Abstract
Secretin has been shown to delay gastric emptying and inhibit gastric motility. We have demonstrated that secretin acts on the afferent vagal pathway to induce gastric relaxation in the rat. However, the efferent pathway that mediates the action of secretin on gastric motility remains unknown. We recorded the response of intragastric pressure to graded doses of secretin administered intravenously to anesthetized rats using a balloon attached to a catheter and placed in the body of the stomach. Secretin evoked a dose-dependent decrease in intragastric pressure. The threshold dose of secretin was 1.4 pmol/kg/h and the ED50 was 5.6 pmol/kg/h. Pretreatment with hexamethonium markedly reduced gastric relaxation induced by secretin (5.6 pmol/kg/h). Bilateral vagotomy also significantly reduced gastric motor responses to secretin. Administration of NG-nitro-L-arginine methyl ester (10 mg/kg) did not affect gastric relaxation induced by secretin. In contrast, intravenous administration of a VIP antagonist (30 nmol/kg) reduced the gastric relaxation response to secretin (5.6 pmol/kg/h) by 89±5%. Indomethacin (2 mg/kg) reduced gastric relaxation induced by secretin (5.6 pmol/kg/h) by 87 ± 5%. Administration of PGE2 (48 mg/kg/h) prevented this inhibitory effect. Indomethacin also reduced gastric relaxation induced by VIP (300 pmol/kg) by 90 ± 7%. These observations indicate that secretin acts through stimulation of presynaptic cholinergic neurons in a vagally mediated pathway. Through nicotinic synapses, secretin stimulates VIP release from postganglionic neurons in the gastric myenteric plexus, which in turn induces gastric relaxation through a prostaglandin-dependent pathway.
Keywords: vagus nerve, gastric relaxation, indomethacin
Secretin has been shown to inhibit gastric contractions and delay gastric emptying of liquids and solids.1-3 However, the mechanisms of secretin’s inhibitory effects on gastric motility remain unclear. The gene expression of secretin receptor has been demonstrated in the rat nodose ganglia.4 We have shown that secretin stimulates a capsaicin-sensitive vagal afferent pathway originating in the gastroduodenal mucosa to induce gastric relaxation.5 We have also shown that the vago-vagal reflex has an important role in mediating secretin-induced gastric relaxation,5 although the final neurotransmitter responsible for secretin-induced gastric relaxation remains unknown.
Pharmacological evidence suggests that there are at least two nonadrenergic, noncholinergic (NANC) inhibitory systems in the gastrointestinal tract. VIP was proposed to be a neurotransmitter for NANC neurons because the intrinsic neurons of the stomach exhibit VIP immunoreactivity.6,7 Moreover, nerve depolarization by KCl or electrical stimulation evokes VIP release.8,9 Vagal stimulation produces a frequency-dependent increase in the amount of VIP released into the portal vein.10,11 These observations support the concept that VIP is an inhibitory NANC transmitter.
Nitric oxide (NO) was also proposed to be an inhibitory NANC neurotransmitter in the gastrointestinal tract. Gastric relaxation induced by stimulation of NANC nerves was shown to be significantly antagonized by the NO biosynthesis inhibitor, NG-nitro-L-arginine (L-NNA) or NG-nitro-L-arginine methyl ester (L-NAME) in the rat stomach.12-14 Exogenous application of NO or sodium nitroprusside, an activator of soluble guanylate cyclase, produces relaxation that mimics NANC-induced relaxation in different regions of the gastrointestinal tract.12,15,16 The presence of NO synthase has been detected in the myenteric plexus.17-19 Further, the NO pathway has been shown to mediate adaptive relaxation in the isolated guinea pig stomach.20
We have shown that vagal stimulation produces two modes of relaxation in the rat stomach: rapid relaxation followed by prolonged relaxation.21 Rapid relaxation is antagonized by a NO inhibitor, and prolonged relaxation is blocked by a VIP antagonist,21 which suggests that different neurotransmitters mediate different modes of relaxation. It is not known which NANC neurotransmitter in the gastric myenteric plexus mediates secretin-induced gastric relaxation. Prostaglandins (PGs) have been shown to mediate the inhibition of gastric acid secretion evoked by secretin.22 Furthermore, indomethacin appears to markedly reduce VIP-induced relaxation of the small arteries of rainbow trout.23 It is therefore conceivable that secretin acts via a VIP/PG pathway to mediate gastric relaxation. The aim of our study is to characterize the vagal efferent pathway mediating secretin-induced gastric relaxation and to investigate the involvement of NO, VIP, and PG in this process.
