Abstract
Pathogenic causes underlying nonischemic cardiomyopathies are increasingly being resolved, yet repair therapies for these commonly heritable forms of heart failure are lacking. A case in point is human dilated cardiomyopathy 10 (CMD10; Online Mendelian Inheritance in Man #608569), a progressive organ dysfunction syndrome refractory to conventional therapies and linked to mutations in cardiac ATP-sensitive K+ (KATP) channel sub-units. Embryonic stem cell therapy demonstrates benefit in ischemic heart disease, but the reparative capacity of this allogeneic regenerative cell source has not been tested in inherited cardiomyopathy. Here, in a Kir6.2-knockout model lacking functional KATP channels, we recapitulated under the imposed stress of pressure overload the gene-environment substrate of CMD10. Salient features of the human malignant heart failure phenotype were reproduced, including compromised contractility, ventricular dilatation, and poor survival. Embryonic stem cells were delivered through the epicardial route into the left ventricular wall of cardiomyopathic stressed Kir6.2-null mutants. At 1 month of therapy, transplantation of 200,000 cells per heart achieved teratoma-free reversal of systolic dysfunction and electrical synchronization and halted maladaptive remodeling, thereby preventing end-stage organ failure. Tracked using the lacZ reporter transgene, stem cells engrafted into host heart. Beyond formation of cardiac tissue positive for Kir6.2, transplantation induced cell cycle activation and halved fibrotic zones, normalizing sarcomeric and gap junction organization within remuscularized hearts. Improved systemic function induced by stem cell therapy translated into increased stamina, absence of anasarca, and benefit to overall survivorship. Embryonic stem cells thus achieve functional repair in nonischemic genetic cardiomyopathy, expanding indications to the therapy of heritable heart failure.
Keywords: ABCC9, ATP-sensitive K+ channels, Dilated cardiomyopathy, Genetics, KCNJ11, Kir6.2
Introduction
Increasingly recognized as primary disorders of heart muscle, nonischemic cardiomyopathies are a major cause of morbidity and mortality worldwide [1]. Considerable genetic heterogeneity suggests that deficits in multiple molecular pathways can lead to progressive decline in myocardial structure and function, causing a heritable form of heart failure [2]. Genetic defects render the myocardium vulnerable to stress, resulting in maladaptation and ultimately poor outcome [3]. The clinical course of cardiomyopathic disease is associated with a mortality rate of up to 50%, halving affected cohorts within 5 years of diagnosis [1]. Although advances in molecular medicine have accelerated our understanding of the gene-environment interrelationship that underlies myocardial susceptibility to the development of a cardiomyopathic phenotype, effective and potentially curative therapies are still lacking, limiting disease management to palliative care [4].
The ontological spectrum of cardiomyopathy-associated mutant genes has traditionally included those encoding contractile, cytoskeletal, or nuclear proteins and was recently expanded to the distinct class of proteins that regulate cardiac ion homeostasis [2, 4]. This latter group includes the ATP-sensitive K+ (KATP) channel, as mutations in the ABCC9-encoded regulatory channel subunit SUR2A have been demonstrated in a subset of patients with dilated cardiomyopathy and ventricular arrhythmia [5]. The clinical entity associated with KATP channelopathy is malignant in nature, and in the spectrum of cardiomyopathic diseases it has been designated CMD10 (Online Mendelian Inheritance in Man #608569; http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=608569) [5]. A link between KATP channel defects and susceptibility to severe myopathic disease has been recapitulated in knockout models, underscoring the vital role of intact channels in cardioprotection [6, 7]. Compromised KATP channel function renders the heart vulnerable to acute or chronic stress, implicating this metabolic sensor in the homeostatic response that would normally prevent or delay progression of heart disease [8–12]. The defective response to metabolic distress signals in the KATP channel knockout sets in motion calcium overload and pathological cardiac remodeling, resulting in fulminant organ failure and death under conditions of stress load [6, 7]. Control of myocardial calcium influx has a prophylactic value in the setting of KATP channel deficiency [6], yet curative strategies capable of reversing the progression of genetic cardiomyopathy have not been established.
