Abstract
Objective
To describe the methodology of evaluating the response of cancer patients to interventions directed at lowering severity of multiple symptoms and compare two arms of a symptom management trial to determine factors associated with response and time to response.
Study Design And Setting
Randomized trial comparing a nurse assisted symptom management (NASM) cognitive behavioral intervention with an automated telephone symptom management (ATSM). Patients in both arms received 6 intervention contacts over 8 weeks. Analyses of the intervention contact data for 190 patients in NASM arm, and 164 patients in the ATSM arm were conducted. Severities of 15 cancer-related symptoms were assessed at each intervention contact and an anchor-based definition of response was adopted. Analyses were carried out using generalized estimating equations and Cox marginal proportional hazard models.
Results
When compared with patients in the NASM, patients in the ATSM had better response to managing anxiety, depression, poor appetite, cough and fatigue. NASM was more successful managing cancer pain. Response and time to response were associated with several patient and disease characteristics.
Conclusion
The approach described here presents an analytic and clinical improvement over methods that examine each symptom separately or use summed scores of severity.
Keywords: Cancer, Symptom Management, Intervention, Symptom Response, Time to Response
What is new?
The evaluation of interventions directed at the management of multiple cancer-related symptoms faces several methodological challenges related to summing severities across multiple symptoms or using absolute or percent change in severity.
A new methodology of evaluating response to symptom management interventions is proposed using anchor-based definition of symptom response.
The application of this methodology to the analysis of a trial of a cognitive behavioral intervention revealed differences between trial arms with respect to symptom response and time to response.
The methodology that incorporates multiple symptom response outcomes can be applied to the analysis of symptom management trials in cancer.
Introduction
Among cancer patients, effective management of multiple symptoms is a key to maintaining therapeutic effective dosing, and improving patients’ quality of life. [1-6] Research has examined single symptoms, [4-15] or groups of symptoms. [1-2, 16-23]. Single symptom assessments may underestimate patients’ total symptom severity burden. Since symptoms may be interrelated as a function of disease, treatment or patient characteristics, [24] the associations among symptoms must be taken into account when responses to interventions are evaluated at the patient level. Some patients may respond differently to interventions directed at different combinations of symptoms thus requiring more or less time to resolve different sets of symptoms.
When multiple symptoms are evaluated, their severities may be summed to produce an index across an array of symptoms. [25-30] The summed symptom severity scores measure the symptom severity burden but have several shortcomings such as equal contribution of symptom ratings toward total burden scores.
Clinically important cut-points separating moderate from mild, and severe from moderate pain, [31-34] and fatigue, [4, 6] have been established demonstrating that increase or decrease of one unit on the rating scale may have different meaning depending on where on the scale the increase occurs. In prior work, [35] the authors established mild, moderate, and severe categories for 15 symptoms experienced by cancer patients undergoing chemotherapy based on their reports of symptom interference with enjoyment of life, relationships with others, general daily activities, and emotions. The cut-points were different for different symptoms, and provided consistent differentiation of the levels of interference over time. [36]
In this work, we define responses to the symptom management interventions based on shifts among the pre-defined mild, moderate and severe categories. These responses are symptom specific and anchor-based. Other definitions of response [37-39] are based on absolute or percent decline in severity. Patients who report a decline in pain from 33% to 50% are considered to have achieved a clinically significant improvement. [40-41] Patients who reported that they were “much improved” or “very much improved” experienced approximately 30% reduction in pain. [37-38] However, such responses include reductions from a severity of 9 to a severity of 6 (33%) on a 0−10 severity scale which is not a satisfactory clinical outcome because pain remains at a distressing level. [42]
Two outcomes are evaluated in this work: a binary response versus non-response, and a continuous time to response for each symptom within a patient. Analyses are conducted by aggregating symptoms to the patient level and accounting for associations among them. Time to event type of outcomes such as survival time, time of disease-free survival, time to recurrence or time to treatment failure have been used in studies testing effects of various therapies and chemotherapeutic agents. [43-44] However psycho-educational or cognitive-behavioral interventions have not included time to response or time to other events as outcomes.
Two interventions are considered in this work: 1) A Nurse Assisted Symptom Management (NASM) intervention, and 2) an Automated Telephone Symptom Management (ATSM) intervention. Their effects on reduction in summed symptom severity are described in detail elsewhere. [45] Briefly, there were no differences between the arms in summed index of symptom severity or in severity of any symptom at the post-intervention interview. Both arms produced clinically significant improvement from baseline to 10 weeks. In this secondary analysis, we look beyond baseline and endpoint observations toward comparing arms according to symptom response and time to response to prescribed interventions.
Our analyses are guided by the following research questions: 1) Compared to ATSM, does the NASM produce better responses to symptom management strategies and shorter time to response at the level of the patient? 2) Do symptom factors such onset time, and patient factors such as comorbidity and the number of moderate and severe symptoms influence response and time to response?
