Abstract
Aim
To measure total retinal blood flow in normal human eyes using Doppler Fourier-domain optical coherence tomography (FD-OCT).
Methods
10 normal people aged 35 to 69 years were measured for the right eye using Doppler FD-OCT. Double circular scans around the optic nerve heads were used. Four pairs of circular scans that transected all retinal branch vessels were completed in 2 s. Total retinal blood flow was obtained by summing the flows in the branch veins. Measurements from the eight scans were averaged. Veins with diameters >33 μm were taken into account.
Results
Total retinal blood flow could be measured in eight of 10 subjects: mean (SD) = 45.6 (3.8) μl/min (range 40.8 to 52.9 μl/min). The coefficient of variation for repeated measurements was 10.5%. Measured vein diameters ranged from 33.3 to 155.4 μm. The averaged flow speed was 19.3 (2.9) mm/s, which did not correlate with vessel diameter. There was no significant difference between flows in the superior and inferior retinal hemispheres.
Conclusions
Double circular scanning using Doppler FD-OCT is a rapid and reproducible method to measure total retinal blood flow. These flow values are within the range previously established by laser Doppler flowmetry.
Many of the leading causes of blindness are related to abnormalities in retinal blood flow, such as diabetic retinopathy and age-related macular degeneration.1 2 Central and branch retinal vein occlusions are also common retinal diseases that are characterised by decreased retinal blood flow. In glaucoma, poor circulation in the retina and optic nerve is thought to be a risk factor for disease progression.3 4 A practical and accurate method for retinal blood-flow measurement may be useful in the diagnosis and management of these diseases.
Optical coherence tomography (OCT)5 provides high-resolution cross-sectional imaging and is commonly used in the diagnosis and management of retinal diseases.6 7 In addition to obtaining morphological images, OCT can also detect the Doppler shift of reflected light, which provides information on blood flow.8 9 For Doppler Fourier-domain optical coherence tomography (FD-OCT),10 11 light reflected by moving blood induces a Doppler frequency shift that is proportional to the velocity component parallel to the axis of the probe beam. This frequency shift introduces a phase shift in the spectral interference pattern that is captured by a line camera. The spectral information is converted into complex axial scans containing both amplitude and phase, using the fast Fourier transform (FFT). The phase differences between sequential axial scans at each pixel are calculated to determine the Doppler shift. Recently, in vivo flow measurements in branch retinal vessels have been reported using Doppler FD-OCT.12-14 We introduced a double circular scan pattern to rapidly measure total retinal blood flow.15 In this paper, we used this new technique to study retinal blood flow in a group of normal human subjects.
MATERIALS AND METHODS
Study population
The research protocol was approved by the institutional review board of the University of Southern California (USC), in accordance with the tenets of the Declaration of Helsinki. Ten healthy human subjects (four male, six female) between the ages of 35 and 69 years (average 57.4 years) participated in the study at the USC Doheny Eye Institute. Written informed consent was obtained from each subject. The right eyes were measured. The following inclusion criteria were met for these normal subjects: (a) no history or evidence of retinal pathology or glaucoma; (b) no history of keratorefractive surgery; (c) normal Humphrey SITA 24-2 visual field: a mean deviation (MD) and pattern standard deviation (PSD) within the 95% confidence limits of normal reference, and glaucoma hemifield test (GHT) within normal limits (97%); (d) intraocular pressure <21 mm Hg; (e) central corneal pachymetry >500 μm; (f) no chronic ocular or systemic corticosteroid use; (g) normal-appearing ONH and nerve fibre layer.
Following pupil dilation with topical tropicamide and phenylephrine, each subject was seated in front of the Doppler FD-OCT scanner and instructed to look at the green flashing cross fixation target. The chin and forehead of the subject rested on a frame in front of the objective lens. Eight measurements were performed in a single session of less than 10 min.
Doppler FD-OCT
The Doppler FD-OCT system was built by coauthor Izatt at Duke University, and its operating software was purchased from Bioptigen (Durham, North Carolina). It used a wavelength of 841 nm. Its axial resolution was 5.4 μm in tissue. The transverse resolution was 20 μm (beam diameter at retinal plane). The OCT scanning optics in the sample arm was mounted on a slit-lamp biomicroscope base. Power incident on the cornea was 500 μW, which was well below the American National Standard Institute limits for extended beam exposure. The measured sensitivity was 107 dB at 200 μm from the zero-path length difference location. The time interval between two sequential axial scans was 56 μs (integration time 50 μs; data transfer time 6 μs). The maximum determinable Doppler shift was 8.9 kHz without phase unwrapping, which gave a maximum axial velocity component in the eye of 2.8 mm/s. The measured phase noise was 51 Hz, which set the minimum determinable speed to 16.3 μm/s.