The emergence of stem cell technology and the opportunity for myocardial repair provide the foundation for a regenerative approach to the therapy of cardiovascular disease [13, 14]. Stem cell transplantation has progressed from preclinical promise to clinical trials, with adult autologous stem cell approaches applied to improve function and rectify damage sustained following myocardial infarction [15–17]. In nonischemic genetic cardiomyopathy, however, more limited information is available on the benefit of adult stem cell therapy [18]. Notably, the patient-derived autologous stem cell genome bears disease-causing mutations, and although phenotypic expression may be lacking at the time of transplantation, cell progeny carries the risk of manifesting myopathic traits compromising long-term therapeutic outcome.
Multiple allogeneic cell types have been isolated from cardiac and noncardiac sources and have shown to display various degrees of cardiogenicity, with embryonic stem cells derived from the inner cell mass of the blastocyst possessing the most widely recognized capacity to yield cardiomyocytes de novo [19–21]. Because of indefinite proliferation in vitro, embryonic stem cells provide a reservoir for extensive tissue regeneration [22]. Differentiation of embryonic stem cells produces progeny that recapitulate the cardiac phenotype-expressing characteristic cardiac markers and demonstrating functional excitation-contraction coupling associated with maturation of the cellular energetic matrix [23–25]. Furthermore, when transplanted into injured hearts, embryonic stem cells generate cardiomyocytes that repopulate significant regions of dysfunctional myocardium and result in improved contractile performance with reduced mortality [26–30]. This transition of pluripotent stem cells to lineage-restricted progeny occurs under the direction of host paracrine signaling that supports cardiac-specific differentiation [31, 32]. The molecular mechanisms of stem cell commitment and integration with host myocardium are increasingly well understood, with therapeutic benefit documented in acquired cardiovascular disease, such as myocardial infarction [33]. Although embryonic stem cells also show promise in preventing developmental cardiac defects in utero [34], limited information is currently available regarding their usefulness in repair of inherited cardiomyopathy in the adult.
Here, we serially monitored the effect of embryonic stem cell therapy in a genetic model of dilated cardiomyopathy, induced by stress load in the KATP channel-null mutant. Whereas the malignant, natural progression of disease led to maladaptive cardiomegaly associated with high mortality, embryonic stem cell transplantation halted myocardial decompensation and restored contractile performance, electrical synchronization, and structural integrity. Fatality-free outcome and prevention of end-stage heart failure identify embryonic stem cell-based repair as a promising strategy in the management of nonischemic heritable cardiomyopathy.
Materials and Methods
Hemodynamic Stress in KATP Channel Gene Knockout
Mice deficient in KATP channels were generated by targeted disruption of the KCNJ11 gene encoding the inwardly rectifying K+ channel Kir6.2 pore and backcrossed for five generations into a C57BL/6 background [35]. Because of the proximity of the mutated KCNJ11 gene with the gene encoding for albino hair color, knockout mice remain white upon backbreeding [10]. Male Kir6.2-null mutants, 8–12 weeks old, underwent transverse aortic constriction. Hemodynamic stress was imposed using a 27-gauge needle to standardize the extent of stenosis and induce continuous pressure overload to the left ventricle [7]. Sham surgery consisted of transverse aortic exposure without constriction. Constricted and sham-operated KATP channel knockout mice were followed up to 150 days following surgery to evaluate progression of stress-induced heart failure in this genetic model.
Cell Therapy Regimen
R1-derived wild-type embryonic stem cells, engineered to express the lacZ reporter transgene [29], were propagated in Glasgow’s Minimum Essential Medium (Lonza, Walkersville, MD, http://www.lonza.com) supplemented with pyruvate (Cellgro; Mediatech Inc., Manassas, VA), nonessential amino acids (Cellgro), mercaptoethanol (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com), 7.5% fetal calf serum (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and leukemia inhibitory factor (ESGRO; Chemicon, Temecula, CA, http://www.chemicon.com) [32]. At 2 weeks following aortic constriction, pressure-overloaded KATP channel knockout hearts were exposed by thoracotomy. Epicardial injection of 200,000 or 3,000,000 embryonic stem cells, in 15 μl of propagation medium, was performed in five separate sites on the anterior wall of the left ventricle under microscopic visualization. Prospective follow-up evaluated multiple therapeutic endpoints, including cardiac and systemic effects of stress-induced heart failure.
