Abstract
Background
Although a majority of patients with amnestic mild cognitive impairment (aMCI) progress to Alzheimer disease, the natural history of nonamnestic MCI (naMCI) is less clear. Noninvasive imaging surrogates for underlying pathological findings in MCI would be clinically useful for identifying patients who may benefit from disease-specific treatments at the prodromal stage of dementia.
Objective
To determine the characteristic magnetic resonance imaging (MRI) and proton MR spectroscopy (1H MRS) profiles of MCI subtypes.
Design
Case-control study.
Setting
Community-based sample at a tertiary referral center.
Patients
Ninety-one patients with single-domain aMCI, 32 patients with multiple-domain aMCI, 20 patients with single- or multiple-domain naMCI, and 100 cognitively normal elderly subjects frequency-matched by age and sex.
Main Outcome Measures
Posterior cingulate gyrus 1H MRS metabolite ratios, hippocampal volumes, and cerebrovascular disease on MRI.
Results
Patients with single-domain aMCI were characterized by small hippocampal volumes and elevated ratios of myo-inositol to creatine levels. Patients with naMCI on average had normal hippocampal volumes and 1H MRS metabolite ratios, but a greater proportion (3 of 20 patients [15%]) had cortical infarctions compared with patients with single-domain aMCI (6 of 91 [7%]). For characterization of MCI subtypes, 1H MRS and structural MRI findings were complementary.
Conclusions
The MRI and 1H MRS findings in singledomain aMCI are consistent with a pattern similar to that of Alzheimer disease. Absence of this pattern on average in patients with naMCI suggests that cerebrovascular disease and other neurodegenerative diseases may be contributing to the cognitive impairment in many individuals with naMCI.
The broad clinical definition of mild cognitive impairment (MCI) includes amnestic MCI (aMCI) and nonamnestic MCI (naMCI), with impairment in a single cognitive domain or multiple cognitive domains such as memory, attention/executive functioning, language, and visuospatial processing.1 Although patients with the aMCI subtype have a higher risk of progression to Alzheimer disease (AD) compared with their cognitively normal peers, the natural history and the pathological underpinnings of naMCI are less clear.2–8 However, longitudinal studies suggest that patients with naMCI also have a higher rate of progression to dementia than do cognitively normal individuals.2–5,8 The most common pathological findings encountered in aMCI include AD, cerebrovascular disease, and Lewy body pathological features.9–13 Noninvasive imaging surrogates for these underlying abnormalities in MCI would be clinically useful for identifying patients who may benefit from disease-specific treatments at the prodromal stage of dementia.
Hippocampal volumetry based on magnetic resonance imaging (MRI) and proton magnetic resonance spectroscopy (1H MRS) metabolite ratios are sensitive markers for AD pathological changes.14–20 Cortical and subcortical infarctions represent cerebrovascular disease, and evidence exists that white matter hyperintensities (WMHs) are related to ischemic vascular disease.18 In cross-sectional 1H MRS studies, the glial activation marker myo-inositol (mI) or the ratio of mI to creatine (mI:Cr ratio) and the membrane integrity marker choline (Cho) or the Cho:Cr ratio are elevated in aMCI, AD, and dementia with Lewy bodies (DLB). The level of the neuronal integrity marker N-acetylaspartate (NAA) tends to be decreased in patients with aMCI, AD, and vascular dementia.21–28 Whereas aMCI has been the focus of much research in identifying imaging markers for prodromal AD, few studies have investigated the imaging characteristics of naMCI.29–33
Because of the complexity of pathological findings underlying dementia, it is conceivable that magnetic resonance markers that are surrogates for various pathological substrates of dementia may provide complementary information concerning the typical underlying causes of dementia in the elderly. Our objective was to determine the characteristic magnetic resonance profiles of the MCI subtypes, including hippocampal volumes, 1H MRS metabolite ratios, and cerebrovascular disease.
METHODS
RECRUITMENT OF SUBJECTS
We identified 91 patients with single-domain aMCI, 32 patients with multiple-domain aMCI, and 20 patients with single-or multiple-domain naMCI who were consecutively recruited to the Mayo Clinic AD Research Center (ADRC) and AD patient registry (ADPR) cohorts34 and who participated in an MRI–1H MRS study. From the same ADRC-ADPR cohort, we identified 100 cognitively normal subjects who were frequency-matched by age and sex to the MCI patients and who underwent MRI during the same period. This study was approved by the Mayo Clinic institutional review board, and informed consent for participation was obtained from every subject and/or an appropriate surrogate.
Individuals participating in the ADRC-ADPR studies undergo approximately annual clinical examinations, structural brain MRI and 1H MRS, routine laboratory tests, and a battery of neuropsychological tests. At the completion of the evaluation, a consensus committee meeting is held involving the behavioral neurologists (R.C.P., B.F.B., and D.S.K.), neuropsychologists (R.J.I. and G.E.S.), nurses, and the geriatrician who performed the evaluations of the subjects to assign a clinical diagnosis to the participant.
