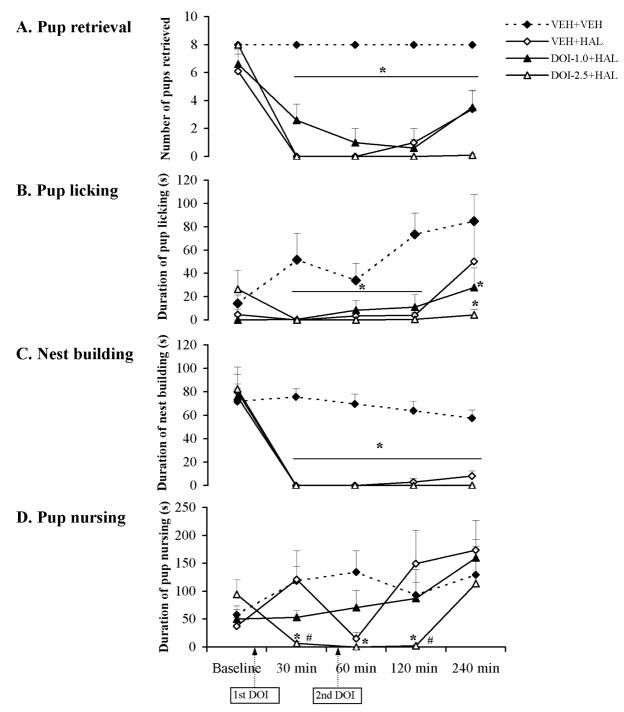
The time course of effects of the 5-HT2A/2C receptor agonist DOI on various HAL-induced maternal behavior deficits in postpartum female rats. Pup retrieval (A), pup licking (B), nest building (C) and pup nursing (D) were tested at baseline, 30, 60, 120 and 240 min after injection of HAL or vehicle. HAL disrupted all the active maternal behaviors tested (A–C), but had no significant effect on pup nursing (D). Neither 1.0 nor 2.5 mg/kg of DOI rescued the HAL-induced deficits on active maternal behaviors (A–C), but 2.5 mg/kg of DOI further suppressed nursing activity in HAL-treated dams (D). Data were represented as mean + SEM. * p < 0.05 versus VEH+VEH control; # p < 0.05 versus VEH+HAL group.
QUI pretreatment dose-dependently improved the haloperidol-induced maternal behavior deficits
As can be seen in Table 1 and Fig. 1A–C, both doses of QUI improved the HAL-induced deficits in pup approach, pup retrieval, pup licking and nest building, as described below.
1. Latency to approach and retrieve pups
QUI shortened the HAL-induced increase in pup approach latency and the first and last pup retrieval latencies (Table 1). Both 0.5 and 1.0 mg/kg doses of QUI were effective in facilitating the HAL-treated dams to initiate contact with their pups and completely restored such behavior to the normal level (e.g., VEH+VEH group) across the testing period. For the pup retrieval latency, it appeared that both doses of QUI shortened the time the HAL-treated rats took to retrieve their first and last pups to the nest site and reinstated the first pup retrieval to the normal level at the 30, 120 and 240 min test points post-injection.
2. Number of Pups retrieved
Both 0.5 and 1.0 mg/kg of QUI increased the number of pups retrieved in the HAL-treated rats and restored it to the normal level at certain time points of testing. Repeated measures ANOVA revealed that there was a significant interaction between the treatment groups and time points [F (20, 168) = 5.97, p < 0.001], a significant main effect of treatment groups [F (5, 42) = 26.30, p < 0.001], and a significant effect of time points [F (4, 168) = 35.22, p < 0.001]. One-way ANOVA and post hoc tests showed that the QUI-0.5+HAL group was significantly different from the VEH+HAL group at the 30 and 120 min time points (Fig. 1A, p < 0.001 for 30 min, p = 0.022 for 120 min), whereas the QUI-1.0+HAL group differed from the VEH+HAL group at the 30, 120 and 240 min test points (Fig. 1A, p < 0.001 for 30 and 120 min, p = 0.014 for 240 min). Further analysis showed that the reversing effect of 0.5 mg/kg of QUI reached the control level at the 30 and 120 min test points while the 1.0 mg/kg of QUI restored the CLZ-induced disruption to the normal level at the 30, 120 and 240 min test points when the two QUI+HAL groups were compared with the VEH+VEH control group (Fig. 1A, all ps > 0.10). It appears that the strongest reversal effect of QUI occurred 120 min after HAL administration (Fig. 1A).
3. Pup licking
QUI produced a reversing effect on HAL-induced pup licking deficit. Repeated measures ANOVA revealed a main effect of treatment groups [F (5, 42) = 4.56, p = 0.002], a significant effect of test time points [F (4, 168) = 7.71, p < 0.001], but no significant interaction between the two factors. One-way ANOVA and post hoc tests showed that both QUI groups differed significantly from the VEH+HAL group at the 120 min test point (Fig. 1B, p = 0.045 for QUI-0.5+HAL group, p = 0.042 for QUI-1.0+HAL group). Further analysis showed that the reversing effect reached the normal level when the two QUI+HAL groups were compared to the VEH+VEH control group (Fig. 1B, p = 0.85 for QUI-0.5+HAL group, p = 0.95 for QUI-1.0+HAL group).
4. Nest building
Similar to the effect on pup retrieval and pup licking, both doses of QUI significantly improved the HAL-induced disruption of nest building activity. Repeated measures ANOVA revealed a significant interaction between the two factors [F (20, 168) = 6.18, p < 0.001], a significant main effect of treatment groups [F (5, 42) = 17.52, p < 0.001], and a significant effect of testing time [F (4, 168) = 35.95, p < 0.001]. One-way ANOVA and post hoc tests showed that the QUI-0.5+HAL group was significantly different from the VEH+HAL group at the 120 and 240 min test points (both ps = 0.021), whereas the QUI-1.0+HAL group differed significantly from the VEH+HAL group at the 30, 120 and 240 min test points post-injection (Fig. 1C, all ps < 0.001). In particular, although both doses of QUI reversed the disruptive effect of HAL at the 120 min test point, only 1.0 mg/kg of QUI was capable of improving it to the normal level when compared to the VEH+VEH group (Fig. 1C, p = 0.34).