Methods
Sample and Setting
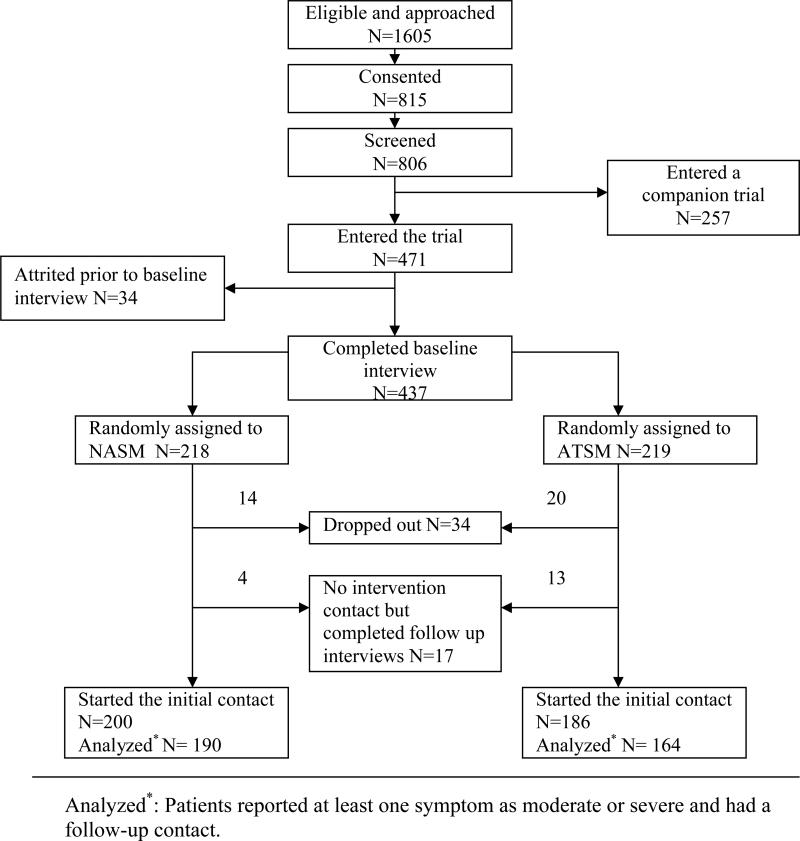
The study was approved by the Institutional Review Board (IRB) of the sponsoring university and IRB's of the participating sites. Inclusion criteria were 1) being 21 years of age or older, 2) having a diagnosis of a solid tumor cancer or non-Hodgkins lymphoma, 3) be undergoing first course of chemotherapy, 4) being able to speak and read English, and 5) having a touchtone telephone. Patients agreeing to participate signed an informed consent form and were screened for symptom severity using an automated voice response version of M.D. Anderson Symptom Inventory. [25] Those who scored 2 or higher on any symptom in screening entered the trial, had an intake interview, received a copy of the Symptom Management Guide (SMG), and were randomized into either the NASM or the ATSM using computer minimization program [46] that balanced the arms with respect to recruitment location and site of cancer. Both arms of the trial received 6 calls over 8 weeks. At 10 weeks, outcome data were obtained through a second interview. In this analysis data collected during intervention contacts were used. We included patients who reported at least one symptom at moderate or severe level with at least one follow-up contact to evaluate the response of the interventions delivered for the symptom(s). Figure 1 summarizes the number of enrolled and attrited patients at each step, the number of cases meeting entry criteria and number analyzed.
Figure 1.
Flowchart of the study.
Intervention
The trial sought to compare the impact ATSM intervention with a NASM protocol on reducing patient reports of severity of 15 prevalent symptoms [16-17, 25-30, 47]: fatigue, pain, dyspnea, insomnia, anxiety, depression, nausea/vomiting, difficulty remembering, lack of appetite, dry mouth, peripheral neuropathy, diarrhea, cough, constipation, and weakness.
Following symptom management guidelines, [42] patients who rated severities of symptoms at 4 or higher at each contact received strategies to manage those symptoms. For patients assigned to the NASM group, nurses delivered up to four strategies for each symptom supplemented with references to the SMG. At each subsequent contact, assigned strategies were evaluated. Successful strategies were continued, and those not tried or tried but not deemed successful were dropped or replaced by different strategies.
In the ATSM arm, patients were called and queried by the automated system to rate severity of each symptom by pressing the appropriate numbers on their telephone keypads. For symptoms rated at 4 or higher, patients were directed to the section of the SMG that informed them about strategies to manage each symptom. For all symptoms with severities above 4 on the prior contact (except first), patients reported if the strategies from SMG were tried, and if tried, if they were helpful.
Measures
Age, sex, site and stage of cancer were obtained from the patients’ medical records, and confirmed in baseline interview.
Comorbid conditions were assessed at baseline using a 15 item questionnaire modified from the one developed by Katz. [48] Number of comorbid conditions was dichotomized at the median into 0−2 versus 3 or more categories.
Severities of 15 symptoms were scored by patients on a scale ranging from absence (0) to the worst severity possible (10) at each of the 6 intervention contacts. Interference-based cut-points established by this team [35-36] were applied to categorize severity of each symptom as mild, moderate, or severe. Patients who reached moderate or severe levels on a symptom were classified as responders to the interventions strategies if they moved from; severe to moderate or to mild (or none), or moderate to mild (or none) between onset time (date of the first contact when symptom was at moderate or severe) and the last contact completed. Patients who went from moderate at onset to moderate or severe at the last contact, or from severe at onset to severe on the last contact, were classified as non-responders. Patients who never had a symptom at a level above mild, did not receive any interventions for the management of that symptom, were not classified as to response, and were not included in the analysis. Similarly, patients who had symptom onset at their last contact were not included in the analysis since no follow-up contacts were available to evaluate their response. Non-responders’ time to response was treated as censored; for responders, time in days from the symptom onset (described above) to the date of the contact when patients reported first sustained improvement (e.g. going from moderate to mild, and staying mild for the remaining contacts) was defined as time to response.
The total symptom burden was assessed by determining the number of symptoms that ever reached moderate or severe during the intervention contacts. This number was dichotomized at the 3rd quartile as less than 8 versus 8 or more symptoms.