Materials and Methods
All procedures were performed in accordance with NIH guidelines and with the approval of The University Committee on Use and Care of Animals at the University of Michigan, Ann Arbor, MI, USA.
Materials
Carbachol, guanethidine, hexamethonium, PGE2 and indomethacin were purchased from Sigma Chemical Co. (St. Louis, MO). Secretin, VIP, and the VIP antagonist [p-chloro-D-Phe14, Leu22]-VIP were purchased from Bachem (Torrance, CA). With the exception of indomethacin and PGE2, all chemicals were dissolved in physiological saline. Indomethacin and PGE2 were dissolved in ethanol in a concentration of 20 mg/ml and 10 mg/ml respectively.
Animal preparation
Male Sprague-Dawley rats weighing between 250 and 300 g were fasted for 24 h with water available ad libitum. All experimental procedures were approved by the University of Michigan University Committee on Use and Care of Animals. The animals were anesthetized with urethan (1.5 g per kg body weight, intraperitoneal). A tracheotomy was performed and a tracheal tube was inserted to facilitate spontaneous inhalation of room air. As previously described,5 a catheter with a rubber balloon attached to it was inserted through a midline incision into the body of the stomach, through an incision in the duodenum, and secured at the pylorus. The right jugular vein of each rat was cannulated with polyethylene tubing.
Measurement of intragastric pressure
Intragastric pressure was measured using the rubber balloon and recorded by a Gould Statham P231D pressure transducer and pen recorder. The balloon was filled with water (1.6–2.0 mL) at 37°C. This volume was determined to be the level necessary to induce an intragastric pressure of 10 cm H2O. The exact location of the balloon was verified after each experiment.
Secretin dose-response studies
Secretin at the following doses, 1.4, 2.8, 5.6, 11.2, 22.4, and 44.8 pmol/kg/h, was administered into the jugular vein, with and without various antagonists. In preliminary experiments, the gastric motor responses to secretin were reproducible up to five times when secretin was applied every 30 min. Therefore, the secretin infusion was performed every 30 min and evaluated in triplicate. Mean values were used to determine the changes in intragastric pressure. After infusion of the various antagonists, secretin infusion studies were repeated and the responses were compared with the control experiments. Only one antagonist was administered to each rat. To investigate if secretin-induced relaxation is mediated through a vagal pathway, subdiaphragmatic bilateral truncal vagotomy was performed 30 min before the secretin infusion studies. At the end of the experiments, the rats were killed by an overdose of pentobarbitone (200 mg/kg, intravenous).
PG and VIP infusion studies
In a similar fashion, we performed PGE2 and VIP infusion studies and evaluated the effects of indomethacin on gastric relaxation induced by PGE2 and VIP. In these studies, PGE2 (48 μg/kg/h) or VIP (300 pmol/kg for 10 s) was infused intravenously and the gastric motor responses were recorded. These studies were repeated with indomethacin (2 mg/kg), which was infused 30 min before PGE2 or VIP administration. The administered doses of PGE2 and VIP reflect the results of preliminary studies, which showed that these doses induced a similar degree of gastric relaxation as that induced by secretin (5.6 pmol/kg/h).
Antagonist studies
To determine the role of nicotinic receptors in the mediation of gastric relaxation induced by secretin, hexamethonium (20 mg/kg bolus) was injected and continuously infused at a rate of 10 mg/kg/h for 20 min before the administration of secretin, as previously described.24 In a similar fashion, guanethidene (5 mg/kg) was administered to examine the role of the sympathetic nervous system in mediating the action of secretin. To determine the role of NO and VIP, L-NAME (10 mg/kg) and a VIP antagonist (30 nmol/kg) were infused 10 min before the infusion of secretin. These specific doses of L-NAME and the VIP antagonist have been shown to abolish rapid and prolonged relaxation, respectively, in response to vagal stimulation in vivo.21,24 To investigate the role of PGs in mediating secretin-induced gastric relaxation, indomethacin (2 mg/kg) was infused 60 min before the administration of secretin.