5. Pup nursing
As can be seen in Fig. 1D, QUI pretreatment had no significant effect on pup nursing. Repeated measures ANOVA revealed a significant interaction between the two factors [F (20, 168) = 2.14, p = 0.005], a significant main effect of treatment groups [F (5, 42) = 4.66, p = 0.002], and a significant effect of testing time [F (4, 168) = 10.53, p < 0.001]. Post hoc tests showed that both QUI groups were not significantly different from the VEH+HAL group (both ps > 0.10).
DOI pretreatment failed to improve the haloperidol-induced maternal behavior deficits
Both 1.0 and 2.5 mg/kg of DOI failed to improve the HAL-induced disruption on pup approach and pup retrieval, as evidenced by no significant improvement in the approach latency nor in first and last pup retrieval latency (Table 1) when the two DOI+HAL groups were compared to the VEH+HAL group. Rather, 2.5 mg/kg of DOI further exacerbated the HAL-induced disruption on the approach latency at the 60 min test point (Table 1, p = 0.005). Post hoc tests on the number of pups retrieved, pup licking and nest building revealed that there was no significant difference between the two DOI+HAL groups and the VEH+HAL group. For pup nursing, one-way ANOVA and post hoc tests indicated that the DOI-2.5+HAL group showed an even more suppressed nursing duration at the 30 and 120 min test points compared to the VEH+HAL group (p = 0.048 for 30 min, p = 0.041 for 120 min) (Fig. 2D). The DOI-1.0+HAL group did not differ significantly from the VEH+HAL group across the testing period.
Experiment 2. Effects of pretreatment with QUI or DOI on clozapine-induced maternal behavior deficits
Clozapine treatment impaired various components of maternal behavior
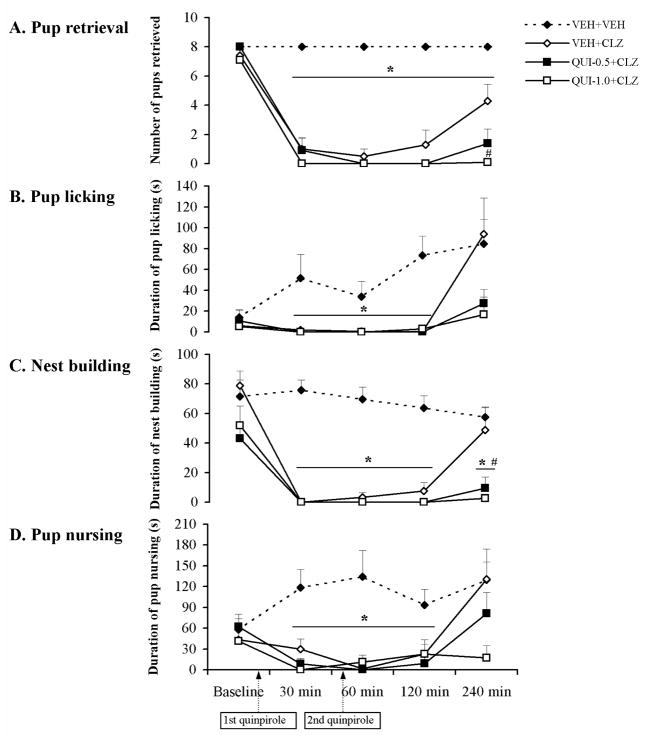
Consistent with our previous findings (Li et al., 2004; Zhao and Li, 2009), the present study showed that a single injection of CLZ significantly disrupted various active components of maternal behavior and maintained such a disruptive effect for about 2 hrs. In comparison with the vehicle-treated ones, rats treated with CLZ took a much longer time to approach and retrieve their first pups into the nest sites (Table 2) at the 30, 60 and 120 min test points (0.001 < all ps < 0.01) and took longer to bring the last pups into the nest with less pups retrieved across the testing period (Table 2, psFigs. 3,4A) (0.001 < all < 0.05). They also spent less time on pup licking, nest building and pup nursing at the 30, 60 and 120 min test points (Figs. 3,4B,C,D) (all ps < 0.05).
Table 2.
Effects of pretreatment with quinpirole or DOI on pup approach latency and pup retrieval latency in postpartum female rats treated with clozapine or vehicle
| Gro ups |
Approach latency (s) | First pup retrieval latency (s) | Last pup retrieval latency (s) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aseline | 0 min | 0 min | 20 min | 40 min | aseline | 0 min | 0 min | 20 min | 40 min | aseline | 0 min | 0 min | 20 min | 40 min | |
| VEH+VEH | .1 | .2 | .5 | .5 | .9 | .8 | .0 | .6 | .1 | .9 | 8.3 | 2.6 | 3.9 | 1.7 | 4.0 |
| 2.0) | 0.7) | 1.2) | 0.8) | 4.6) | 87.0) | 0.9) | 1.4) | 0.7) | 0.5) | 104.0) | 9.7) | 7.7) | 9.8) | 14.4) | |
| VEH+CLZ | .8 | 8.5a | 90.8a | 1.8a | 0.2 | .9 | 80.0a | 80.0a | 80.0a | 3.0 | 0.8 | 80.0a | 80.0a | 80.0a | 15.4a |
| 1.7) | 357.3) | 474.6) | 477.2) | 43.7) | 10.4) | 357.1) | 0.0) | 346.9) | 154.8) | 130.8) | 0.0) | 0.0) | 0.0) | 420.6) | |
| QUI-0.5+CLZ | .7 | 77.2a | 02.9a | 68.2a | .7 | 4.3 | 80.0a | 80.0a | 80.0a | 80.0a,b | 5.7 | 80.0a | 80.0a | 80.0a | 80.0a |
| 16.2) | 473.2) | 330.3) | 432.7) | 7.6) | 20.6) | 0.0) | 0.0) | 0.0) | 339.1) | 146.3) | 0.0) | 0.0) | (0.0) | 0.0) | |
| QUI-1.0+CLZ | .3 | 59.9a | 29.0a | 19.3a | 01.4a,b | 0.4 | 80.0a | 80.0a | 80.0a | 80.0a,b | 43.4 | 80.0a | 80.0a | 80.0a | 80.0a |
| 9.1) | 460.3) | 240.4) | 464.0) | 442.5) | 75.1) | 0.0) | 0.0) | 0.0) | 0.0) | 408.2) | 0.0) | 0.0) | 0.0) | 0.0) | |
| DOI-1.0+CLZ | .2 | .9b | .8b | .7b | .2 | .7 | .0a | .8b | .4 | .2 | 07.3 | 91.4a | 8.3b | 14.7b | 4.8 |
| 1.1) | 38.4) | 24.5) | 1.2) | 5.3) | 168.0) | 363.1) | 358.7) | 441.4) | 360.7) | 442.8) | 440.3) | 453.3) | 452.0) | 456.8) | |
| DOI-2.5+CLZ | .6 | .8b | .3b | .3b | .8 | .7 | 1.8a | .5b | .4b | .2 | 7.13 | 80.0a | 6.93b | 5.13b | 4.4b |
| 2.6) | 0.4) | 7.2) | 34.6) | 4.9) | 1.7) | 371.5) | 63.4) | 34.8) | 3.8) | 32.0) | 396.9) | 372.2) | 104.7) | 54.7) | |
Data are represented as median ± interquartile range.
