Abstract
Inhibiting peripheral nerve function can be useful for many studies of the nervous system or motor control. Accomplishing this in a temporary fashion in animal models by using peripheral nerve blocks permits studies of the immediate effects of the loss, and/or any resulting short-term changes and adaptations in behavior or motor control, while avoiding the complications commonly associated with permanent lesions, such as sores or self-mutilation. We have developed a method of quickly and repeatedly inducing temporary, controlled motor deficits in rhesus macaque monkeys via a chronically implanted drug delivery system. This assembly consists of a nerve cuff and a subdermal injection dome, and has proved effective for delivering local anesthetics directly to peripheral nerves for many months. Using this assembly for median and ulnar nerve blocks routinely resulted in over 80% losses in hand and wrist strength for rhesus monkeys. The assembly was also effective for inducing ambulatory motor deficits in rabbits through blocks of the sciatic nerve. Interestingly, while standard anesthetics were sufficient for the rabbit nerve blocks, the inclusion of epinephrine was essential for achieving significant motor blockade in the monkeys.
Keywords: Peripheral nerve block, Rhesus macaque, Nerve cuff, Lidocaine, Limb movement, Paralysis
1. Introduction
Temporarily disrupting the function of peripheral nerves to prevent normal sensory and/or motor function is useful for studies of neurophysiology, behavior, motor control, and, more recently, neural interfaces. Our group has used this method to develop a cortically-controlled functional electrical stimulation (FES) system to restore hand function during paralysis (Pohlmeyer et al., 2007). Unlike a nerve transection or other chronic method of inducing paralysis, a temporary pharmacological block allows repeated comparisons between the normal and blocked state. Furthermore, long-lasting deafferentation or paralysis can also lead to long-term complications in the animals’ health. These risks are greatly reduced by a temporary block.
A peripheral nerve block is typically done by a percutaneous injection of local anesthetic directly to the nerve at an area in which it is relatively superficial or otherwise accessible. The nerve can be located by stimulating electrically through a needle as it is inserted. The stimulation is reduced in intensity as the needle approaches the nerve until the current strength and magnitude of the physical response indicates close proximity. Ideally the needle will not actually touch the nerve, in order to avoid making an intraneural injection (Capdevila et al., 2004; Raj, 1991; Selander et al., 1977; Sung, 2004). It is important for the subject to remain still during this procedure, as even small movements can prevent proper injections. This can be particularly difficult with an awake animal. It is possible to use a general anesthetic for restraint, but the anesthesia can then confound the effects of the desired experiment.
Given the difficulties involved in performing reliable peripheral nerve blocks (PNBs) in animals, we developed a method that uses a fully implantable drug delivery assembly. This assembly consists of a chronically implanted nerve cuff connected to a subdermal injection dome. A similar nerve cuff was used to deliver a controlled anesthetic dosage to selectively block the gamma input to muscle spindles during locomotion in the cat (Hoffer and Loeb, 1983). In that system, however, the proximal end of the cannula was brought out through the skin. We chose to use subdermal injection domes so that the entire assembly could be implanted to reduce the risk of infection, and the likelihood of damage when the animal is in its home cage. Other nerve cuff applications have included studies involving nerve recording, stimulation, or the application of growth factors, anesthetics or other drugs to nerves (Andreasen and Struijk, 2003; Grill and Mortimer, 1998; Haugland et al., 1999; Hoffer and Kallesoe, 2000; Kroin et al., 1986; McDonald and Zochodne, 2003; Strange and Hoffer, 1999; Tarler and Mortimer, 2004). The extensive use of nerve cuffs has made information regarding appropriate cuff sizing and materials readily available, and has provided ample evidence that they can be safely used over long periods (Cuoco and Durand, 2000; Grill and Mortimer, 2000; Larsen et al., 1998; Stein et al., 1977).
Macaque monkeys have frequently been used in studies of spinal nerve blocks (Denson et al., 1981; Denson et al., 1984) and tests of the toxicity of regional anesthetics (Munson et al., 1977; Munson et al., 1975; Rosen et al., 1983), which suggested that standard regional anesthetics, such as lidocaine and bupivacaine, would also be effective for PNBs. Recently, work by (Moritz et al., 2008) that focused on brain-machine interfaces used peripheral nerve blocks using either chloroprocaine or lidocaine (with epinephrine) delivered either through a catheter inserted into the epineurium or in some cases, with nerve cuffs and a percutaneous catheter similar to that used previously in cats (Hoffer and Loeb, 1983).
2. Materials and Methods
2.1. Nerve Cuff Assembly
The drug delivery assembly described here consisted of a Silastic cuff that was placed around the nerve and connected to a subdermal injection dome via a short cannula. Anesthetics injected into the dome were thus delivered directly to the nerve. Since all the components were located beneath the skin, the chance of infection was greatly reduced.
2.1.1. Rabbit Nerve Cuffs
The basic design of the rabbit cuffs consisted of a Silastic tube (slit open lengthwise to permit placement around a nerve) that incorporated three stimulating wires, and was attached to a cannula as shown in Figure 1A. The wires were sewn into the proximal end of the cuff, the cuff’s exterior at that location being insulated with Silastic adhesive (Silbione MED ADH 4300 RTV) to prevent current spread to the surrounding tissue (Hoffer and Loeb, 1983; Stein et al., 1977). These wires were used to evoke muscle twitches to test the effectiveness of the nerve block.
