Abstract
The NADPH oxidase (NOX) family of enzymes, which catalyze the reduction of O2 to form reactive oxygen species (ROS), have increased in number during eukaryotic evolution1,2. Seven isoforms of the NOX gene family have been identified in mammals; however, specific roles of NOX enzymes in mammalian physiology and pathophysiology have not been fully elucidated3,4. The best established physiological role of NOX enzymes is in host defense against pathogen invasion in diverse species, including plants5,6. The prototypical member of this family, NOX2 (gp91phox), is expressed in phagocytic cells and mediates microbicidal activities7,8. Here, we report a role for the NOX4 isoform in tissue repair functions of myofibroblasts and fibrogenesis. Transforming growth factor-β1 (TGF-β1) induces NOX4 expression in lung mesenchymal cells by a SMAD3-dependent mechanism. NOX4-dependent generation of hydrogen peroxide (H2O2) is required for TGF-β1-induced myofibroblast differentiation, extracellular matrix (ECM) production, and contractility. NOX4 is upregulated in lungs of mice subjected to non-infectious injury and in human idiopathic pulmonary fibrosis (IPF). Genetic or pharmacologic targeting of NOX4 abrogates fibrogenesis in two different murine models of lung injury. These studies support a novel function for NOX4 in tissue fibrogenesis and provide proof-of-concept for therapeutic targeting of NOX4 in recalcitrant fibrotic disorders.
Tissue repair in mammals involves the integrated actions of growth factors and matrix molecules that orchestrate cell-cell interactions9–11. Fibrosis of diverse tissues occurs when this process is dysregulated by impaired re-epithelialization in association with myofibroblast activation9,11. Myofibroblast differentiation and activation are critically dependent on TGF-β1, matrix signaling, and biomechanical tension12–14. We have previously reported that myofibroblast differentiation by TGF-β1 is associated with the activation of a flavoenzyme that generates extracellular H2O215–17. NOX4 has been implicated in the differentiation of cardiac fibroblasts to myofibroblasts18. However, physiological and pathophysiological roles for NOX4 in tissue repair and fibrogenesis are not well defined.
We identified NOX4 as one of the most highly induced genes by whole-genome Affymetrix analysis in human fetal lung mesenchymal cells (hFLMCs) stimulated with TGF-β1; other members of the NOX gene family were not affected at the mRNA level (Fig. 1a). The upregulation of NOX4 mRNA by TGF-β1 was confirmed by RT-PCR (Supplementary Fig. 1a) and NOX4 protein expression was induced in a time-dependent manner (Fig. 1b and Supplementary Fig. 1b). To define the specific role of NOX4, an RNA interference (RNAi) approach utilizing small interfering RNA (siRNA) targeting NOX4 was employed. Two of four siRNA duplexes, duplex 3 and duplex 4, efficiently blocked NOX4 induction by TGF-β1 (Supplementary Fig. 1c). The NOX4 siRNA duplex 4 was utilized in subsequent in vitro studies designed to examine the role for NOX4 in myofibroblast differentiation and activation. RNAi-mediated knockdown of NOX4 significantly inhibited TGF-β1-induced H2O2 production in hFLMCs (Fig. 1c), implicating NOX4 as the primary enzymatic source of extracellular H2O2 generation by TGF-β1-differentiated myofibroblasts.
Figure 1. Identification of NOX4 as the enzymatic source of extracellular H2O2 production by myofibroblasts and its role in mediating myofibroblast differentiation and contractility.