p < 0.05 relative to the VEH+VEH group.
p < 0.05 relative to the VEH+CLZ group.
Fig. 3.
The time course of effects of the dopamine D2/D3 receptor agonist quinpirole on various CLZ-induced maternal behavior deficits in postpartum female rats. Pup retrieval (A), pup licking (B), nest building (C) and pup nursing (D) were tested at baseline, 30, 60, 120 and 240 min after injection of CLZ or vehicle. CLZ disrupted all maternal behaviors tested (A–D). Both doses of quinpirole (0.5 and 1.0 mg/kg) failed to improve the maternal behavior deficits induced by CLZ (A–D), but rather worsened pup retrieval (A) and nest building (C) deficits. Data were represented as mean + SEM. * p < 0.05 versus VEH+VEH control; # p < 0.05 versus VEH+CLZ group.
Fig. 4.
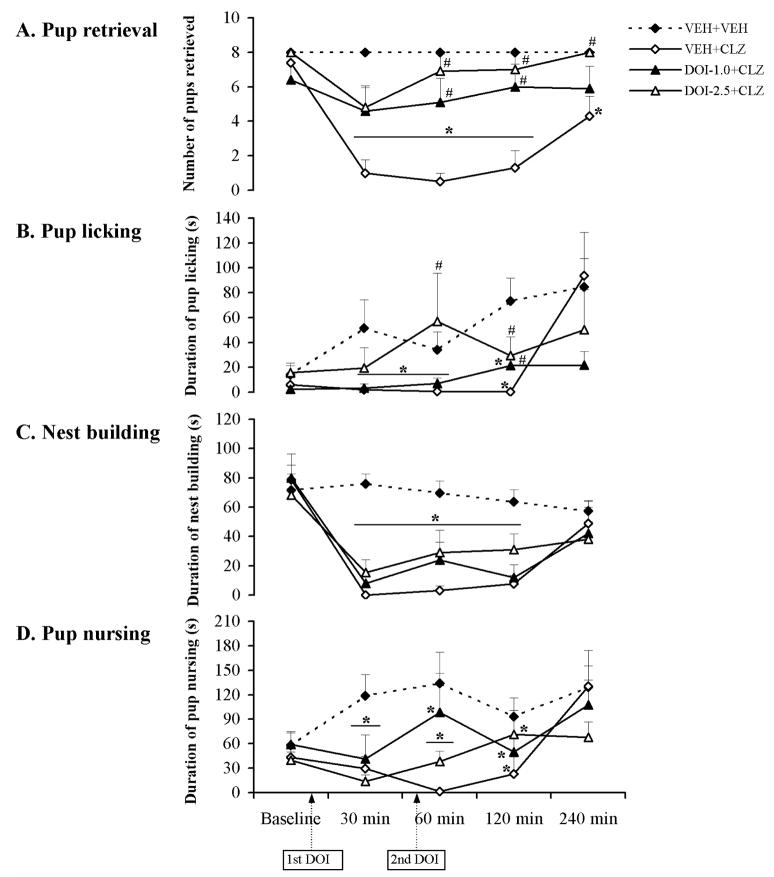
The time course of effects of the 5-HT2A/2C receptor agonist DOI on various CLZ-induced maternal behavior deficits in postpartum female rats. Pup retrieval (A), pup licking (B), nest building (C) and pup nursing (D) were tested at baseline, 30, 60, 120 and 240 min after injection of CLZ or vehicle. CLZ disrupted all maternal behaviors tested (A–D). Both doses of DOI (1.0 and 2.5 mg/kg) significantly improved the pup retrieval and pup licking deficits induced by CLZ (A, B). However, DOI did not improve the CLZ-induced disruption on nest building and pup nursing (C, D). Data were represented as mean + SEM. * p < 0.05 versus VEH+VEH control; # p < 0.05 versus VEH+CLZ group.