Data Analysis
The baseline equivalence and equivalence of attrition by the arms of the trial were established earlier. [45] To explain response versus non-response across multiple symptoms, a Generalized Estimating Equations (GEE) model with compound symmetry correlation structure [49] was used. Patient symptom was the unit of analysis. For the covariates in the model; trial arm, comorbidity, total symptom burden, and onset time of each symptom, dummy variables were created to allow beta coefficients to vary across multiple symptoms to model possibly different effects of covariates across symptoms. The equality of beta coefficients across symptoms was tested for each of the covariates in the model using generalized score tests. [50-51] Because of reported unticonservatism of score tests, [53] we evaluated the symptom-specific coefficients to note any effects that may not be significantly different from other symptoms statistically, but represent clinically meaningful findings. The final “hybrid” model was built that included covariates with coefficients that differed across symptoms, and covariates with coefficients set to be equal across multiple symptoms. The GEE model was fit using GENMOD procedure, SAS version 9.1. [54] Odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated for trial arm and covariates of interest.
Time to response analysis was carried out using marginal Cox proportional hazard models implemented in TPHREG procedure in SAS. The marginal approach of Wei, Lin, and Weissfeld [55] was used for the patient level analysis that aggregated multiple symptoms and was carried out using maximum partial likelihood estimates of regression parameters and a robust sandwich covariance matrix estimate to account for the intracluster dependence (symptoms clustered within patient). [56-57] Similar to the response versus non-response analyses, the effects of the covariates were tested for equality of betas across multiple symptoms using Wald's test, and the symptom-specific coefficients were examined. The final “hybrid” model included covariates with coefficients that differed across symptoms, and covariates with the coefficients set to be equal across an array of symptoms.
Results
Table 1 presents characteristics of the sample by trial arm. Among solid tumors, breast cancer was the most prevalent site of cancer; the majority of patients had advanced (late stage) disease and all were undergoing first course of chemotherapy at the time of enrollment. By the 10 week interview, 64% of patients in the NASM, and 61% of patients in the ATSM arm were still on chemotherapy. Those who finished or discontinued chemotherapy by the 10 week interview were on average 5 weeks away from the last infusion.
Table 1.
Characteristics of the sample by trial arm.
| Variable | Category | NASM Arm |
ATSM Arm |
||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Patient Age | 25 ∼ 44 | 33 | 15.14 | 31 | 14.22 |
| 45 ∼ 54 | 57 | 26.15 | 62 | 28.44 | |
| 55 ∼ 64 | 72 | 33.03 | 74 | 33.94 | |
| 65 ∼ 74 | 38 | 17.43 | 27 | 12.39 | |
| |
75 + |
18 |
8.26 |
24 |
11.01 |
| Education | High school or below |
71 |
32.57 |
67 |
30.59 |
| Some college or technical training |
72 |
33.03 |
66 |
30.14 |
|
| College |
41 |
18.81 |
42 |
19.18 |
|
| |
Graduate professional degree |
34 |
15.60 |
44 |
20.09 |
| Cancer Site | Colon |
30 |
13.76 |
33 |
15.07 |
| Breast |
88 |
40.37 |
86 |
39.27 |
|
| Lung |
39 |
17.89 |
37 |
16.89 |
|
| |
Other |
61 |
27.98 |
63 |
28.77 |
| Cancer Stage | Early | 53 | 24.65 | 66 | 30.84 |
| |
Late |
162 |
75.35 |
148 |
69.16 |
| Metastatic Cancer | Yes | 131 | 60.93 | 118 | 55.14 |
| |
No |
84 |
39.07 |
96 |
44.86 |
| Comorbidity | 0∼2 |
139 |
63.76 |
139 |
63.47 |
| |
3+ |
79 |
36.24 |
80 |
36.53 |
| Number of Symptoms that Reached Moderate or Severe | Less than 8 |
126 |
66.32 |
104 |
63.41 |
| 8 and above | 64 | 33.68 | 60 | 36.59 | |
The interference-based categories of symptom severity for each of 15 symptoms are presented in Table 2. Patient symptom cases not included in this analysis did not differ by arm of the trial, including those who went from mild to none. More than half of the patients reported their symptoms at the first intervention contact at moderate or severe levels.
Table 2.
Mild, moderate and severe categories for symptom severity, symptom responses and onset times by trial arm.