Analysis of data
Results were expressed as means ± SE. Statistical analysis was performed using the paired t test. P < 0.05 was considered statistically significant.
Results
The response of intragastric pressure to graded doses of secretin was recorded in anesthetized rats using a balloon attached to a catheter placed in the body of the stomach. Intravenous infusion of secretin at 1.4 to 22.4 pmol/kg/h decreased intragastric pressure in a dose-dependent manner (Fig.1). The threshold dose was 1.4 pmol/kg/h; maximal relaxation was observed at 22.4 pmol/kg/h; and the ED50 was 5.6 pmol/kg/h.
Fig. 1.

Effect of secretin on intragastric pressure as measured in cm H20. Intragastric pressure was set at 10 cm H20 with balloon distension. Infusion of secretin produced a dose-dependent decrease in intragastric pressure. Values are means ± SE, n = 12, *P < 0.05.
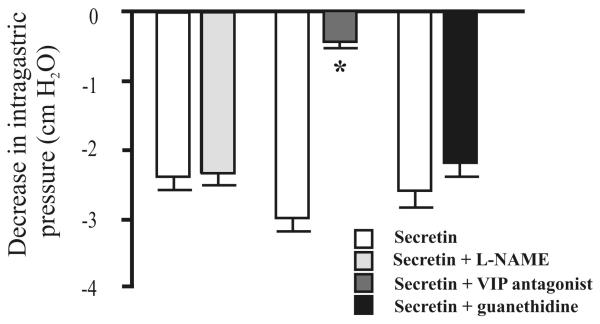
Subsequent studies were performed using secretin at the ED50 value of 5.6 pmol/kg/h. Pretreatment with hexamethonium markedly reduced gastric relaxation in response to secretin (5.6 pmol/kg/h). Bilateral vagotomy reduced basal tone about 20%. After a rapid return to baseline (i.e., within 5 min), the effect of secretin on gastric motility was tested. Bilateral vagotomy also significantly reduced the gastric motor response to secretin (5.6 pmol/kg/h) (Fig. 2). In contrast, secretin (5.6 pmol/kg/h) had no effect on gastric contraction evoked by carbachol (30 nmol/kg/h) (data not shown). Guanethidine (5 mg/kg) did not affect gastric relaxation evoked by secretin (Fig. 3). These observations indicate that secretin acts through stimulation of presynaptic cholinergic neurons in a vagally mediated pathway.
Fig. 2.
Effects of hexamethonium and vagotomy on gastric relaxation induced by secretin (5.6 pmol/kg/h). Pretreatment with hexamethonium markedly reduced gastric relaxation induced by secretin. Bilateral vagotomy also significantly reduced gastric motor responses induced by secretin. Values are means ± SE, n = 8, *P < 0.05 versus control.
Fig. 3.
Effects of L-NAME (10 mg/kg), a VIP antagonist (30 nmol/kg), and guanethidine (5 mg/kg) on gastric relaxation induced by secretin (5.6 pmol/kg/h). Administration of L-NAME and guanethidine had no effect on gastric relaxation induced by secretin. In contrast, intraarterial administration of the VIP antagonist markedly reduced the secretin-induced gastric relaxation. Values are means ± SE, n = 12, *P < 0.05 versus control.
To investigate the role of NO and VIP in mediating the action of secretin, we examined the effects of L-NAME and a potent VIP antagonist. NO and VIP have been shown to mediate vagal-evoked gastric relaxation.21 Administration of L-NAME (10 mg/kg), which abolished the rapid transient relaxation evoked by electrical vagal stimulation,21 had no effect on gastric relaxation induced by secretin (Fig. 3). In contrast, intravenous administration of the VIP antagonist (30 nmol/kg) significantly reduced the gastric relaxation in response to secretin (5.6 pmol/kg/h) (Fig. 3). The VIP antagonist, at the same dose of 30 nmol/kg, completely prevented gastric relaxation induced by VIP (300 pmol/kg).