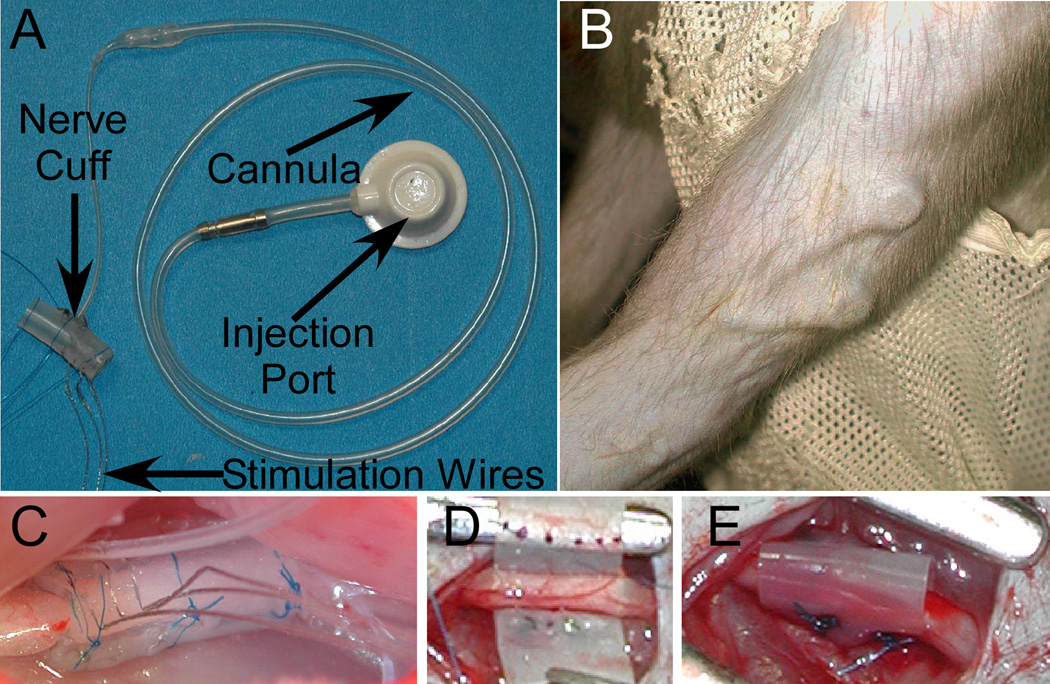
Figure 1.
A: Nerve cuff, cannula, and injection port used in rabbit experiments. B: The appearance of the injection dome beneath the skin in the upper arm of monkey M2. C: A nerve cuff around the rabbit sciatic nerve. The 3 stimulating wires are visible in the foreground. D-E show the implantation of a cuff around the ulnar nerve in M2 before (D) and after (E) the silastic has been shaped into a tube around the nerve.
The cannulae and cuffs were all made from laboratory grade Dow Corning tubing. The cuffs themselves were typically 0.132” ID; 0.183” OD, although slightly different diameters were occasionally used depending on the size of the sciatic nerve in each animal. Using only a single type of tubing, (0.040” ID; 0.0854” OD), to connect the cuff directly to the injection dome limited the flexibility of the assembly. Thus, the cuff was joined to the injection dome using a short piece of thin, flexible tubing, (0.020” ID; 0.037” OD), attached to the thicker tubing, as shown in Figure 1A. Several different methods of attaching the cannulae to the cuffs were explored, but none appeared to be superior to the others. In most cases, the cannula was simply attached to the middle of the cuff by slitting about 5 mm of the distal end of the cannula lengthwise, inserting the split ends of the cannula through a slit in the wall of the cuff, and suturing them to the inside of the cuff with 4-0 Neurolon. The cannula was cemented in place with more Silastic adhesive.
2.1.2. Monkey Nerve Cuffs
For the monkey cuffs, the cannula was connected to a small rectangular sheet of Silastic (thickness: 0.007”) at a 90° angle in the same manner as the attachment to the cuff described above. The Silastic sheet was then wrapped around the nerve at the time of surgery (Figure 1D, E). This approach was used to provide a more flexible, custom-fitted cuff design to address concerns about joint motion and limited implant space at the elbow. Since the effectiveness of the nerve block in the monkeys was established using behavioral tasks, no stimulating wires were needed.
2.1.3. Subdermal Injection Domes
Mentor Injection Domes (350-DOMPK) were used for the injections. Since two or more domes were implanted in the arm of the monkeys, the smaller “micro” domes were used (Figure 1B shows a pair of domes after implantation), while for the rabbits the larger standard size was adequate. The fill volumes of the combined dome and cannula were approximately 0.5 ml for the rabbit assemblies, and 0.3 ml for the monkey. While the domes were only guaranteed for 10 and 20 uses (micro and standard sizes, respectively), leakage through the injection area did not appear to be a problem, even for the micro domes that underwent many dozens of injections. A standard Mentor coupler was used to join the cannulae to the injection domes.
2.2. Surgical Implantation
All animal care, surgical, and research procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Northwestern University. All surgical procedures were conducted under sterile conditions using Isoflurane anesthesia. Each of the animals was given Buprenex and antibiotics (monkeys: Baytril or Cephalexin, rabbits: Baytril) as appropriate.
2.2.1. Rabbit Implantation
Six female New Zealand White rabbits (3.1–4.8 kg) were fitted with nerve cuffs and injection domes. The cuff was placed around the sciatic nerve (Figure 1C), proximal to the bifurcation of the peroneal and tibial nerves. Separating the gluteus and biceps muscles at the hip exposed the nerve, which was then dissected free of the surrounding tissue and elevated. A nerve cuff was selected whose diameter would comfortably contain the nerve, and the tube was positioned underneath the nerve and opened, allowing the nerve to drop inside. The cuff was closed with sutures and the cannula routed subcutaneously to the animal’s back. There, a small pocket was formed between the skin and the muscle to hold the injection dome. The dome was placed in the pocket and sutured to the underlying fascia. The electrode leads were tunneled to the head, where the connector was anchored to the skull with bone screws and dental acrylic. Five rabbits quickly recovered from the implant surgeries, and showed no signs of any motor deficits. One rabbit had a chronic limp after the implant procedure, and was not used for any of the successive tests.
2.2.2. Monkey Implantation
Three male rhesus macaque monkeys (9, 10, and 8 kg respectively) were fitted with cuffs and subdermal injection domes. One received implants for both the median and ulnar nerves in the right arm (M1-R), and 12 months later, median and ulnar implants in the left arm (M1-L). Another monkey (M2) received median and ulnar implants in one arm and a 3rd monkey (M3) received implants in the median, ulnar, and radial nerves. During each procedure the cuffs were placed around the nerves at, or just proximal to, the elbow. The nerves were freed from the surrounding tissue and elevated, and the Silastic sheet slid underneath. The sheet was trimmed to approximately 10 mm along the length of the nerve, and curled into a tube (Figure 1D–E). The opposing edges of the sheet were sutured together to form a tube loosely fitted to the nerve to allow for expansion of as much as 33% following surgery (Cuoco and Durand, 2000; Hoffer and Kallesoe, 2000; Stein et al., 1977). The ends of our cuffs were left open so that excess fluid could flow out during infusions to avoid increases in pressure.
