Abstract
Background
Liver transplantation is currently the only established treatment for end-stage liver disease, but it is limited by a severe shortage of viable donor livers. Donors after cardiac death (DCD) are an untapped source that could significantly increase the pool of available livers. Preservation of these DCD livers by conventional static cold storage (SCS) is associated with an unacceptable risk of primary non-function and delayed graft failure. Normothermic extracorporeal liver perfusion (NELP) has been suggested as an improvement over SCS.
Methods
Livers recovered from male Lewis rats were subjected to 1hr of warm ischemia and preserved with 5hrs of SCS or NELP, and transplanted into syngeneic recipients. As additional controls, non-ischemic livers preserved with 6hrs of SCS or NELP and unpreserved ischemic livers were transplanted.
Results
Following NELP, ischemically damaged livers could be orthotopically transplanted into syngeneic recipients with 92% survival (N=13) after 4 weeks, which was comparable to control animals which received healthy livers preserved by SCS (N=9) or NELP (N=11) for 6hrs. On the other hand, animals from ischemia/SCS control group all died within 12hrs post-operatively (N=6). Similarly, animals that received ischemic livers without preservation all died within 24hrs after transplantation (N=6).
Conclusions
These results suggest that NELP has the potential to reclaim warm ischemic livers that would not be transplantable otherwise. The rat model in this study is a useful platform to further optimize NELP as a method of recovery and preservation of DCD livers.
Keywords: transplantation, reperfusion injury, machine perfusion, preservation, preconditioning
Introduction
Transplantation is currently the only established treatment for end-stage liver disease, but it is limited by the shortage of available organs. Extending liver graft criteria to include marginal livers, such as those obtained from donors after cardiac death (DCD), could alleviate this problem (1). It is estimated that about 6,000 ischemic livers (1, 2) could be reconditioned for transplantation, effectively doubling the availability of grafts. However, conventional static cold storage (SCS) of these marginal organs leads to unsatisfactory transplant outcome (1); they exhibit a higher risk of primary non-function as well as delayed graft failure, especially due to biliary complications such as stricture (3). It is thought that warm ischemic damage experienced by DCD livers leads to increased sensitivity to subsequent cold ischemia and rewarming injury associated with SCS.
Both hypothermic and normothermic machine perfusion have been suggested as methods to improve the preservation of DCD livers. The advantages of hypothermic perfusion over SCS have been previously demonstrated (4-8). Recently, functional recovery of ischemically damaged rat livers was shown using a combination of SCS followed by short term hypothermic machine perfusion (9, 10). However, extended hypothermic machine perfusion can cause endothelial damage (11), which may limit organ viability.
Normothermic extracorporeal liver perfusion (NELP) has been suggested as a method to avoid the problems associated with SCS and hypothermic perfusion (12-14). Near-normothermic machine perfusion has been successfully used in experimental kidney preservation (15-17), and recently normothermic perfusion was shown to be superior to SCS in the preservation of DCD livers (13, 14, 18). A survival benefit after transplantation of DCD livers preserved normothermically has been demonstrated in one study using a porcine model (13).
The complexity and high cost of large animal models limits the number of thorough studies that can be conducted, making systematic characterization and optimization of NELP difficult. To provide an alternative model that is more amenable to research and development, we developed a small-scale NELP system where rat livers can be successfully transplanted after 6 hours of normothermic perfusion (19). Herein, we investigated the potential of NELP to recover warm ischemic livers. We show that rat livers that underwent 60 minutes of ex-vivo warm ischemia (34°C) and then preserved by 5 hours of NELP could be successfully transplanted into syngeneic recipients. By contrast, recipients of similar livers stored by SCS for 5 hours, as well as those transplanted directly without having undergone preservation, did not survive.
Experimental Procedures
Isolation of donor livers
Experiments were performed using male Lewis rats weighing 250-300g (Charles River Labs, Wilmington, MA). The animals were maintained in accordance with National Research Council guidelines and the experimental protocols were approved by the Subcommittee on Research Animal Care, Massachusetts General Hospital. All animals were anesthetized with isoflurane using a Tech 4 vaporizer (Surgivet, Waukesha, WI) under sterile conditions. The donor liver surgery and is described in detail elsewhere (19,20).
Warm ischemia induction
After isolation from the donor, the liver was weighed and then placed in a temperature controlled chamber filled with saline and maintained at 34±0.1°C for one hour. During this period the PV and IVC were cuffed as previously described (19).
Normothermic liver perfusion
The perfusate and dialysate comprised phenol red-free Williams Medium E (Sigma Chemical, St. Louis, MO) supplemented with: 2 u/l insulin (Humulin, Eli Lily, Indianapolis, IN), 100,000 u/l penicillin, 100 mg/l streptomycin sulfate (Gibco, Invitrogen, Grand Island, NY), 0.292 g/l l-glutamine (Gibco), 10 mg/l hydrocortisone (Solu-Cortef, Pharmacia & Upjohn, Kalamazoo, MI), and 1000 u/l heparin (APP, Schaumberg, IL). Fresh frozen rat plasma (25% v/v), and erythrocytes (18-20% v/v) were collected earlier (19) and added to the perfusate only. The total perfusate volume was 55-60 ml.
The perfusion system consisted of a primary liver perfusion circuit and a critical secondary dialysis circuit (19). Briefly, the primary circuit included perfusate that recirculated via a peristaltic pump through a jacketed perfusion chamber, a membrane oxygenator, a heat exchanger and bubble trap. The oxygenator was gassed with a mixture of 74% N2/21% O2/5% CO2 and 100% O2 to maintain a constant pH. A fraction of the perfusate was diverted to the secondary circuit through a hollow fiber dialyzer with a 2200 cm2 membrane area and a 30 kD nominal molecular weight cut-off (Spectrum Labs, Rancho Dominguez, CA) at a rate of 3 ml/min/g wet liver weight. The secondary circuit dialyzed the perfusate by counter-current exposure to 450ml of dialysate. The volumes of perfusate and dialysate were kept constant by varying the flow of dialysate through the dialyzer in the secondary circuit. Temperature within the system was maintained at 37.5°C.