For blood-flow measurements, a double circular scanning pattern was employed to determine the angle between the probe beam and blood flow.15 The scanning circles were centred at the optic nerve head (ONH) and had radii of r1 = 1.8 mm and r2 = 2.0 mm. The frame rate for Doppler FD-OCT imaging was 4.2 circles/s (2.1 double circles/s).
Image sampling and processing
Each retinal blood-flow measurement was made from a set of four double circular scans (eight circles) acquired in approximately 2 s. There were 3000 axial scans sampled in each circle. Each circle transects all branch retinal arteries and veins (fig 1). Total retinal blood flow was measured by summing flow in the veins. The veins were identified by their centripetal flow direction. The locations of the vessels were selected by a human operator. Phase unwrapping and flow profile calculation were then performed by the software that we developed.12
Figure 1.
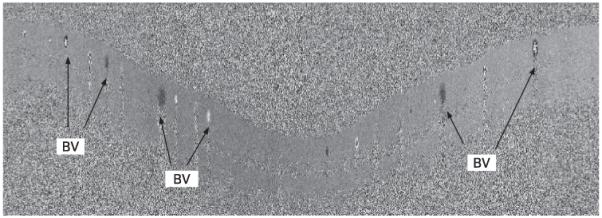
Doppler optical coherence tomography image. The 2.0 mm radius cylindrical section of the retinal is unwrapped, and the Doppler shift is shown in grey scale. BV, branch vein.
Volumetric blood flow in each vein was calculated by integrating the velocity over the vessel cross-section and includes steps to account for background motion, Doppler angle, sampling step size and pulsation.15 The background axial motion of the retina was measured by the Doppler shift of the tissue between the inner retina boundary and the vessel wall. This background was subtracted to obtain the net Doppler shift induced by blood flow. The Doppler shift measured by the OCT system only corresponds to the axial component of the blood flow velocity. To obtain the total velocity, the axial component is divided by the cosine of the incidence angle between the OCT beam and flow direction. The incidence angle was computed by the relative axial position of the vein in the two circular scans of different radii. The standard error of Doppler angle measurements was 1.4°. Thus, if the incidence angle was less than 2.8° from perpendicular, the data are rejected to prevent excessive amplification of angle error from the cosine calculation. We found that in the 1.8 to 2.0 mm radius circles, retinal vessels sloped upward as they emerged from the disc, and the incidence angles were usually acceptable for flow measurements. Because the Doppler shift is measured between consecutive axial scans, the transverse movement of the OCT beam between adjacent scans causes a partial phase decorrelation and reduces the measured Doppler shift. Based on previous studies, we divided the measured Doppler shift by a factor of 0.683 to correct for a transverse sampling step of 4.0 μm.15 The average flow and pulsation factor (ratio between peak and average flow) were measured from the eight time points over 2 s for each vessel. For some veins, if the Doppler flow signal was too weak for accurate reading at diastole, the pulsation factor in the adjacent veins was used to estimate the average flow from peak flow.
The inner diameters of veins were measured from the Doppler shifted flow regions in the OCT images. For each eye, the total retinal venous cross-section area (TVCA) was calculated by summing all branch veins.
STATISTICAL ANALYSIS
Linear mixed models16 or a generalised estimating equation (GEE)17 were used to account for intrasubject correlations for repeated measurements for different scans or vessels. The reproducibility was assessed from the pooled standard deviation (SD) and coefficient of variation (CV).
A paired t test was used to compare flow-rate differences between the superior and inferior retinas. The correlation between retinal blood flow rate (y) and vessel diameter (x) used log-log regression analysis with log10 transformations based on the relationship: y = cxβ. A similar analysis was done for the correlations between velocity and vessel diameter. Linear regression analysis was used for total retinal venous blood flow rate versus TVCA. The level of significance was set at 0.05. Statistical analyses used SAS 9.1 software.