The cannulae were tunneled to the upper arm and attached to the subdermal domes. For all implants except M1-L, the domes were placed in subcutaneous pockets on the medial or lateral sides of the biceps, and sutured to the underlying fascia. The domes for M1-L were not anchored to fascia; instead a very small pocket was formed on the medial side of the biceps just above the cuffs, and the domes simply slid inside. All monkeys had full use of the arm and hand within a few days of the procedure.
2.3. Evaluation of Peripheral Nerve Block Effectiveness
2.3.1. Nerve Block Injections and Cuff Maintenance
When using the cuff assemblies for PNBs, a local anesthetic was injected and the resulting motor and/or sensory effects noted. At the end of an experiment, the assemblies were flushed with heparinized saline (1.0–1.5 ml; 50 units:ml) to expel any remaining anesthetic. The assemblies were also routinely flushed with heparinized saline when not being tested to ensure continued patency (Hoffer and Kallesoe, 2000; Hoffer and Loeb, 1983). For the first month after implantation this was typically done daily, with a gradual reduction in frequency over time. For the monkey cuffs, flushing was reduced to three times a week by about three months post-implant, and to once a week or less, after about six months. Larger volumes or additional flushes were used if any resistance to the injections was noted. All injections for the monkey assemblies were done using 24G non-coring (Huber) needles.
2.3.2. Anesthetic Agents
Lidocaine was the primary anesthetic used for the peripheral nerve blocks. In the majority of the tests the anesthetic was given as a single bolus, although the benefit of administering the drug in two injections, separated by 15–45 minutes, was briefly explored. We also investigate the effects of including epinephrine (1:100,000) and varying the injection concentration (2–5%) and volume (1–4 ml). Sodium bicarbonate (1:10) was used for the monkey tests to raise the pH to avoid any stinging sensation during the injection. It may also serve to increase the speed of nerve blockade (Bedder et al., 1988; Candido et al., 1995). During several rabbit tests, injections of saline (1–5 ml) were done to distinguish any effects of injection pressure from the effects of the lidocaine.
2.3.3. Rabbit Nerve Block Tests
Effectiveness of the block in rabbits was quantified by comparing the amount of stimulation current required to evoke muscle twitches before and after injection. To find the threshold stimulation, bipolar, monophasic, 100 µsec current pulses were delivered. The current was increased until a muscle response was obtained in either the foot or ankle. Baseline was determined by averaging five threshold measurements taken during several different postures prior to the injection of anesthetic. The pair of stimulating wires that yielded the lowest baseline threshold was used throughout each experiment. After the injection, the response threshold was periodically measured (typically every 5–10 minutes), and the ratio of the baseline to the blocked threshold (the “nerve conduction”) was calculated for each of those measurements. The decrease in nerve conduction appeared to correspond to several observed behavioral effects, including sciatic palsy (lack of a toe spread response) (Altschul and Turner, 1942; Ready et al., 1985), difficulties in balancing, dragging of toes during locomotion, and excessive foot washing.
2.3.4. Monkey Nerve Block Tests
For the monkeys M1 and M2, the nerve cuffs were placed on the median and ulnar nerves at the elbow. At this level, these nerves innervate muscles that flex the wrist and fingers, including the flexor carpi radialis and ulnaris, palmaris longus, flexor digitorum superficialis and profundus, as well as intrinsic hand muscles, such those of the thenar eminence, the lumbricals and the interossei (Hartman and Strauss, 1933). Therefore, these monkeys’ strength when flexing the wrist and fingers was used to evaluate the PNBs. Blocking all three nerves in M3 yielded complete paralysis of all the muscles in the wrist and hand. Similar tests of wrist flexion and extension were used in this animal.
For M1, hand strength was tested by several different devices that required either a palmar or lateral grasp, or a precision pinch between the first finger and thumb. The force that could be generated by flexing the wrist was measured for all monkeys. For all of these tests, force was displayed as a cursor on a computer monitor. The monkeys were trained to generate forces that matched specific targets. Their maximum strength was measured by encouraging them to match increasingly high targets by giving additional rewards, and rescaling the force feedback to mask the higher required force. In this fashion, high force requirements were intermixed with lower forces using targets at the same apparent position. Importantly, similar methods were used under both normal and PNB conditions. The nerve block effects were then quantified using the ratio of the maximum force each of the monkeys could match after the nerve block to their maximum baseline force prior to the block.
Each of the different tasks was effective in measuring a loss in hand strength. Unfortunately, the monkeys often learned methods of “cheating” in the blocked condition. For example, when the monkeys were unable to grip a device properly, they could still register force by simply impacting their fist or palm on one of the force sensors. Similarly, the wrist task device would detect small inertial forces generated by extending the elbow, rotating the forearm, or swinging the arm at the shoulder. Thus, while the behavioral tasks showed great loss in strength during the blocks, the measured force was rarely zero, even when the fingers appeared to be completely unusable. As such, the true losses in muscle strength that occurred under PNB conditions were likely to have been greater than the values measured by these methods.
Several other more qualitative evaluations of the effects of the blocks on the hand were employed. These included tests for sensation in the fingers and palm, the monkeys’ ability to grasp small food treats, and finger dexterity during motor tests such the removal of food from small wells. All of these tasks showed substantial motor and sensory deficits during the blocks that corresponded well to the measured reductions in wrist and grasp strength.