Neither vagotomy nor pretreatment with hexamethonium affected gastric relaxation induced by VIP (300 pmol/kg), which suggests that VIP acts directly on smooth muscle cells.
To assess the role of PGs in gastric relaxation induced by secretin, motility studies were repeated 60 min after administration of the cyclooxygenase inhibitor, indomethacin (2 mg/kg). Indomethacin reduced secretin (5.6 pmol/kg/h) -induced gastric relaxation by 87 ± 5% (Fig. 4). This inhibitory effect was abolished by administration of PGE2 (48 mg/kg/h) (Fig. 4). Similarly, indomethacin blocked the gastric relaxation induced by VIP (300 pmol/kg) (Fig. 5). However, indomethacin did not affect gastric relaxation induced by PGE2 (48 mg/kg/h) (Fig. 5). To demonstrate the specificity of the action of indomethacin, we showed that indomethacin did not affect gastric relaxation induced by sodium nitroprusside (10-6 mol/L) (data not shown).
Fig. 4.
Effects of indomethacin (2 mg/kg) and PGE2 (48 mg/kg/h) on gastric relaxation induced by secretin (5.6 pmol/kg/h). Indomethacin reduced secretin-induced gastric relaxation by 87 ± 5%. The inhibitory effect of indomethacin on secretin-induced gastric relaxation was prevented by the administration of PGE2. Values are means ± SE, n = 12, *P < 0.05 versus control.
Fig. 5.
Effects of indomethacin (2 mg/kg) on gastric relaxation induced by VIP (300 pmol/kg) and PGE2 (48 mg/kg/h). Indomethacin significantly reduced the gastric relaxation induced by VIP (values are means ± SE, n = 5, *P < 0.05 versus control), but had no effect on gastric relaxation induced by PGE2 (values are means ± SE, n = 7).
Discussion
A number of studies have shown that secretin inhibits gastric acid secretion stimulated by pentagastrin or in response to a meal.22,25 It has also been shown that secretin has an inhibitory effect on gastric emptying and gastric motility.3,5 The inhibitory actions of secretin, both on gastric acid secretion and on gastric motility, appear to be mediated by a vago-vagal reflex.5,26 Li and colleagues26 demonstrated that truncal vagotomy or perivagal capsaicin treatment abolished the inhibitory action of secretin on gastric acid secretion stimulated by pentagastrin. Because periceliac ganglionic application of capsaicin did not affect the inhibitory action of secretin on gastric acid secretion, they concluded that the inhibition by secretin of pentagastrin-stimulated acid secretion is mediated through a capsaicin-sensitive vagal afferent pathway and not through a splanchnic afferent pathway.26 We have shown that perivagal capsaicin treatment abolishes secretin-induced gastric relaxation and that gastroduodenal mucosal application of capsaicin also markedly reduces the gastric response to secretin.5 Direct evidence that secretin activates vagal primary afferent neurons was obtained from in vivo electrophysiological concentrations of secretin stimulated a subpopulation of nodose ganglia neurons which are responsive to luminal serotonin.27
Vagal afferent fibers, which may terminate in the mucosa or muscle layer of the gastrointestinal tract,28 transmit sensory information to the central nervous system and play an important role in the vago-vagal reflex. Tension receptors usually are located in the serosa/muscle layers and mechanoreceptors are found in the mucosa.28 Perivagal capsaicin treatment markedly reduces neuron labeling in the nodose ganglia if the dye is applied to the mucosa, but it has little effect on labeling if the dye is injected into the serosa/muscle layer,29 which suggests that most vagal afferent fibers transmitting sensory information from the mucosa are capsaicin-sensitive.