The operational definition of MCI was based on clinical judgment through a careful history obtained from the patient and preferably a collateral source without reference to MRI results, using the following criteria for the broad definition of MCI1: cognitive complaint, cognitive function not normal for age, decline in cognition, essentially normal functional activities, and no dementia. The patients with MCI were further classified into 1 of the following 4 MCI subtypes1: (1) single-domain aMCI, if the impairment was only in the memory domain; (2) multiple-domain aMCI, if the impairment was in the memory domain plus 1 or more other domains such as language, attention/executive function, and visuospatial processing; (3) singledomain naMCI, if the impairment was in 1 or more non-memory domains with relative preservation of memory; and (4) multiple-domain naMCI, if the impairment was in more than 1 domain with relative preservation of memory. We grouped patients with naMCI and impairment in single and multiple domains in this study because of the small number of patients with multiple-domain naMCI. We excluded patients with structural abnormalities that could impair cognitive function other than cerebrovascular lesions, such as tumor, subdural hematoma, and contusion due to a previous head trauma, and patients with addictions, psychiatric diseases, or treatments that would affect cognitive function. Subjects were not excluded for the presence of infarctions and leukoaraiosis; thus, the full range of ischemic cerebrovascular disease was included.
Cognitively normal subjects were recruited from the community but underwent evaluation in the same manner as patients with MCI. The cognitively normal group did not have any neurological or psychiatric conditions, did not have a cognitive concern, had normal results of the neurological and neurocognitive examinations, and were not taking psychoactive medications in doses that would affect cognition. To ensure that the cognitively normal group did not include subjects with preclinical dementia, we included subjects only if they remained cognitively normal for at least 2 years during longitudinal clinical follow-up.
Apolipoprotein E genotype is determined by established polymerase chain reaction techniques. Participants with genotypes 2\4, 3\4, and 4\4 were labeled ε4 carriers; those with the other genotypes were labeled ε4 noncarriers.
NEUROPSYCHOLOGICAL TESTING
Cognitive testing was completed within 4 months of the MRI and 1H MRS studies. Memory was evaluated by means of free-recall percentage of retention scores computed after a 30-minute delay for the Wechsler Memory Scale-Revised logical memory and visual reproduction subtests and the Rey Auditory Verbal Learning Test.35 Language tests measured naming to confrontation (ie, the Boston Naming Test)36 and category fluency (ie, naming animals, fruits, and vegetables).37 The attention/executive measures included the Trail-Making Test parts A and B38 and the Wechsler Adult Intelligence Scale-Revised digit symbol subtest. Visuospatial processing was examined by means of the picture completion and block design subtests of the Wechsler Adult Intelligence Scale-Revised. All tests were administered by experienced psychometrists and supervised by clinical neuropsychologists (R.J.I. and G.E.S.). All raw scores were converted to Mayo Older Americans Normative Studies (MOANS) age-adjusted scaled scores that are normally distributed and that have a mean (SD) score of 10 (3) in cognitively healthy subjects, on whom each test was normed.37,39,40 In each cognitive domain, we computed a mean MOANS age-corrected scaled score for every participant. We note that the patients’ mean MOANS scores within a certain domain did not strictly define their MCI category, although cognitive tests were used to inform the clinical consensus diagnosis.
MRI AND 1H MRS STUDIES
All subjects underwent MRI and 1H MRS studies on a 1.5-T scanner (Signa; GE Medical Systems, Milwaukee, Wisconsin). Hippocampal volume measurements were derived from a T1-weighted 3-dimensional volumetric spoiled gradient-recalled echo sequence with 124 continuous partitions, 1.6-mm section thickness, a 22 × 16.5-cm or 24 × 18.5-cm field of view, 192 views, and 25° flip angle. Hippocampal volumes were measured by manually tracing their anatomical boundaries for each image section sequentially from posterior to anterior.41 The intrarater test-retest coefficient of variation of hippocampal volume measurements is 0.97% for the image analyst (M.M.S.), who manually traced each hippocampus and was masked to all clinical information. Volumes were then converted to normal deviates, referred to as W scores, using age- and sex-specific normal percentiles based on a previous study.42 Among cognitively normal subjects, a value of 0 corresponds to the 50th percentile; 1.64, to the 95th percentile; and −1.64, to the 5th percentile.
A fluid-attenuated inversion recovery (FLAIR) pulse sequence (repetition time, 16 000 milliseconds; time following inversion pulse, 2600 milliseconds; echo time, 140 milliseconds; 256 × 160 matrix; 1 repetition; 22-cm field of view; and 3-mm interleaved images of the whole head) was used for the assessment of cerebrovascular disease. A radiologist (K.K.) blinded to all clinical information assessed the WMHs and hemispheric cortical and lacunar infarctions. The WMH volume was estimated by visually comparing the subject’s FLAIR images with a bank of 10 FLAIR image templates with increasing WMH volumes (from 1 to 100 cm3) determined with an automated image segmentation algorithm.43 A continuous scale with a slider bar was used to estimate each subject’s WMH volume by matching to the WMH templates.44 The WMH volume estimation algorithm was previously validated against quantification using the automated image segmentation of the WMH volume (concordance correlation coefficient, 0.88; 95% confidence interval, 0.83–0.94).45 Intrarater reliability for assessment of the WMHs is excellent (intraclass correlation coefficient, 0.98; 95% confidence interval, 0.97–0.98). Hemispheric cortical infarctions were defined as areas of elevated signal intensity involving the cortical gray mantle and immediately subjacent white matter that exceed 1 cm in the largest diameter on FLAIR images. Subcortical infarctions were defined as discrete subcortical lesions of more than 3 mm in diameter with intensity that is equivalent to cerebrospinal fluid on FLAIR images and accompanying hyperintense gliotic rim. Intrarater reliability for assessment of subcortical infarctions (proportion in agreement, 0.94) and cortical infarctions (proportion in agreement, 0.98) is excellent.
