Abstract
Background:
Class III β-tubulin (βIII-tubulin) is expressed in tissues of neuronal lineage and also in several human malignancies, including non-small-cell lung carcinoma, breast and ovarian cancer. Overexpression of βIII-tubulin in these tumours is associated with an unfavourable outcome and resistance to taxane-based therapies. At present, βIII-tubulin expression remains largely uncharacterised in prostate cancer.
Methods:
In this report, we evaluated the expression of βIII-tubulin in 138 different human prostate tumour specimens by immunohistochemistry from patients with hormone-treated or hormone-untreated prostate cancer. βIII-tubulin expression was also examined in various prostatic cancer cell lines including in androgen-sensitive human prostate cancer cells, LNCaP, grown in androgen-depleted medium in 2D cultures or as tumour xenografts when the host mouse was castrated.
Results:
Whereas moderate-to-strong βIII-tubulin expression was detected in only 3 out of 74 (4%) hormone-naive tumour specimens obtained from patients who never received hormone therapy, 6 out of 24 tumour specimens (25%) from patients treated for 3 months with neoadjuvant hormone therapy and 24 out of 40 (60%) castration-resistant tumour specimens from chronic hormone-treated patients were found to express significant levels of βIII-tubulin. These findings were supported by in vitro and in vivo settings.
Conclusion:
Our data indicate that βIII-tubulin expression is augmented in prostate cancer by androgen ablation and that the expression of this β-tubulin isoform is associated with the progression of prostate cancer to the castration-resistant state, a stage largely responsible for mortality from prostate cancer.
Keywords: class III β-tubulin, prostate cancer, hormone therapy, disease progression, therapeutic resistance
Prostate carcinoma (PCa) is the most common malignancy and the second leading cause of cancer death in men (Ferlay et al, 2007; Jemal et al, 2008). Despite the current widespread use of prostate-specific antigen (PSA) screening that increases the detection of PCa at an early stage when it is localised to the prostate, ∼30% of men treated with surgical or radiation therapies will relapse (Han et al, 2001). These men and those who present with locally advanced or overt metastatic PCa are treated by hormonal therapies that deplete circulating androgen levels (Hellerstedt and Pienta, 2002). For most men with advanced PCa, hormone therapy provides a median progression-free survival of 12–33 months until the emergence of a castration-resistant prostate cancer (CRPC) that is refractory to the low androgen levels of the hormone-treated patient. As CRPC generally responds poorly to alternate therapeutics, this form of the disease is overwhelmingly responsible for mortality from PCa.
Recently, however, treatments involving docetaxel-based chemotherapy have shown some benefit for CRPC, although the survival advantage remains relatively limited (Petrylak et al, 2004; Tannock et al, 2004). Taxanes, of which docetaxel is a derivative, target the microtubules of cancer cells to disrupt the mitotic apparatus leading to cancer cell death. Microtubules are composed of polymers of α- and β-tubulin heterodimers (Orr et al, 2003; Seve and Dumontet, 2008). Both α- and β-tubulins exist as multiple isotypes with a complex pattern of distribution among different tissues. Class III β-tubulin (βIII-tubulin) is normally expressed in neuronal tissues and in neuroendocrine (NE) cells, and this isoform of β-tubulin is also expressed in some solid tumours, including tumours of neuronal origin and non-small-cell lung, gastric, breast and ovarian carcinomas. For these latter tumours, the expression of βIII-tubulin correlates with a poor overall survival as well as with reduced response to taxanes (Ferrandina et al, 2006; Gan et al, 2007; Galmarini et al, 2008; Seve and Dumontet, 2008). Indeed, resistance to taxanes can be induced in cultured human tumour cells by transfection with βIII-tubulin, whereas βIII-tubulin depletion in cells resulted in the sensitisation to taxanes (Lu and Luduena, 1993; Panda et al, 1994; Kavallaris et al, 1999; Kamath et al, 2005; Shalli et al, 2005; Gan et al, 2007). These findings suggest that the presence of βIII-tubulin in cancer cell microtubules alters their sensitivity to taxane-based agents.