Under physiological conditions, the increase in plasma secretin in response to acid or lipid in the rat duodenum is small (i.e., <10 M).22 Studies in rats have shown that the infusion of secretin at 5.6 pmol/kg/h produces plasma levels of secretin of 6–7 pM, similar to that produced by oleic acid.22 We therefore considered doses of 5.6 pmol/kg/h or less to be physiological. We have shown that physiological doses of secretin act mainly via stimulation of the vagal afferent pathway, whereas supraphysiological doses act via an extravagal pathway.5 In this study, we showed that the ED50 dose of secretin to induce gastric relaxation is 5.6 pmol/kg/h. This dose was used for all subsequent studies to elucidate the mechanisms responsible for secretin-induced gastric relaxation. We showed that hexamethonium and vagotomy significantly reduced secretin-induced gastric relaxation, suggesting that secretin-induced gastric relaxation is mediated through vagal fibers and nicotinic synapses. It has previously been shown that the preganglionic fibers in the vagal trunk are connected through nicotinic synapses to at least three types of postganglionic neurons that contain ACh, NO, and VIP, which serve as neurotransmitters to mediate gastric contraction and relaxation in the rat stomach.21 In the human stomach, approximately 40% and 20% of the myenteric plexus contain NOS and VIP immunoreactivities respectively.30 Similar findings have been reported in the guinea pig.31 In the guinea pig stomach most of the NOS-immunoreactive neurons also contain VIP. However much less co-localization between NOS and VIP occurs in the human stomach.30 This emphasizes the importance of species specificity in the neurochemical coding of the myenteric neurons. It appears that gastric distension evokes a vago-vagal reflex and preferentially stimulates NO neurons, but not VIP neurons, in the gastric myenteric plexus.24 Grundy and colleagues32 have also shown that the increase in gastric pressure in response to ramp distension (20 mL) is not affected by VIP immunoneutralization in the ferret stomach. Therefore, the NANC inhibitory mechanism that regulates corpus tone to accommodate gastric distension may involve NO neurons, but not VIP neurons, of the gastric myenteric plexus.
In our present study, neither L-NAME nor guanethidine affected gastric relaxation induced by secretin. In contrast, administration of the VIP antagonist markedly reduced the gastric relaxation induced by secretin. This suggests that secretin-induced gastric relaxation is mediated by a VIP pathway. NO and sympathetic pathways appear to play little or no role in secretin’s action. On the other hand, hyperglycemia stimulates vagal afferents, which in turn, activates vagal efferent cholinergic pathways synapsing with intragastric NO- and VIP-containing neurons to mediate gastric relaxation. Hence different agents which act via the vagal afferent pathways may utilize different NANC inhibitory neurotransmitters to mediate gastric motility.33 In both the stomachs of human and rodents34 many of the VIP containing neurons also express NOS. It is therefore also conceivable that different stimuli may cause differential release of inhibitory neurotransmitters.
Prostaglandins have been shown to mediate the inhibition of gastric acid secretion evoked by secretin. Indomethacin completely abolished the inhibitory action of secretin on pentagastrin-stimulated acid secretion in rats.22 To assess the role of PGs in gastric relaxation induced by secretin, motility studies were repeated 60 min after administration of indomethacin. Indomethacin reduced secretin-induced gastric relaxation by 87 ± 5%. The inhibitory effect of indomethacin on secretin-induced gastric relaxation was prevented by the administration of PGE2.
It is generally accepted that VIP relaxes gastric muscle cells with a concomitant increase in intracellular cyclic AMP.35 In addition, it seems likely that VIP uses an intracellular PG pathway. It has been shown that indomethacin markedly reduces VIP-induced relaxation of small arteries of the rainbow trout and shifts the VIP concentration-response curve significantly to the right.23 Our current investigation in rats showed that indomethacin markedly reduces gastric relaxation induced by secretin. Furthermore, VIP-induced gastric relaxation was also blocked by indomethacin. This suggests that VIP-induced relaxation is mediated largely by PG release. This may be explained by the observation that VIP stimulated the formation of PG via the phospholipase A2/arachidonic acid cascade in gastric smooth muscle.36 Alternatively, the interstitial cells of Cajal in the stomach wall may be the source of the cyclooxygenase produced in response to VIP stimulation. Further studies are needed to clarify this issue.
In conclusion, our studies show that the vago-vagal reflex plays a prominent role in mediating secretin-induced gastric relaxation in rats. It involves a capsaicin-sensitive vagal afferent pathway that transmits sensory information from tension receptors located in the mucosal layers. The vagal efferent pathway that uses VIP as a final neurotransmitter of the gastric myenteric plexus mediates secretin-induced gastric relaxation. The relaxant effect of VIP is largely mediated through a PG-dependent pathway (Fig. 6).