RESULTS
Total venous blood flow
In this study, retinal blood flow could be determined for eight of the 10 subjects. We were unable to obtain retinal blood-flow measurements from two subjects; one was due to poor fixation, and the other was due to a tilted disc. Table 1 lists the total venous blood flow, the number of measurable vein branches and the TVCA for individual subjects. The total venous blood flow ranged from 40.8 to 52.9 μl/min. Table 2 lists the statistics of these measurements. The mean (SD) value for the flow results for eight normal subjects was 45.6 (3.8) μl/min. The CV for total venous flow was 10.5%. The TVCA ranged from 0.033 to 0.065 mm2. Figure 2 shows a scatter plot of the total venous flow rates vs TVCA for normal subjects, which gives a significant correlation.
Table 1.
Retinal venous flow measurements in eight normal human subjects
| Subjects | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th |
|---|---|---|---|---|---|---|---|---|
| Total blood flow (μl/min) | 44.2 | 45.9 | 44.5 | 40.8 | 46.7 | 48.2 | 42.0 | 52.9 |
| No of branch veins | 5 | 5 | 5 | 6 | 6 | 5 | 5 | 7 |
| Total retinal venous cross-section area (mm2) | 0.043 | 0.046 | 0.037 | 0.043 | 0.034 | 0.048 | 0.033 | 0.065 |
Table 2.
Distribution and reproducibility statistics of retinal vein measurements
| Parameters | Distribution |
Reproducibility |
|
|---|---|---|---|
| Mean (SD), range | Pooled SD | Coefficient of variation (%) | |
| Total venous flow rate (μl/min) | 45.6 (3.8), 40.8 to 52.9 | 4.8 | 10.5 |
| Total retinal venous cross-section area (mm2) | 0.044 (0.010), 0.033 to 0.065 | 0.0031 | 7.64 |
Figure 2.
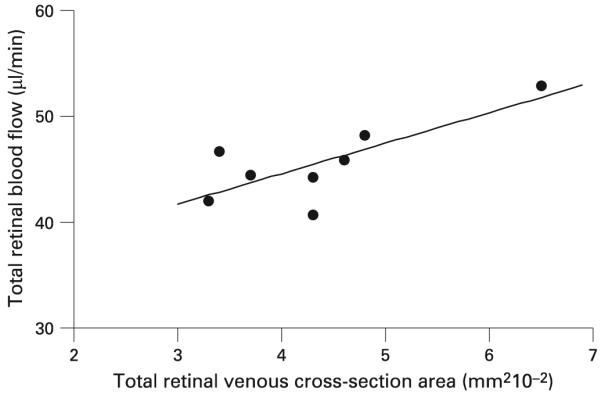
Total retinal blood flow is plotted against total venous cross-sectional area. The best fit line (solid line) was y = 33.04+288.74x (R2 = 0.57; p = 0.03).
Regional differences in blood flow
We examined the blood flow in the superior (Fsup) and inferior (Finf) retinal hemispheres separately. Venous flow had a mean (SD) of 23.5 (3.0) μl/min for the superior retina and 22.2 (2.6) μl/min for the inferior retina. The flow difference was: ΔF = Fsup-Finf = 1.32 (4.13) μl/min. There was no statistically significant difference (p = 0.4) between the flows for the superior and inferior retinal hemispheres based on a paired t test.
Relationship between blood flow rate and vessel diameter
The inner diameters (D) of retinal veins were measured from the Doppler OCT images and ranged from 33.3 to 155.4 μm. We calculated the relationship between blood flow and D by linear regression for each subject on a log-log scale. The slopes ranged from 1.52 to 2.54, and the mean (SD) was 1.90 (0.40). Linear regression analysis of the combined data from all eight subjects (fig 3) gave a slope of 1.97 (R2 = 0.86; p<0.0001). This indicated that flow was proportional to vessel area.
Figure 3.
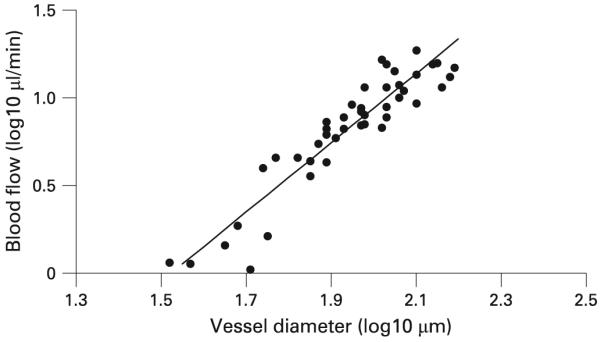
Blood volume flow rate versus blood-vessel diameter. Results are on a log-log scale. The solid line is the best fit result of linear regression: y = -3.00+1.97x (R2 = 0.86; p<0.001).