After the warm ischemic period, the liver was flushed with 10ml of warm saline and immersed in perfusate in the perfusion chamber. The liver was perfused at a constant flow rate via the portal vein and effluent flowed freely from the suprahepatic and inferior vena cava into the surrounding medium. While the recipient hepatectomy was prepared, the liver was disconnected from the circuit, rinsed in a bowl of saline at room temperature and weighed again prior to transplantation. The operating parameters of the perfusion system were: Flow rate: 1.84±0.05 ml/min/g; Portal hydrostatic pressure: 12-16 cm H2O (8-12 mmHg); Hematocrit: 17.8%±0.8; Inlet pO2: 128.4±8.1 mmHg; Outlet pO2: 47.9±1.7 mmHg; Inlet pCO2: 30.1±1.1 mmHg; Outlet pCO2: 34.6±1.6 mmHg.
Analysis of perfusate levels of metabolites and liver enzymes
Perfusate samples (1ml) were collected prior to placing the liver in the perfusion system and hourly thereafter. For each sample, 100μl aliquots were immediately analyzed using a Piccolo comprehensive metabolic panel (Abaxis, Union City, CA) for alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, electrolytes and glucose. The remainder was stored at -80°C for later analysis. Dialysate samples (1ml) were collected at the same times and stored at -80°C.
For analysis of the hepatic oxygen uptake rate (HOUR), 200 μl samples were taken from the PV and IVC of the liver every 10 minutes for the first hour of the perfusion and every hour subsequently. Samples were analyzed immediately using a blood gas analyzer (Rapidlab, Chiron Diagnostics, Norwood, MA). The total concentration of O2 (ml/dl) in the samples was determined according to the formula:
[O2] = 1.39 × [Hb] × FO2Hb + 0.00314 × pO2
where [Hb] is the hemoglobin concentration in g/dl, FO2Hb is the fraction oxygenated hemoglobin and pO2 is the partial pressure of oxygen in mmHg. HOUR was determined as:
HOUR = (([O2] in - [O2] out)/100) × flow rate/weight of liver.
Bile was collected continuously in pre-weighed microfuge tubes that were exchanged every hour.
Recipient surgery
The cuff technique developed by Kamada and Calne (20-22) was implemented and is described in detail elsewhere (19). All recipient surgery was carried out by the same microsurgeon (H.T.). The anhepatic phase of the procedure was typically 13- 15 minutes and did not exceed 17 minutes. Animals were hydrated with 8ml/kg of warm (37°C) lactated Ringer's solution with 5% dextrose and 2ml/kg of NaHCO3 7%w/v (Abbott, North Chicago, IL) by penile vein injection.
The animals were put in single clean cages, allowed to recover from anesthesia under an infrared lamp for half an hour, and subsequently returned to regular housing. The first 12 hours post-operatively animals were checked every 2 hours and subsequently every 8 hours for one week.
Post-operative blood sampling
To determine the post-operative levels of AST, ALT and total bilirubin, 100-200μl of blood were drawn from the tail vein under isoflurane anesthesia on post-operative days 1, 3, 5, 7, 14, 21, 28 and immediately analyzed using a Piccolo blood chemistry analyzer. For these studies n was ≥4 for each group.
Simple cold storage
Warm ischemic livers (n=6) and freshly isolated livers (n=6) were flushed with 20ml of ice-cold (0°C) UW solution and placed on melting ice in a bowl containing UW solution for the duration of the SCS period; these livers were not perfused.
Diluted Whole Blood Reperfusion
For detailed evaluation of the graft response in the very early phase (0-2hrs) after transplantation, we employed a diluted whole-blood reperfusion model. This method was preferable as manipulation of animals for sampling immediately after transplantation could further stress the animals, affect survival, and introduce artefactual findings. The reperfusion circuit was identical to the normothermic perfusion system, but contained no secondary dialysis circuit. The livers were reperfused for 120 minutes and inflow (portal vein) and outflow (infrahepatic vena cava) sampling was performed every 15 minutes. The operating conditions for the reperfusion system were: Flow rate: 1.74±0.15 ml/min/g; Hematocrit 13.8±8.2; Inlet pO2: 263.5±111.9 mmHg; Outlet pO2: 75.1±49.7 mmHg; Inlet pCO2: 40.4± 14.9 mmHg; Outlet pCO2: 43.4±15.9 mmHg.
Histology
Liver tissue slices were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Apoptosis was evaluated through TUNEL staining (Roche, Indianapolis, IN).
Statistical Analysis
Data presented are means ± SE. All statistical analysis for differences performed with ANOVA at significance level of α=0.1.
Results
Integrity and Function of Liver during Perfusion
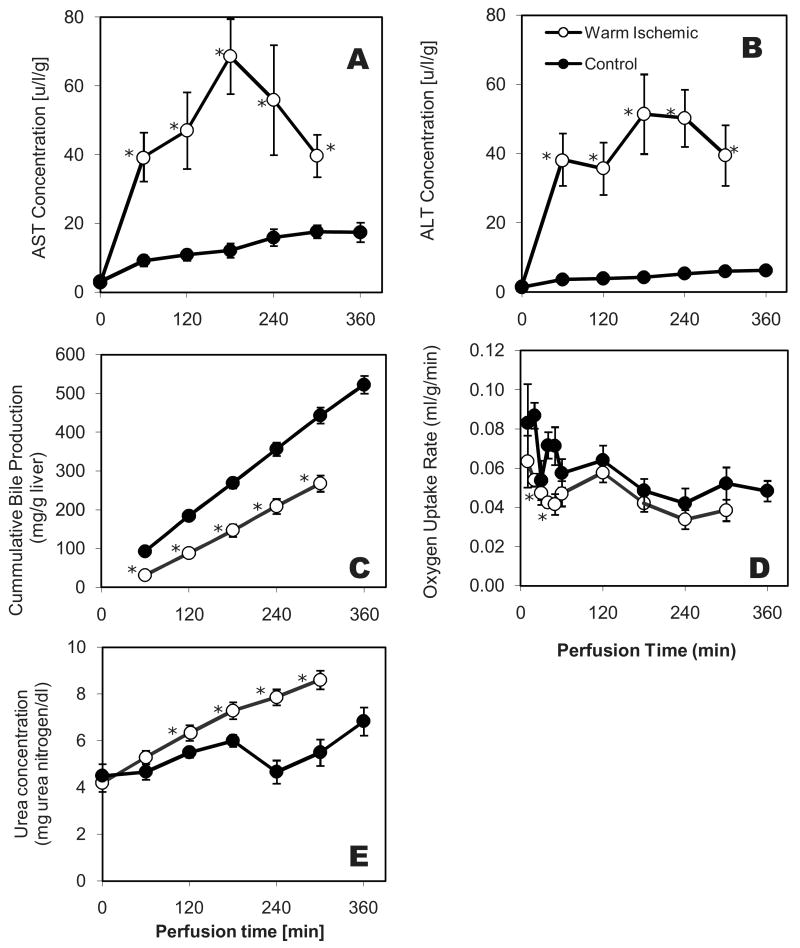
ALT and AST activities as indicators of hepatocellular damage are shown in Figure 1A & B; both AST and ALT accumulated during the first 180 min of perfusion and then decreased. These values were several fold higher than those previously reported for freshly isolated livers not subjected to any warm ischemia (19). Neither ALT nor AST were detected in the dialysate (data not shown).