(a) RNA was isolated from human fetal lung mesenchymal cells (hFLMCs) treated with/without TGF-β1 (2 ng/ml) for 18 h and analyzed by Affymetrix (U133A) microarray for members of the NOX/DUOX gene family. Values represent mean ± S.D., n = 3 per group. *P < 0.001 compared to control. ND indicates “not detected” (below threshold). (b) hFLMCs were treated with/without TGF-β1 (2 ng/ml) for the times indicated and cell lysates subjected to SDS-PAGE and Western immunoblotting for NOX4 and GAPDH. (c) Effect of NOX4 siRNA (duplex 4) on extracellular release of H2O2 by hFLMCs treated with/without TGF-β1 (2 ng/ml for 16 h). (d) hFLMCs were pretreated with pharmacologic inhibitors against ALK5 receptor kinase (SB431542; 1 μM), MEK (PD98059; 20 μM), p38 MAPK (SB203580; 6 μM), JNK (SP600125; 100 nM), and then stimulated with TGF-β1 (2 ng/ml × 16 h) prior to measurement of extracellular H2O2 release. (e) Effect of SMAD3 siRNA knockdown on TGF-β1-induced NOX4 expression in hFLMCs, as determined by Western immunoblotting. (f) Effect of siRNA-mediated knockdown of SMAD3 on extracellular H2O2 production stimulated by TGF-β1 (2 ng/ml × 16 h) in hFLMCs. (g) hFLMCs in 3-D collagen matrix were stimulated with/without TGF- β1 (2 ng/ml × 16 h) in the presence/absence of catalase (750 U/ml) and effects on α-smooth muscle actin (α-SMA), fibronectin, and β-actin were determined by Western immunoblotting. (h) Effect of siRNA-mediated silencing of NOX4 in 3D-collagen matrix-embedded hFLMCs on cellular expression of α-SMA, fibronectin, and procollagen-1 treated with/without TGF-β1 (2.5 ng/ml × 72 h), as determined by Western immunoblotting. (i,j) Effect of exogenous catalase (750 U/ml) (i), and siRNA-mediated NOX4 silencing (j) on TGF-β1-induced contractility in 3D-collagen matrices. Values represent mean ± S.E.M.; n = 4. *P < 0.001 compared to controls.
TGF-β1 signals via two heterodimeric transmembrane receptors, the type II and type I (ALK5) receptors. To define upstream mechanisms of TGF-β1-induced NOX4 induction and H2O2 generation in myofibroblasts, we tested the effect of pharmacologic inhibitors of ALK5 and canonical MAPK pathways. Of these, only ALK5 inhibition attenuated the induction of H2O2 production by hFLMCs (Fig. 1d). The ALK5 receptor is known to activate SMAD2 and SMAD3; however, pro-fibrotic effects TGF-β1/ALK5 signaling have been largely attributed to SMAD3 signaling19. We employed an RNAi strategy to determine if SMAD3 is required for NOX4 induction and H2O2 generation in hFLMCs; SMAD3 siRNA knockdown inhibited TGF-β1-induced NOX4 inducibility (Fig. 1e) and H2O2 production (Fig. 1f). Similarly, a requirement for SMAD3 signaling in TGF-β1-induced NOX4 expression was observed in primary mesenchymal cells isolated from lungs of human subjects with IPF (Supplementary Fig. 1d), a chronic fibrosing and ultimately fatal lung disease. These data support a role for TGF-β1 signaling via ALK5/SMAD3 in the induction and activation of NOX4 in myofibroblasts.
Myofibroblasts contribute to the tissue repair by secreting ECM proteins and remodeling/contracting the ECM9,11. We utilized a 3D-collagen matrix cell culture system to determine if fibronectin synthesis and contractile functions of myofibroblasts are regulated by NOX4 activation and extracellular H2O2 generation. The upregulation of α-smooth muscle actin (α-SMA), a cytoskeletal component of contractile actin stress fibers, and fibronectin synthesis induced by TGF-β1 were inhibited by addition of catalase (Fig. 1g), an enzyme which reduces H2O2 to H2O, indicating a role for H2O2 in mediating these effects. Similar to the effects of catalase, endogenous suppression of NOX4 by siRNA knockdown inhibited TGF-β1-induced expression of α-SMA, fibronectin, and procollagen-I (Fig. 1h). Next, we studied whether NOX4-dependent H2O2 production by myofibroblasts is required for their capacity to contract 3D-collagen gels. Both the exogenous introduction of catalase and RNAi-mediated knockdown of NOX4 inhibited TGF-β1-induced collagen gel contractility (Fig. 1i,j). Together, these studies indicate a critical role for NOX4-dependent H2O2 in conferring synthetic and contractile properties to myofibroblasts that differentiate under the influence of TGF-β1.