3. Results
3.1 Rabbit Peripheral Nerve Blocks
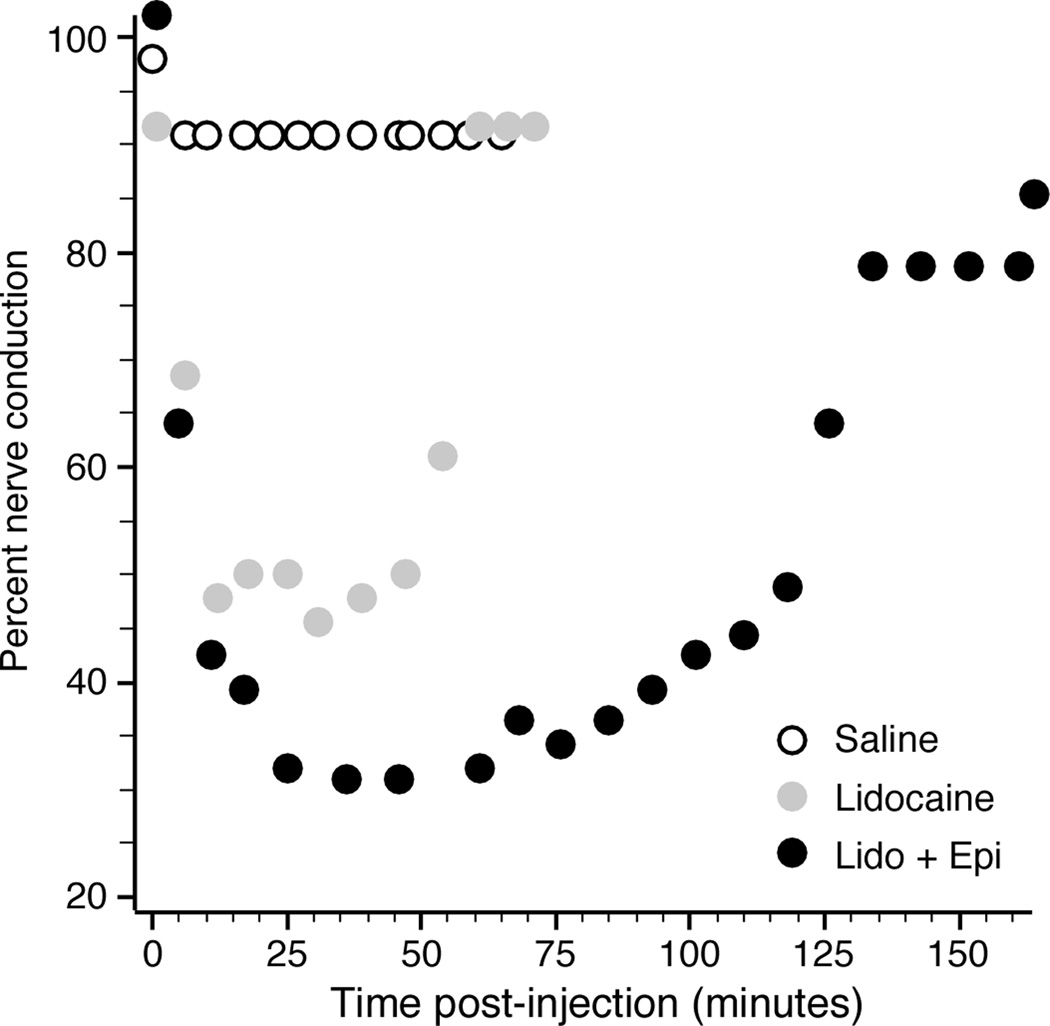
In the rabbits, the PNB effects were quantified by the nerve conduction. Low values reflected the relatively small muscle twitch evoked by a given current relative to normal. Figure 2 shows nerve conduction as a function of time following a single lidocaine injection (2.5 ml of 2% lidocaine with and without epinephrine) together with a control injection of saline. The lidocaine caused a substantial decrease in nerve conduction for nearly 60 minutes, with epinephrine both further suppressing nerve conduction and extending the duration of the effect.
Figure 2.
Effect of peripheral nerve block on sciatic nerve conduction for rabbit R5. Injection of 2% lidocaine substantially decreased conduction, shown as the reciprocal of the normalized current strength required to evoke a contraction. Combination with epinephrine (1:100,000) further increased the effect, while saline had very little effect.
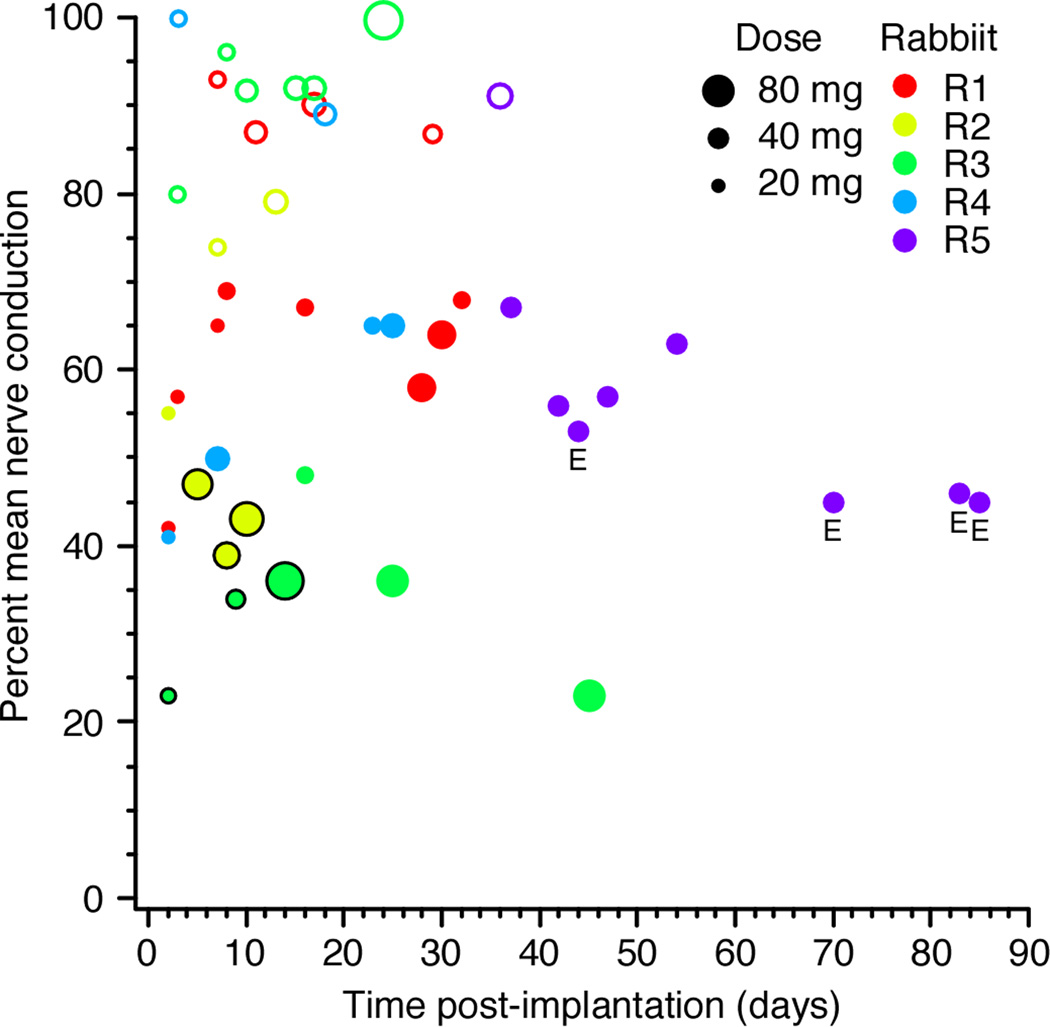
The effect of each experiment’s PNB was summarized by averaging all the nerve conduction measurements taken from the time of the injection until the current threshold returned within 10% of its baseline value. For example, the averaged nerve conductions over this time period for the PNBs shown in Figure 2 were 0.91 (saline), 0.57 (lidocaine), and 0.46 (lidocaine + epinephrine). Figure 3 shows these average nerve conduction values for PNBs performed across the life of the cuff assembles for several rabbits. The size of each marker reflects the dose of lidocaine used for that PNB. In general, the PNBs were most effective in the days to weeks immediately following the cuff implantation. On average, for lidocaine tests with no epinephrine, about 120 minutes passed before nerve conduction returned within 10% of baseline, with an overall average nerve conduction of 0.47 during that time period.
Figure 3.