At present, the expression of βIII-tubulin remains relatively uncharacterised in PCa. One study did assess βIII-tubulin expression in a small group of localised PCa specimens by immunohistochemistry and reported that βIII-tubulin was moderately expressed in both benign and malignant tissues cells of the prostate (Ranganathan et al, 1997). However, this evaluation did not include analysis of castration-resistant disease, which is the actual candidate for standard chemotherapeutic intervention. In this study, we provide evidence, on the basis of immunostaining profiles of different classes of prostate tumours, that βIII tubulin expression is highly upregulated in prostate cancer cells during the transition from hormone-sensitive to the castration-resistant state. Whereas this characterisation might help to better explain the more aggressive and therapeutic-resistant nature of CRPC, it also has implications for the use of alternative microtubule-targeting therapeutic agents that might be more effective than docetaxel in the treatment of CRPC.
Materials and methods
Human prostate tissue samples
Formalin-fixed, paraffin-embedded prostate tissues were obtained from the Department of Pathology at the Henri Mondor Hospital and from the Cancer Centre in Montpellier, France. Pathological specimens of hormone-naive (HNPC; n=74) and hormone-therapy-treated (HTPC; n=24) prostate cancers used in this study were obtained from patients treated for localised disease by radical prostatectomy. Hormone-therapy-treated prostate cancer cases comprised patients who had been treated with 3 months of neoadjuvant therapy with LHRH agonists before surgery. Metastatic CRPC (n=6) tissues were liver biopsy samples collected from patients diagnosed with liver metastases as the first site of metastatic disease. Primary CRPC specimens (n=34) were collected at the time of the trans-urethral resection of the prostate in patients who experienced disease recurrence after an initial response to hormone therapy. Recurrence was defined as patients having PSA progression despite a complete androgen blockade therapy and a castrated level of testosterone. All samples were obtained with institutional review board approval from the respective institutions.
Immunohistochemical analysis
Immunohistochemistry was carried out on tissue microarrays (TMAs) containing samples (primary CRPC and HNPC cases) and regular sections (HNPC (10 cases), primary CRPC, HTPC and biopsy samples). For TMA, four replicate cores (with diameter of 0.6 mm) were collected from the donor block. βIII-tubulin protein expression was assessed after standard biotin–avidin complex immunohistochemistry using a monoclonal antibody against βIII-tubulin (1 : 500; clone TUJ1; Covance, Emeryville, CA, USA). Protein expression was scored as null (0), weak (1), moderate (2) and strong (3). In this analysis, a case was considered positive only when the score was ⩾2 in more than 10% of cancer cells, whereas cases with <10% staining or those that scored <2 were considered negative. βIII-tubulin expression was evaluated separately by one pathologist (YA) and by two non-pathologists (ST and GP). The staining assessment was highly reproducible. Immunostains obtained from regular sections of CRPC and HNPC samples were constantly comparable with those obtained in the TMA analysis. Positivity in nerves and axons present in the sections served as positive control. Negative controls were included in each experiment in which the primary antibody was omitted. The absence of immunostaining in red cells was also used as a negative control as previously described (Ranganathan et al, 1997). The following antibodies were used to assess the NE characteristics of the specimens: polyclonal antibodies to chromograninA (CgA; Dako, Trappes, France; A0430); monoclonal antibodies to synaptophysin (Snp88, Biogenex, San Ramon, CA, USA) and NSE (BBS/NC/VI-H14, Dako).
Cell culture
Human prostate cancer cell lines LNCaP (clone FGC), 22Rv1, PC3 and DU145 were obtained from American Type Culture Collection (Manassas, VA, USA), and the cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. For androgen-reduced conditions, LNCaP cells were cultured in phenol red-free RPMI supplemented with 10% charcoal-stripped FBS for the indicated time in the presence or absence of Dihydrotestosterone (DHT, Sigma-Aldrich, Saint-Quetin Fallavier, France).
Western blot analysis
Protein lysates were prepared in the RIPA buffer (radioimmunoprecipitation assay lysis buffer) supplemented with protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland) and phosphatase inhibitors (25 mmol l−1 orthovanadate and 50 mmol l−1 NaF) (Sigma-Aldrich). The total protein concentration of the soluble extract was determined using the Bicinchoninic Acid Kit (Sigma-Aldrich). Each protein sample (30 μg) was resolved to SDS–PAGE, transferred onto a polyvinylidene difluoride membrane (Millipore, Molsheim, France) and incubated with a monoclonal antibody against βIII-tubulin (1 : 10 000) or β-actin (1 : 16 000; AC-15; Sigma-Aldrich). Immune complexes were visualised by enhanced chemiluminescence detection (ECL plus kit, GE Healthcare, Little Chalfont, UK).