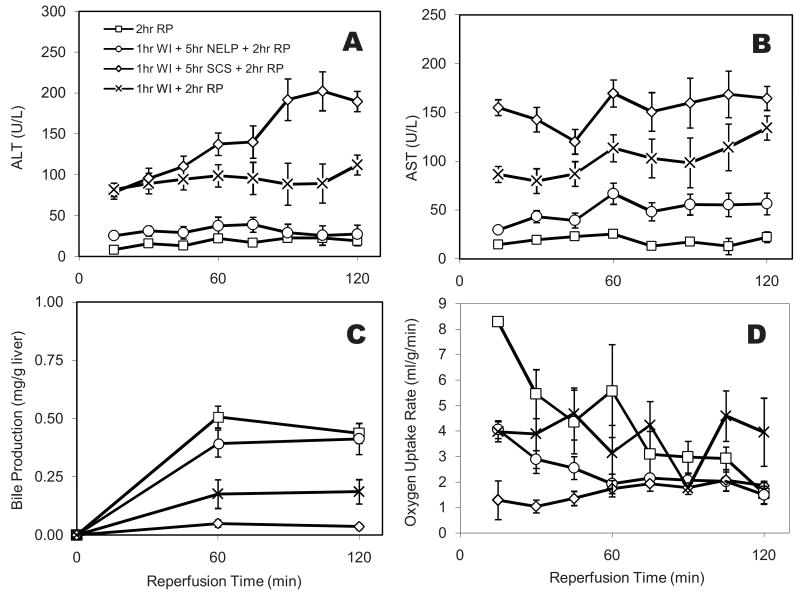
Figure 1.
Function and Integrity of warm ischemic livers during normothermic perfusion. (A) Asparate aminotransferase (AST) and (B) Alanine Aminotransferase (ALT) levels in perfusate samples collected hourly from the primary circuit; (C) Total bile accumulation normalized to wet liver weight; (D) Oxygen uptake rate normalized to wet liver weight. Data shown are averages of 6 ischemic livers ± Standard Error. Values for the warm ischemic livers are significantly lower than the controls for bile and oxygen uptake, and significantly higher for urea (p<0.01 by Analysis of Variance). Data for the control group (normothermic perfusion of non-ischemic livers) are from Tolboom et al. (19). * indicates statistical difference compared to healthy perfused livers at p<0.1.
Bile secretion and oxygen consumption describe the metabolic state of the liver. Bile was produced at a constant rate throughout the perfusion (Figure 1C). This rate was 40% lower than that previously reported for freshly isolated livers. The HOUR of warm ischemic livers declined rapidly during the first 60 minutes of the perfusion and then remained stable (Figure 1D). This behavior was very similar to that observed for freshly isolated livers. The HOUR's of the perfused warm ischemic and freshly isolated livers were very similar in the plateau region beyond 60 min.
The urea level in the perfusate showed a steady increase from 4.20 mg/dl at t=0 to 8.60 mg/dl at t=300 minutes, indicating a constant rate of urea production. This rate was consistently higher than that observed in perfused healthy livers (Figure 1E).
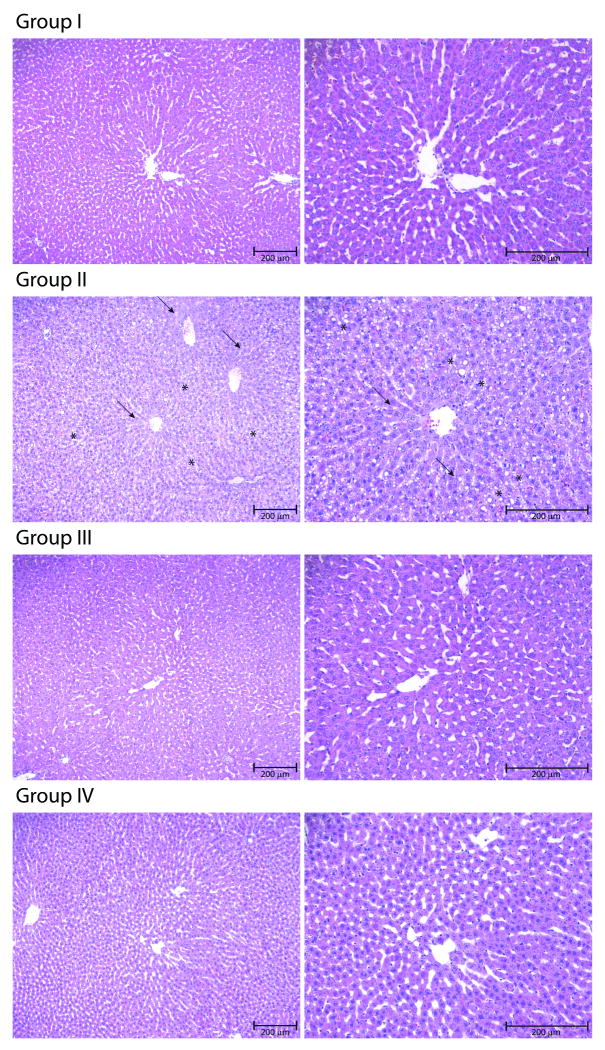
Figure 2 shows the histological appearance of warm ischemic livers after 5 hours of NELP. Ischemic livers treated with NELP show minimal to no damage compared to freshly isolated livers preserved either by SCS or NELP. In contrast, livers subjected to 1 hour of warm ischemia and subsequently preserved by SCS show swelling of hepatocytes (indicated by the arrows in the figure), widespread vacuolization and destruction of liver architecture (as indicated by the asterisks).