Effects of lidocaine PNBs of the rabbit sciatic nerve across post-implantation time. The size of the markers is proportional to the dose of lidocaine (volume * concentration). Open circles mark saline injections, and correspond to the volume of a 2% lidocaine injection. Injections that included epinephrine are marked with an “E”.
Figure 2 and Figure 3 show that even large volumes of saline had only small, transient effects on nerve conduction. Furthermore, the rabbits did not show any qualitative signs of motor deficits (such as limping or sciatic palsy) following the saline injections. For comparison purposes, in Figure 3 a marker for a 2 ml injection of saline is shown as the same size as a 2 ml dose of 2% lidocaine, with the markers of larger saline injections scaled appropriately.
3.2 Monkey Peripheral Nerve Blocks
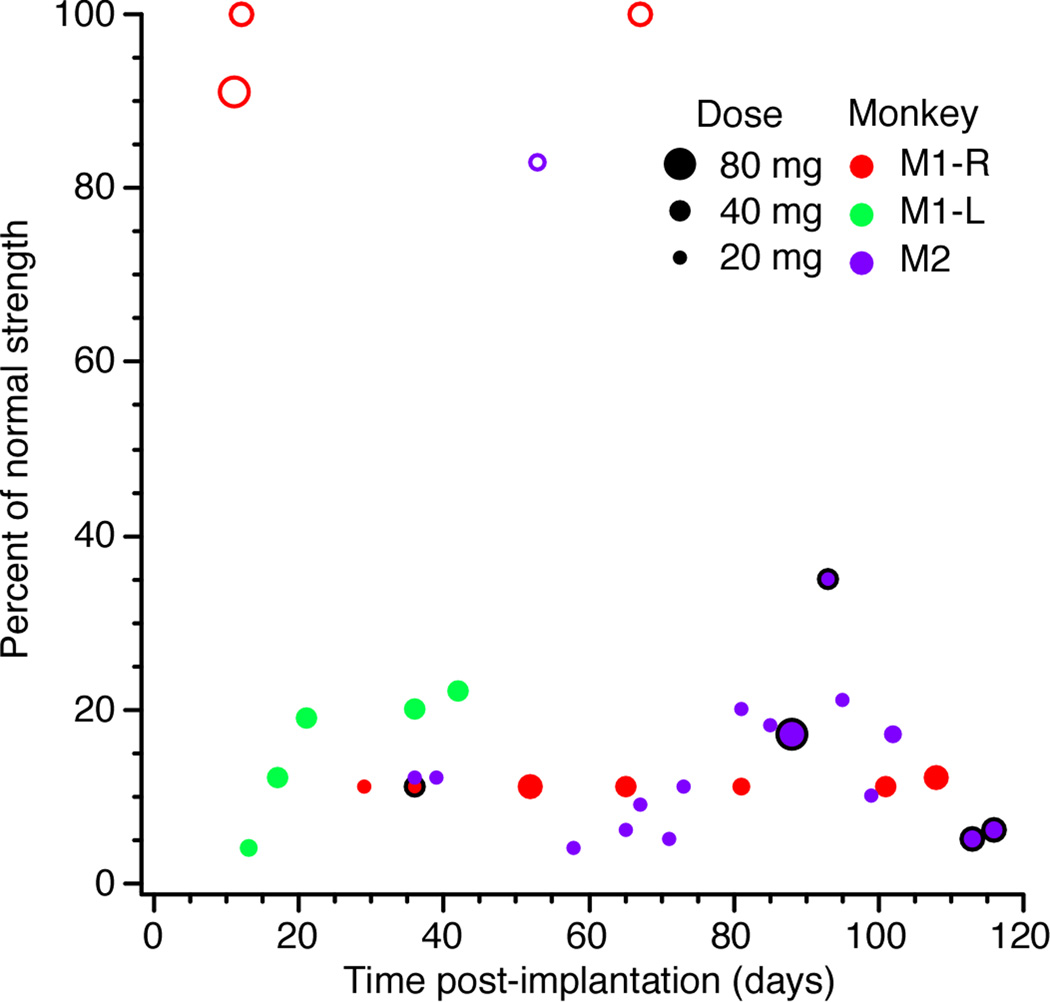
Figure 4 shows the reduction in wrist and grasp strength that occurred during lidocaine (1–5%) PNBs for the monkeys M1 and M2, as a function of post-implant time. The effects on the key and pinch grips are not shown, as they closely paralleled the palmar grip measures. Tests with M3 were done in conjunction with an experiment designed to use functional electrical stimulation to restore the monkey’s ability to control muscle contractions following peripheral nerve block. Thus the effectiveness of the block was carefully monitored for the duration of those experiments, but all the tests described here were not used systematically. As long as epinephrine was included, appreciable motor deficits occurred in all monkeys, even when using small (1 ml) doses of 1% lidocaine. Without epinephrine, little to no effects were observed, even when using 5% lidocaine. For almost all of the tests in Figure 4, both the median and ulnar nerves were blocked. However, the ulnar cuff became occluded for M1-R after 37 days, thus the tests beyond that point reflect only median nerve blocks.