cDNA synthesis and real-time PCR
Quantitative PCR was carried out using SYBR Green dye on an Applied Biosystems 7000 Real Time PCR system (Applied Biosystems, Foster City, CA, USA). The conditions for RT–PCR have been described previously (Azoulay et al, 2008). The amount of βIII-tubulin mRNA levels relative to the housekeeping gene Ribosomal Protein, large, P0 (RPLP0) was determined on the basis of the comparative threshold cycle CT method (2−ΔΔCT). The primer sequences for βIII-tubulin and RPLP0 have been described previously (Azoulay et al, 2008; Frigo and McDonnell, 2008).
Tumour xenografts in athymic mice
2 × 106 LNCaP cells at low passage (P29) were inoculated s.c. with 0.1 ml of Matrigel (Becton Dickinson Labware, Le-Pont-de-Claix, France) in the flank region of 6-week-old male athymic nude mice (Elevage Janvier – Le Genest-Saint-Isle, France) under anaesthesia. Mice bearing tumours between 200 and 300 mm3 were killed or were surgically castrated through scrotal incision. At the indicated days, LNCaP xenografts were processed after killing to evaluate βIII-tubulin expression. All animal procedures were carried out according to local guidelines on animal care and with appropriate institutional certification.
Statistical analysis
χ2 or Fisher's exact tests were used to assess associations between hormone and βIII-tubulin status in human specimens. For post hoc comparisons, the Bonferroni correction was applied. To evaluate trend over hormone status, χ2 for trend was calculated. Immunochemistry scoring results of xenografts LNCaP tumours stained for βIII-tubulin were analysed using Kruskal–Wallis and Mann–Whitney tests. All statistical tests used a two-tailed α=0.05 level of significance and were conducted using SAS statistical software, version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Expression of βIII tubulin is predominantly expressed in castration-resistant human prostate cancer
A total of 138 different cases of prostatic carcinoma were evaluated in this study. The tumours included 74 specimens of HNPC found in prostatectomy specimens obtained from patients who had never received any form of hormonal therapy, 24 cases of HTPC specimens from patients who received 3 months of adjuvant hormonal therapy before radical prostatectomy and 40 cases of CRPC specimens of which 34 were surgically removed from the prostate to relieve bladder outlet obstruction and 6 cases of castration-resistant liver metastases. All specimens were present in formalin-fixed and paraffin-embedded surgical tissues. The tissues were sectioned and were subsequently immunostained for βIII-tubulin using a standard immunoperoxidase-based procedure.
In the HNPC group, accounting for more than half of the specimens, the presence of βIII-tubulin expression in cancer cells was relatively rare (Table 1 and Figure 1A). Of these 74 cases, 67 were scored as completely negative (0) and only 3 cases (4%) were considered to be positive, scoring from moderate-to-strong for βIII-tubulin in the cancer cells. With the exception of occasional stained cells in the basal compartment, βIII-tubulin expression was not detected in either normal basal cells or luminal prostate epithelial cells present in these specimens. The extremely small number of positively stained specimens in this group prevented us from deriving any statistically reliable association with other patient prognostic factors. Of the 24 cases of HTPC evaluated in our study, 6 (25%) were considered to be positive with βIII-tubulin immunoreactivity in the moderate-to-strong range in more than 10% of cancer cells (Table 1). Nine cases of HTPC showed complete negative (0) staining, whereas nine other cases in this category were scored as weak or contained only rare (<10%) tumour cells positively stained, and this later group was also recorded as negative. For the overall HTPC group, βIII-tubulin expression was found to be significantly higher when compared with the HNPC group as evaluated by Fisher's exact test applying the Bonferroni correction (P=0.0186).
Table 1. βIII-tubulin expression before and after hormone therapy.
| βIII-tubulin negative | βIII-tubulin positive | |
|---|---|---|
| Prostate carcinoma | No. of samples (%) | No. of samples (%) |
| Hormone-naive PCa (HNPC) | 71 (95.9) | 3 (4.1) |
| Hormone-therapy-treated PCa (HTPC) | 18 (75) | 6 (25) |
| Castration-resistant PCa (CRPC) | 16 (40) | 24 (60) |
| Pearson's χ2 test: | P<0.00001 | |
| Fisher's exact test: | ||
| HNPC/HTPC | P=0.0186 | |
| HTPC/CRPC | P=0.0285 | |
| HNPC/CRPC | P<0.000001 | |
Abbreviation: PCa=prostate carcinoma.