The T1-weighted images in the sagittal plane were obtained for localizing the 1H MRS voxel. The 1H MRS studies were performed with an automated single-voxel MRS package (Proton Brain Examination/Single Voxel; GE Medical Systems).46 Point-resolved spectroscopy pulse sequences (repetition time, 2000 milliseconds; echo time, 30 milliseconds; 2048 data points; and 128 excitations) were used for the examinations. An 8-cm3 (2 × 2 × 2-cm) voxel, prescribed on a midsagittal T1-weighted image, included the right and left posterior cingulate gyri and inferior precunei.26 We analyzed the metabolite intensity ratios, using Cr as an internal reference metabolite.
STATISTICAL ANALYSIS
We compared the cognitively normal subjects and patients with the 3 MCI subtypes on categorical patient characteristics and MRI findings such as the presence of cortical and subcortical infarctions using the χ2 test, or, if expected cell counts were less than 5, the Fisher exact test. We used Kruskal-Wallis tests to compare groups on age, education, MOANS scores for the 4 cognitive domains, and quantitative magnetic resonance measurements. Pairwise group comparisons among the clinical groups on quantitative imaging measures were performed using Wilcoxon rank sum tests because of skewness. These tests were all 2 sided.
To assess the effect of imaging measures on the estimated probability of cognitively normal, single-domain aMCI, multipledomain aMCI, or naMCI findings, we used generalized logit multinomial models.47 These models extend binary logistic regression to the situation where the response is an unordered categorical variable with 3 or more levels. Models included age, sex, and education as adjustment variables and examined the effects of a single imaging predictor. We performed a likelihood ratio test to evaluate whether adding the imaging predictor significantly improved model fit (and therefore discrimination) compared with a model with age, sex, and education only (ie, a model without imaging information). We graphically illustrated the effect of the imaging predictor on the estimated probability of group membership by showing relative probabilities as a function of the imaging predictor. The relative probability is the estimated probability of group membership at a given imaging value divided by the estimated probability at the median or at these estimates. We arbitrarily set sex to male, age to 77 years, and education to 14 years, with covariate levels representing the most common category or the median value in our sample.
We also used multinomial modeling to fit what we call 2-imaging predictor models that included age, sex, education, and hippocampal W score plus 1 of the following: NAA:Cr ratio, mI:Cr ratio, Cho:Cr ratio, the log of WMH, and cortical infarctions. These models were used to evaluate whether a measurement improved model fit and discrimination beyond that obtained from an age-, sex-, and education-adjusted model based on hippocampal volume only. The significance of the second imaging predictor was evaluated using a likelihood ratio test. We did not adjust for multiple comparisons because the statistical tests address questions of distinct albeit related clinical interest.48,49 We used commercially available software (SAS, version 8.2 [SAS Institute Inc, Cary, North Carolina] and R, version 2.7.0 [R Foundation for Statistical Computing, Vienna, Austria]) for these analyses.
RESULTS
Cognitively normal subjects and patients with MCI did not differ significantly in age, sex, and years of education. As expected, a greater proportion of patients with MCI than cognitively normal subjects were apolipoprotein E ε4 carriers. Table 1 demonstrates that, although general cognitive function as indicated by Mini-Mental State Examination and Clinical Dementia Rating sum of boxes scores are identical in terms of median value across the MCI subtypes, the specific domains that are impaired as indicated by lower MOANS scores differ significantly by MCI subtype.
Table 1.
Demographic Characteristics and Cognitive Features of the Cohorta
| Subject Groups | |||||
|---|---|---|---|---|---|
| CN (n = 100) |
SD-aMCI (n = 91) |
MD-aMCI (n = 32) |
naMCI (n = 20) |
P Valueb |
|
| Women, No. (%) | 42 (42) | 40 (44) | 14 (44) | 7 (35) | .90 |
| Age, median (IQR), y | 76 (71–81) | 78 (71–83) | 78 (72–83) | 76 (66–80) | .17 |
| Education, y | 14 (12–16) | 15 (12–18) | 13 (12–16) | 12 (12–15) | .28 |
| APOE ε4 carriers, No. (%) | 26 (26) | 41 (45) | 13 (41) | 8 (40) | .01 |
| Memory MOANS score | 11.0 (10.3–12.0) | 6.4 (4.8–7.8) | 7.7 (5.9–8.5) | 9.1 (8.3–10.1) | <.00 |
| Language MOANS score | 10.8 (9.7–12.7) | 9.3 (8.0–11.0) | 7.8 (6.3–9.3) | 8.8 (7.2–10.0) | .00 |
| Attention/executive MOANS score | 11.0 (10.3–12.0) | 9.8 (8.7–11.0) | 7.3 (6.3–9.0) | 7.8 (6.7–9.4) | <.00 |
| Visuospatial processing MOANS score | 11.0 (9.8–12.5) | 10.5 (9.0–12.0) | 7.0 (6.5–9.0) | 8.5 (6.8–10.0) | <.00 |
| MMSE | 29 (28–30) | 27 (25–29) | 27 (25–28) | 27 (26–29) | .42 |
| CDR sum of boxes | 0 (0–0) | 0.5 (0.5–1.5) | 0.5 (0.5–1.0) | 0.5 (0.5–2.0) | .92 |
Abbreviations: APOE, apolipoprotein E; CDR, Clinical Dementia Rating; CN, cognitively normal; IQR, interquartile range; MD-aMCI, multiple-domain amnestic mild cognitive impairment (MCI); MMSE, Mini-Mental State Examination; MOANS, Mayo Older Americans Normative Studies; naMCI, nonamnestic MCI SD-aMCI, single-domain aMCI.
Except where indicated, values shown are median (interquartile range).