Figure 4.
Effects on palmar grip strength of lidocaine PNBs of the median and ulnar nerves for rhesus monkey across post-implantation time. Open circles mark those injections without epinephrine, which were largely ineffective. Tests beyond 40 days for M1-R included only median nerve block. Symbols denoting experiments in which the dose was split into two injections are circled in black.
Figure 4 shows that, while effectiveness may have been somewhat greater immediately following implant, the impact of the PNBs quickly settled to fairly consistent levels. However, Figure 4 does not reflect the fact that PNBs began to take as long as 40 minutes to become effective, (compared to 20–25 minutes in early PNBs), and that the motor deficits were not as long lasting. Typically we would compensate for these effects by increasing the initial dosage of lidocaine, or by administering the anesthetic as two smaller injections rather than a single large bolus, as was done for M2-L at days 113 and 116. These gradual drops in PNB effectiveness may be attributable to tissue encapsulation of the cuffs, which isolated the nerve from the anesthetic. Furthermore, at around 80 days post-implant, M2-L learned to generate small amounts of output for the wrist task by extending and twisting the forearm. By doing this, he made the PNB appear to be less effective. Similar observations were made for the other monkeys. While data obtained when a monkey was clearly cheating was not analyzed, some of the fluctuations in effectiveness in Figure 4 may still be due to such behavior.
3.3 Nerve Cuff Effective Lifetimes
All of the cuffs used in the rabbit tests were still effective when the animals were sacrificed (from 19 to 85 days). Upon removal, the cuffs typically were encapsulated with fibrous tissue, both within the cuff and surrounding the nerve. However, ink injected during post-mortem examination reached, and at least partially filled the cuffs. These results, together with those from the monkey subjects, are summarized in table 2.
The M1-R median nerve cuff was used successfully for over three months, at which point experiments were halted. Although no more PNBs were done, saline flushes done at bi-weekly or longer intervals suggested that it remained unobstructed until the animal was sacrificed 15 months post-implantation. While an unobstructed cannula is necessary, it is not sufficient to guarantee an effective block. Tissue accumulated around the nerve might have prevented penetration of the anesthetic without necessarily clogging the catheter. The M1-R ulnar cuff became occluded and nonfunctional after 37 days. Several unsuccessful attempts were made to recover it by injections of heparanized saline. While it is possible that there was a blockage within the cuff or cannula, none was apparent on post-mortem inspection. Rather, it appeared the cannula had become kinked at the junction between the narrow and wider cannula tubes. When both cuffs were ultimately removed, tissue encapsulation of each was very similar to that of the rabbit implants.
It was only possible to test the M1-L implants for 42 days, as shortly thereafter, the cuffs had to be removed because of the persistent presence of fluid around the median cuff and dome, and the eventual formation of lesions in the skin. These cuffs had been made with thicker, stiffer tubing, which may have caused greater mechanical irritation of the nerves. In addition, the domes were not sutured to the fascia, which lead to noticeably more movement, irritation, and accumulation of fluid in the subcutaneous pocket. These surgical methods were avoided in subsequent implants.
Both the M2 cuffs remained functional for four months, at which point regular testing was stopped. Two later tests using qualitative evaluations indicated that they were still effective at seven months. The three cuffs implanted in M3 remain fully effective in experiments that are on-going five months after implant.
4. Discussion
The fully implanted nerve cuff assembly described here proved to be an effective means of eliciting reproducible peripheral nerve blocks for both the rabbit sciatic nerve, and a variety of nerves in the monkey forearm. The subdermal injection domes made the injections straightforward even in awake monkeys, making it possible to arbitrarily extend the duration of anesthesia with a supplemental anesthetic injection. This technique could be useful for a wide range of experiments requiring regional anesthesia. Since the block is temporary, the likelihood of self injurious behavior is minimal, unlike animal models that involve chronic nerve lesions.
With the exception of one rabbit subject that suffered an immediate post-surgical complication, all five successful rabbit implants continued to be effective through the duration of the experiment, ranging from 19 – 85 days. Of the nine cuff assemblies tested in the three monkeys, only three had useful lifetimes of less than 20 weeks. The probable cause of failure of two of these three was an altered surgical procedure that is likely to have lead to excessive inflammation at the site of the dome implants for M1-L. Likewise, the apparent cause of early failure of the ulnar implant in M1-R was a kinked cannula, not occlusion caused by an accumulation of fibrous tissue.
An unexpected, but potentially interesting difference between the results in monkey and rabbit was the effect of epinephrine. Appreciable motor deficits for the monkeys were only achieved with epinephrine. In numerous tests with M1 and M2 lidocaine alone did not cause any significant motor deficit, even at 5% concentrations. Lidocaine can affect motor and sensory fibers differently (Mink and Thach, 1991; Nakamura et al., 2003), and it is possible that there were sensory effects with lidocaine alone that our motor tests did not reveal. However, simple tests of sensation in the hand and fingers failed to reveal any loss of sensation in the absence of epinephrine either. Similarly, several nerve blocks were attempted with 0.5% and 0.75% bupivacaine, with no motor deficits observed unless epinephrine was included. The necessity of epinephrine was evidently not due to the use of the nerve cuffs. Among several blocks attempted by percutaneous injection of lidocaine directly to the median and ulnar nerves, motor deficits were again only ever achieved in combination with epinephrine.