χ2 and Fisher's exact tests were used to assess associations between hormone and βIII-tubulin status. For post hoc comparisons, the Bonferroni correction was applied. All statistical tests used a two-tailed α=0.05 level of significance.
Figure 1.
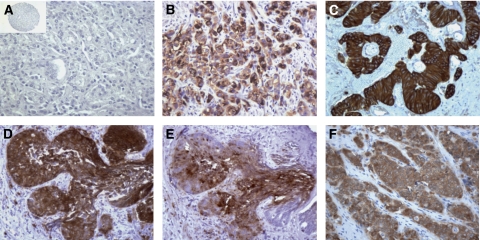
βIII-tubulin expression in prostate cancers. (A–C) Representative tissue microarray element, regular section or biopsy sample stained with antibody to βIII-tubulin with immunostains showing the absence of staining in hormone-naive prostate cancer (panel A) and strong staining in primary (panel B) and metastatic (panel C) castration-resistant refractory prostate cancers. (D and E) Representative consecutive sections stained with antibodies to βIII-tubulin (panel D) or NSE (panel E). Immunostainings show concomitant βIII-tubulin and NSE expression in prostate cancer cells. (F) Prostatic small cell carcinoma showing strong immunoreactivity for βIII-tubulin. Original magnification × 200; inset, × 25.
Finally, of the 40 specimens of CRPC that were analysed, the majority (24 or 60%) was considered to be positive (Figure 1A and C). The predominant staining pattern for CRPC specimens was strong and diffuse. Only three cases of CRPC showed a complete absence of staining (see Supplementary Table 1). Statistical analysis of the data showed that the expression of βIII-tubulin was significantly correlated with the castration-resistant phenotype (P<0.000001). A further analysis with a trend test also reached statistical significance (P<0.0001). It is noteworthy that, βIII-tubulin protein was also expressed in cancer cells of metastatic CRPC lesions present in the liver of patients (Figure 1C). Indeed, the fact that we were able to detect βIII-tubulin-positive cancer cells in at least five of six cases of this class suggests that the deregulated expression of βIII-tubulin in CRPC disease is not restricted to recurrent lesions localised to the prostate. This finding is also important because metastatic lesions are mainly responsible for both morbidity and mortality of CRPCs. Cumulatively, these results indicate that βIII-tubulin expression in human prostate cancer cells is upregulated by hormone therapy and increased further when tumours progress to a castration-resistant stage.
Expression of βIII tubulin is upregulated by hormone therapy in human prostate cancer LNCaP cells and LNCaP tumours
βIII-tubulin is commonly expressed in normal neural tissues and in NE cells (Katsetos et al, 2000, 2003). In line with this information, the results showing that βIII-tubulin expression that was upregulated in PCa cells obtained from both acute and chronic hormone-treated patients might be a manifestation of the NE trans-differentiation phenomenon that has been associated with prostate cancer cells capable of surviving in a low-androgen environment (Yuan et al, 2007). For further exploration of this event, 22 βIII-tubulin-positive CRPC specimens were immunostained with antibodies directed against NE markers; namely CgA, synaptophysin (SYN) and NSE. In 6 out of 22 cases analysed, we were able to detect areas showing strong positivity for both βIII-tubulin and NSE (Figure 1D and E). In line with this finding, 2 out of 10 evaluable cases demonstrated concomitant βIII-tubulin and SYN expression. However, we failed to find any co-expression pattern when using antibodies directed against CgA (eight evaluable cases). It is noteworthy that, βIII-tubulin expression was also examined in one case of prostatic small cell carcinoma, an uncommon subtype of PCa with predominance of the NE phenotype (Yao et al, 2006). In this case, 100% of cancer cells expressed high levels of βIII-tubulin (Figure 1F).