The average flow velocity in the veins was 19.6 (2.9) mm/s for the combined data from all subjects. Linear regression did not show any significant correlation between flow velocity and vein diameter.
DISCUSSIONS
Using Doppler FD-OCT, we measured total venous blood flow in normal human subjects. Since arterial and venous flows must be balanced, this is equivalent to measuring the total retinal flow. Measurements by laser Doppler flowmeter (LDF) had confirmed that the difference in the total retinal blood flow measured from arteries compared with veins did not exceed 9%.18 The average measured total venous flow in our study, 45.71 μl/min, was within the range of previously published values of 34.0 (6.3) μl/min19 to 64.9 (12.8) μl/min using LDF.20
In our study, the volumetric flow rate varied with the vessel diameter with a power coefficient of 1.97, which indicates that flow speed was not affected by vein diameter. This logarithmic slope value previously reported for LDF was 2.76∼3.35,19 20 which indicated faster flows in larger veins. The difference might be due to the vessel diameter measurement methods. In our study, the inner diameters of the veins were measured from the OCT Doppler shift image. For LDF, vessel diameters were measured from a fundus camera image and might be slightly larger.
With regard to superior versus inferior retinal blood flow rates, we did not observe a significant difference. This agrees with other investigators who also found no statistical differences between superior and inferior retinal flows.18-20 We also observed that the total venous flow rate correlated with TVCA in normal subjects. This agrees with previous LDF results.19
The precision of total blood-flow measurement in this study was 10.5% as assessed by CV. The primary limitation to precision was the speed of OCT scanning. A faster FD-OCT system would decrease the motion error in incidence angle calculation and the sampling error due to cardiac pulsation. A faster system would also allow reliable measurement of the faster flow in retinal arteries by reducing phase wrapping and signal loss due to washout of interference fringes.
The measurement of total blood flow could provide an objective tool to monitor treatment approaches that target retinal vascular conditions. This has led many investigators to study retinal blood flow as a surrogate marker of retinal function.21-25 However, none of the previously studied blood flow imaging modalities has found its way to widespread clinical application, except for fluorescein angiography (FA), which cannot provide quantitative measurements of volumetric flow. LDF can provide quantitative blood-flow measurement.19 20 23-25 However, it is laborious to use because each blood vessel is measured one at a time, and LDF’s accuracy is limited by the lack of direct information on the flow-velocity profile and blood-vessel dimensions.
In Doppler OCT, flow profile can be characterised across both depth and transverse dimensions. Total flow is measured by integrating the flow profile over the vessel cross-section and does not require any assumptions regarding vessel shape or flow profile. The measured results in volume flow units can be compared for different subjects.
For clinical applications, the chair time is of great practical importance. Using a circular scanning method, total retinal venous blood flow can be calculated with the data sampled within 2 s. In our study, the scanning session for each subject was less than 10 min for eight measurements. This is much shorter than the reported 1 h session time for LDF.20
Finally, FD-OCT retinal scanners are becoming widely used in ophthalmology. Doppler blood-flow measurement only requires additional software for scan acquisition and analysis, and no additional hardware modification of the FD-OCT scanner is required. Thus, Doppler OCT may provide widely available clinical blood-flow evaluations with minimal additional cost.
In summary, we present the first clinical study of retinal blood flow using Doppler FD-OCT in a group of normal subjects. Volumetric flow in the branch veins around the optic nerve head was calculated with the data sampled within 2 s. The average total retinal blood flow in eight normal subjects was 45.6 (3.8) μl/min. This method can be used to measure total retinal blood flow without relying on any assumption for vessel sizes or flow profiles. The precision of total blood-flow measurement could be further improved using a faster FD-OCT system. Larger studies are needed to validate clinical application in health and disease.
Acknowledgements
This work was supported by NIH grants R01 EY013516 and P30 EY03040 and by a grant from Research to Prevent Blindness.
Footnotes
Competing interests: DH receives royalty from the Massachusetts Institute of Technology derived from an OCT patent licensed to Carl Zeiss Meditec and receives stock options, travel support, research grants, and patent royalties from Optovue. JAI is a cofounder and corporate officer of Bioptigen; YW: Optovue.
Ethics approval: Ethics approval was provided by the institutional review board of the University of Southern California.