To investigate a potential role for NOX4 in a human fibrotic disease, lung tissue sections from IPF were examined. NOX4 is highly expressed in myofibroblastic foci of the remodeled IPF lung, as determined by immunohistochemical (IHC) staining (Fig. 2a). Additionally, lung mesenchymal cells isolated from explants of IPF lung tissue (IPF-MCs) were studied ex vivo. Similar to our findings in hFLMCs, NOX4 was found to be induced and necessary for TGF-β1-stimulated H2O2 production (Fig. 2b); NOX4 was also required for the induction of α-SMA and fibronectin mRNA (Fig. 2c e) and protein expression (Fig. 2f) by TGF-β1. Differentiated myofibroblasts are known to secrete higher amounts of extracellular collagen. We evaluated the role of NOX4 on collagen secretion by IPF-MCs. Constitutive and TGF-β1-induced secretion of soluble collagen by IPF-MCs were inhibited by siRNA-mediated knockdown of NOX4 (Fig. 2g). We examined the role of NOX4 in IPF-MC proliferation in response to serum; siRNA-mediated knockdown of NOX4 inhibited serum-stimulated proliferation of IPF-MCs (Fig. 2h,i). These data support a critical role for NOX4 in myofibroblast differentiation and proliferation of human IPF-MCs.
Figure 2. NOX4 is expressed in lungs of human subjects with idiopathic pulmonary fibrosis (IPF) and mediates H2O2 production, myofibroblast differentiation, and serum-stimulated proliferation of IPF-derived mesenchymal cells.
(a) Immunohistochemical staining demonstrating expression of NOX4 in myofibroblastic foci in lungs of a representative human subject with IPF. Length bar = 100 μm. (b–i) Mesenchymal cells isolated from IPF lung tissues (IPF-MCs) by explant tissue culture and analyzed at passage 2–5. (b) IPF-MCs were transfected with nontargeting (control) siRNA or NOX4 siRNA and treated with/without TGF-β1 (2 ng/ml) for 16 h and analyzed for NOX4 protein (inset) and extracellular H2O2 production. (c–f) The effect of siRNA knockdown of NOX4 in IPF-MCs with/without TGF-β1 (2 ng/ml) on the expression of α-SMA mRNA (c) and protein (f); fibronectin mRNA (d) and protein (f); and NOX4 mRNA (e) and protein (f), as determined by real-time PCR (at 24 h) and Western immunoblotting (at 48 h). (g) Control (nontargeting) and NOX4 siRNA transfected IPF-MCs were treated with/without TGF-β1 (2 ng/ml) for 48 h and conditioned culture media was collected and analyzed for acid-soluble collagen using the Sircol assay. (h,i) The effect of siRNA knockdown of NOX4 on proliferation of IPF-MCs treated with/without serum was determined at 24 h by BrdU incorporation assay (h) and at 48 h by assessment of cell numbers using a coulter counter (i). Values represent mean ± S.E.M.; n = 3–5. *P < 0.001 compared to control (without TGF-β1 or serum) and nontargeting siRNA.
To define the in vivo role of NOX4 in the reparative response to injury of the mammalian lung, a murine model of acute lung injury was employed. In this model, direct airway instillation of the chemotherapeutic drug, bleomycin, causes epithelial injury with subsequent mesenchymal cell activation and fibrosis. Several key features of fibrotic reactions in mammalian tissues, including TGF-β1 upregulation/activation and myofibroblast differentiation/activation are recapitulated in this animal model20. We first determined whether NOX4 expression is induced during the fibrogenic phase of bleomycin-induced lung injury. NOX4 was induced in a time-dependent manner, increasing from day 7 up to day 28 (Fig. 3a), supporting a temporal relationship between NOX4 expression, myofibroblast activation and fibrosis following lung injury. In contrast, expression of the NOX2 isoform, which is predominantly expressed in phagocytic cells, was increased on day 7 and returned to baseline levels at later time-points when inflammatory responses have subsided (Fig. 3a).
Figure 3. NOX4 is induced during the fibrogenic phase of bleomycin-induced lung injury in mice and inhibition of NOX4 expression/activity attenuates pulmonary fibrosis.