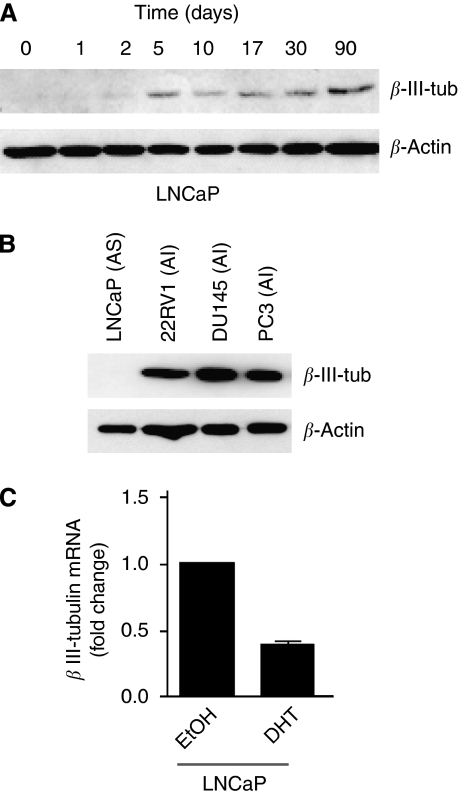
A trans-differentiation phenomenon is observed in cultured androgen-sensitive PCa cells when they are maintained in a medium depleted of androgens (Burchardt et al, 1999), and therefore we examined, using western blot analysis, whether androgen-sensitive human prostate cancer LNCaP cells upregulate βIII-tubulin when they are switched to androgen-deficient medium. Figure 2A shows that the steady-state levels of βIII-tubulin protein were strikingly elevated in the days following growth in the androgen-depleted medium with persistent expression detected up to 3 months under these conditions. The ability of growth in the androgen-depleted medium to upregulate βIII-tubulin protein expression was independent of the passage number of the LNCaP cells, as we obtained similar results using either low- (P25) or high-passage (P80) cells (data not shown). In addition, βIII-tubulin protein was expressed in various androgen-independent human PCa cell lines, including 22Rv1, PC3 and DU145 after western blot analysis of protein extracts from these cells (Figure 2B). These data agree with a previous finding that βIII-tubulin protein expression could be upregulated in LNCaP cells cultured in the androgen-deficient medium for 4 days or after transient suppression of androgen receptor expression using siRNA (Wright et al, 2003). We then tested the effect of the AR agonist, DHT on βIII-tubulin mRNA expression. To this end, LNCaP cells were incubated for 10 days in an androgen-depleted medium supplemented with 10 nM DHT or with vehicle (ethanol, EtOH). The expression of βIII-tubulin was partially abrogated in chronic DHT-treated cells (Figure 2C).
Figure 2.
βIII-tubulin expression is regulated by androgen depletion in LNCaP cultures and is expressed in androgen-independent PCa lines. (A) Time-course expression of βIII-tubulin in LNCaPs cultivated in androgen-reduced medium. At day 0, monolayer cultured LNCaP cells were grown in the androgen-reduced medium. Each protein sample (30 μg) was resolved to SDS–PAGE, transferred onto a polyvinylidene difluoride membrane (Millipore) and incubated with a monoclonal antibody against βIII-tubulin or β-actin as an internal control. (B) The same procedure was applied to examine βIII-tubulin expression in various PCa cell lines (LNCaP, 22Rv1, DU145 and PC3) maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), AS, androgen-sensitive; AI, androgen-independent. (C) βIII-tubulin mRNA is reduced on 10 days of stimulation by 10 nM DHT in LNCaP cells relative to the expression in cells maintained in the androgen-reduced medium. Columns, mean±s.e.m.
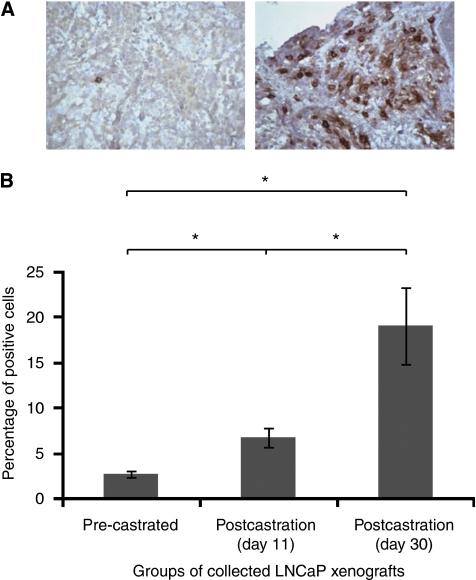
Finally, we sought to extend this observation by analysing βIII-tubulin expression in LNCaP tumours after xenografting into athymic male nude mice. Five mice bearing tumours between 200 and 300 mm3 were killed and the tumour specimens were collected, fixed and embedded for sectioning. In all, 10 other mice with tumours of the same size were surgically castrated and then killed at day 11 or 30 post castration to retrieve and process the tumours for immunostaining. Both the pre-castrated and castrated levels of βIII-tubulin were immunohistochemically analysed in various LNCaP tumours using the same parameters that were used for human tissues. Our analysis showed that the percentage of βIII-tubulin-positive staining cells was significantly elevated in tumour cells from castrated groups in a time-dependent manner. Although only 2.7% of the tumour cells were found to be βIII-tubulin positive in the tumour specimens obtained from the pre-castrated group, 6.8 or 19.1% of tumour cells were considered to be positive for βIII-tubulin at 11 or 30 days after castration, respectively (Figure 3A and B). In summary, this study of cultured and engrafted human prostate cancer cells supports the idea that hormone therapy leading to the development of CRPC increases the expression of βIII-tubulin in cancer cells.