REFERENCES
- 1.Klaver CWC, Wolfs CWR, Vingerling RJ, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population. Arch Ophthalmol. 2007;116:653–8. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 2.West KS, Klein R, Rodriguez J, et al. Diabetes and diabetic retinopathy in a Mexican-American population. Diabetes Care. 2001;24:1204–9. doi: 10.2337/diacare.24.7.1204. [DOI] [PubMed] [Google Scholar]
- 3.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 4.Kerr J, Nelson P, O’Brien C. A comparison of ocular blood flow in untreated primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 1998;126:42–51. doi: 10.1016/s0002-9394(98)00074-9. [DOI] [PubMed] [Google Scholar]
- 5.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–29. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 7.Shuman JS, Hee MR, Arya AV, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6:89–95. doi: 10.1097/00055735-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Wang XJ, Milner TE, Nelson JS. Characterization of fluid flow velocity by optical Doppler tomography. Opt Lett. 1995;20:1337–9. doi: 10.1364/ol.20.001337. [DOI] [PubMed] [Google Scholar]
- 9.Izatt JA, Kulkarni MD, Yazdanfar S, et al. In vivo bidirectional color Doppler flow imaging of picoliter blood volumes using optical coherence tomography. Opt Lett. 1997;22:1439–41. doi: 10.1364/ol.22.001439. [DOI] [PubMed] [Google Scholar]
- 10.White BR, Pierce MC, Nassif N, et al. In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical Doppler tomography. Opt Express. 2003;11:3490–7. doi: 10.1364/oe.11.003490. [DOI] [PubMed] [Google Scholar]
- 11.Leitgeb RA, Schmetterer L, Hitzenberger CK, et al. Real-time measurement of in vitro flow by Fourier-domain color Doppler optical coherence tomography. Opt Lett. 2004;29:171–3. doi: 10.1364/ol.29.000171. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Bower BA, Izatt JA, et al. In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007;12:041215–22. doi: 10.1117/1.2772871. [DOI] [PubMed] [Google Scholar]
- 13.Makita S, Fabritius T, Yasuno Y. Quantitative retinal-blood flow measurement with three dimensional vessel geometry determination using ultrahigh-resolution Doppler optical coherence angiography. Opt Lett. 2008;33:836–8. doi: 10.1364/ol.33.000836. [DOI] [PubMed] [Google Scholar]
- 14.Wehbe H, Ruggeri M, Jiao S, et al. Automatic retinal blood flow calculation using spectral domain optical coherence tomography. Opt Express. 2007;15:15193–206. doi: 10.1364/oe.15.015193. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Bower BA, Izatt JA, et al. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence. J Biomed Optics. 2008;13:064003-1–9. doi: 10.1117/1.2998480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 17.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 18.Feke GT, Tagawa H, Deupree DM, et al. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci. 1989;30:58–65. [PubMed] [Google Scholar]
- 19.Riva CE, Grunwald JE, Sinclair SH, et al. Blood Velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985;26:1124–32. [PubMed] [Google Scholar]
- 20.Garcia JPS, Jr, Garcia PT, Rosen RB. Retinal blood flow in the normal human eye using the canon laser blood flowmeter. Ophthalmic Res. 2002;34:295–9. doi: 10.1159/000065600. [DOI] [PubMed] [Google Scholar]
- 21.Langham ME, To’mey KF. A clinical procedure for the measurement of the ocular pulse-pressure relationship and the ophthalmic arterial pressure. Exp Eye Res. 1978;27:17–25. doi: 10.1016/0014-4835(78)90049-0. [DOI] [PubMed] [Google Scholar]
- 22.Flower RW. Extraction of choriocapillaris hemodynamic data from ICG fluorescence angiograms. Invest Ophthalmol Vis Sci. 1993;34:2720–9. [PubMed] [Google Scholar]
- 23.Cuypers CPJ, Chung HS, Kagemann L, et al. New neuroretinal rim blood flow evaluation method combining Heidelberg retina flowmetry and tomography. Br J Ophthalmol. 2001;85:304–9. doi: 10.1136/bjo.85.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris A, Jonescu-Cuypers PC, Kagemann L, et al. Atlas of ocular blood flow—vascular anatomy, pathophysiology, and metabolism. Butterworth-Heinemann; Philadelphia: 2003. pp. 25–32. [Google Scholar]
- 25.Riva C, Ross B, Benedek GB. Laser Doppler measurements of blood flow in capillary tubes and retinal arteries. Invest Ophthalmol. 1972;11:936–44. [PubMed] [Google Scholar]