Figure 2.
Microscopic appearance of livers after preservation. A) Group I: Warm ischemic livers after 5 hours of Normothermic Extracorporeal Liver Perfusion (NELP). B) Group II: Warm ischemic livers after 5 hours of Static Cold Storage (SCS) in University of Wisconsin (UW) solution (Arrows indicate cell swelling and asterisks vacuolization and tissue destruction). C) Group III: Freshly isolated livers after 6 hours of NELP. D) Group IV: Freshly isolated livers after 6 hours of SCS in UW solution. Bar = 200 μm.
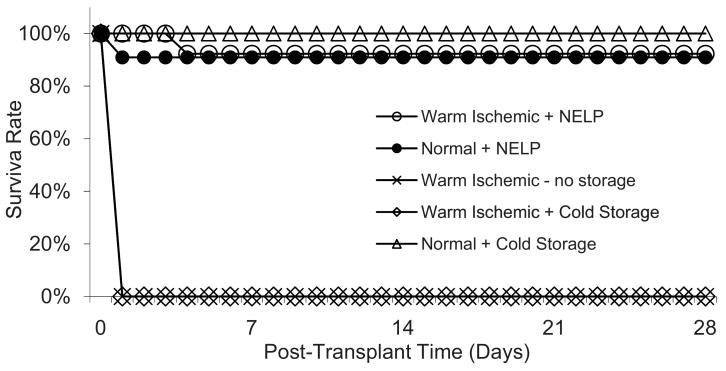
Survival after Transplantation
Warm ischemic livers were transplanted into recipient rats after 5 hours of NELP (n=13) or 5 hours of SCS in UW solution at 0°C (n=6). In addition, freshly isolated livers not subjected to any warm ischemia were transplanted after 6 hours of SCS (n=6) or NELP (n=11) and ischemic livers were transplanted directly without having undergone preservation (n=9).
Transplantation of NELP treated ischemic livers was uneventful in all but one case, where bleeding at the anastomosis occurred. All animals recovered from anesthesia rapidly. The animal that bled during surgery died on day 4 postoperatively. The other recipient animals survived beyond one month and did not exhibit external signs of liver failure, such as jaundice
No surgical complications occurred during transplantation of ischemic livers preserved by SCS and recipients recovered rapidly from anesthesia, but within 6 hours all developed symptoms and died within 12 hours. Autopsy revealed patchy livers and serous fluid in the abdomen.
All recipients of directly transplanted ischemic livers died in a similar way within 24 hours post-operatively.
All controls that received freshly isolated livers preserved for 6 hours by SCS recovered rapidly from surgery and survived beyond one month (Figure 3).
Figure 3.
Survival curves of recipient rats after transplantation of perfused warm ischemic livers, warm ischemic cold-stored livers, compared to healthy perfused livers, and healthy cold-stored livers. Data for the Normal+ Normothermic Extracorporeal Liver Perfusion (NELP) group are from Tolboom et al. (19).
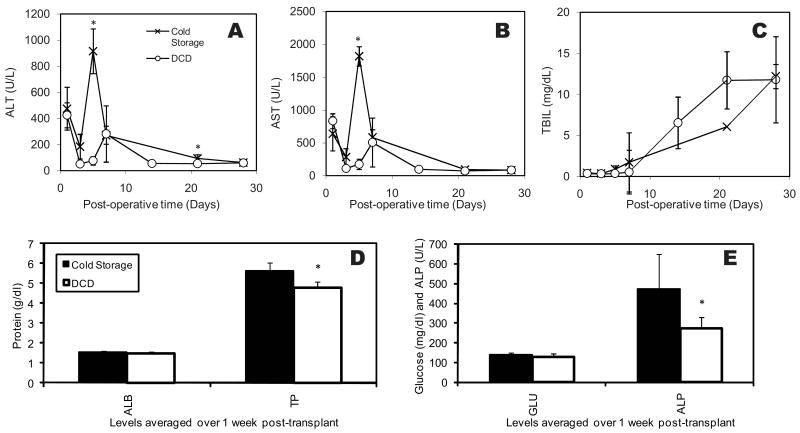
Post-operative liver enzymes and bilirubin
The levels of both AST and ALT (Figures 4A and B) were elevated on day 1 post-operatively similar to the levels found in recipients of healthy cold stored livers. Overall, values of recipients for healthy cold-stored livers and DCD livers showed similar and normal levels implying successful transplantation. The AST levels were significantly lower for the perfused warm ischemic livers as compared to the healthy cold-stored livers on post-operative day 5. Both values were comparable to those observed in a hypothermic machine perfusion study (23).
Figure 4.
Values of A) Asparate aminotransferase (AST), (B) Alanine Aminotransferase (ALT) (C) Total Bilirubin (TBIL) measured on days 1, 3, 5, 7, 14, 21 and 28 after transplantation of perfused warm ischemic livers compared to healthy cold-stored livers. (D) and (E): comparison of the serum levels of Albumin (ALB), Total Protein (TP), Glucose (GLU) and Alkaline Phosphatase (ALP) within 1 week after transplant. * indicates statistical difference compared to cold stored livers at p<0.1
The total bilirubin level, an indicator of liver function, was similar in both groups post-operatively and showed an increasing trend (Figure 4C). The increasing bilirubin value is within expected ranges and is likely an artifact of non-rearterialization and ensuing histopathologically observable bile duct proliferation (24). It is worth noting that the elevated bilirubin levels are reported (24) to normalize after 6 weeks and survival is minimally affected. There was no statistical difference of bilirubin levels between the groups on any day.