QUI pretreatment failed to improve the clozapine-induced maternal behavior deficits
In contrast to its effects on HAL-induced maternal behavior deficits, both doses of QUI had little effect on the CLZ-induced disruptions of pup approach, pup retrieval latency (Table 2), the number of pups retrieved and nest building (Fig. 3A,C). Contrarily, QUI further potentiated the disruptive effect on these behaviors (Table 2, Fig. 3A,C). Repeated measures ANOVA on the number of pups retrieved and nest building revealed a significant interaction between the treatment groups and test time points [the number of pups retrieved: F (20, 168) = 9.56, p < 0.001; nest building: F (20, 168) = 2.77, p < 0.001], a significant main effect of treatment groups [the number of pups retrieved: F (5, 42) = 20.41, p < 0.001; nest building: F (5, 42) = 13.61, p < 0.001], and a significant effect of test time points [the number of pups retrieved: F (4, 168) = 61.43, p < 0.001; nest building: F (4, 168) = 38.33, p < 0.001]. One-way ANOVA and post hoc tests showed that the QUI-1.0+CLZ group differed significantly from the VEH+CLZ group in the number of pups retrieved and the duration of nest building at the 240 min test point (Fig. 3A,C, p = 0.001 for the number of pups retrieved, p = 0.007 for the nest building), while the QUI-0.5+CLZ group differed from the VEH+CLZ group in nest building at the 240 min test point (Fig. 3C, p = 0.026). QUI pretreatment also did not improve the CLZ-induced deficits in pup licking and pup nursing activities (Fig. 3B,D) at any test points. Repeated measures ANOVA on pup licking and pup nursing revealed a significant interaction between the two factors [pup licking: F (20, 168) = 1.68, p = 0.04; pup nursing: F (20, 168) = 2.05, p = 0.007], a significant main effect of treatment groups [pup licking: F (5, 42) = 4.47, p = 0.002; pup nursing: F (5, 42) = 5.52, p = 0.001], and a significant effect of test time points [pup licking: F (4, 168) = 8.02, p < 0.001; pup nursing: F (4, 168) = 6.72, p < 0.001]. Post hoc tests on pup licking and pup nursing revealed that there was no significant difference between the two QUI+CLZ groups and the VEH+CLZ group.
DOI pretreatment dose-dependently reversed the clozapine-induced maternal behavior deficits
As shown in Table 2 and Fig. 4A,B, pretreatment of both doses of DOI (1.0 and 2.5 mg/kg) significantly improved the CLZ-induced disruption on pup approach, pup retrieval, and pup licking. However, the reversing effect of DOI on CLZ-induced disruption of nest building and pup nursing was not apparent (Fig. 4C,D).
1. Latency to approach and retrieve pups
As shown in Table 2, both 1.0 and 2.5 mg/kg of DOI shortened the prolonged approach latency induced by CLZ when the two DOI+CLZ groups were compared to the VEH+CLZ group and restored it to the normal level compared to the VEH+VEH group at the 30, 60 and 120 min test points (0.001 < all ps < 0.01). It also reduced the CLZ-induced deficits of the first pup retrieval and restored it to the normal level at the 60 min test point (p = 0.035) at a dose of 1.0 mg/kg as well as at the 60 and 120 min test points (both ps < 0.01) at a dose of 2.5 mg/kg. For the last pup retrieval latency, both doses of DOI completely rescued the disruption at the 60 and 120 min (0.001 < all ps < 0.01) test points. Furthermore, 2.5 mg/kg of DOI shortened the last pup retrieval latency at the 240 min test point (p = 0.001).
2. Number of pups retrieved
Both doses of DOI increased the number of pups retrieved in the CLZ-treated rats. One-way ANOVA and post hoc tests showed that there was a significant difference between the DOI-1.0+CLZ group and the VEH+CLZ group at the 60 and 120 min test points (p = 0.006 for 60 min, p = 0.008 for 120 min), whereas the DOI-2.5+CLZ group differed significantly from the VEH+CLZ group even at the 240 min test point (Fig. 4A, 0.001 < all ps < 0.05). Further analysis showed that the reversing effect of 1.0 mg/kg of DOI reached the normal level at the 60 and 120 min test points while the 2.5 mg/kg of DOI restored the CLZ-induced disruption to the normal level at 60, 120 and 240 min test points (Fig. 4A, all ps > 0.05). It appears that the strongest reversal effect of DOI occurred 120 min after CLZ administration (Fig. 4A).
3. Pup licking
Both doses of DOI alleviated the CLZ-induced disruption on pup licking. One-way ANOVA and post hoc tests showed that there was a significant difference between the DOI-1.0+CLZ group and the VEH+CLZ group at the 120 min test point (p = 0.049), while the DOI-2.5+CLZ group differed significantly from the VEH+CLZ group at the 60 and 120 min test points (Fig. 4B, p = 0.034 for 60 min, p = 0.015 for 60 min). Further analysis showed that only 2.5 mg/kg of DOI restored this behavior to the normal level at the 60 and 120 min test points when the two DOI+CLZ groups were compared with the VEH+VEH control group (Fig. 4B, both ps > 0.05).
4. Nest building and pup nursing
Compared to the reversing effects on pup retrieval and pup licking, DOI at the currently tested doses appeared to be ineffective in reversing the CLZ-induced deficits on nest building and pup nursing. Post hoc tests on nest building and pup nursing revealed that there was no significant difference between the two DOI+CLZ groups and the VEH+CLZ group (Fig. 4C,D, all ps > 0.05).
Discussion
The present study demonstrates an interesting double dissociation between dopamine and serotonin receptor mechanisms in the mediation of HAL- and CLZ-induced maternal behavior deficits in postpartum rats. Acute treatment of HAL and CLZ disrupted various components of rat maternal behavior. Pretreatment of QUI, a selective D2/D3 dopaminergic receptor agonist, but not DOI, a selective 5-HT2A/2C serotonergic receptor agonist, dose-dependently improved HAL-induced disruption of pup approach, pup retrieval, pup licking and nest building, whereas pretreatment of DOI, but not QUI, dose-dependently improved CLZ-induced disruption of pup approach, pup retrieval and pup licking. These data strongly suggest that the HAL-induced maternal deficits are primarily mediated by its blockade of D2 dopamine receptors, whereas the CLZ-induced maternal deficits are primarily mediated by the blockade of 5-HT2A/2C receptors.