Figure 3.
βIII-tubulin expression increases after androgen depletion in LNCaP xenografts. (A) Representative immunostains in LNCaP xenografts with antibody to βIII-tubulin. Immunohistochemical stains show weak or absent staining for LNCaP tumours from a non-castrated mouse (left), while a high proportion of positive cells with intense staining is detected 30 days after castration (right); original magnification, × 200. (B) Means of positive cells for βIII-tubulin in each group. Kruskal–Wallis test (P=0.002) and Mann–Whitney test were used for statistical analysis of the immunohistochemistry scoring results. Columns mean; bars, s.e. *P<0.05.
Discussion
This study for the first time provided definitive evidence that βIII-tubulin is differentially expressed at the different stages of PCa progression. One notable aspect of our observations with regard to a relationship between βIII-tubulin expression and hormone therapy for PCa is the possibility that βIII-tubulin expression contributes to the aggressiveness of hormone-treated or castration-resistant PCa similar to the reports for other solid tumours. However, studies investigating the functional ablation of βIII-tubulin functions in this setting are required to demonstrate conclusively its functional requirement for the emergence of castration-resistant tumours. Additional studies are also required to better define the mechanisms monitoring βIII-tubulin expression. Advanced PCa and castration-resistant PCa have been associated with an attenuated androgen signalling signature (Tomlins et al, 2007). This information further supports androgen regulation and the increased expression of βIII-tubulin in castration-resistant tumours. Interestingly, one recent study provided evidence for a potential role of oestradiol and oestrogen receptor (ER) in regulating βIII-tubulin in breast cancer cells (Saussede-Aim et al, 2009). Although it remains to be determined as to whether this regulation implies a genomic or a non-genomic effect of ER, this observation could be of interest in PCa as oestrogen-dependent signature has been linked to aggressive characteristics in this tissue (Bonkhoff et al, 2001; Setlur et al, 2008). Future work will explore these questions. Recent data also indicate that hypoxia may stimulate βIII-tubulin expression in the A2780 ovarian cancer cells (Raspaglio et al, 2008). We previously observed that hypoxia response can be altered in CRPC tumours and cell lines (Anastasiadis et al, 2002). This may highlight an alternative mechanism through which βIII-tubulin expression is increased in those tumours.
In the hormone-naive PCa group, βIII-tubulin expression in cancer cells was found to be restricted to a small number of prostate cancer samples. Our results contrast with a previous report showing that βIII-tubulin was consistently detectable in HNPC, although at a relatively low level of expression that was comparable with normal prostate epithelial cells (Ranganathan et al, 1997). This difference might be attributable to a more stringent immunostaining protocol. Future studies including larger patient cohorts would help to determine whether βIII-tubulin-positive tumours could represent a particular subtype of PCa. In addition, it will be of particular interest to examine the potential value of βIII-tubulin in predicting response to microtubule-targeting agents in patients with prostate cancer. This knowledge may be useful for a better management of patients in that it could help to refine decision criteria and to better select patients who are more likely to benefit from chemotherapy regimens. We also believe that one appealing aspect of the association between βIII-tubulin expression and hormone therapy for PCa is the indication that anti-cancer chemotherapeutic drugs that preferentially target microtubules with a βIII-tubulin component might have greater value for the treatment of CRPC than would docetaxel, which is currently recommended. Whereas we already know that docetaxel therapy does provide some benefit for CRPC patients, this benefit is generally limited to a few months of additional survival (Petrylak et al, 2004; Tannock et al, 2004). Clearly, the evidence that βIII-tubulin can modulate the sensitivity of cancer cells to standard taxane-based cancer therapy gives reason to consider whether other microtubule-targeting drugs with greater efficacy against cancer cells that express βIII-tubulin might be more effective in this regard. One candidate for this type of approach may be the epothilone B analogue, ixabepilone, which is being tested against various solid tumours with βIII-tubulin expression (Rivera et al, 2008; Dumontet et al, 2009) and that has already demonstrated activity in CRPCs, although it was with no consideration of βIII-tubulin status (Galsky et al, 2005). Our results give strong reason to consider βIII-tubulin status and to further test this therapeutic for CRPC patients.