Fig. 6.
Proposed mechanisms of action of secretin to inhibit gastric contraction. Secretin acts through stimulation of presynaptic cholinergic neurons in a vagally stimulated pathway. Through nicotinic synapses, secretin stimulates VIP release from postganglionic neurons in the gastric myenteric plexus, which induces gastric relaxation through a PG-dependent pathway.
Acknowledgments
This investigation was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grants RO1 DK-32838, DK-48419 and P30 DK-34933.
References
- 1.Herzig KH. Cholecystokinin- and secretin-releasing peptides in the intestine—a new regulatory interendocrine mechanism in the gastrointestinal tract. Regul Pept. 1988;73:89–94. doi: 10.1016/s0167-0115(97)01062-8. [DOI] [PubMed] [Google Scholar]
- 2.Jin HO, Lee KY, Chang TM, et al. Secretin: a physiological regulator of gastric emptying and acid output in dogs. Am J Physiol. 1994;267:G702–G708. doi: 10.1152/ajpgi.1994.267.4.G702. [DOI] [PubMed] [Google Scholar]
- 3.Murthy SN, Ganiban G. Effect of the secretin family of peptides on gastric emptying and small intestinal transit in rats. Peptides. 1988;9:583–588. doi: 10.1016/0196-9781(88)90168-4. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Wang L, Wu SV, et al. Peripheral secretin-induced Fos expression in the rat brain is largely vagal dependent. Neuroscience. 2004;128:131–141. doi: 10.1016/j.neuroscience.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Owyang C. Secretin at physiological doses inhibits gastric motility via a vagal afferent pathway. Am J Physiol. 1995;268:G1012–G1016. doi: 10.1152/ajpgi.1995.268.6.G1012. [DOI] [PubMed] [Google Scholar]
- 6.Fahrenkrug J, Haglund U, Jordal M, et al. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiology implications. J Physiol. 1978;284:291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson LI, Fahrenkrug J, Schaffalitzky De Muckadell O, et al. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci USA. 1976;73:3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agoston DV, Conlon JM, Whittaker VP. Selective depletion of the acetylcholine and vasoactive intestinal polypeptide of the guinea pig myenteric plexus by differential mobilization of distinct transmitter pools. Exp Brain Res. 1988;72:535–542. doi: 10.1007/BF00250599. [DOI] [PubMed] [Google Scholar]
- 9.Grider JR, Makhlouf GM. Prejunctional inhibition of vasoactive intestinal peptide release. Am J Physiol. 1987;253:G7–G12. doi: 10.1152/ajpgi.1987.253.1.G7. [DOI] [PubMed] [Google Scholar]
- 10.Reid AM, Shulkes A, Titchen DA. Effects of the vagus nerves on gastric motility and release of vasoactive intestinal polypeptide in the anesthetized lamb. J Physiol. 1988;396:11–24. doi: 10.1113/jphysiol.1988.sp016946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasui A, Naruse S, Yanaihara C, et al. Corelease of PHI and VIP by vagal stimulation in the dog. Am J Physiol. 1987;253:G13–G19. doi: 10.1152/ajpgi.1987.253.1.G13. [DOI] [PubMed] [Google Scholar]
- 12.Boeckxstaens GE, Pelckmans PA, Bogers JJ, et al. Release of nitric oxide upon stimulation of non-adrenergic non-cholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther. 1991;256:441–447. [PubMed] [Google Scholar]
- 13.D’Amato M, Curro D, Montuschi P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. J Auton Nerv Syst. 1992;37:175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- 14.Shimamura K, Fujisawa A, Toda N, et al. Effects of NG-nitro-l-arginine on electrical and mechanical responses to stimulation of non-adrenergic, non-cholinergic inhibitory nerves in circular muscle of the rat gastric fundus. Eur J Pharmacol. 1993;231:103–109. doi: 10.1016/0014-2999(93)90690-j. [DOI] [PubMed] [Google Scholar]