The dopamine systems have been well documented to play an important role in the regulation of maternal behavior. For example, systemic (e.g., haloperidol, a D2 antagonist) or microinfusion of dopamine receptor antagonists such as SCH-23390 (a D1 antagonist), pimozide (a D2 antagonist) and cis-flupenthixol (a mixed D1/D2 antagonist) into the specific brain regions implicated in maternal behavior (e.g., medial preoptic area, nucleus accumbens) disrupts various active maternal behaviors (Byrnes et al., 2002; Giordano et al., 1990; Keer and Stern, 1999; Li et al., 2004; Miller and Lonstein, 2005; Numan et al., 2005; Numan and Stolzenberg, 2009; Silva et al., 2001, 2003; Stern and Taylor, 1991). 6-OHDA lesion of the ventral striatum and ventral tegmental area disrupts pup retrieval (Hansen, 1994; Hansen et al., 1991a, 1991b). Additionally, mother-pup separation, which putatively enhances maternal motivation (Hansen, 1994), stimulates dopamine release in the ventral striatum of maternal rats (Hansen et al., 1993). Because HAL is primarily a D2 receptor antagonist, we hypothesized that it disrupts maternal behavior by blocking D2 dopamine receptors. We tested this hypothesis by co-administrating QUI, a selective D2/D3 agonist together with HAL and examined whether QUI could reverse the effects of HAL. As expected, QUI did significantly improve the HAL-induced maternal behavior deficits. HAL rats pretreated with QUI showed shortened pup approach and retrieval latency than those pretreated with vehicle. This result is in general agreement with a previous study showing that apomorphine, a mixed D1/D2 receptor agonist with a preferential affinity and potency for the D2 over D1 receptor subtype (Kebabian and Calne, 1979), reversed HAL-induced pup retrieval deficits in postpartum rats (Giordano et al., 1990). We extended this line of research by showing that HAL specifically acts on D2 receptors to achieve its disruptive effect. Of note, this finding does not necessarily rule out the involvement of the D1 receptor mechanism because HAL does have a weak D1 antagonism (Miyamoto et al., 2005), and other studies also find that D1 receptors are involved in the regulation of maternal behavior (Miller and Lonstein, 2005; Numan et al., 2005; Numan and Stolzenberg, 2009).
The lack of the effect of DOI treatment on HAL-induced maternal behavior deficits is not surprising given the fact that DOI is a selective 5-HT2A/2C agonist and HAL lacks an action on these serotonergic receptor systems. Interestingly, DOI pretreatment further decreased the duration of pup nursing in the HAL-treated rats (see Fig. 2D). Informal observations indicate that after the pups were returned to the cage, the HAL rats pretreated with 2.5 mg/kg of DOI did not approach and retrieve their pups, but stayed in the corner of the cage with no apparent movement. This may explain the reduction of pup nursing by DOI. In light that DOI itself reduces locomotor activity (Hameleers et al., 2007; Krebs-Thomson and Geyer, 1996; Mittman and Geyer, 1991; Wing et al., 1990), it is possible that DOI-induced hypolocomotor activity may have contributed to its disruptive effect on pup nursing.
Our original hypothesis that QUI may also attenuate the CLZ-induced disruption to some extent, as CLZ also possesses a D2 antagonism, was not supported by the present findings. In contrast, QUI actually worsened some maternal behavior deficits, such as on pup retrieval and nest building. These findings indicate that CLZ-induced maternal deficits are not primarily mediated by its weak antagonism of D2 receptor. Our preliminary work (data not shown) finds that QUI at a dose of 1.0 mg/kg by itself disrupts pup retrieval and nest building. Therefore, it is possible that a combination of clozapine and QUI may provide an additive disruption on the CLZ-induced maternal deficits.
The most striking finding from the present study was that DOI pretreatment rescued most, if not all, maternal behavior deficits induced by CLZ. We found that CLZ rats pretreated with DOI took a much shorter time to initiate contact with pups and retrieve their pups into the nest sites (Table 2), retrieved more pups (Fig. 4A) and spent more time licking their pups than CLZ rats pretreated with vehicle (Fig. 4B). CLZ has a unique and multifaceted pharmacological, behavioral and clinical profile in comparison with other antipsychotic drugs (Matsubara et al., 1993; Meltzer, 1989; Safferman et al., 1991; Schmauss et al., 1989) due to its multiple-receptor action, including a relatively high affinity for D4, histaminergic (H1), muscarinic, serotoninergic (5-HT2) and alpha-1-adrenergic receptors (Baldessarini et al., 1992; Lane et al., 1988; Meltzer, 1989; Meltzer et al., 1989a; Miller and Hiley, 1974; Seeman, 1992; Zavitsanou et al., 2007), moderate affinity for D1, D2, D5, alpha-2-adrenergic and 5-HT3 receptors (Ellenbroek et al., 1991; Matsubara et al., 1993; Miyamoto et al., 2005; Murray and Waddington, 1990; Perry et al., 1983; Remington and Kapur, 2000). The unique antipsychotic property of CLZ (e.g., low EPS and less prolactin elevation) has been correlated with its low affinity and fast dissociation at the D2 receptor (Kapur and Remington, 2001; Kapur and Seeman, 2001; Seeman, 2002). This profile makes it difficult to pinpoint the exact mechanisms mediating the antipsychotic and behavioral effects of CLZ. Our finding that DOI dose-dependently improved the CLZ-induced maternal behavior deficits suggests that CLZ exerts its disruptive effect on maternal behavior through a 5-HT2A/2C receptor-mediated mechanism, and that this effect thus might not be directly related to its antipsychotic efficacy, which is mediated by blockade of D2 receptors (Seeman, 2002).
There are several possible mechanisms underlying the reversing effect of DOI on CLZ-induced maternal behavior deficits. First, DOI may directly activate the 5-HT2A/2C receptors localized in the brain regions important for maternal behavior, such as the nucleus accumbens (NA) and the ventral tegmental area (VTA), etc. There is considerable evidence demonstrating that moderate to high levels of 5-HT2A/2C receptor immunoreactivity (Bubar et al., 2005; Clemett et al., 2000; Cornea-Hebert et al., 1999; Jakab and Goldman-Rakic, 1998) and mRNAs (Eberle-Wang et al., 1997; Pompeiano et al., 1994; Wright et al., 1995) are expressed in the NA and VTA, thus, DOI may antagonize CLZ’s blockade on 5-HT2A/2C receptors through competitive binding to 5-HT receptors in these areas to alleviate the CLZ-induced maternal behavior deficits.