The recipient serum analysis is displayed in Figures 4D and E. Albumin levels were similar for both SCS and WI-NELP groups (1.52 and 1.46 mg/dL respectively), though lower than systemic levels we previously obtained in vivo (1.91±.23 g/dl) (25). The total protein was higher in the SCS group (5.63 vs. 4.76 g/dl, both similar to the in vivo levels of 4.96±0.64 g/dl), suggestive of immunoglobulin elevation. Glucose levels were similar and within the normal in vivo range (241.64±.119.81 mg/dl), as were the electrolyte levels (results not shown). ALP, indicative of general tissue damage, was beyond the normal rat values (208.54±.66.019 U/L) for cold stored organs (472.75 U/L). NELP-treated ischemic livers were statistically lower than healthy DCD livers (273.36 U/L) and well within the normal range. These results are in agreement with slightly increased ALT and AST levels in recipients of cold stored organs, though the difference is statistically significant only for day 5. These results overall indicate that the function of NELP-perfused DCD livers is slightly better than cold stored healthy organs. This could be either due to avoiding the cold injury (11), or ischemic preconditioning effects of warm ischemia (26).
Short-term graft function
In order to evaluate the very-short-term graft function post-transplantation, we employed a diluted whole-blood reperfusion model. This was preferred to repetitive blood sampling shortly after transplantation., as the animals would not tolerate well additional manipulations.
Figure 5 displays ALT and AST as markers of cellular damage, bile secretion as a viability indicator, as well as liver oxygen uptake rate as base-line indicator of metabolic activity. ALT levels for WI+NELP group was lower than WI-only and WI+SCS groups at all time points, and indifferent from normal livers after the first 45 minutes. Very similar trend were observed for AST, although the difference between WI+NELP and freshly isolated livers was statistically significant at all time points except t=45min. These results suggest that NELP improves early graft function and viability.
Figure 5.
Reperfusion results: (A) Alanine Aminotransferase (ALT), (B) Asparate aminotransferase (AST), (C) bile synthesis, and (D) oxygen uptake rate measured during reperfusion. See text for statistical analysis.
It was observed that bile secretion in the reperfusion system was well correlated with the survival results (Figure 5C). Post-transplant bile secretion has been previously shown to be strongly correlated to graft survival (27-29) and to cellular ATP levels (30, 31). The average bile production during reperfusion of normal livers and NELP-treated WI livers was statistically not different (p=0.21); secretion for both groups was higher than the other two WI groups (p≪0.01). Further, WI + SCS group showed lower bile secretion than WI-only group. Overall these results were as anticipated: it is known that substrate depletion causes reduction in bile synthesis, and the degree of reduction is proportional to ischemic injury (32).
As displayed in Figure 5D, reperfusion results in oxygen uptake rates that are different between groups. The average oxygen uptake was highest for the freshly isolated livers, statistically higher than that of WI+ NELP and WI +SCS groups, but not different from 1hr WI alone. There was no difference between WI+NELP and WI+SCS groups. Interestingly the WI-only livers displayed oxygen uptakes comparable to healthy livers. While oxygen uptake can be considered a bottom-line figure for respiration and metabolism, these results suggest that there is limited correlation between survival and early oxygen uptake rates.
Figure 6 displays the TUNEL staining results at the end of reperfusion. Apoptosis was absent in healthy and NELP-treated ischemic livers, and limited in WI-only livers. By comparison, WI+SCS group demonstrated significant staining. These results confirm that NELP is an effective method for preservion of ischemic livers. However, the absence of apoptosis in the WI-only livers that result in primary non-function when transplanted, suggest that apoptosis is not a determinant factor for graft survival.
Figure 6.
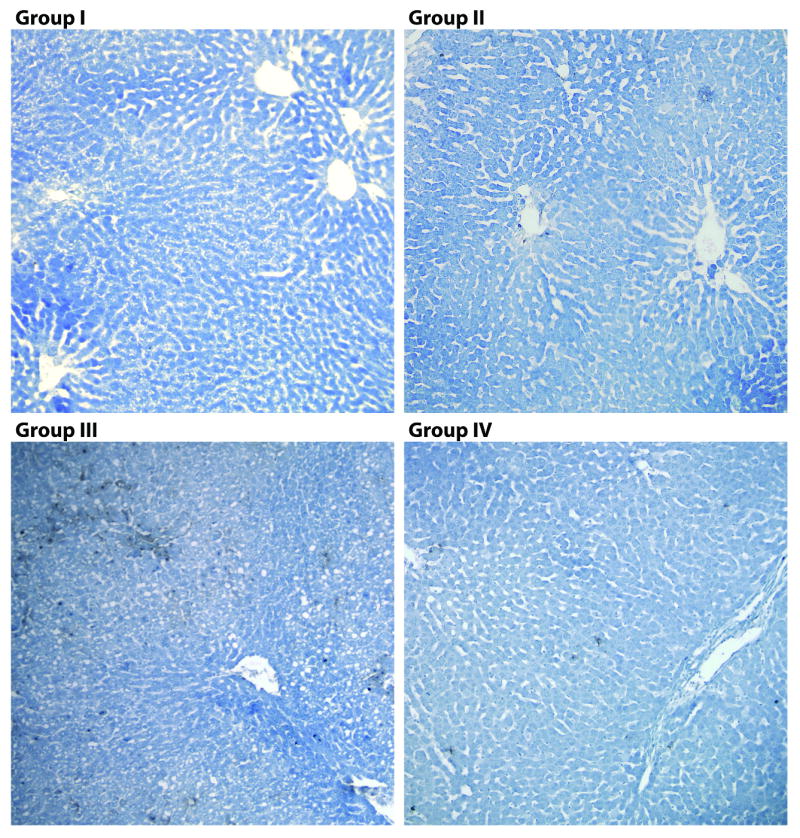
TUNEL of livers after preservation and reperfusion. A) Group I: Freshly isolated liver after reperfusion. B) Group II: Warm ischemic livers after reperfusion. C) Group III: Warm ischemic livers after 5 hours of Static Cold Storage (SCS) in University of Wisconsin (UW) solution after reperfusion. D) Group IV: Warm ischemic livers after 5 hours of Normothermic Extracorporeal Liver Perfusion (NELP) followed by reperfusion. Magnification (10×).