P values for sex, age, education, and APOE genotype compare all 4 groups, whereas P values for cognitive variables compare MCI subtypes. Sex and APOE genotype are tested using a χ2 test, whereas other variables are tested with a Kruskal-Wallis test.
The patients with single-domain aMCI had smaller hippocampal W scores than did cognitively normal subjects (P < .001) and patients with multiple-domain aMCI (P = .009) and naMCI (P = .008). Similarly, patients with single-domain aMCI had elevated mI:Cr ratios on 1H MRS compared with cognitively normal subjects (P < .001) and patients with multiple-domain aMCI (P =.02) and naMCI (P =.03). Patients with multiple-domain aMCI had a trend toward smaller hippocampal W scores (P = .046) and lower NAA:Cr ratios on 1H MRS (P = .06) compared with cognitively normal subjects. Patients with naMCI had normal hippocampal volumes and 1H MRS metabolite ratios (Table 2).
Table 2.
MRI and 1H MRS Findings in Cognitively Normal Subjects and MCI Subtypesa
| Subject Groups | |||||
|---|---|---|---|---|---|
| CN (n=100) |
SD-aMCI (n = 91) |
MD-aMCI (n = 32) |
naMCI (n = 20) |
P Valueb |
|
| Hippocampal W score | 0.29 (−0.50 to 0.74) | −1.01 (−1.93 to −0.01) | −0.38 (−0.73 to 0.58) | 0.21 (−0.85 to 0.75) | <.001 |
| NAA:Cr ratio | 1.52 (1.46 to 1.59) | 1.51 (1.43 to 1.58) | 1.50 (1.42 to 1.53) | 1.53 (1.44 to 1.58) | .28 |
| mI:Cr ratio | 0.67 (0.62 to 0.71) | 0.70 (0.66 to 0.74) | 0.67 (0.61 to 0.72) | 0.65 (0.62 to 0.72) | .003 |
| Cho:Cr ratio | 0.64 (0.60 to 0.70) | 0.68 (0.64 to 0.73) | 0.68 (0.63 to 0.72) | 0.67 (0.60 to 0.74) | .012 |
| WMH volume, cm3 | 10 (6 to 17) | 16 (6 to 25) | 11 (7 to 23) | 14 (7 to 30) | .02 |
| No. (%) of subjects with any subcortical infarctions | 10 (10) | 9 (10) | 3 (9) | 2 (10) | >.99 |
| No. (%) of subjects with any cortical infarctions | 0 | 6 (7) | 5 (16) | 3 (15) | .17 |
Abbreviations: CN, cognitively normal; Cho, choline; Cr, creatine; 1H MRS, proton magnetic resonance spectroscopy; MCI, mild cognitive impairment; MD-aMCI, multiple-domain amnestic MCI; mI, myo-inositol; MRI, magnetic resonance imaging; NAA, N-acetylaspartate; naMCI, nonamnestic MCI; SD-aMCI, single-domain aMCI; WMH, white matter hyperintensity.
Except where indicated, values shown are median (interquartile range).
P values for continuous imaging measures are tested using a Kruskal-Wallis test among all 4 groups, whereas infarction variables are tested using a Fisher exact test among MCI subtypes only.
A greater observed proportion of patients with multiple-domain aMCI (16%) and with naMCI (15%) had cortical infarctions compared with patients with single-domain aMCI (7%). Although the proportion of the patients with cortical infarctions was twice as high in multiple-domain aMCI and naMCI compared with single-domain aMCI, this difference was statistically a trend (P =.17). If the true rates of cortical infarctions were 0.07 in the single-domain aMCI group, 0.16 in the multiple-domain aMCI group, and 0.15 in the naMCI group (ie, what we observed), then we would have approximately 38% power to detect a significant difference using the Fisher exact test. On the other hand, the proportion of patients with subcortical infarctions was similar across MCI subtypes and cognitively normal subjects. The average estimated volume of WMH in patients with single-domain aMCI was higher than in cognitively normal subjects (P = .002). Despite the fact that, on average, the patients with multiple-domain aMCI had lower WMH volumes than did those with single-domain aMCI and naMCI subtypes, the 3 MCI groups did not differ on WMH volume, which may be a result of the small sample sizes of the multiple-domain aMCI and naMCI groups (Table 2).