| Mild, moderate and severe categories of symptoms | Intervention Arm | Patient Response to Symptom Management | Not included in the analysis of response | Onset time2 | ||||
|---|---|---|---|---|---|---|---|---|
| Response | Non-Response | Remained mild | Not present | Onset on last contact1 | 1st contact | 2nd or later contact | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Fatigue 0 1, 2−4, 5−10 | NASM | 82 (41.00) | 95 (47.50) | 3 (1.50) | 6 (3.00) | 14 (7.00) | 144 (81.36) | 33 (18.64) |
| ATSM | 95 (51.08) | 63 (33.87) | 10 (5.38) | 8 (4.30) | 10 (5.38) | 148 (93.67) | 10 (6.33) | |
| Weakness 0 1−2, 3−4, 5−10 | NASM | 79 (39.50) | 27 (13.50) | 20 (10.00) | 65 (32.50) | 9 (4.50) | 76 (71.70) | 30 (28.30) |
| ATSM | 61 (33.52) | 32 (17.58) | 38 (20.88) | 43 (23.63) | 8 (4.40) | 70 (75.27) | 23 (24.73) | |
| Depression 0 1, 2−3, 4−10 | NASM | 66 (33.00) | 33 (16.50) | 16 (8.00) | 78 (39.00) | 7 (3.50) | 62 (62.63) | 37 (37.37) |
| ATSM | 69 (37.50) | 17 (9.24) | 19 (10.33) | 67 (36.41) | 12 (6.52) | 68 (79.07) | 18 (20.93) | |
| Difficulty Remembering 0 1, 2−4, 5−10 | NASM | 63 (31.50) | 41 (20.50) | 18 (9.00) | 71 (35.50) | 7 (3.50) | 77 (74.04) | 27 (25.96) |
| ATSM | 46 (25.27) | 30 (16.48) | 24 (13.19) | 73 (40.11) | 9 (4.95) | 60 (78.95) | 16 (21.05) | |
| Pain 0 1, 2−4, 5−10 | NASM | 68 (34.00) | 29 (14.50) | 10 (5.00) | 76 (38.00) | 17 (8.50) | 61 (62.89) | 36 (37.11) |
| ATSM | 45 (32.14) | 36 (25.71) | 21 (15.00) | 30 (21.43) | 8 (5.71) | 58 (71.60) | 23 (28.40) | |
| Insomnia 0 1−3, 4−6, 7−10 | NASM | 64 (32.00) | 22 (11.00) | 52 (26.00) | 53 (26.50) | 9 (4.50) | 63 (73.26) | 23 (26.74) |
| ATSM | 56 (30.77) | 19 (10.44) | 62 (34.07) | 38 (20.88) | 7 (3.85) | 61 (81.33) | 14 (18.67) | |
| Poor Appetite 0 1−3, 4−5, 6−10 | NASM | 69 (34.50) | 19 (9.50) | 27 (13.50) | 74 (37.00) | 11 (5.50) | 51 (57.95) | 37 (42.05) |
| ATSM | 71 (38.38) | 11 (5.95) | 53 (28.65) | 38 (20.54) | 12 (6.49) | 61 (74.39) | 21 (25.61) | |
| Anxiety 0 1−3, 4−5, 6−10 | NASM | 55 (27.50) | 19 (9.50) | 61 (30.50) | 58 (29.00) | 7 (3.50) | 45 (60.81) | 29 (39.19) |
| ATSM | 56 (30.43) | 9 (4.89) | 66 (35.87) | 46 (25.00) | 7 (3.76) | 48 (73.85) | 17 (26.15) | |
| Dyspnea 0 1−2, 3−6, 7−10 | NASM | 38 (19.00) | 24 (12.00) | 25 (12.50) | 108 (54.00) | 5 (2.50) | 37 (59.68) | 25 (40.32) |
| ATSM | 43 (23.12) | 18 (9.68) | 32 (17.20) | 79 (43.41) | 10 (5.49) | 41 (67.21) | 20 (32.79) | |
| Dry Mouth 0 1−4, 5−8, 9−10 | NASM | 52 (26.00) | 9 (4.50) | 81 (40.50) | 52 (26.00) | 6 (3.00) | 34 (55.74) | 27 (44.26) |
| ATSM | 38 (20.88) | 8 (4.40) | 62 (34.07) | 70 (38.46) | 4 (2.20) | 28 (60.87) | 18 (39.13) | |
| Constipation 0 1−3, 4−6, 7−10 | NASM | 36 (18.00) | 10 (5.00) | 35 (17.50) | 113 (56.50) | 6 (3.00) | 29 (63.04) | 17 (36.96) |
| ATSM | 52 (28.26) | 9 (4.89) | 55 (29.89) | 60 (32.61) | 8 (4.35) | 46 (75.41) | 15 (24.59) | |
| Cough 0 1−2, 3−4, 5−10 | NASM | 37 (18.50) | 20 (10.00) | 35 (17.50) | 103 (51.50) | 5 (2.50) | 37 (64.91) | 20 (35.09) |
| ATSM | 45 (24.46) | 4 (2.17) | 46 (25.00) | 82 (44.57) | 7 (3.80) | 30 (61.22) | 19 (38.78) | |
| Peripheral Neuropathy 0 1−3, 4−7, 8−10 | NASM | 23 (11.50) | 24 (12.00) | 43 (21.50) | 103 (51.50) | 7 (3.50) | 28 (59.57) | 19 (40.43) |
| ATSM | 40 (21.98) | 14 (7.69) | 55 (30.22) | 66 (36.26) | 7 (3.85) | 28 (51.85) | 26 (48.15) | |
| Nausea/Vomiting 0 1−3, 4−6, 7−10 | NASM | 36 (18.00) | 5 (2.50) | 39 (19.50) | 114 (57.00) | 6 (3.00) | 19 (46.34) | 22 (53.66) |
| ATSM | 38 (20.88) | 12 (8.79) | 51 (28.02) | 76 (41.76) | 5 (2.75) | 33 (66.00) | 17 (34.00) | |
| Diarrhea 0 1−3, 4−5, 6−10 | NASM | 27 (13.50) | 5 (2.50) | 31 (15.50) | 132 (66.00) | 5 (2.50) | 12 (37.50) | 20 (62.50) |
| ATSM | 23 (12.57) | 6 (3.28) | 64 (34.97) | 87 (47.54) | 3 (1.64) | 17 (58.62) | 12 (41.38) | |
Onset on last contact1: Symptom reached moderate or severe for the first time at the last contact, no follow-up contact.
Onset Time2: Intervention contact when symptom reached moderate or severe level for the first time.