Acknowledgments
This work was supported by INSERM, the ‘Conseil Général du Val de Marne’, the ‘Université Paris 12 Val-de-Marne’, grants from the ARTP (to ST, GP and FV) and the ‘Association pour la Recherche sur le Cancer’ (to FV and GP). We are grateful to Pascale Soyeux (Université Paris 12) and Lyna Guilbault (Laval University, Canada) for technical support critical to this work. We thank Julie Cognet and Rachid Souktani (small animal facility, INSERM, U955, Université Paris 12) and Xavier Decrouy (imaging facility, INSERM, U955, Université Paris 12) for providing technical assistance and advice.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Anastasiadis AG, Ghafar MA, Salomon L, Vacherot F, Benedit P, Chen MW, Shabsigh A, Burchardt M, Chopin DK, Shabsigh R, Buttyan R (2002) Human hormone-refractory prostate cancers can harbor mutations in the O(2)-dependent degradation domain of hypoxia inducible factor-1alpha (HIF-1alpha). J Cancer Res Clin Oncol 128: 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay S, Terry S, Chimingqi M, Sirab N, Faucon H, Gil Diez de Medina S, Moutereau S, Maille P, Soyeux P, Abbou C, Salomon L, Vacherot F, de La Taille A, Loric S, Allory Y (2008) Comparative expression of Hedgehog ligands at different stages of prostate carcinoma progression. J Pathol 216: 460–470 [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Fixemer T, Hunsicker I, Remberger K (2001) Progesterone receptor expression in human prostate cancer: correlation with tumor progression. Prostate 48: 285–291 [DOI] [PubMed] [Google Scholar]
- Burchardt T, Burchardt M, Chen MW, Cao Y, de la Taille A, Shabsigh A, Hayek O, Dorai T, Buttyan R (1999) Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J Urol 162: 1800–1805 [PubMed] [Google Scholar]
- Dumontet C, Jordan MA, Lee FF (2009) Ixabepilone: targeting {beta}III-tubulin expression in taxane-resistant malignancies. Mol Cancer Ther 8: 17–25 [DOI] [PubMed] [Google Scholar]
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 18: 581–592 [DOI] [PubMed] [Google Scholar]
- Ferrandina G, Zannoni GF, Martinelli E, Paglia A, Gallotta V, Mozzetti S, Scambia G, Ferlini C (2006) Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res 12: 2774–2779 [DOI] [PubMed] [Google Scholar]
- Frigo DE, McDonnell DP (2008) Differential effects of prostate cancer therapeutics on neuroendocrine transdifferentiation. Mol Cancer Ther 7: 659–669 [DOI] [PubMed] [Google Scholar]
- Galmarini CM, Treilleux I, Cardoso F, Bernard-Marty C, Durbecq V, Gancberg D, Bissery MC, Paesmans M, Larsimont D, Piccart MJ, Di Leo A, Dumontet C (2008) Class III beta-tubulin isotype predicts response in advanced breast cancer patients randomly treated either with single-agent doxorubicin or docetaxel. Clin Cancer Res 14: 4511–4516 [DOI] [PubMed] [Google Scholar]
- Galsky MD, Small EJ, Oh WK, Chen I, Smith DC, Colevas AD, Martone L, Curley T, Delacruz A, Scher HI, Kelly WK (2005) Multi-institutional randomized phase II trial of the epothilone B analog ixabepilone (BMS-247550) with or without estramustine phosphate in patients with progressive castrate metastatic prostate cancer. J Clin Oncol 23: 1439–1446 [DOI] [PubMed] [Google Scholar]
- Gan PP, Pasquier E, Kavallaris M (2007) Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res 67: 9356–9363 [DOI] [PubMed] [Google Scholar]
- Han M, Partin AW, Pound CR, Epstein JI, Walsh PC (2001) Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am 28: 555–565 [DOI] [PubMed] [Google Scholar]
- Hellerstedt BA, Pienta KJ (2002) The current state of hormonal therapy for prostate cancer. CA Cancer J Clin 52: 154–179 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58: 71–96 [DOI] [PubMed] [Google Scholar]