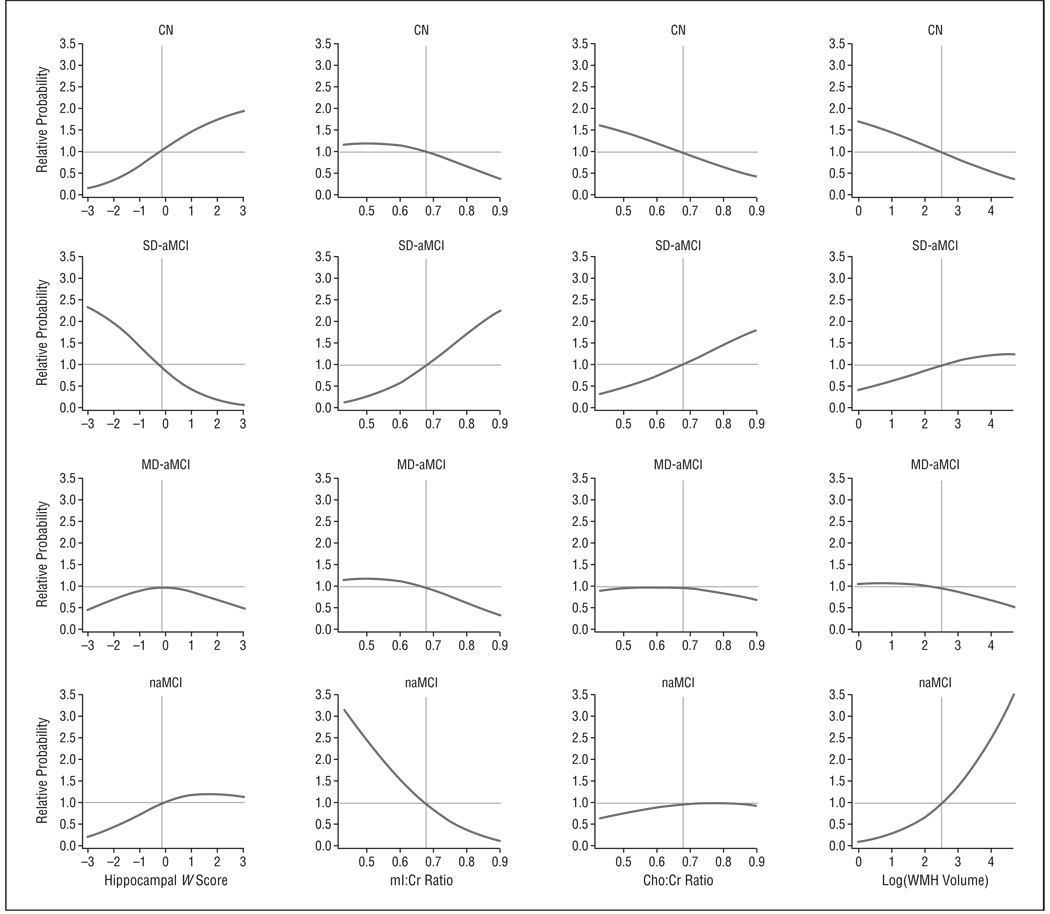
The Figure illustrates that among the cognitively normal subjects and MCI subtypes, after adjusting for age, sex, and education, the estimated probability of being in the single-domain aMCI group was higher at lower hippocampal W scores and higher mI:Cr ratios than in the cognitively normal subjects and the patients with naMCI (generalized logistic multinomial models, P < .001). Whereas the estimated probabilities in single-domain aMCI and naMCI were in general in the opposite direction of those for the cognitively normal individuals, quantitative imaging markers did not seem to affect the probability of membership in the multiple-domain aMCI group.
Figure 1.
Estimated relative probability of group membership as a function of 4 imaging measures. The relative probabilities shown are the estimated probability of group membership obtained from the multinomial model divided by the estimated probability at the median of the imaging measure. The gray reference lines intersect at the median for the measure on the x-axis and 1.0 on the y-axis. For all estimates we assumed a 77-year-old man with 14 years of education. Cho indicates choline; CN, cognitively normal; Cr, creatine; MD-aMCI, multiple-domain amnestic mild cognitive impairment (MCI); mI, myo-inositol; naMCI, nonamnestic MCI; SD-aMCI, single-domain aMCI; and WMH, white matter hyperintensity.
Multinomial modeling to fit the 2 imaging-predictor models that included age, sex, education, and a single imaging marker were used to evaluate whether a second imaging measurement improved model fit and discrimination beyond that obtained from an age-, sex-, and education-adjusted model based on a single imaging marker only. We included cognitively normal subjects and each of the MCI subtypes in the first model and included only the MCI subtypes in the second model to determine whether MRI and 1H MRS variables were complementary in distinguishing MCI subtypes. Higher mI:Cr and Cho:Cr ratios and greater WMH volume improved the discrimination among all clinical groups (MCI subtypes and cognitively normal subjects) when controlled for hippocampal W scores or other imaging markers, demonstrating that hippocampal W scores, mI:Cr and Cho:Cr ratios, and WMHs are complementary in characterizing patients with MCI and cognitively normal subjects. On the other hand, only higher mI:Cr ratios improved the discrimination among the MCI subtypes when we controlled for hippocampal W scores and WMH volume (Table 3).
Table 3.
Likelihood Ratio Tests Evaluating the Additive Effect of Each of 2 Predictors Given That the Other Predictor Is in the Model After Adjusting for Age, Sex, and Education
| Predictor 1 | Predictor 2 | Additive Effect (P Value) | |||
|---|---|---|---|---|---|
| Predictor 1 | Predictor 2 | ||||
| All Groupsa | MCI Onlyb | All Groupsa | MCI Onlyb | ||
| Hippocampal W score | NAA:Cr ratio | 54 (<.001) | 14 (.001) | 7.4 (.06) | 1.6 (.46) |
| mI:Cr ratio | 50 (<.001) | 14 (.001) | 18 (<.001) | 14 (.001) | |
| Cho:Cr ratio | 52 (<.001) | 15 (<.001) | 11 (.01) | 3.6 (.16) | |
| Log(WMH volume) | 54 (<.001) | 15 (<.001) | 18 (<.001) | 5.4 (.07) | |
| Cortical infarctionsc | … | 13 (.002) | … | 1.5 (.47) | |
| NAA:Cr ratio | mI:Cr ratio | 4.7 (.20) | 2.4 (.31) | 19 (<.001) | 15 (<.001) |
| Cho:Cr ratio | 7.7 (.053) | 1.5 (.46) | 13 (.005) | 2.6 (.27) | |
| Log(WMH volume) | 5.2 (.16) | 1.8 (.42) | 16 (.001) | 4.8 (.09) | |
| Cortical infarctionsc | … | 1.7 (.43) | … | 2.7 (.26) | |
| mI:Cr ratio | Cho:Cr ratio | 14 (.003) | 13 (.002) | 7.2 (.07) | 0.74 (.69) |
| Log(WMH volume) | 21 (<.001) | 16 (<.001) | 18 (<.001) | 5.7 (.06) | |
| Cortical infarctionsc | … | 13 (.001) | … | 1.2 (.56) | |
| Cho:Cr ratio | Log(WMH volume) | 9.0 (.03) | 2.5 (.28) | 14 (.002) | 4.6 (.10) |
| Cortical infarctionsc | … | 2.4 (.30) | … | 2.3 (.31) | |
| Log(WMH volume) | Cortical infarctionsc | … | 5.2 (.07) | … | 3.1 (.21) |
Abbreviations: Cho, choline; Cr, creatine; mI, myo-inositol; MCI, mild cognitive impairment; NAA, N-acetylaspartate; WMH, white matter hyperintensity; ellipses not reported.