Based on the tests of the equality of betas across multiple symptoms in response versus non-response model (Table 3), the trial arm and symptom onset time were entered as symptom-specific, that is the final model included 15 dummy variables (one for each symptom) to model group effect, and 15 dummy variables to model onset time. The number of comorbid conditions, and number of symptoms that ever reached moderate or severe levels during the intervention were treated as patient-level variables with the same coefficients across symptoms.
Table 3.
The test of equality of coefficients across symptoms in GEE model of response versus non-response.
| Covariates | Levels | Degrees of freedom | Wald Chi-Square | p-value |
|---|---|---|---|---|
| Trial arm | ATSM vs. NASM | 14 | 31.03 | <0.01 |
| Number of comorbid conditions | 3 and above vs. less than 3 | 14 | 21.26 | 0.10 |
| Number of symptoms that reached moderate or severe | 8 and above vs. less than 8 | 14 | 21.26 | 0.10 |
| Onset time | 1st contact vs. 2nd or later contact | 14 | 38.24 | <0.01 |
Compared to the NASM, the ATSM arm was more successful in achieving response for anxiety, depression, poor appetite, cough and fatigue (Table 4). The NASM arm produced a greater response to managing pain. Varying magnitudes of ORs for arm of the trial support the decision to model trial arm using different coefficients for different symptoms.
Table 4.
Adjusted odds ratios of response derived from GEE model of response versus non-response for multiple symptoms.
| Covariates | Symptom | Level | Ref. Level | Adjusted odds ratio (95% CI) | Chi-square p-value |
|---|---|---|---|---|---|
| Trial arm | Cough | ATSM | NASM | 6.91 (1.89, 25.22) | <0.01 |
| Anxiety | 2.65 (1.21,5.84) | 0.02 | |||
| Poor Appetite | 2.44 (1.19, 5.03) | 0.02 | |||
| Depression | 2.16 (1.11, 4.19) | 0.02 | |||
| Dry Mouth | 1.91 (0.78, 4.69) | 0.16 | |||
| Constipation | 1.85 (0.84, 4.06) | 0.12 | |||
| Fatigue | 1.75 (1.09, 2.80) | 0.02 | |||
| Diarrhea | 1.72 (0.57, 5.23) | 0.34 | |||
| Peripheral Neuropathy | 1.66 (0.80, 3.42) | 0.17 | |||
| Dyspnea | 1.24 (0.61, 2.51) | 0.56 | |||
| Nausea/Vomiting | 1.01 (0.44, 2.34) | 0.98 | |||
| Difficulty Remembering | 0.96 (0.53, 1.76) | 0.91 | |||
| Insomnia | 0.91 (0.44, 1.85) | 0.79 | |||
| Weakness | 0.64 (0.37, 1.10) | 0.11 | |||
| Pain | 0.48 (0.26, 0.88) | 0.02 | |||
| Number of comorbid conditions | 3 and above | Less than 3 | 0.58 (0.44, 0.76) | <0.01 | |
| Number of symptoms that reached moderate or severe | 8 and above | Less than 8 | 0.60 (0.46, 0.79) | <0.01 | |
| Onset time | Nausea/Vomiting | 1st contact | 2nd or later contact | 1.67 (0.72, 3.86) | 0.23 |
| Dry Mouth | 1.51 (0.75, 3.05) | 0.25 | |||
| Insomnia | 1.50 (0.83, 2.70) | 0.18 | |||
| Constipation | 1.40 (0.75, 2.62) | 0.29 | |||
| Weakness | 1.27 (0.81, 2.00) | 0.30 | |||
| Diarrhea | 1.26 (0.47, 3.39) | 0.64 | |||
| Poor Appetite | 1.15 (0.67, 1.96) | 0.62 | |||
| Anxiety | 1.05 (0.58, 1.88) | 0.88 | |||
| Pain | 0.95 (0.56, 1.59) | 0.84 | |||
| Cough | 0.79 (0.39, 1.58) | 0.50 | |||
| Dyspnea | 0.78 (0.43, 1.41) | 0.41 | |||
| Depression | 0.73 (0.44, 1.22) | 0.23 | |||
| Difficulty Remembering | 0.59 (0.37, 0.93) | 0.02 | |||
| Peripheral Neuropathy | 0.44 (0.22, 0.86) | 0.02 | |||
| Fatigue | 0.28 (0.20, 0.40) | <0.01 |
In both arms of the trial, patients with fewer than 3 comorbid conditions were more likely to respond compared to patients with 3 or more; patients with 8 or more symptoms that reached moderate or severe levels during intervention period had lower probability of response compared to those with less than 8 symptoms. Onset time was associated with the probability of response for fatigue, peripheral neuropathy and difficulty remembering: presence of these symptoms on the first intervention contact was associated with smaller probability of response compared to reaching moderate or severe levels for the first time on the second or later contacts.
In the analysis of the time to response, only the coefficients for trial arm were set to differ across symptoms based on the results of tests of equality of betas presented in Table 5.
Table 5.
The test of equality of coefficients across symptoms in marginal Cox proportional hazard model of time to response.
| Covariates | Levels | Degrees of freedom | Wald Chi-Square | p-value |
|---|---|---|---|---|
| Trial arm | ATSM vs. NASM | 14 | 36.65 | <0.01 |
| Number of comorbid conditions | 3 and above vs. less than 3 | 14 | 16.88 | 0.26 |
| Number of symptoms that reached moderate or severe | 8 and above vs. less than 8 | 14 | 10.53 | 0.72 |
| Onset time | 1st contact vs. 2nd or later contact | 14 | 17.96 | 0.21 |
The ATSM group produced a shorter time to response for poor appetite, depression, cough, fatigue and peripheral neuropathy (Table 6). Greater number of comorbid conditions, and greater number of symptoms that reached moderate or severe levels were associated with longer time to response. In addition, symptoms present on the first intervention contact took longer to resolve compared to “new’ symptoms that first presented on second or later contact.