The reason epinephrine was needed is unclear. It is commonly thought to improve nerve blocks by constricting the blood vessels and slowing systemic absorption, thus allowing the anesthetic more time to diffuse through the epineurium (Covino, 1986). However, it is not typically believed to be essential, and its benefits are controversial (Chambers et al., 1981; Chambers et al., 1982; Chiu et al., 1995; Kito et al., 1998). It is possible that the metabolism of the rhesus macaque is sufficiently high as to prevent any significant amount of anesthetic to cross the epineurium without the extra time provided by epinephrine. Alternatively, the monkeys’ epineurium may be relatively impermeable, such that it is harder for anesthetics to cross it.
5. Conclusions
The nerve cuff assembly presented here has proven effective for easily inducing peripheral nerve blocks in the sciatic nerve of rabbits and the median, ulnar and radial nerves of the rhesus macaque. Anesthetic injections were accomplished using a subdermal injection dome, meaning that no part of the drug delivery assembly penetrated the skin. This greatly reduced the risks of infection, and the assemblies have been functional for many months. While lidocaine alone was effective for sciatic nerve blocks in the rabbits, it was only effective for nerve blocks in the monkeys when combined with epinephrine.
Table 1.
Summary of the location, effectiveness, and longevity of cuffs implanted in each rabbit and monkey subject. Duration reflects the longest time point at which the effectiveness of blocks was tested either as part of systematic tests, or at a later date following cessation of regular testing.
| Species | ID | location | Duration | Comments |
|---|---|---|---|---|
| Rabbit | R1 | sciatic | 0days | Nerve damage following implant |
| Rabbit | R2 | sciatic | 19 days | Mean nerve conduction = 0.45 |
| Rabbit | R3 | sciatic | 45 days | Mean nerve conduction = 0.31 |
| Rabbit | R4 | sciatic | 32 days | Mean nerve conduction = 0.60 |
| Rabbit | R5 | sciatic | 32 days | Mean nerve conduction = 0.53 |
| Rabbit | R6 | sciatic | 85 days | Mean nerve conduction = 0.60 |
| Monkey | M1L | median | 6wks | Cuffs removed following edematous inflammatory response |
| Monkey | M1L | ulnar | ||
| Monkey | M1R | median | >12 wks | Testing ended at 12 weeks. Unobstructed at time of last saline injection at 64 weeks |
| Monkey | M1R | ulnar | 5wks | Cannula probably kinked |
| Monkey | M2 | median | 43 wks | Systematic testing ended at 24 weeks |
| Monkey | M2 | ulnar | ||
| Monkey | M3 | median | >20 wks | All cuffs remained functional at the time of submission |
| Monkey | M3 | ulnar | ||
| Monkey | M3 | radial |
Acknowledgements
The authors gratefully acknowledge the assistance of Michael Johnson in the initial rabbit implants. Dr. Jon Kim assisted in the implantation of the first set of cuffs in monkey M1. Finally, Emily R. Oby assisted in the testing of M2 and M3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul R, Turner KP. The Innervation of the musculi interossei and the toe spreading reflex. Am J Physiol. 1942;137:247–250. [Google Scholar]
- Andreasen LN, Struijk JJ. Skin contact forces extracted from human nerve signals--a possible feedback signal for FES-aided control of standing. IEEE transactions on bio-medical engineering. 2003;50:1320–1325. doi: 10.1109/TBME.2003.819848. [DOI] [PubMed] [Google Scholar]
- Bedder MD, Kozody R, Craig DB. Comparison of bupivacaine and alkalinized bupivacaine in brachial plexus anesthesia. Anesth Analg. 1988;67:48–52. [PubMed] [Google Scholar]
- Candido KD, Winnie AP, Covino BG, Raza SM, Vasireddy AR, Masters RW. Addition of bicarbonate to plain bupivacaine does not significantly alter the onset or duration of plexus anesthesia. Reg Anesth. 1995;20:133–138. [PubMed] [Google Scholar]
- Capdevila X, Lopez S, Bernard N, Dadure C, Motais F, Biboulet P, Choquet O. Percutaneous electrode guidance using the insulated needle for prelocation of peripheral nerves during axillary plexus blocks. Reg Anesth Pain Med. 2004;29:206–211. doi: 10.1016/j.rapm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Chambers WA, Littlewood DG, Logan MR, Scott DB. Effect of added epinephrine on spinal anesthesia with lidocaine. Anesth Analg. 1981;60:417–420. [PubMed] [Google Scholar]
- Chambers WA, Littlewood DG, Scott DB. Spinal anesthesia with hyperbaric bupivacaine: effect of added vasoconstrictors. Anesth Analg. 1982;61:49–52. [PubMed] [Google Scholar]
- Chiu AA, Liu S, Carpenter RL, Kasman GS, Pollock JE, Neal JM. The effects of epinephrine on lidocaine spinal anesthesia: a cross-over study. Anesth Analg. 1995;80:735–739. doi: 10.1097/00000539-199504000-00015. [DOI] [PubMed] [Google Scholar]
- Covino BG. Pharmacology of local anaesthetic agents. Br J Anaesth. 1986;58:701–716. doi: 10.1093/bja/58.7.701. [DOI] [PubMed] [Google Scholar]
- Cuoco FA, Jr, Durand DM. Measurement of external pressures generated by nerve cuff electrodes. IEEE Trans Rehabil Eng. 2000;8:35–41. doi: 10.1109/86.830947. [DOI] [PubMed] [Google Scholar]
- Denson DD, Bridenbaugh PO, Phero JC, Raj PP, Turner PA, Ohlweiler DF. Evaluation of lidocaine spinal anesthesia in the rhesus monkey. Anesth Analg. 1981;60:756–759. [PubMed] [Google Scholar]