Cardiac Electrophysiology and Echocardiography
Continuous cardiac electrical activity was monitored in the conscious state with implantable telemetry devices (Data Sciences International, St. Paul, MN, http://www.datasci.com) [7]. Electrical vulnerability was evaluated under isoproterenol challenge (10 mg/kg i.p.) [36]. Surface electrocardiogram was recorded using four-limb-lead electrocardiography (MP150; Biopac, Goleta, CA, http://www.biopac.com) under light anesthesia (1.5% isoflurane). Left ventricular function and structure were quantified in vivo by transthoracic echocardiography (15L8 transducer, Sequoia 512; Siemens, Malvern, PA, http://www.medical.siemens.com) in lightly anesthetized mice [37]. Percentage of left ventricular fractional shortening was calculated as [(LVDd − LVDs)/LVDd] × 100, where LVDd is left ventricular end-diastolic dimension (mm), and LVDs is left ventricular end-systolic dimension (mm). Velocity of left ventricular circumferential shortening was calculated as [(LVDd − LVDs)/LVDd]/Et, where Et is ejection time determined from the actual pulsed-wave Doppler tracings. Ejection fraction (%) was calculated as [(LVVd − LVVs)/LVVd] × 100, where LVVd is left ventricular end-diastolic volume (μl), and LVVs is left ventricular end-systolic volume (μl). Left ventricular weight (mg) was derived as [(LVDd + IVST + PWT)3 − LVDd3] × 1.055, where IVST is interventricular septum thickness (mm), and PWT is posterior wall thickness (mm) [6]. Relative wall thickness was calculated as the sum of IVST and PWT divided by LVDd, and it was used to monitor the evolution of the heart geometry.
Functional Capacity and Post-Mortem Evaluation
Exercise tolerance, lung congestion, and survivorship were collectively used to assess systemic effects of stress-induced heart failure and the impact of cell therapy [7]. The treadmill exercise protocol (Columbus Instruments, Columbus, OH, http://www.colinst.com) included a stepwise increase in incline and velocity at 3-minute intervals. Workload (J), a composite parameter incorporating time, speed, and incline, was calculated as the sum of kinetic (Ek = mv2/2) and potential (Ep = mgvtsinθ) energy, where m represents animal mass, v is treadmill velocity, g is acceleration due to gravity, t is elapsed time at a protocol level, and θ is angle of incline [8]. Post-mortem examination included weight examination of lung and heart and histological survey of hematoxylin-eosin-stained heart, lung, liver, and spleen [26, 32]. Transplanted embryonic stem cells were tracked by β-galactosidase expression (1:5,000; Abcam, Cambridge, MA, http://www.abcam.com) in conjunction with cardiac-specific sarcomeric α-actinin (1:200; Sigma-Aldrich) [29]. Stem cell engraftment was confirmed by Kir6.2 expression within an otherwise KATP channel-deficient host myocardium using specific antibodies (1:1,000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com). Laser confocal microscopy (Zeiss LSM 510 Axiovert; Carl Zeiss, Thornwood, NY, http://www.zeiss.com) was used to visualize immunofluorescence along with 4′,6′-diamidino-2-phenylindole (Molecular Probes, Eugene, OR, http://probes.invitrogen.com) nuclear staining. Cell proliferation was evaluated with the Ki67 antibody (1:500; clone MIB-1; Diagnostic Biosystems, Pleasanton, CA, http://www.dbiosys.com) [32]. Cardiac interstitial fibrosis was quantified by computerized imaging analysis (MetaMorph; Molecular Devices Corp., Union City, CA, http://www.moleculardevices.com) of 0.5-μm-thick, paraffin-embedded Masson’s trichrome-stained sections [6, 7]. Ultrastructural evaluation was performed by transmission electron microscopy (JEOL 1200 EXII; Jeol Ltd., Tokyo, http://www.jeol.com) in ventricular tissue sections fixed in phosphate-buffered saline containing 1% glutaraldehyde and 4% formaldehyde (pH 7.2) [26].
Statistical Analysis
Data are presented as means ± SEM or medians with 95% confidence intervals. Student’s t test or nonparametric test was used to evaluate significance (JMP 6; SAS Institute, Cary, NC, http://www.sas.com). Kaplan-Meier analysis with log-rank testing was applied for survival analysis. A p value <.05 was predetermined.