Table 6.
Adjusted hazard ratios of response derived from Cox proportional hazard model of time to response for multiple symptoms.
| Covariates | Symptom | Level | Ref. Level | Adjusted hazard ratio (95% CI) | Chi-square p-value |
|---|---|---|---|---|---|
| Trial arm | Cough | ATSM | NASM | 2.11 (1.39, 3.20) | <0.01 |
| Peripheral Neuropathy | 1.83 (1.13, 2.98) | 0.01 | |||
| Depression | 1.60 (1.17, 2.19) | <0.01 | |||
| Fatigue | 1.49 (1.12, 1.99) | 0.01 | |||
| Poor Appetite | 1.38 (1.01, 1.88) | 0.04 | |||
| Anxiety | 1.34 (0.96, 1.88) | 0.09 | |||
| Constipation | 1.31 (0.88, 1.97) | 0.19 | |||
| Dyspnea | 1.23 (0.81, 1.85) | 0.33 | |||
| Difficulty Remembering | 1.09 (0.76, 1.58) | 0.63 | |||
| Diarrhea | 0.99 (0.59, 1.66) | 0.97 | |||
| Dry Mouth | 0.93 (0.63, 1.37) | 0.72 | |||
| Weakness | 0.93 (0.68, 1.27) | 0.67 | |||
| Nausea/Vomiting | 0.92 (0.60, 1.41) | 0.70 | |||
| Insomnia | 0.92 (0.66, 1.29) | 0.64 | |||
| Pain | 0.78 (0.54, 1.13) | 0.19 | |||
| Number of comorbid conditions | 3 and above | Less than 3 | 0.77 (0.66, 0.91) | <0.01 | |
| Number of symptoms that reached moderate or severe | 8 and above | Less than 8 | 0.69 (0.60, 0.80) | <0.01 | |
| Onset time | 1st contact | 2nd or later contact | 0.75 (0.67, 0.85) | <0.01 |
Discussion
The results presented in this paper underscore the importance of conducting the analyses of multiple symptoms experienced by cancer patient undergoing chemotherapy at a patient level where multiple symptoms are aggregated, correlations among them are accounted for, and the effects of patient, disease and treatment characteristics are summarized across an array of symptoms. When separate analyses for each symptom are conducted, there is a possibility, given the number of tests, that the findings could occur by chance alone. Also, while fatigue was reported at moderate or severe levels during the intervention by approximately 90% of patients, other symptoms such as diarrhea, vomiting, and cough have much lower prevalence, and sample size becomes an issue when investigating response and time to response for each symptom separately. When data are aggregated across symptoms to the level of the patient, symptoms are nested within patients, and patients with at least one symptom at moderate or severe level are included in the analysis ensuring larger sample size compared to separate symptom by symptom analyses, and the possibility of type I errors due to multiple testing no longer exists.
A possible explanation for the differences in response to the management of some symptoms is that the automated system delivered interventions that are direct, and are tailored to patient needs by referring patients to the specific sections of the symptom management guide. These interventions may be easier to follow for patients in contrast to the more numerous and potentially shifting interventions delivered by nurses. However, a symptom of pain is difficult to manage, [39] and the skills of nurses in tailoring interventions to specific patients resulted in better response compared to an automated system. When time to response was examined, no significant differences between trial arms were found for pain. One possible explanation is that even with nurses’ skills, pain took fairly long to resolve.
Shorter time to response for poor appetite, cough, depression, and fatigue in the ATSM arm compared to NASM was to be expected since for these symptoms the rate of response was better in the ATSM, and therefore there were more censored observations in the NASM arm. When arms of a trial are equal in response to symptom management, time to response may provide an important distinction, and this was the case with peripheral neuropathy.
The associations found between patient and symptom characteristics and response and time to response suggests that future behavioral trials may adjust the intensity of dosing regimens for patients who enter trials with more comorbid conditions and experience more symptoms. The finding that fatigue, peripheral neuropathy and difficulty remembering that presented on the first contact had lower probability of response compared to onset on later contacts may be counter-intuitive since patients in both arms received interventions for these symptom from the very first contact, so greater probability of response may be expected. However, our results suggest otherwise.
Our definition of response is anchor-based: it uses the decrease in symptom severity between mild, moderate and severe categories. In this research transitions from severe to mild corresponded to at least 50% reduction in symptom severity. Moreover, transitions from moderate to mild correspond to at least 33% for cough, depression, dyspnea, fatigue, pain, difficulty remembering, and weakness. Thus, implicit in these responses are clinically significant reductions in symptom severity produced by both arms of this trial. [40]
Several limitations of this work should be noted. The trial did not include a control group. Time to response was measured using dates between intervention contacts and not the actual dates when the symptom first reached moderate or severe or improved.. Despite these limitations, the approach of evaluating symptom responses presented here represents both an analytic and a clinical improvement over methods that use distributional measures of improvement, or use severity measures that sum severities of multiple symptoms into an index. Using response categories, clinicians can evaluate symptom severity interventions appreciating both clinical and statistical referents of success.
ACKNOWLEDGMENT OF RESEARCH SUPPORT
This research supported by Grant CA79280 from the National Cancer Institute and in affiliation with the Walther Cancer Institute, Indianapolis Indiana.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alla Sikorskii, Michigan State University Department of Statistics and Probability College of Natural Science A423 Wells Hall East Lansing, MI 48824 Phone: 517−353−2963 alla.sikorskii@hc.msu.edu.