Discussion
We have demonstrated that livers subjected to 60 min of ex-vivo warm ischemia can be resuscitated with NELP and transplanted with excellent graft function and long term survival of the recipient, comparable to that of recipients of perfused fresh livers and fresh livers preserved with cold storage. Animals that received ischemic livers that were either preserved with cold storage or experienced no storage time at all, died within 24 hours of transplantation.
Diluted whole-blood reperfusion experiments were performed to assess early graft function. It was observed that function of NELP-treated ischemic livers matched that of freshly isolated livers, whereas the function of untreated ischemic grafts was significantly worse. The transaminase levels and bile trends in the reperfusion system correlated very well with our survival results. In addition, the results indicate that the reperfusion system can be used to simulate liver transplantation for rapid optimization of NELP conditions.
Hypothermic machine perfusion provides the organ with constant supply oxygen and nutrients while waste is removed. However, the basic approach to preservation still relies upon slowing down metabolic rates, and herein does not differ from SCS. Under hypothermic conditions, a delicate equilibrium exists between maintaining perfusate flow sufficient to ensure adequate tissue oxygenation and damage of the sinusoidal endothelium due to barotrauma and shear stress that may limit it usefulness (33-35).
Normothermic machine perfusion is fundamentally different from hypothermic perfusion because its aim is to not only re-establish perfusion of the liver, but also closely mimic the in-vivo conditions and maintain the liver in a metabolically active state. The organ's metabolic activity can be continuously monitored throughout the preservation period, making it possible to assess its viability and function, providing potential markers that could be used to predict viability after transplantation. Furthermore, once oxidative metabolism has been sufficiently restored and intracellular energy supplies have been replenished, induction of repair and even regenerative processes might be possible. Other applications that have been suggested for normothermic machine perfusion include preconditioning, such as the induction of heat-shock proteins, and immunomodulation, such as induction of resistance against recurrent hepatitis C infection of the liver graft and possibly the reversal of hepatic steatosis (36, 37).
Although previous NELP efforts have predominantly used larger animal models, the rat was our model of choice in order to keep our approach simple and inexpensive. Since the blood supply of the rat liver is mostly venous (38, 39), we have chosen to perfuse via the portal vein only, as usually done in the traditional isolated perfused rat liver systems (38, 40), which further helped to simplify the setup. For the same reason, the orthotopic liver transplant without reconstruction of the hepatic artery was performed using the cuff technique first described by Kamada (20, 21). By using an inbred strain of rats, issues associated with immunoreactivity during perfusion and after transplantation were avoided. A limitation of this approach is the lack of rearterialization, which is known to introduce certain artifacts, including histopathologically observable biliary proliferation (24). The recipient rats in our studies displayed the same complications (results not shown), as well as the increased serum bilirubin which is known to return to normal levels after 6 weeks in this model (24); however, survival was not affected by this phenomenon. This artifact makes it difficult to identify other biliary complications, such as biliary strictures, which is an important long term issue with DCD transplantation.
In order to model DCD we subjected livers to ex-vivo warm ischemia in a homeothermically controlled bath filled with warm saline as previously described (5). The benefit of this method is precise control of the ischemic time and temperature due to the fact that the explantation of the organ occurred before the period of ischemia. We have chosen 60 minutes as clinically relevant time scale of prolonged ischemia. The temperature of 34 C was chosen to simulate a degree of reduction of the core temperature after cardiac arrest. Although we have also tested the effect of warm ischemia prior to liver explantation (41) we found that this approach introduces more variability due to poor control of the temperature history of the liver and variation of the duration of the donor surgery. Additionally we found that body-core temperature in the rat dropped much faster that what could be expected in a larger animal model or in humans, reducing the impact of the ischemia. We have chosen to heparinize animals before explantation for the same reason of consistency.
During normothermic perfusion of the ischemic livers, the initial peak in the release of both AST and ALT suggests that hepatocellular damage occurred during the period of warm ischemia but stopped upon perfusion of the liver. Post-operative values of the AST, ALT were comparable, if not lower than those of recipients of fresh livers preserved with either NELP or SCS for a similar period. The lower bile secretion of the ischemic livers suggests that the damage sustained during warm ischemia may affect the biliary epithelium more than the hepatic parenchyma. Interestingly, the urea production of the ischemic livers was higher than that of freshly resected livers, which may reflect an increased nitrogen availability caused by proteolysis secondary to cellular damage. The fact that the oxygen uptake rate was similar to that of fresh livers indicates that the machinery responsible for oxidative metabolism was largely intact and mitochondrial function maintained in warm ischemic livers.
One of the possible hypotheses to explain the beneficial effect of NELP in reconditioning DCD organs is reduced apoptosis, which could be through reduced Kupffer cell activity (due to presence of hydrocortisone in the perfusate) which is known to be correlated to improved graft survival (42) or ROS reduction (through glutathione which is present in Williams E) which was also found to correlate with graft viability (43). However, the TUNEL results of reperfused livers displayed that WI-only and WI+NELP groups had both very limited apoptosis, and yet the survival in recipients of WI-only livers were nil. This result indicates that suppression of Kupffer cells is an unlikely cause of survival in our system. However, it is possible that other inflammatory mechanisms not present in our model are involved. On the other hand, the correlation between bile secretion and survival suggests that restoration of metabolic activity, perhaps ATP levels and/or other metabolites, may play a role in the protective effects of NELP.
Conclusions
The goal of this study was to evaluate the possibility of resuscitating livers after warm ischemia with normothermic perfusion in a modified isolated perfused rat liver system. We have shown that livers subjected to one hour of ex vivo warm ischemia can be reclaimed using warm perfusion technology. Post transplant survival of rats that received these perfused livers was far superior to that of animals that received ischemic livers that did not undergo any preservation, and those preserved with traditional SCS. Our system provides an effective to investigate the various aspects of warm perfusion for preservation and resuscitation of the DCD liver grafts, as well as a model to study liver metabolism (44). We envision that a similar, scaled up version of our system could be used in a clinical setting enabling the use of DCD livers for transplantation.
In addition, this study establishes that the dilute whole-blood reperfusion system can be used as a model simulating rat liver transplantation, and transaminase levels and bile synthesis are all adequate markers of viability.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (R01 DK43371, R01 DK59766, K99 DK080942), the Shriners Hospitals for Children (Grants 8450, 8460 and 8490 and the Harvard University William F. Milton Fund.