Based on a 3-df χ2 test.
Based on a 2-df χ2 test.
Because absence of cortical infarctions was among the inclusion criteria for the cognitively normal group, the results among all 4 groups are not reported
COMMENT
There were distinct groupwise differences in MRI and 1H MRS findings between single-domain aMCI and naMCI subtypes. Patients with single-domain aMCI tended to have smaller hippocampal volumes and elevated mI:Cr ratios compared with patients with naMCI and cognitively normal subjects. On the other hand, patients with naMCI had normal hippocampal volumes and normal mI:Cr ratios, but a greater proportion of these patients in our sample had cortical infarctions compared with the patients with single-domain aMCI. Both hippocampal atrophy and elevated mI:Cr ratios are sensitive markers of early AD pathological changes, and the severity of these abnormalities correlate with pathological severity of AD.14–20 For this reason, hippocampal atrophy and elevated mI:Cr ratios most likely represent a high frequency of early AD pathological changes in the patients with single-domain aMCI. On average, hippocampal volumes and mI:Cr ratios in the naMCI subtype suggest that underlying pathological substrates may include abnormalities other than AD in some patients with naMCI. Mixed brain abnormalities, including AD cerebrovascular and Lewy body disease, underlie most cases of dementia in the community.50 It appears likely from the higher prevalence of cortical infarctions in patients with naMCI that large-vessel cerebrovascular disease was one of the pathological contributors to naMCI, in agreement with a previous study showing that a greater proportion of patients with naMCI experienced transient ischemic attacks and stroke compared with patients with aMCI32 (Table 4).
Table 4.
MRI and 1H MRS Characteristics of MCI Subtypes and the Pattern of Imaging Abnormalities That Relate to AD or CVD
| MCI Subtype | Hippocampal Volumes |
1H MRS, mI:Cr Ratio |
Cortical Infarctions |
Pattern | |
|---|---|---|---|---|---|
| AD-Like | CVD More Common | ||||
| Single-domain aMCI | Atrophy | Elevated | Less frequent | Yes | No |
| naMCI | Normal | Normal | More frequent | No | Yes |
| Multiple-domain aMCI | Mild atrophy | Normal | More frequent | Some | Yes |
Abbreviations: AD, Alzheimer disease; aMCI, amnestic mild cognitive impairment (MCI); Cr, creatine; CVD, cerebrovascular disease; 1H MRS, proton magnetic resonance spectroscopy; mI, myo-inositol; MRI, magnetic resonance imaging; naMCI, nonamnestic MCI.
On the other hand, we found a similar apolipoprotein Eε4 frequency in the naMCI and aMCI groups, yet we demonstrate that patients with naMCI on average do not have the magnetic resonance features of AD, and by definition they present with an early AD phenotype (ie, predominantly memory impairment). One possible way to reconcile these seemingly contradictory findings, although speculative, is that some patients with naMCI have AD pathological features but in an atypical anatomical distribution, thus accounting for the non–AD-like magnetic resonance findings and clinical presentation. In support of this, autopsy studies have documented the presence of AD pathological characteristics in an atypical distribution in subjects who present with a non–AD-like clinical profile.51,52 However, this will require follow-up, preferably to autopsy.
Multinomial modeling demonstrated that elevated mI:Cr and Cho:Cr ratios and increased WMH volumes were complementary to smaller hippocampal volumes in distinguishing the clinical groups. This is in agreement with a previous finding that 1H MRS metabolites improve discrimination of cognitively impaired nondemented individuals from their cognitively normal peers, when considered together with hippocampal volumes.25 Furthermore, 1H MRS findings and hippocampal volume are independent and complementary predictors of verbal memory on neuropsychometric testing in nondemented older adults.53 The added value of 1H MRS metabolites, hippocampal volumes, and WMH volumes in characterizing the MCI subtypes suggests that 1H MRS scans may complement structural MRI findings as a prognostic marker in MCI; however, this will require longitudinal studies for verification.
The magnetic resonance findings in patients with multiple-domain aMCI showed some similarities to those of patients with single-domain aMCI and naMCI. These patients had some hippocampal atrophy, but this was less than the hippocampal atrophy observed in patients with single-domain aMCI. Furthermore, they typically had normal mI:Cr ratios on 1H MRS findings, suggesting that if these patients had pathological AD, it would be on average less severe than in patients with single-domain aMCI. On the other hand, in our sample, the proportion of patients with multiple-domain aMCI and cortical infarctions was more than twice as high as that for patients with single-domain aMCI. For this reason, our data suggest that AD and cerebrovascular disease, in variable amounts, are some of the underlying abnormalities in the patients with multiple-domain aMCI in this study.