Charles W. Given, Michigan State University Department of Family Medicine College of Human Medicine B108 Clinical Center East Lansing, MI 48824 Phone: 517−353−0851 x420 givenc@msu.edu.
Mei You, Michigan State University College of Nursing East Lansing, MI 48824 Phone: 517−432−8352 mei.you@hc.msu.edu.
Sangchoon Jeon, Yale University School of Nursing 100 Church Street South, PO Box 9740 New Haven, CT 06536−0704 Phone: 203−785−6280 sangchoon.jeon@yale.edu.
Barbara Given, Michigan State University College of Nursing B515B West Fee Hall East Lansing, MI 48824 Phone: 517−353−0306 Barb.Given@hc.msu.edu.
References
- 1.Miaskowski C. Symptom clusters: establishing the link between clinical practice and symptom management research. Support Care Cancer. 2006;14:792–794. doi: 10.1007/s00520-006-0038-5. [DOI] [PubMed] [Google Scholar]
- 2.Miaskowski C, Dodd M, Lee K. Symptom clusters: the new frontier in symptom management research. J NCI Monographs. 2004;32:17–21. doi: 10.1093/jncimonographs/lgh023. [DOI] [PubMed] [Google Scholar]
- 3.Chang V, Thaler H, Polyak T, Kornblith A, et al. Quality of Life and Survival: The role of multidimensional symptom assessment. Cancer. 1998;83:173–179. doi: 10.1002/(sici)1097-0142(19980701)83:1<173::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza T, Wang X, Cleeland C, Morrisey M, et al. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Curt G, Breitbart W, Cella D, Groopman J, Horning S, Itri L, Johnson D, Miaskowski C, Scherr S, Portenoy R, Vogelzang N. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y, Lee J, Lee W, Lee K, Bang S, Wang X, Mendoza T, Cleeland C, Yun Y. Assessment of clinical relevant fatigue level in cancer. Support Care Cancer. 2007 Jul;15(7):891–6. doi: 10.1007/s00520-007-0219-x. [DOI] [PubMed] [Google Scholar]
- 7.Jordan K, Kasper C, Scholl H. Chemotherapy-induced nausea and vomiting: current and new standards in the antiemetic prophylaxis and treatment. Eur J Cancer. 2005;41(2):199–205. doi: 10.1016/j.ejca.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Jordan K, Hinke A, Grothey A, Voigt W, Arnold D, Wolf H, Schmoll H. A meta-analysis comparing the efficacy of four 5-HT3-receptor antagonists for acute chemotherapy-induced emesis. Support Care Cancer. 2007 Sep;15(9):1023–33. doi: 10.1007/s00520-006-0186-7. [DOI] [PubMed] [Google Scholar]; Cleeland C. Assessment of Pain in Cancer. Measurement Issues. Advances in Pain Research and Therapy. 1990;16:47–55. [Google Scholar]
- 9.Anderson K, Getto C, Mendoza T, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25(4):307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 10.Berger A. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncology Nursing Forum. 1998;25(1):51–61. [PubMed] [Google Scholar]
- 11.Cella D. Factors influencing quality of life in cancer patients: Anemia and Fatigue. Seminars in Oncology. 1998;25:43–46. [PubMed] [Google Scholar]
- 12.Cella D, Davis K, Breitbart W, Kurt G. Cancer-related fatigue: Prevalence pf proposed diagnostic criteria in the United States sample of cancer survivors. J Clin Oncology. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 13.Piper B, Dibble S, Dodd M, Weiss M, et al. The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncology Nursing Forum. 1998;25:677–684. [PubMed] [Google Scholar]
- 14.Vogelzang N, Breitbart W, Cella D, Curt G, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey; The Fatigue Coalition. Seminars in Hematology. 1997;34:4–12. [PubMed] [Google Scholar]
- 15.Yellen S, Cella D, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain and Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 16.Given B, Given C, McCorkle R, Kozachik S, Cimprich B, Rahbar H, Wojcik C. Pain and fatigue management: results of a nursing randomized clinical trial. Oncology Nursing Forum. 2002;29(6):949–956. doi: 10.1188/02.ONF.949-956. [DOI] [PubMed] [Google Scholar]
- 17.Given C, Given B, Azzouz F, et al. Predictors of pain and fatigue in the year following diagnosis in elderly patients with cancer. J Pain Symptom Manage. 2001;21:456–466. doi: 10.1016/s0885-3924(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, Tseng H. Symptom clusters in cancer patients. Support Care Cancer. 2006;14(8):825–830. doi: 10.1007/s00520-006-0019-8. [DOI] [PubMed] [Google Scholar]
- 19.Dodd M, Miaskowski C, Paul S. Symptom clusters and their effect on the functional status of patients with cancer. Oncology Nursing Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 20.Cleeland C, Bennet G, Dantzer R, Dougherty P, Dunn A, Meyers C, Miller A, Payne R, Reuben J, Wang X, Lee B. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 21.Dodd M, Miaskowski C, Lee K. Occurrence of symptom clusters. J National Cancer Institute Monographs. 2004;(32):76–78. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- 22.Barsevick A, Whitmer K, Nail L, Beck S, Dudley W. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–94. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, Link MP, Lustig C, McLaughlin J, Reid LD, Turrisi AT, 3rd, Unutzer J, Vernon SW, National Institutes of Health State-of-the-Science Panel Symptom management in cancer: pain, depression, and fatigue. J Natl Cancer Inst Monographs. 2004;(32):9–16. doi: 10.1093/jncimonographs/djg014. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health State-of-the-Science Conference Statement Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15−17, 2002 National Institutes of Health State-of-the-Science Panel. Special article. J of NCI. 2003;95(15):1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 25.Cleeland C, Mendoza T, Wang X, et al. Assessing symptom distress in cancer patients. Cancer. 