Results
KATP Channel Gene Knockout Predisposes to Stress-Induced Malignant Heart Failure
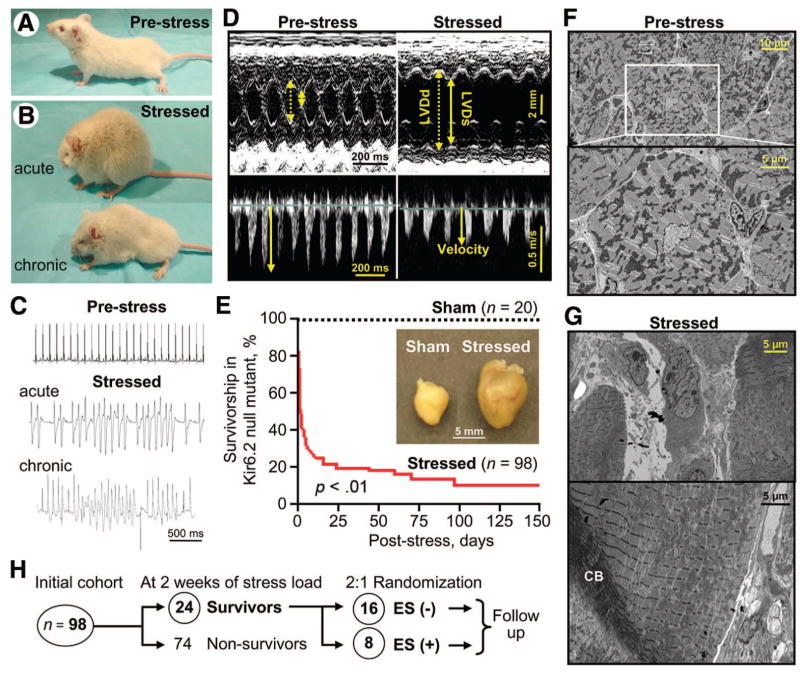
Stress imposed by transverse aortic constriction, in mice lacking cardioprotective KATP channels through gene deletion of the Kir6.2 pore, induced acutely physical signs of biventricular failure with systemic fluid retention and anasarca (Fig. 1A, 1B, acute). The resultant congestive heart failure chronically reduced ambulation (Fig. 1B, chronic), increased susceptibility to ventricular arrhythmia (Fig. 1C), and compromised contractility with ventricular dilatation (Fig. 1D), features of the human dilated CMD10 phenotype associated with KATP channel gene mutations [5]. Consistent with the decline in cardiac function, aortic-constricted Kir6.2-KO mice (n = 98) displayed cachexia (Fig. 1B, chronic), with a significant survival disadvantage compared with their sham-operated counterparts (n = 20; p < .01; Fig. 1E). In fact, following imposition of stress load in the Kir6.2-null mutant, mortality rapidly progressed to 59% at 2 days, 71% at 7 days, 76% at 14 days, 79% at 21 days, 84% at 60 days, and 90% at 150 days of follow-up, in contrast to absence of fatality in the sham cohort (Fig. 1E). Poor outcome correlated on autopsy with excessive remodeling and cardiomegaly (Fig. 1E, inset) in hearts from stressed Kir6.2 knockouts, along with ultrastructural damage characterized by pathognomonic contraction banding (Fig. 1F, 1G). Thus, the KATP channel knockout recapitulated, under the imposed stress of pressure overload, the gene-environment cardiomyopathic substrate of the CMD10 heart failure syndrome.
Figure 1.
KATP channel knockout model recapitulates human cardiomyopathy. KATP channel Kir6.2-null mutants prior to (prestress) (A) and following transverse aortic constriction (stressed) (B). Under hemodynamic overload, stressed Kir6.2-null mutants displayed signs of biventricular failure and anasarca ([B], acute) with reduced ambulation and cachexia ([B], chronic). Predisposition to ventricular tachyar-rhythmia was demonstrated by telemetry either spontaneously (acute) or under isoproterenol challenge (chronic) (C), and left ventricular dilatation with compromised contractility was documented by echocardiography (D). In (D), dotted line, LVDd; solid line, LVDs; velocity, peak velocity at the left ventricular outflow tract. Continuous stress induced a significant increase in left ventricular mass ([E], inset), with survivorship curves demonstrating a poor survival of the stressed cohort (E). On electron microscopy (F, G), CB were present in cardiomyopathic stressed myocardium displaying sarcomeric disarray (G). From the initial cohort of 98 Kir6.2-null mutants, following 2 weeks of stress, 24 surviving cardiomyopathic mutants were randomized 2:1, in ES(−) or ES(+) treatment groups (H). Numbers of animals per group (n) are indicated in (E, H). Abbreviations: CB, contraction band; ES, embryonic stem cell; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; ms, millisecond.