Second, DOI may improve CLZ-induced maternal deficits through its indirect action on the dopaminergic systems. The mesolimbic dopamine system, originating in the VTA and terminating in the NA has been implicated in the appetitive aspects of maternal behavior (Hansen 1994; Hansen et al. 1991a, 1991b; Hansen et al. 1993; Numan 2007; Numan et al. 2005). Recently, 5-HT2A/2C receptors were found to be localized on the dopamine neurons in the VTA (Bubar and Cunningham 2007; Doherty and Pickel 2000; Ji et al. 2006; Nocjar et al. 2002). This finding provides evidence in support of the possible interaction between the serotonin and dopamine systems in the VTA (Alex and Pehek 2007; Di Matteo et al. 2001). More importantly, a growing body of evidence shows that 5-HT2 receptors play a prominent role in the modulation of mesolimbic dopamine-mediated function. For example, in the NA, activation of 5-HT2 receptor appears to facilitate dopamine release that can be blocked by local injection of a 5-HT2 receptor antagonist (Parsons and Justice, 1993). DOI, a mixed 5-HT2A/2C agonist, has been shown to increase extracellular levels of dopamine in the posterior NA (Bowers et al., 2000). Thus, it is possible that DOI may reverse the effect of CLZ by regulating the DA release in the NA and thus indirectly regulate maternal behavior via the DA system. Future research should directly address the neural pathways that mediate the modulatory effects of 5-HT2A/2C receptors on the mesolimbic dopamine systems.
Third, at the behavioral level, the diminished sedation could be a contributing factor in reversing CLZ-induced maternal deficits by DOI. In the present study, we noted that DOI treatment significantly alleviated CLZ-induced sedative effect as those rats showed increased motor activity, no sign of closed eyes or bowed head. It has been reported that acute and repeated injection of CLZ at a dose of 10.0 mg/kg produces substantial sedation in animals (Chesler and Salamone, 1996). The CLZ-induced sedative effect has also been reported in the clinic (Burke and Sebastian, 1993; Safferman et al., 1991). Moreover, recent work from our laboratory shows that the sedative effect of CLZ contributes to its disruption on maternal behavior (Zhao and Li, 2009). At this point, it is still premature to suggest that DOI reverses CLZ’s disruption on maternal behavior by alleviating the sedative effect of CLZ, because CLZ is not known to cause sedation by blocking 5-HT2A/2C receptors, but rather by blocking alpha-adrenergic and histamine receptors (Lieberman et al., 1989; Safferman et al., 1991). More research is needed to examine whether CLZ can cause sedation via action on 5-HT2A/2C receptors.
Because improvement of CLZ-induced disruption of nest rebuilding was not apparently observed after DOI pretreatment, it is possible that other neurotransmitter systems may be involved in CLZ-induced nest building deficit. One such system could involve the dopamine D2/D3 receptors, as our results show that HAL-induced disruption of nest building was reversed and restored to the normal level by QUI, a selective D2/D3 receptor agonist. Other systems may include dopamine D1, D4 and D5 receptors. CLZ has high affinity for D4 and moderate affinity for D1 and D5 receptors. Consistent with this hypothesis, previous studies have shown that D1 receptor activity is critical for the regulation of maternal behavior. Microinjection of a dopamine D1 receptor antagonist SCH-23390 into the medial preoptic area (MPOA) or nucleus accumbens (NA) greatly impairs pup retrieval in postpartum rats (Miller and Lonstein, 2005; Numan et al., 2005). Microinfusion of a dopamine D1 receptor agonist SKF-38393 into either the MPOA or the NA promotes the onset of maternal behavior in pregnancy-terminated rats (Stolzenberg et al., 2007). However, it is unknown whether the D4 and/or D5 receptors are involved in the regulation of maternal behavior due to lack of evidence. Alternatively, nest building activity may still be mediated by 5-HT2A/2C receptors, but is more sensitive and vulnerable to pharmacological intervention (Li et al., 2004; Silva et al., 2001; Zhao and Li, 2009). Future work should address this issue in detail.
The present study provides strong evidence supporting the involvement of 5-HT2A/2C receptors in the mediation of rat maternal behavior. Previous work on this issue is inadequate and inconsistent. For example, Brunner et al. (1999) reported that 5-HT1B receptor knockout mother mice did not display pup retrieval deficits. More recently, Lerch-Haner et al. (2008) reported that transgenic mouse dams with a specific disruption in serotonin neuron development displayed profound maternal deficits (e.g., pup retrieval, pup nursing, nest building). This study, however, did not answer which subtypes of 5-HT receptor are implicated in maternal activity. Other studies have found that 5-HT1B and 5-HT2A/2C receptors are involved in maternal aggressive behavior (De Almeida et al., 2005, 2006a, 2006b; Olivier et al., 1995; Veiga et al., 2007). For example, intracerebroventricular injections of DOI, a 5-HT2A/2C agonist, inhibited maternal aggression in postpartum female rats (De Almeida and Lucion, 1994). To the best of our knowledge, the present study was the first to demonstrate that 5-HT2A/2C receptors may be important in regulating rat maternal behavior.
Consistent with our previous work (Li et al., 2004), we found that HAL and CLZ had different time courses of action. HAL tended to produce a prolonged disruption, whereas CLZ produced a transient one. For instance, the HAL-induced disruption of nest building was still apparent even at the 240 min test point (4 hr post-injection), whereas the effect of CLZ was diminished (Figs. 1,3, C). It appears that the reversal effect of QUI on HAL-induced maternal deficits also differed in regard to time course of action from that of DOI on CLZ-induced deficits. For example, the reversal effect of DOI on CLZ-induced disruption of pup retrieval was somewhat delayed in onset when compared to that of quinpirole on HAL-induced impairment (Figs. 1,4A), which may be attributable to the rapid peak plasma concentration of quinpirole (Whitaker and Lindstrom, 1987).