(a) C57BL/6 mice were subjected to acute lung injury by airway (intra-tracheal) administration of bleomycin or saline/control on day 0. Following bleomycin injury, mice were euthanized at the indicated time intervals, whole lungs were harvested, and tissue homogenates analyzed by SDS-PAGE and Western immunoblotting for NOX4, NOX2, and β-actin. (b–e) NOX4 siRNA or a nontargeting control siRNA was instilled directly down the trachea of mice at the time of bleomycin injury (day 0), and lungs were analyzed on day 14 or 21. (b) NOX4 expression on day 21 was determined by Western immunoblotting of whole lung homogenates. (c) Fibrosis was assessed on day 14 by H & E staining and Masson’s trichrome blue staining for collagen (top panels); NOX4 and α-SMA expression were assessed by immunohistochemical (IHC) analysis (bottom panels); length bar = 100 μm. (d,e) Whole lung homogenates were analyzed on day 21 for hydroxyproline content (d), and on day 14 for acid-soluble collagen using the Sircol assay (e). Values represent mean± S.E.M.; n = 4–6; *P < 0.01 compared to all other groups. (f) C57BL/6 mice were administered intra-tracheal (IT) bleomycin on day 0. Diphenyleneiodonium (DPI; 1.6 mg/kg) or vehicle control was administered by daily intraperitoneal (IP) injections starting on day 7 for 14 days. Whole lung homogenates were analyzed for acid-soluble collagen using the Sircol assay on day 21. Values represent mean ± S.E.M.; n = 6; *P < 0.05 compared to saline control.
The effects of targeted suppression of NOX4 induction employing an in vivo RNAi approach in two different animal models of lung injury/fibrosis were examined. We first confirmed that the murine siRNA homologous to the human NOX4 siRNA duplex 4 (Supplementary Table 1) efficiently knocked down NOX4 in cultured primary murine mesenchymal cells (Supplementary Fig. 2a). In the first animal model, NOX4 siRNA or nontargeting control siRNA was instilled directly down the trachea of mice at the time of bleomycin injury (day 0), and tissues were analyzed at day 14 or 21. NOX4 siRNA was effective in inhibiting NOX4 induction in injured lung tissue at 21 days as determined by Western immunoblotting (Fig. 3b), and at day 14 as determined by IHC analysis (Fig. 3c). IHC analysis confirmed NOX4 expression localized to fibrotic foci surrounding remodeled alveolar structures on day 14 post-lung injury (Fig. 3c). NOX4 knockdown mediated a marked anti-fibrotic effect as determined by histopathology, trichrome staining for collagen, IHC analysis of α-SMA (Fig. 3c), and by biochemical analyses of hydroxyproline content (Fig. 3d) and acid-soluble collagen (Fig. 3e) in whole lung homogenates.
Pharmacological approaches that specifically target fibrogenic processes are currently lacking. To determine if pharmacologic blockade of NOX/flavoenzyme activity during the post-injury reparative phase protects against fibrogenic tissue responses, the flavoenzyme inhibitor, diphenyleneiodonium chloride (DPI), was employed. DPI, which blocks NOX4 activity in myofibroblasts at a relatively low IC50 (<0.5 μM; data not shown), was administered by daily intra-peritoneal injection on days 8 to 21 following bleomycin lung injury. We delayed DPI administration until day 8 to minimize effects of the drug on inflammatory responses that typically subside following the first week bleomycin lung injury in mice21,22. Mice receiving DPI demonstrated significant protection from fibrosis, as measured by acid-soluble collagen in whole lung (Fig. 3f); this is associated with reduced fibrosis as determined by histopathology, Masson’s trichrome staining for collagen, as well as reduced expression of NOX4 and α-SMA-expressing myofibroblasts (Supplementary Fig. 2b).