Contrary to the evidence of varying prevalence rates of cortical infarctions, we did not observe any difference in the proportion of patients with subcortical infarctions among cognitively normal subjects and the 3 MCI subtypes. The possibility that cortical and subcortical infarctions have a differential effect on future progression to dementia is currently unclear. One study found that subcortical infarctions did not increase the risk of progression to dementia in MCI.54 Others have shown that silent infarctions, most of which were presumably subcortical, increase the risk of future dementia in cognitively normal elderly individuals.55
The patients with single-domain aMCI had a greater WMH volume compared with cognitively normal elderly subjects. On the other hand, we did not identify a difference in the estimated WMH volume among the MCI subtypes. Because the frequency of subcortical infarctions was also similar across the MCI subtypes, our data suggest that subcortical vascular disease is a common but not a differentiating feature of the MCI subtypes. This is consistent with the previous reports that WMH is associated with an increased risk of MCI and that there is no association between subcortical hyperintensity load and different MCI subtypes.30,56,57
This study has several limitations. First, we classified infarctions as cortical and subcortical and did not further investigate the effects of the location, number, and size of the infarctions on cognitive function in MCI. This was a deliberate choice because the small number of subjects with infarctions prevented any coherent grouping of infarctions by detailed anatomical criteria for group-wise analysis. Second, cognitively normal subjects were neurologically healthy individuals who did not have a clinical history of stroke or cortical infarctions. They were included in this study to test for the abnormalities in quantitative magnetic resonance markers in MCI subtypes without the confounding effects of overt known neurological diseases. It is possible, although not common, for cognitively normal subjects to have cortical infarctions. Therefore, the absence of cortical infarctions in the cognitively normal subjects of this study cannot be generalized to the population. Third, although it is expected, based on pathological series, that DLB is one of the underlying abnormalities in MCI subtypes,50,58 the presence of DLB-related imaging changes was not evaluated in this study because there are no established magnetic resonance markers for prodromal DLB. Normal hippocampal volumes and 1H MRS in naMCI do not rule out the presence of Lewy body disease because patients with DLB on average have normal hippocampal volumes and normal posterior cingulate gyrus NAA:Cr and mI:Cr ratios.21,59 Although patients with DLB have elevated Cho:Cr ratios on 1H MRS findings, it is not clear whether the Cho:Cr ratio elevation in the posterior cingulate gyrus is a feature of prodromal DLB. For these reasons, DLB may be one of the underlying pathological findings in MCI subtypes.
Our data indicate that specific constellations of magnetic resonance findings are complementary in characterizing MCI subtypes and cognitively normal individuals. The MRI and 1H MRS findings in patients with single-domain aMCI are characterized by an AD-like pattern of elevated mI:Cr ratios in the posterior cingulate gyrus and hippocampal atrophy. On the other hand, patients with naMCI do not have the magnetic resonance features of AD and are more likely to have cortical infarctions. Magnetic resonance findings in patients with multiple-domain aMCI show similarities to those in patients with single-domain aMCI and naMCI. Clinical follow-up will demonstrate the combinations of MRI and 1H MRS findings that are useful in determining which MCI patients will experience progression to specific dementia syndromes in the future.
Acknowledgments
Funding/Support: This study was supported by Paul B. Beeson Career Development Award in Aging K23 AG030935, from the National Institutes of Health (NIH)/National Institute on Aging (NIA); Alzheimer’s Association New Investigator Research Grant 03-4842; NIH Roadmap Multidisciplinary Clinical Research Career Development awards KL2 RR024151 from the NIH/National Center for Research Resources (Dr Kantarci), P50 AG16574 and U01 AG06786 from the NIH/NIA (Dr Petersen), and R01 AG11378 from the NIH/NIA (Dr Jack); the Alexander Family Professorship in Alzheimer’s Research (Dr Jack); and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
Footnotes
Author Contributions: Dr Kantarci had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Kantarci, Whitwell, and Jack. Acquisition of data: Kantarci, Shiung, Whitwell, Ivnik, Knopman, Smith, and Jack. Analysis and interpretation of data: Kantarci, Petersen, Przybelski, Weigand, Negash, Boeve, and Jack. Drafting of the manuscript: Kantarci. Critical revision of the manuscript for important intellectual content: Petersen, Przybelski, Weigand, Shiung, Whitwell, Negash, Ivnik, Boeve, Knopman, Smith, and Jack. Statistical analysis: Kantarci, Przybelski, and Weigand. Obtained funding: Kantarci, Petersen, and Jack. Administrative, technical, and material support: Kantarci, Shiung, Ivnik, Boeve, Smith, and Jack. Study supervision: Jack.
Financial Disclosure: None reported.