- Kamath K, Wilson L, Cabral F, Jordan MA (2005) BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem 280: 12902–12907 [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Herman MM, Mork SJ (2003) Class III beta-tubulin in human development and cancer. Cell Motil Cytoskeleton 55: 77–96 [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Kontogeorgos G, Geddes JF, Herman MM, Tsimara-Papastamatiou H, Yu Y, Sakkas LI, Tsokos M, Patchefsky AS, Ehya H, Cooper HS, Provencio J, Spano AJ, Frankfurter A (2000) Differential distribution of the neuron-associated class III beta-tubulin in neuroendocrine lung tumors. Arch Pathol Lab Med 124: 535–544 [DOI] [PubMed] [Google Scholar]
- Kavallaris M, Burkhart CA, Horwitz SB (1999) Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to Taxol. Br J Cancer 80: 1020–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Luduena RF (1993) Removal of beta III isotype enhances taxol induced microtubule assembly. Cell Struct Funct 18: 173–182 [DOI] [PubMed] [Google Scholar]
- Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB (2003) Mechanisms of taxol resistance related to microtubules. Oncogene 22: 7280–7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D, Miller HP, Banerjee A, Luduena RF, Wilson L (1994) Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci USA 91: 11358–11362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara Jr PN, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351: 1513–1520 [DOI] [PubMed] [Google Scholar]
- Ranganathan S, Salazar H, Benetatos CA, Hudes GR (1997) Immunohistochemical analysis of beta-tubulin isotypes in human prostate carcinoma and benign prostatic hypertrophy. Prostate 30: 263–268 [DOI] [PubMed] [Google Scholar]
- Raspaglio G, Filippetti F, Prislei S, Penci R, De Maria I, Cicchillitti L, Mozzetti S, Scambia G, Ferlini C (2008) Hypoxia induces class III beta-tubulin gene expression by HIF-1alpha binding to its 3′ flanking region. Gene 409: 100–108 [DOI] [PubMed] [Google Scholar]
- Rivera E, Lee J, Davies A (2008) Clinical development of ixabepilone and other epothilones in patients with advanced solid tumors. Oncologist 13: 1207–1223 [DOI] [PubMed] [Google Scholar]
- Saussede-Aim J, Matera EL, Ferlini C, Dumontet C (2009) Beta3-tubulin is induced by estradiol in human breast carcinoma cells through an estrogen-receptor dependent pathway. Cell Motil Cytoskeleton 66: 378–388 [DOI] [PubMed] [Google Scholar]
- Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, Sboner A, Pawitan Y, Andren O, Johnson LA, Tang J, Adami HO, Calza S, Chinnaiyan AM, Rhodes D, Tomlins S, Fall K, Mucci LA, Kantoff PW, Stampfer MJ, Andersson SO, Varenhorst E, Johansson JE, Brown M, Golub TR, Rubin MA (2008) Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst 100: 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seve P, Dumontet C (2008) Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol 9: 168–175 [DOI] [PubMed] [Google Scholar]
- Shalli K, Brown I, Heys SD, Schofield AC (2005) Alterations of beta-tubulin isotypes in breast cancer cells resistant to docetaxel. FASEB J 19: 1299–1301 [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351: 1502–1512 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM (2007) Integrative molecular concept modeling of prostate cancer progression. Nat Genet 39: 41–51 [DOI] [PubMed] [Google Scholar]
- Wright ME, Tsai MJ, Aebersold R (2003) Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol 17: 1726–1737 [DOI] [PubMed] [Google Scholar]
- Yao JL, Madeb R, Bourne P, Lei J, Yang X, Tickoo S, Liu Z, Tan D, Cheng L, Hatem F, Huang J, Anthony di Sant’Agnese P (2006) Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol 30: 705–712 [DOI] [PubMed] [Google Scholar]
- Yuan TC, Veeramani S, Lin MF (2007) Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer 14: 531–547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.