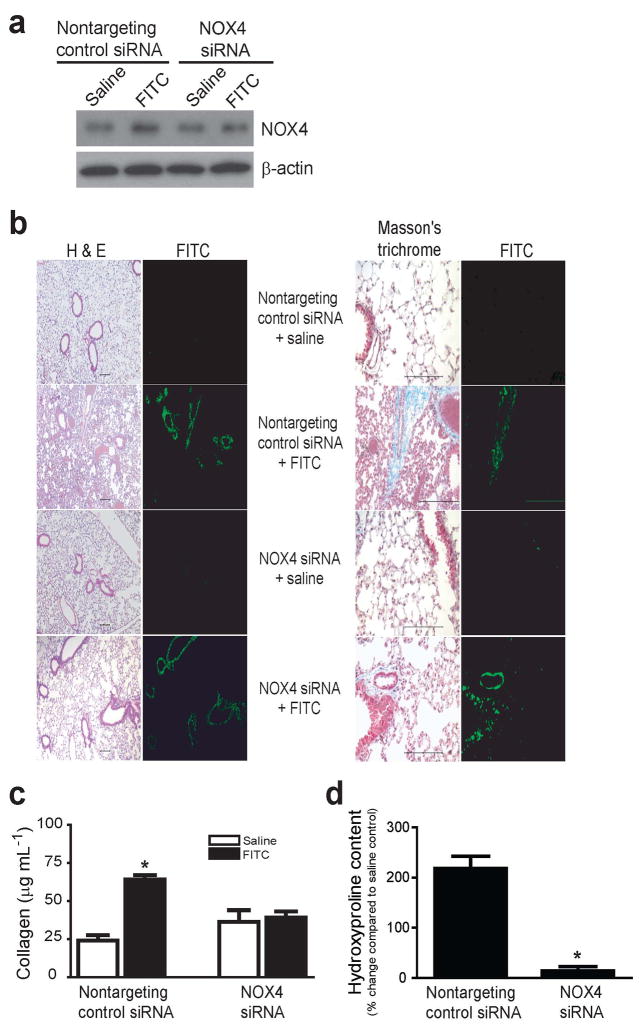
We also employed a hapten (fluorescein isothiocyanate; FITC)-driven lung injury/fibrosis murine model to test the role of NOX4 in fibrogenesis23. In this model, NOX4 siRNA was instilled directly down the trachea of mice at the time of FITC injury (day 0), and tissues were analyzed on day 14 or 21. This RNAi approach was effective in inhibiting NOX4 induction at day 21 post-lung injury (Fig. 4a). The fibrotic response surrounding airways where FITC is deposited was found to be markedly attenuated in mice receiving NOX4 siRNA versus nontargeting siRNA, as determined by histopathology and trichrome staining for collagen (Fig. 4b); this was confirmed by analyzing whole lung homogenates for acid-soluble collagen by Sircol assay (Fig. 4c) and acid-insoluble collagen by hydroxyproline assay (Fig. 4d).
Figure 4. RNAi-mediated knockdown of NOX4 attenuates fibrosis in mice subjected to fluorescein isothiocyanate-induced lung injury.
C57BL/6 mice were administered intra-tracheal fluorescein isothiocyanate (FITC) or saline/control on day 0 with nontargeting control siRNA or NOX4 siRNA and lung tissues were analyzed on day 14 or 21. (a) NOX4 expression on day 21 was determined by Western immunoblotting of whole lung homogenates. (b) Fibrosis was assessed by H & E staining and Masson’s trichrome blue staining for collagen; length bar = 100 μm. (c,d) Whole lung homogenates were analyzed on day 14 for acid-soluble collagen using the Sircol assay (c), and on day 21 for hydroxyproline content (d). Values represent mean± S.E.M.; n = 4–6; *P < 0.01 compared to saline, nontargeting (control) siRNA.
In this study, we demonstrate a functional role for NOX4 in myofibroblast differentiation and activation ex vivo and in fibrogenic responses to lung injury in vivo. The pro-fibrogenic mediator, TGF-β1, specifically induces mRNA/protein expression and enzymatic activation of the NOX4 isoform in differentiated myofibroblasts. NOX4-dependent H2O2 generation is required for myofibroblast differentiation, synthesis of ECM proteins, and contractility mediated by TGF-β1. NOX4 is expressed in myofibroblastic foci of remodeled IPF lung tissue, supporting a role for this NOX isoform in the induction and activation of myofibroblasts in this human pulmonary disorder. In two different murine models of pulmonary fibrosis, genetic or pharmacologic strategies targeting NOX4 induction or activity protects against fibrosis. We utilized an RNAi strategy to suppress NOX4 expression by administering NOX4 siRNA at the time of bleomycin or FITC injury. It is likely that the disruption of the airway-alveolar epithelial barrier immediately following bleomycin/FITC injury facilitated the transduction of siRNA to epithelial or mesenchymal precursor cells that prevented their differentiation into activated myofibroblasts, thus protecting from lung fibrosis.