REFERENCE
- 1.Petersen RC. Mild cognitive impairment. Continuum. 2004;10(1):9–28. [Google Scholar]
- 2.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68(4):288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 3.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41(7):1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 4.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 5.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 7.Bowen J, Teri L, Kukull W, McCormick W, McCurry SM, Larson EB. Progression to dementia in patients with isolated memory loss. Lancet. 1997;349(9054):763–765. doi: 10.1016/S0140-6736(96)08256-6. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22(4):312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 9.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 10.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63(5):665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 12.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63(5):674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001;17(2):101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 14.Barkhof F, Polvikoski TM, van Straaten EC, et al. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology. 2007;69(15):1521–1527. doi: 10.1212/01.wnl.0000277459.83543.99. [DOI] [PubMed] [Google Scholar]
- 15.Bobinski M, Wegiel J, Tarnawski M, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(4):414–420. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002;58(10):1476–1482. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]
- 17.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63(1):72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarow C, Vinters HV, Ellis WG, et al. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol. 2005;57(6):896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantarci K, Knopman DS, Dickson DW, et al. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248(1):210–220. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarci K, Petersen RC, Boeve BF, et al. 1H MR spectroscopy in common dementias. Neurology. 2004;63(8):1393–1398. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metastasio A, Rinaldi P, Tarducci R, et al. Conversion of MCI to dementia: role of proton magnetic resonance spectroscopy. Neurobiol Aging. 2006;27(7):926–932. doi: 10.1016/j.neurobiolaging.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Shonk TK, Moats RA, Gifford P, et al. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology. 1995;195(1):65–72. doi: 10.1148/radiology.195.1.7892497. [DOI] [PubMed] [Google Scholar]
- 24.Jessen F, Block W, Traber F, et al. Proton MR spectroscopy detects a relative decrease of N-acetylaspartate in the medial temporal lobe of patients with AD. Neurology. 2000;55(5):684–688. doi: 10.1212/wnl.55.5.684. [DOI] [PubMed] [Google Scholar]
- 25.Chao LL, Schuff N, Kramer JH, et al. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology. 2005;64(2):282–289. doi: 10.1212/01.WNL.0000149638.45635.FF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuff N, Capizzano AA, Du AT, et al. Selective reduction of N-acetylaspartate in medial temporal and parietal lobes in AD. Neurology. 2002;58(6):928–935. doi: 10.1212/wnl.58.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chantal S, Braun CM, Bouchard RW, Labelle M, Boulanger Y. Similar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer disease. Brain Res. 2004;1003(1–2):26–35. doi: 10.1016/j.brainres.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 29.Whitwell JL, Petersen RC, Negash S, et al. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 2007;64(8):1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bombois S, Debette S, Delbeuck X, et al. Prevalence of subcortical vascular lesions and association with executive function in mild cognitive impairment subtypes. Stroke. 2007;38(9):2595–2597. doi: 10.1161/STROKEAHA.107.486407. [DOI] [PubMed] [Google Scholar]
- 31.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanetti M, Ballabio C, Abbate C, Cutaia C, Vergani C, Bergamaschini L. Mild cognitive impairment subtypes and vascular dementia in community-dwelling elderly people: a 3-year follow-up study. J Am Geriatr Soc. 2006;54(4):580–586. doi: 10.1111/j.1532-5415.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 33.Mariani E, Monastero R, Ercolani S, et al. ReGAl Study Group. Vascular risk factors in mild cognitive impairment subtypes: findings from the ReGAl Project. Dement Geriatr Cogn Disord. 2007;24(6):448–456. doi: 10.1159/000110653. [DOI] [PubMed] [Google Scholar]
- 34.Petersen RC, Kokmen E, Tangalos E, Ivnik RJ, Kurland LT. Mayo Clinic Alzheimer’s Disease Patient Registry. Aging (Milano) 1990;2(4):408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- 35.Rey A. L’examen clinique en psychologie. Paris: Universitaires de France; 1964. [Google Scholar]
- 36.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 37.Lucas JA, Ivnik RJ, Smith GE, et al. Mayo’s Older Americans Normative Studies: category fluency norms. J Clin Exp Neuropsychol. 1998;20(2):194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- 38.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 39.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. Clin Neuropsychol. 1996;10(3):262–278. [Google Scholar]
- 40.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6(suppl 1):1–104. [Google Scholar]
- 41.Jack CR, Jr, Bentley MD, Twomey CK, Zinsmeister AR. MR imaging-based volume measurements of the hippocampal formation and anterior temporal lobe: validation studies. Radiology. 1990;176(1):205–209. doi: 10.1148/radiology.176.1.2353093. [DOI] [PubMed] [Google Scholar]
- 42.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49(3):786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR, Jr, O’Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14(6):668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu CC, Mungas D, Petkov CI, et al. Brain structure and cognition in a community sample of elderly Latinos. Neurology. 2002;59(3):383–391. doi: 10.1212/wnl.59.3.383. [DOI] [PubMed] [Google Scholar]
- 45.Murray ME, Shiung MM, Weigand SD, et al. Mayo MRI visual grading scale: validation studies. Presented at: Annual Meeting of the Society for Neuroscience; November 5, 2007; San Diego, CA: [Google Scholar]
- 46.Webb PG, Sailasuta N, Kohler SJ, Raidy T, Moats RA, Hurd RE. Automated single-voxel proton MRS: technical development and multisite verification. Magn Reson Med. 1994;31(4):365–373. doi: 10.1002/mrm.1910310404. [DOI] [PubMed] [Google Scholar]
- 47.Agresti A. Categorical Data Analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc; 2002. [Google Scholar]
- 48.O’Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39(3):787–794. [PubMed] [Google Scholar]
- 49.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 51.Josephs KA, Whitwell JL, Duffy JR, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70(1):25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray ME, Dickson DW. Alzheimer’s disease with relative hippocampal sparing: a distinct clinicopathological variant [abstract] Alzheimers Dement. 2008;4(4 suppl 2):T106. [Google Scholar]
- 53.Zimmerman ME, Pan JW, Hetherington HP, et al. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology. 2008;70(18):1594–1600. doi: 10.1212/01.wnl.0000306314.77311.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63(2):220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 56.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58(4):643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 57.Luchsinger J, Brickman A, Reitz C, et al. Cerebrovascular disease in mild cognitive impairment [abstract] Alzheimers Dement. 2008;4(4 suppl 2):T131. [Google Scholar]
- 58.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 59.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130(pt 3):708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]