Abbreviations
- ALB
Albumin
- ALT
Alanine Aminotransferase
- ALP
Alkaline Phosphotase
- ANOVA
Analysis of Variance
- AST
Aspartate Aminotransferase
- CBD
Common Bile Duct
- DCD
Donors after Cardiac Death
- GLU
Glucose
- HA
Hepatic Artery
- IVC
Inferior Vena Cava
- NELP
Normothermic Extracorporeal Liver Perfusion
- PV
Portal Vein
- ROS
Reactive Oxygen Species
- SCS
Static Cold Storage
- SEM
Standard Error of the Mean
- SHVC
Suprahepatic Vena Cava
- TBIL
Total Bilirubin
- TP
Total Protein
- WI
Warm Ischemia
References
- 1.Reddy S, Zilvetti M, Brockmann J, McLaren A, Friend P. Liver transplantation from non-heart-beating donors - current status and future prospects. Liver Transplantation. 2004;10(10):1223. doi: 10.1002/lt.20268. [DOI] [PubMed] [Google Scholar]
- 2.Abt PL, Desai NM, Crawford MD, et al. Survival following liver transplantation from non-heart-beating donors. Annals of Surgery. 2004;239(1):87. doi: 10.1097/01.sla.0000103063.82181.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abt PL, Fisher CA, Singhal AK. Donation after cardiac death in the US: History and use. Journal of the American College of Surgeons. 2006;203(2):208. doi: 10.1016/j.jamcollsurg.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Lee CY, Zhang JX, Jones JW, Jr, Southard JH, Clemens MG. Functional recovery of preserved livers following warm ischemia: improvement by machine perfusion preservation. Transplantation. 2002;74(7):944. doi: 10.1097/00007890-200210150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Lee CY, Jain S, Duncan HM, et al. Survival transplantation of preserved non-heart-beating donor rat livers: preservation by hypothermic machine perfusion. Transplantation. 2003;76(10):1432. doi: 10.1097/01.TP.0000088674.23805.0F. [DOI] [PubMed] [Google Scholar]
- 6.Bessems M, Doorschodt BM, van Marle J, Vreeling H, Meijer AJ, van Gulik TM. Improved machine perfusion preservation of the non-heart-beating donor rat liver using Polysol: a new machine perfusion preservation solution. Liver Transpl. 2005;11(11):1379. doi: 10.1002/lt.20502. [DOI] [PubMed] [Google Scholar]
- 7.Bessems M, Doorschodt BM, van Vliet AK, van Gulik TM. Machine perfusion preservation of the non-heart-beating donor rat livers using polysol, a new preservation solution. Transplant Proc. 2005;37(1):326. doi: 10.1016/j.transproceed.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Bessems M, Doorschodt BM, van Vliet AK, van Gulik TM. Improved rat liver preservation by hypothermic continuous machine perfusion using Polysol, a new, enriched preservation solution. Liver Transplantation. 2005;11(5):539. doi: 10.1002/lt.20388. [DOI] [PubMed] [Google Scholar]
- 9.Dutkowski P, Furrer K, Tian Y, Graf R, Clavien PA. Novel Short-term Hypothermic Oxygenated Perfusion (HOPE) System Prevents Injury in Rat Liver Graft From Non-Heart Beating Donor. Ann Surg. 2006;244(6):968. doi: 10.1097/01.sla.0000247056.85590.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutkowski P, Graf R, Clavien PA. Rescue of the cold preserved rat liver by hypothermic oxygenated machine perfusion. Am J Transplant. 2006;6(5 Pt 1):903. doi: 10.1111/j.1600-6143.2006.01264.x. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Lee CY, Clemens MG, Zhang JX. Pronlonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation. 2004;77(11):1676. doi: 10.1097/01.tp.0000129644.23075.71. [DOI] [PubMed] [Google Scholar]
- 12.Schon MR, Hunt CJ, Pegg DE, Wight DG. The possibility of resuscitating livers after warm ischemic injury. Transplantation. 1993;56(1):24. doi: 10.1097/00007890-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Schon MR, Kollmar O, Wolf S, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surgery. 2001;233(1):114. doi: 10.1097/00000658-200101000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imber CJ, St Peter SD, Lopez de Cenarruzabeitia I, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002;73(5):701. doi: 10.1097/00007890-200203150-00008. [DOI] [PubMed] [Google Scholar]
- 15.Stubenitsky BM, Booster MH, Brasile L, Araneda D, Haisch CE, Kootstra G. Amelioration of ischemic damage by ex vivo warm perfusion. Transplantation. 2000;69(8):S205. doi: 10.1097/00007890-200103270-00005. [DOI] [PubMed] [Google Scholar]
- 16.Brasile L, Stubenitsky BM, Booster MH, et al. Overcoming severe renal ischemia: The role of ex vivo warm perfusion. Transplantation. 2002;73(6):897. doi: 10.1097/00007890-200203270-00011. [DOI] [PubMed] [Google Scholar]
- 17.Brasile L, Stubenitsky B, Haisch CE, Kon M, Kootstra G. Potential of repairing ischemically damaged kidneys ex vivo. Transplantation Proceedings. 2005;37(1):375. doi: 10.1016/j.transproceed.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 18.St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89(5):609. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 19.Tolboom H, Pouw R, Uygun K, et al. A Model for Normothermic Preservation of the Rat Liver. Tissue Eng. 2007;13(8):2143. doi: 10.1089/ten.2007.0101. [DOI] [PubMed] [Google Scholar]
- 20.Delriviere L, Gibbs P, Kobayashi E, Goto S, Kamada N, Gianello P. Detailed modified technique for safer harvesting and preparation of liver graft in the rat. Microsurgery. 1996;17(12):690. doi: 10.1002/(SICI)1098-2752(1996)17:12<690::AID-MICR6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Delriviere L, Gibbs P, Kobayashi E, Goto S, Kamada N, Gianello P. Technical details for safer venous and biliary anastomoses for liver transplantation in the rat. Microsurgery. 1998;18(1):12. doi: 10.1002/(sici)1098-2752(1998)18:1<12::aid-micr4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28(1):47. [PubMed] [Google Scholar]