2000;89:634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Portenoy R, Thaler H, Kornblith A, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 27.Portenoy R, Thaler H, Kornblith A, McCarthy L, et al. Symptom prevalence, characteristics and distress in a cancer population. Quality of Life Research. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 28.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 29.Bruera E, Kuehn N, Miller M, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliative Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 30.De Hayes J, van Knippenberg F, Neijt J. Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer. 1990;62:1034–1038. doi: 10.1038/bjc.1990.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrar J, Portenoy R, Berlin J, Kinman J, Strom B. Defining the clinically important difference in pain outcome measures. Pain. 88:287–294. doi: 10.1016/S0304-3959(00)00339-0. 200. [DOI] [PubMed] [Google Scholar]
- 32.Paul S, Zelman D, Smith M, Miaskowski C. Categorizing the severity of cancer pain: further exploration of the established cutpoints. Pain. 2005;113:37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Serlin R, Mendoza T, Nakamura Y, Edwards K, Cleeland C. When cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 34.Zelman DC, Dukes E, Brandenburg N, Bostrom A, Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain. 2005;115(1−2):29–36. doi: 10.1016/j.pain.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Given B, Given C, Sikorskii A, Jeon S, McCorkle R, Champion V, Decker D. Establishing mild, moderate and severe scores for cancer related symptoms: How consistent and clinically meaningful are interference based severity cut-points? Journal of Pain and Symptom Management. 2008;35(2):126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon S, Given C, Sikorskii A, Given B. Do interference based cut-points differentiate mild, moderate and severe levels of 16 cancer related symptoms over time? Journal of Pain and Symptom Management. 2008 Jul 9; doi: 10.1016/j.jpainsymman.2008.01.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important differences in pain outcome measures. Journal of Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 38.Farrar JT, Young JP, Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of chances in chronic pain intensity measured on an 11-point numerical pain rating scale. Journal of Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 39.Miaskowski C, Dodd M, West C, Paul SM, Schumacher K, Tripathy D, Koo P. The use of responder analysis to identify differences in patient outcomes following a self-care intervention to improve cancer pain management. Pain. 2007;129:55–63. doi: 10.1016/j.pain.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guyatt GH, Norman GR, Juniper EF, Griffith LE. A critical look at transition ratings. J Clin Epidemiol. 2002;55(9):900–908. doi: 10.1016/s0895-4356(02)00435-3. [DOI] [PubMed] [Google Scholar]
- 41.McQuay HJ, Barden J, Moore R. Clinically important changes – what's important and whose change is it anyway? Journal of Pain and Symptom Management. 2003;25(5):395–396. doi: 10.1016/s0885-3924(03)00099-x. [DOI] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network [September 28, 2007];Clinical practice guidelines in Oncology. 2006 from http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf http://www.nccn.org/professionals/physician_gls/PDF/pain.pdf.
- 43.Allegra C, Blanke C, Buyse M, Goldberg R, Grothey A, Meropol NJ, Saltz L, venook A, Yothers G, Sargent D. End points in advanced colon cancer clinical trials: A review and proposal. Journal of Clinical Oncology. 2007;25(24):3572–3575. doi: 10.1200/JCO.2007.12.1368. [DOI] [PubMed] [Google Scholar]
- 44.Punt CJA, Buyse M, Kohne C-H, Hohenberger P, Labianca R, Schmoll HJ, Pahlman L, Sobrero A, Douillard J-Y. Endpoints in adjuvant treatment trials: A systematic review of the literature in colon cancer and proposed definitions for future trials. JNCI. 2007;99(13):998–1003. doi: 10.1093/jnci/djm024. [DOI] [PubMed] [Google Scholar]
- 45.Sikorskii A, Given C, Given B, Jeon S, Decker V, Decker D. Symptom management for cancer patients: A trial comparing two multimodal interventions. Journal of Pain and Symptom Management. 2007;34(3):253–264. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clinical Pharmacological Therapy. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 47.Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. Journal of Clinical Oncology. 2004;22(3):507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 48.Katz JN, Chang LC, Sangha O, Fossel A, Bates D. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 50.McCullagh P, Nelder J. Generalized Linear Models. Chapman and Hall; London: 1989. [Google Scholar]
- 51.Boos D. On generalized score tests. The American Statistician. 1992;46:327–333. [Google Scholar]
- 52.Rotnitzky A, Jewell NP. Hypothesis testing of regression parameters in semiparametric generalized linear models for cluster correlated data. Biometrika. 1990;77:485–497. [Google Scholar]
- 53.Harrell FE. Regression Modeling Strategies. Springer-Verlag; New York: 2001. [Google Scholar]
- 54. SAS software, Version 9 of the SAS System for Windows. Copyright © 2002−2003 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
- 55.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. Journal of the American Statistical Association. 1989;84:1065–1073. [Google Scholar]
- 56.Lee E, Wei L, Amato D. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. Kluwer Academic Publishers; Netherlands: 1992. pp. 237–247. [Google Scholar]
- 57.Lin D. Cox regression analysis of multivariate failure time data: The marginal approach. Statistics in Medicine. 1994;13:2233–2247. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]