Taken together, the present study demonstrates an interesting double dissociation receptor mechanism underlying the HAL and CLZ-induced maternal behavior deficits. Our data suggest that the HAL-induced maternal deficits are primarily mediated by the blockade of D2 dopamine receptors, whereas the CLZ-induced maternal deficits are primarily mediated by the blockade of 5-HT2A/2C receptors. Because not all components of CLZ-induced maternal behavior deficits were reversed by DOI, other receptor mechanisms such as the D1 receptor may also be involved, prompting the need for further investigation.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (5R03MH080822-02). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. We would like to thank Wei He for his administrative and technical help with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini RJ, Huston-Lyons D, Campbell A, Marsh E, Cohen BM. Do central antiadrenergic actions contribute to the atypical properties of clozapine? Br J Psychiatry Suppl. 1992;17:12–6. [PubMed] [Google Scholar]
- Bowers BJ, Henry MB, Thielen RJ, McBride WJ. Serotonin 5-HT(2) receptor stimulation of dopamine release in the posterior but not anterior nucleus accumbens of the rat. J Neurochem. 2000;75:1625–33. doi: 10.1046/j.1471-4159.2000.0751625.x. [DOI] [PubMed] [Google Scholar]
- Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146:286–97. doi: 10.1016/j.neuroscience.2006.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Seitz PK, Thomas ML, Cunningham KA. Validation of a selective serotonin 5-HT(2C) receptor antibody for utilization in fluorescence immunohistochemistry studies. Brain Res. 2005;1063:105–13. doi: 10.1016/j.brainres.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Burke M, Sebastian CS. Treatment of clozapine sedation. Am J Psychiatry. 1993;150:1900–1. doi: 10.1176/ajp.150.12.1900. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacol Biochem Behav. 2002;73:869–75. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Salamone JD. Effects of acute and repeated clozapine injections on cholinomimetic-induced vacuous jaw movements. Pharmacol Biochem Behav. 1996;54:619–24. doi: 10.1016/0091-3057(95)02280-5. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–32. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Lucion AB. Effects of intracerebroventricular administration of 5-HT receptor agonists on the maternal aggression of rats. Eur J Pharmacol. 1994;264:445–8. doi: 10.1016/0014-2999(94)00548-6. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ. Maternal aggression in Wistar rats: effect of 5-HT2A/2C receptor agonist and antagonist microinjected into the dorsal periaqueductal gray matter and medial septum. Braz J Med Biol Res. 2005;38:597–602. doi: 10.1590/s0100-879x2005000400014. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ. The effect of 5-HT(2a/2c) receptor agonist microinjected into central amygdaloid nucleus and median preoptic area on maternal aggressive behavior in rats. Rev Bras Psiquiatr. 2006a;28:130–4. doi: 10.1590/s1516-44462006000200011. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA. 5-HT(1B) receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology (Berl) 2006b;185:441–50. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Duman RS. The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: cellular localization and pharmacological characterization. Neuroscience. 1996;70:377–89. doi: 10.1016/0306-4522(95)00357-6. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol Sci. 2001;22:229–32. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Pickel VM. Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res. 2000;864:176–85. doi: 10.1016/s0006-8993(00)02062-x. [DOI] [PubMed] [Google Scholar]
- Dollinger MJ, Holloway WR, Denenberg VH. Parturition in the rat (Rattus norvegicus): normative aspects and the temporal patterning of behaviours. Behavioural Processes. 1980;5:21–37. doi: 10.1016/0376-6357(80)90046-7. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson GS, Faull RL, Robertson HA, Jansen K. D2 dopamine receptor antagonists induce fos and related proteins in rat striatal neurons. Neuroscience. 1990;37:287–94. doi: 10.1016/0306-4522(90)90399-o. [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF. Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol. 1997;384:233–47. [PubMed] [Google Scholar]
- Ellenbroek BA, Artz MT, Cools AR. The involvement of dopamine D1 and D2 receptors in the effects of the classical neuroleptic haloperidol and the atypical neuroleptic clozapine. Eur J Pharmacol. 1991;196:103–8. doi: 10.1016/0014-2999(91)90414-l. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C. Factors influencing maternal responsiveness in humans: usefulness of an animal model. Psychoneuroendocrinology. 1988;13:189–212. doi: 10.1016/0306-4530(88)90014-5. [DOI] [PubMed] [Google Scholar]
- Galef BG., Jr Development of olfactory control of feeding-site selection in rat pups. J Comp Physiol Psychol. 1981;95:615–22. doi: 10.1037/h0077792. [DOI] [PubMed] [Google Scholar]
- Giordano AL, Johnson AE, Rosenblatt JS. Haloperidol-induced disruption of retrieval behavior and reversal with apomorphine in lactating rats. Physiol Behav. 1990;48:211–4. doi: 10.1016/0031-9384(90)90288-f. [DOI] [PubMed] [Google Scholar]
- Hameleers R, Blokland A, Steinbusch HW, Visser-Vandewalle V, Temel Y. Hypomobility after DOI administration can be reversed by subthalamic nucleus deep brain stimulation. Behav Brain Res. 2007;185:65–7. doi: 10.1016/j.bbr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav. 1994;55:615–20. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991a;105:588–98. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991b;39:71–7. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–6. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Meltzer HY. DOI, a 5-HT2A/2C receptor agonist, potentiates amphetamine-induced dopamine release in rat striatum. Brain Res. 1995;698:204–8. doi: 10.1016/0006-8993(95)00865-n. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Dai J, Meltzer HY. DOI, a 5-HT2A/2C receptor agonist, attenuates clozapine-induced cortical dopamine release. Brain Res. 2001;907:151–5. doi: 10.1016/s0006-8993(01)02596-3. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–40. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji SP, Zhang Y, Van Cleemput J, Jiang W, Liao M, Li L, Wan Q, Backstrom JR, Zhang X. Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat Med. 2006;12:324–9. doi: 10.1038/nm1349. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med. 2001;52:503–17. doi: 10.1146/annurev.med.52.1.503. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics? : A new hypothesis. Am J Psychiatry. 2001;158:360–9. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–6. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–69. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. The role of 5-HT(1A) receptors in the locomotor-suppressant effects of LSD: WAY-100635 studies of 8-OH-DPAT, DOI and LSD in rats. Behav Pharmacol. 1996;7:551–9. [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J. 5-HT 2A receptor stimulation by DOI, a 5-HT 2A/2C receptor agonist, potentiates amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2003;972:216–21. doi: 10.1016/s0006-8993(03)02516-2. [DOI] [PubMed] [Google Scholar]