- 23.Lee CY, Jain S, Duncan HM, et al. Survival transplantation of preserved non-heartbeating donor rat livers: Preservation by hypothermic machine perfusion. Transplantation. 2003;76(10):1432. doi: 10.1097/01.TP.0000088674.23805.0F. [DOI] [PubMed] [Google Scholar]
- 24.Imamura H, Rocheleau B, Cote J, Huet PM. Long-term consequence of rat orthotopic liver transplantation with and without hepatic arterial reconstruction: A clinical, pathological, and hemodynamic study. Hepatology. 1997;26(1):198. doi: 10.1002/hep.510260126. [DOI] [PubMed] [Google Scholar]
- 25.Izamis M, Uygun K, Berthiaume F, Yarmush M. In vivo metabolic fluxes in rat livers: effect of burn injury. Metab Eng. 2008 submitted. [Google Scholar]
- 26.Suzuki S, Inaba K, Konno H. Ischemic preconditioning in hepatic ischemia and reperfusion. Current Opinion in Organ Transplantation. 2008;13(2):142. doi: 10.1097/MOT.0b013e3282f6a164. [DOI] [PubMed] [Google Scholar]
- 27.Sumimoto K, Inagaki K, Yamada K, Kawasaki T, Dohi K. Reliable Indices For The Determination Of Viability Of Grafted Liver Immediately After Orthotopic Transplantation Bile Flow Rate And Cellular Adenosine Triphosphate Level. Transplantation. 1988;46(4):506. doi: 10.1097/00007890-198810000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Rojas A, Chen L, Bartlett RH, Arenas JD. Assesment of liver function during extracorporeal membrane oxygenation in the non-heart beating donor swine. Transplantation Proceedings. 2004;36(5):1268. doi: 10.1016/j.transproceed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Adham M, Peyrol S, Chevallier M, et al. The isolated perfused porcine liver: Assessment of viability during and after six hours of perfusion. Transplant International. 1997;10(4):299. doi: 10.1007/s001470050061. [DOI] [PubMed] [Google Scholar]
- 30.Furuyashiki S, Sumimoto K, Oku JI, et al. The significance of bile secretion after the transplantation of long-preserved livers in the rat. Surgery Today. 1994;24(1):59. doi: 10.1007/BF01676887. [DOI] [PubMed] [Google Scholar]
- 31.Kamiike W, Nakahara M, Nakao K, et al. Correlation Between Cellular Atp Level And Bile Excretion In The Rat-Liver. Transplantation. 1985;39(1):50. doi: 10.1097/00007890-198501000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Imber C, St Peter S, de Cenarruzabeitia I, et al. Optimisation of bile production during normothermic preservation of porcine livers. Am J Transplant. 2002;2(7):593. doi: 10.1034/j.1600-6143.2002.20703.x. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Lee CY, Clemens MG, Zhang JX. Prolonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation. 2004;77(11):1676. doi: 10.1097/01.tp.0000129644.23075.71. [DOI] [PubMed] [Google Scholar]
- 34.Jain S, Xu H, Duncan H, et al. Ex-vivo study of flow dynamics and endothelial cell structure during extended hypothermic machine perfusion preservation of livers. Cryobiology. 2004;48(3):322. doi: 10.1016/j.cryobiol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 35.t Hart NA, van der Plaats A, Leuvenink HG, et al. Hypothermic machine perfusion of the liver and the critical balance between perfusion pressures and endothelial injury. Transplant Proc. 2005;37(1):332. doi: 10.1016/j.transproceed.2004.12.090. [DOI] [PubMed] [Google Scholar]
- 36.Brasile L, Buelow R, Stubenitsky BM, Kootstra G. Induction of heme oxygenase-1 in kidneys during ex vivo warm perfusion. Transplantation. 2003;76(8):1145. doi: 10.1097/01.TP.0000081044.37318.E3. [DOI] [PubMed] [Google Scholar]
- 37.Imber CJ, St Peter SD, Handa A, Friend PJ. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8(5):415. doi: 10.1053/jlts.2002.32275. [DOI] [PubMed] [Google Scholar]
- 38.Gores GJ, Kost LJ, Larusso NF. The Isolated Perfused-Rat-Liver - Conceptual and Practical Considerations. Hepatology. 1986;6(3):511. doi: 10.1002/hep.1840060331. [DOI] [PubMed] [Google Scholar]
- 39.Daemen M, Thijssen HHW, Vanessen H, et al. Liver Blood-Flow Measurement in the Rat - the Electromagnetic Versus the Microsphere and the Clearance Methods. Journal of Pharmacological Methods. 1989;21(4):287. doi: 10.1016/0160-5402(89)90066-1. [DOI] [PubMed] [Google Scholar]
- 40.Bessems M, t Hart NA, Tolba R, et al. The isolated perfused rat liver: standardization of a time-honoured model. Lab Anim. 2006;40(3):236. doi: 10.1258/002367706777611460. [DOI] [PubMed] [Google Scholar]
- 41.Dutkowski P, Furrer K, Tian YH, Graf R, Clavien PA. Novel short-term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non-heart beating donor. Annals of Surgery. 2006;244(6):968. doi: 10.1097/01.sla.0000247056.85590.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monbaliu D, van Pelt J, De Vos R, et al. Primary graft nonfunction and Kupffer cell activation after liver transplantation from non-heart-beating donors in pigs. Liver Transplantation. 2007;13(2):239. doi: 10.1002/lt.21046. [DOI] [PubMed] [Google Scholar]
- 43.Schauer RJ, Bilzer M, Kalmuk S, et al. Microcirculatory failure after rat liver transplantation is related to Kupffer cell-derived oxidant stress but not involved in early graft dysfunction. Transplantation. 2001;72(10):1692. doi: 10.1097/00007890-200111270-00022. [DOI] [PubMed] [Google Scholar]
- 44.Yarmush ML, Banta S. Metabolic engineering: Advances in modeling and intervention in health and disease. Annual Review of Biomedical Engineering. 2003;5(1):349. doi: 10.1146/annurev.bioeng.5.031003.163247. [DOI] [PubMed] [Google Scholar]