- 15.Bult H, Boeckxstaens GE, Pelckmans PA, et al. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- 16.Osthaus LE, Galligan JJ. Antagonists of nitric oxide synthesis inhibit nerve-mediated relaxations of longitudinal muscle in guinea pig ileum. J Pharmacol Exp Ther. 1992;260:140–145. [PubMed] [Google Scholar]
- 17.Aimi Y, Kimura H, Kinoshita T, et al. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993;53:553–560. doi: 10.1016/0306-4522(93)90220-a. [DOI] [PubMed] [Google Scholar]
- 18.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 19.Costa M, Furness JB, Pompolo S, et al. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neurosci Lett. 1992;148:121–125. doi: 10.1016/0304-3940(92)90819-s. [DOI] [PubMed] [Google Scholar]
- 20.Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Owyang C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J Physiol. 1995;484:481–492. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee JC, Chang TM, Lee KY, et al. Mechanism of oleic acid-induced inhibition on gastric acid secretion in rats. Am J Physiol. 1991;260:G564–G570. doi: 10.1152/ajpgi.1991.260.4.G564. [DOI] [PubMed] [Google Scholar]
- 23.Kagstrom J, Holmgren S. VIP-induced relaxation of small arteries of the rainbow trout, Oncorhynchus mykiss, involves prostaglandin synthesis but not nitric oxide. J Auton Nerv Syst. 1997;63:68–76. doi: 10.1016/s0165-1838(96)00138-5. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chey WY, Kim MS, Lee KY, et al. Secretin is an enterogastrone in the dog. Am J Physiol. 1981;240:G239–G244. doi: 10.1152/ajpgi.1981.240.3.G239. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Chang TM, Chey WY. Secretin inhibits gastric acid secretion via a vagal afferent pathway in rats. Am J Physiol. 1998;275:G22–G28. doi: 10.1152/ajpgi.1998.275.1.G22. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Wu XY, Yao H, Owyang C. Secretin activates vagal primary afferent neurons in the rat: evidence from electrophysiological and immunohistochemical studies. Am J Physiol (Gastrointest Liver Physiol) 2005;289:G745–G752. doi: 10.1152/ajpgi.00039.2005. [DOI] [PubMed] [Google Scholar]
- 28.Grundy D. Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. J Auton Nerv Syst. 1988;22:175–180. doi: 10.1016/0165-1838(88)90104-x. [DOI] [PubMed] [Google Scholar]
- 29.Wang LM, Li Y, Owyang C. Gastroduodenal mucosal afferent fibers mediate physiological action of cholecystokinin on pancreatic secretion. Gastroenterology. 1994;106:A849. [Google Scholar]
- 30.Pimont S, Bruley Des Vamannes S, LeNeel JC, et al. Neurochemical coding of myenteric neurones in the human gastric fundus. Neurogastroenterol Motil. 2003;15:655–662. doi: 10.1046/j.1350-1925.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 31.Vanden Berghe P, Coulie B, Tack J, et al. Neurochemical coding of myenteric neurons in the guinea-pig antrum. Cell Tissue Res. 1999;297:81–90. doi: 10.1007/s004410051335. [DOI] [PubMed] [Google Scholar]
- 32.Grundy D, Gharb-Naseri MK, Hutson D. Effect of immunization against VIP and vagotomy in the ferret. Am J Physiol Gastrointest Liver Physiol. 1993;265:G432–39. doi: 10.1152/ajpgi.1993.265.3.G432. [DOI] [PubMed] [Google Scholar]
- 33.Zhou SY, Lu YX, Owyang C. Gastric relaxation induced by hyperglycemia is mediated by vagal afferent pathways in the rat. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1158–64. doi: 10.1152/ajpgi.00067.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furness JB. Types of neurones in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 35.Bitar KN, Makhlouf GM. Relaxation of gastric smooth muscle cells by vasoactive intestinal polypeptide. Science. 1982;216:531–533. doi: 10.1126/science.6176025. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Tsunoda Y, Owyang C. Intracellular mechanism responsible for VIP action on gastric relaxation: interaction between cyclic AMP and prostaglandin pathways. Gastroenterology. 1995;108:A986. [Google Scholar]