- Lane RF, Blaha CD, Rivet JM. Selective inhibition of mesolimbic dopamine release following chronic administration of clozapine: involvement of alpha 1-noradrenergic receptors demonstrated by in vivo voltammetry. Brain Res. 1988;460:398–401. doi: 10.1016/0006-8993(88)90390-3. [DOI] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–3. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Davidson P, Budin R, Kapur S, Fleming AS. Effects of typical and atypical antipsychotic drugs on maternal behavior in postpartum female rats. Schizophr Res. 2004;70:69–80. doi: 10.1016/j.schres.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Li M, Budin R, Fleming AS, Kapur S. Effects of novel antipsychotics, amisulpiride and aripiprazole, on maternal behavior in rats. Psychopharmacology (Berl) 2005a;181:600–10. doi: 10.1007/s00213-005-0091-7. [DOI] [PubMed] [Google Scholar]
- Li M, Budin R, Fleming AS, Kapur S. Effects of chronic typical and atypical antipsychotic drug treatment on maternal behavior in rats. Schizophr Res. 2005b;75:325–36. doi: 10.1016/j.schres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Kane JM, Johns CA. Clozapine: guidelines for clinical management. J Clin Psychiatry. 1989;50:329–38. [PubMed] [Google Scholar]
- Matsubara S, Matsubara R, Kusumi I, Koyama T, Yamashita I. Dopamine D1, D2 and serotonin2 receptor occupation by typical and atypical antipsychotic drugs in vivo. J Pharmacol Exp Ther. 1993;265:498–508. [PubMed] [Google Scholar]
- Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology (Berl) 1989;99 (Suppl):S18–27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull. 1989a;25:390–2. [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989b;251:238–46. [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Hiley CR. Anti-muscarinic properties of neuroleptics and drug-induced Parkinsonism. Nature. 1974;248:596–7. doi: 10.1038/248596a0. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine d1 and d2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav Neurosci. 2005;119:1072–83. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Dissociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology (Berl) 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Murray AM, Waddington JL. The interaction of clozapine with dopamine D1 versus dopamine D2 receptor-mediated function: behavioural indices. Eur J Pharmacol. 1990;186:79–86. doi: 10.1016/0014-2999(90)94062-3. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Roth BL, Pehek EA. Localization of 5-HT(2A) receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience. 2002;111:163–76. doi: 10.1016/s0306-4522(01)00593-0. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. Springer; New York: 2003. pp. 236–7. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van Oorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry. 1995;28 (Suppl 2):80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res. 1993;606:195–9. doi: 10.1016/0006-8993(93)90984-u. [DOI] [PubMed] [Google Scholar]
- Perry BD, Simon PR, U’Prichard DC. Interactions of neuroleptic compounds at alpha 2-adrenergic receptor affinity states in bovine caudate nucleus. Eur J Pharmacol. 1983;95:315–8. doi: 10.1016/0014-2999(83)90654-4. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–78. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Remington G, Kapur S. Atypical antipsychotics: are some more atypical than others? Psychopharmacology (Berl) 2000;148:3–15. doi: 10.1007/s002130050017. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Lehrman DS. Maternal behavior in the laboratory rat. In: Rheingold HL, editor. Maternal behavior in mammals. John Wiley and Sons; New York: 1963. pp. 8–57. [Google Scholar]
- Safferman A, Lieberman JA, Kane JM, Szymanski S, Kinon B. Update on the clinical efficacy and side effects of clozapine. Schizophr Bull. 1991;17:247–61. doi: 10.1093/schbul/17.2.247. [DOI] [PubMed] [Google Scholar]
- Schmauss M, Wolff R, Erfurth A, Rüther E. Tolerability of long term clozapine treatment. Psychopharmacology (Berl) 1989;99 (Suppl):S105–8. doi: 10.1007/BF00442572. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology. 1992;7:261–84. [PubMed] [Google Scholar]
- Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–9. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Silva MR, Bernardi MM, Felicio LF. Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacol Biochem Behav. 2001;68:461–8. doi: 10.1016/s0091-3057(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Silva MR, Bernardi MM, Cruz-Casallas PE, Felicio LF. Pimozide injections into the Nucleus accumbens disrupt maternal behaviour in lactating rats. Pharmacol Toxicol. 2003;93:42–7. doi: 10.1034/j.1600-0773.2003.930106.x. [DOI] [PubMed] [Google Scholar]
- Stern JM, Taylor LA. Haloperidol inhibits maternal retrieval and licking, but facilitates nursing behavior and milk ejection in lactating rats. J Neuroendocrinol. 1991;3:591–6. doi: 10.1111/j.1365-2826.1991.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Stern JM, Keer SE. Maternal motivation of lactating rats is disrupted by low dosages of haloperidol. Behav Brain Res. 1999;99:231–9. doi: 10.1016/s0166-4328(98)00108-9. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav Neurosci. 2007;121:907–19. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, Almeida RM. Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Braz J Med Biol Res. 2007;40:825–30. doi: 10.1590/s0100-879x2006005000113. [DOI] [PubMed] [Google Scholar]
- Whitaker NG, Lindstrom TD. Disposition and biotransformation of quinpirole, a new D-2 dopamine agonist antihypertensive agent, in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1987;15:107–13. [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl) 1990;100:417–25. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–73. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Nguyen VH, Han M, Huang XF. Effects of typical and atypical antipsychotic drugs on rat brain muscarinic receptors. Neurochem Res. 2007;32:525–32. doi: 10.1007/s11064-006-9266-9. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li M. Sedation and disruption of maternal motivation underlie the disruptive effects of antipsychotic treatment on rat maternal behavior. Pharmacol Biochem Behav. 2009;92:147–56. doi: 10.1016/j.pbb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]