- Denson DD, Turner PA, Bridenbaugh PO, Thompson GA. Pharmacokinetics and neural blockade after subarachnoid lidocaine in the rhesus monkey. III. Effects of phenylephrine. Anesth Analg. 1984;63:129–133. [PubMed] [Google Scholar]
- Grill WM, Mortimer JT. Neural and connective tissue response to long-term implantation of multiple contact nerve cuff electrodes. J Biomed Mater Res. 2000;50:215–226. doi: 10.1002/(sici)1097-4636(200005)50:2<215::aid-jbm17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Grill WM, Mortimer JT. Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Trans Rehabil Eng. 1998;6:364–373. doi: 10.1109/86.736150. [DOI] [PubMed] [Google Scholar]
- Hartman CG, Strauss WL, editors. The Anatomy of the Rhesus Monkey (Macaca mulatto) Balitmore: Williams and Wilkins Co; 1933. [Google Scholar]
- Haugland M, Lickel A, Haase J, Sinkjaer T. Control of FES thumb force using slip information obtained from the cutaneous electroneurogram in quadriplegic man. IEEE Trans Rehabil Eng. 1999;7:215–227. doi: 10.1109/86.769412. [DOI] [PubMed] [Google Scholar]
- Hoffer JA, Kallesoe K. Nerve cuffs for nerve repair and regeneration. Prog Brain Res. 2000;128:121–134. doi: 10.1016/S0079-6123(00)28012-6. [DOI] [PubMed] [Google Scholar]
- Hoffer JA, Loeb GE. A technique for reversible fusimotor blockade during chronic recording from spindle afferents in walking cats. Exp.Brain Res. Sup. 1983;7:272–279. [Google Scholar]
- Kito K, Kato H, Shibata M, Adachi T, Nakao S, Mori K. The effect of varied doses of epinephrine on duration of lidocaine spinal anesthesia in the thoracic and lumbosacral dermatomes. Anesth Analg. 1998;86:1018–1022. doi: 10.1097/00000539-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Kroin JS, Penn RD, Levy FE, Kerns JM. Effect of repetitive lidocaine infusion on peripheral nerve. Experimental neurology. 1986;94:166–173. doi: 10.1016/0014-4886(86)90280-3. [DOI] [PubMed] [Google Scholar]
- Larsen JO, Thomsen M, Haugland M, Sinkjaer T. Degeneration and regeneration in rabbit peripheral nerve with long-term nerve cuff electrode implant: a stereological study of myelinated and unmyelinated axons. Acta Neuropathol (Berl) 1998;96:365–378. doi: 10.1007/s004010050907. [DOI] [PubMed] [Google Scholar]
- McDonald DS, Zochodne DW. An injectable nerve regeneration chamber for studies of unstable soluble growth factors. Journal of neuroscience methods. 2003;122:171–178. doi: 10.1016/s0165-0270(02)00319-9. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia motor control. II. Late pallidal timing relative to movement onset and inconsistent pallidal coding of movement parameters. Journal of neurophysiology. 1991;65:301–329. doi: 10.1152/jn.1991.65.2.301. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–642. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson ES, Paul WL, Embro WJ. Central-nervous-system toxicity of local anesthetic mixtures in monkeys. Anesthesiology. 1977;46:179–183. doi: 10.1097/00000542-197703000-00004. [DOI] [PubMed] [Google Scholar]
- Munson ES, Tucker WK, Ausinsch B, Malagodi MH. Etidocaine, bupivacaine, and lidocaine seizure thresholds in monkeys. Anesthesiology. 1975;42:471–478. doi: 10.1097/00000542-197504000-00018. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Popitz-Bergez F, Birknes J, Strichartz GR. The critical role of concentration for lidocaine block of peripheral nerve in vivo: studies of function and drug uptake in the rat. Anesthesiology. 2003;99:1189–1197. doi: 10.1097/00000542-200311000-00028. [DOI] [PubMed] [Google Scholar]
- Pohlmeyer EA, Perreault EJ, Slutzky MW, Kilgore KL, Kirsch RF, Taylor DM, Miller LE. Real-Time Control of the Hand by Intracortically Controlled Functional Neuromuscular Stimulation; IEEE Proc 10th international conference on rehab robotics; 2007. [Google Scholar]
- Raj PP. Clinical Practice of Regional Anesthesia. Churchill Livingstone Inc; 1991. [Google Scholar]
- Ready LB, Plumer MH, Haschke RH, Austin E, Sumi SM. Neurotoxicity of intrathecal local anesthetics in rabbits. Anesthesiology. 1985;63:364–370. [PubMed] [Google Scholar]
- Rosen MA, Baysinger CL, Shnider SM, Dailey PA, Norton M, Curtis JD, Collins M, Davis RL. Evaluation of neurotoxicity after subarachnoid injection of large volumes of local anesthetic solutions. Anesth Analg. 1983;62:802–808. [PubMed] [Google Scholar]
- Selander D, Dhuner KG, Lundborg G. Peripheral nerve injury due to injection needles used for regionalanesthesia. An experimental study of the acute effects of needle point trauma. Acta Anaesthesiol Scand. 1977;21:182–188. doi: 10.1111/j.1399-6576.1977.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Stein RB, Nichols TR, Jhamandas J, Davis L, Charles D. Stable long-term recordings from cat peripheral nerves. Brain Res. 1977;128:21–38. doi: 10.1016/0006-8993(77)90233-5. [DOI] [PubMed] [Google Scholar]
- Strange KD, Hoffer JA. Restoration of use of paralyzed limb muscles using sensory nerve signals for state control of FES-assisted walking. IEEE Trans Rehabil Eng. 1999;7:289–300. doi: 10.1109/86.788466. [DOI] [PubMed] [Google Scholar]
- Sung DH. Locating the target nerve and injectate spread in rabbit sciatic nerve block. Reg Anesth Pain Med. 2004;29:194–200. doi: 10.1016/j.rapm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four contact nerve-cuff electrode. IEEE Trans Neural Syst Rehabil Eng. 2004;12:251–257. doi: 10.1109/tnsre.2004.828415. [DOI] [PubMed] [Google Scholar]