These are the first studies, to our knowledge, to definitively implicate a specific NOX isoform in tissue repair functions and fibrogenesis. We speculate that NOX4 may have been selected during metazoan evolution to execute tissue repair functions critical for the survival of more complex multicellular eukaryotes24. In support of this notion, NOX4 is almost exclusively expressed in chordates25. Furthermore, NOX4 activation in myofibroblasts and tissue fibrogenesis may represent yet another example of antagonistic pleiotropy, whereby genes that confer a survival advantage during early reproductive life mediate potential harmful effects in later life26. Fibrosis is typically a complication of failed tissue regeneration and ineffective epithelial repair in diverse organ systems, often with an age-dependent increase in incidence27. Fibrosis in mammalian tissues is perhaps best viewed as an initial adaptive response executed by mesenchymal cells to restore tissue barrier function while secreting a provisional matrix to facilitate re-epithelialization; however, persistent mesenchymal activation and failed re-epithelialization results in unrestrained and progressive fibrosis. Targeting NOX4 may prove to be an effective therapeutic strategy for an otherwise treatment-unresponsive and ultimately fatal group of human fibrotic disorders.
METHODS
Reagents
We purchased porcine platelet-derived TGF-β1 was obtained from R&D Systems, Minneapolis, MN; protease inhibitor cocktail set III from Calbiochem, San Diego, CA. We purchased monoclonal antibodies to fibronectin (clone IST-4) and β-actin (clone AC-15) from Sigma, St. Louis, MO; monoclonal antibody to α-SMA (clone 1A4) from Dako, Carpinteria, CA; antibody to SMAD3 from Cell Signaling Technology, Danvers, MA; antibody to procollagen-I from Cederlane laboratories, Hornby, Ontario, Canada; and rabbit polyclonal antibody to GAPDH antibody from Abcam Inc., Cambridge, MA. We purchased all other reagents from Sigma unless otherwise specified.
Cell culture
We obtained human fetal lung mesenchymal cells (hFLMCs; IMR-90 cells) from Coriell Cell Repositories, Institute for Medical Research, Camden, NJ. We isolated primary mesenchymal cells from the lungs of C57BL/6 mice as previously described22. We obtained primary lung mesenchymal cells isolated by explant cultures from lung tissues of human subjects with IPF (IPF-MCs) under an approved protocol by the Institutional Review Board at the University of Michigan. Informed consent was obtained from all individuals enrolled in the Lung Tissue Research Consortium of the National Institutes of Health. We cultured all mesenchymal cells in DMEM (Life Technologies, Inc.) supplemented with 10% fetal calf serum (Hyclone Laboratories, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1.25 μg/ml amphotericin B, and incubated cells at 37°C in 5% CO2, 95% air.
3D-collagen gels
We reconstituted collagen gels by mixing 1 part of 3 mg/ml neutralized rat tail collagen type 1 and two parts of cell suspension in serum-free media. We seeded cell suspensions at a density of 200,000 cells per ml into 24-well tissue culture plates and allowed the gels to polymerize at 37°C for 1 h before adding 1 ml of media. We incubated gels overnight prior to treatments, and gently detached the edge of the gels from the walls of the well using a sterile spatula. We photographed images of gels and measured gel area using ImageJ (NIH) software.
Murine model of bleomycin/FITC lung injury
We anesthetized C57BL/6J mice (6–8 weeks of age; Jackson Laboratories, Bar Harbor, ME) with intraperitoneal injection of ketamine and xylazine. We administered intra-tracheal bleomycin (0.025 U) or FITC (4.67 mg/ml suspension in PBS) to induce lung injury as previously described22. The University of Michigan Committee on the Use and Care of Animals approved the animal protocols.
RNA interference
For in vitro RNAi, we transfected cells with single duplexes targeting NOX4 or nontargeting control siRNA (100 nM) using Lipofectamine-2000 reagent. For in vivo RNAi studies, we administered NOX4 siRNA or control nontargeting siRNA (Dharmacon Inc.) at a dose of ~50 μg per mouse by intratracheal injection with bleomycin, FITC, or saline, in a total volume of 50 μl using a 26-gauge needle.
Real-time PCR
We isolated total RNA from cells using the RNeasy® Mini Kit (Qiagen) and reverse transcribed using M-MLV RT First-Strand Synthesis (Invitrogen) as per manufacturers’ protocols. We performed real-time PCR reactions for each cDNA sample in triplicate using SYBR® Green PCR Master Mix (Applied Biosystems) and gene specific primer pairs for α-actin, NOX4, α-SMA, and fibronectin (Supplementary Table 1). We carried out reactions for 40 cycles (95°C for 15 sec, 60°C for 1 min) in a 7300 Real Time PCR System (Applied Biosystems, Foster City, CA). We expressed semi-quantitative real-time PCR data for each target gene as 2−ΔΔCt Relative Quantitation (RQ) versus endogenous β-actin, with error bars representing the standard error of the mean for triplicate reactions. We performed a 2-way analysis of variance (ANOVA) with Bonferroni post-test on grouped data.
Gene microarray analyses
We hybridized RNA isolates on microarray Affymetrix U133A chips with 22976 probe-pairs and the University of Michigan Microarray Core facility performed the statistical analyses.
Western immunoblotting
We prepared cell lysates in RIPA buffer, subjected them to SDS-PAGE under reducing conditions and performed western immunoblotting as previously described28.
Measurement of H2O2 production
We assayed extracellular H2O2 release from cultured cells as previously described15.
BrdU incorporation assay and coulter counting
We serum-starved cells cultured in 96-well plates for 24 h followed by BrdU pulse in media with/without 10% serum for 24 hours. We measured BrdU incorporation using a kit from Calbiochem (Cat #QIA58). We measured cell counts using a coulter counter (model ZM, Coulter Electronic, Hialeah, FL).
Sircol assay for collagen
We assessed acid-soluble collagen in cell culture supernatants or whole lung homogenates using the Sircol assay as previously described22.
Hydroxyproline content of whole lung
We homogenized murine whole lungs in PBS, then acidified (by adding an equal volume of 12 N HCl), hydrolyzed (by heating at 120°C for 24 h), and processed samples for hydroxyline measurements as previously described29.
Lung histology and immunohistochemical staining
We processed paraffin embedded tissue sections for lung histology and immunohistochemical staining as previously described22.
Statistical Analysis
We expressed data from various groups as mean ± S.E.M. We made statistical comparisons using the Student’s t test for unpaired samples. We analyzed Affymetrix data using two-sample t test of log data.
Supplementary Material
Acknowledgments
We thank D. Lambeth, Department of Biochemistry, Emory University, Atlanta, GA, for providing the rabbit polyclonal antibody to NOX4 and A. Jesaitis, Department of Microbiology, Montana State University, Bozeman, MT for the mouse monoclonal antibody to NOX2. We thank D. Arenberg, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, for providing primary lung mesenchymal cells from IPF subjects (IPF-MCs). This work was supported by grants from the National Institutes of Health, R01 HL067967 (to V.J.T.), K08 HL081059 (to J.C.H.), and by an NIH-sponsored Lung Tissue Research Consortium grant, N01 HR046162 (to F.J.M.).
Footnotes
AUTHOR CONTRIBUTIONS
L.H. and R.V. designed, conducted, and supervised experiments, and contributed to manuscript preparation; T.J. and R.J. conducted experiments and contributed to primer design and analysis of gene expression data; T.R.L. contributed to animal studies; J.C.H. contributed to RNAi studies and protein expression data; S.P. contributed to manuscript preparation; F.J.M. contributed to the human IPF studies; V.J.T conceived, designed and supervised the project, and L.H. and V.J.T. wrote the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare no conflict of interest and there are no financial relationships with commercial entities which have interest in the subject of this manuscript.
References
- 1.Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 4.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 6.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 7.Ahluwalia J, et al. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature. 2004;427:853–858. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Quie PG, White JG, Holmes B, Good RA. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967;46:668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 10.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 11.Hinz B, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serini G, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 16.Thannickal VJ, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 17.Waghray M, et al. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. Faseb J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 18.Cucoranu I, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 19.Bonniaud P, et al. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 21.Thrall RS, Barton RW, D’Amato DA, Sulavik SB. Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1982;126:488–492. doi: 10.1164/arrd.1982.126.3.488. [DOI] [PubMed] [Google Scholar]
- 22.Vittal R, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts SN, et al. A novel model for human interstitial lung disease: hapten-driven lung fibrosis in rodents. J Pathol. 1995;176:309–318. doi: 10.1002/path.1711760313. [DOI] [PubMed] [Google Scholar]
- 24.Thannickal VJ. Oxygen in the evolution of complex life and the price we pay. Am J Respir Cell Mol Biol. 2009;40:507–510. doi: 10.1165/rcmb.2008-0360PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. Febs J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 26.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz JC, et al. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hattori N, et al. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.