Safety Profile of Embryonic Stem Cell Transplantation
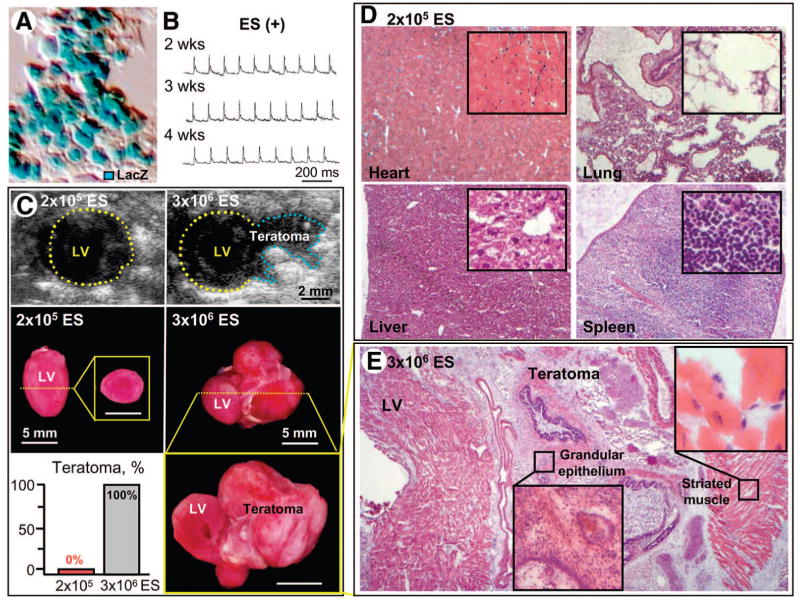
At 2 weeks following aortic constriction, surviving cardiomyopathic Kir6.2-null mutants (n = 24) were randomized 2:1 into groups without or with embryonic stem cell treatment to evaluate stem cell-based therapy during the course of disease (Fig. 1H). Following thoracotomy, the treatment protocol included multiple direct epicardial injections into the left ventricular wall. Within 1 month of treatment with 200,000 lacZ-labeled embryonic stem cells (Fig. 2A), cardiomyopathic mice were alert and physically active with no signs of cachexia, in contrast to their untreated, moribund counterparts. At this dose regimen (n = 8), stem cell transplantation did not produce cardiac rhythm disturbances (Fig. 2B), and signs of uncontrolled growth were not detectable on either in vivo imaging (Fig. 2C, top left) or ex vivo macroscopic (Fig. 2C, middle left) and microscopic (Fig. 2D) system evaluations of the heart, lung, liver, or spleen. This dose circumvented tumorigenic risk (Fig. 2C, bottom left), typically observed at higher cell loads [28, 32]. Indeed, all Kir6.2-null mutants treated separately with 3,000,000 embryonic stem cells demonstrated teratoma formation (n = 5; Fig. 2C, bottom left) documented by echocardiography (Fig. 2C, top right) and on tissue gross inspection (Fig. 2C, middle and bottom right), with histology indicating differentiation into multiple lineages (Fig. 2E). Because of a favorable safety profile, the target dose of 200,000 cells per heart was thus selected for efficacy follow-up.
Figure 2.
Safety profile of ES transplantation. (A): Wild-type ES were engineered to express the lacZ reporter transgene visualized by optical analysis. (B): Cardiomyopathic Kir6.2-knockout mice treated with 200,000 ES per heart (ES(+); unless otherwise indicated) had no arrhythmic events on four-limb electrocardiography throughout the follow-up period. (C): Uncontrolled growth was not detectable at this dose on echocardiography (top left) or macroscopic (middle left and bottom left) evaluation. (D): Microscopy confirmed the absence of multiorgan seeding in heart, lung, liver, and spleen following treatment with 200,000 ES per heart. Treatment with 3,000,000 ES, however, regularly demonstrated teratoma formation ([C], right panels) containing multiple lineages (E). Scale bars = 5 mm. Abbreviations: ES, embryonic stem cell; LV, left ventricle; ms, millisecond; wks, weeks.
Benefit of Stem Cell Therapy on Contractile and Electrical Function
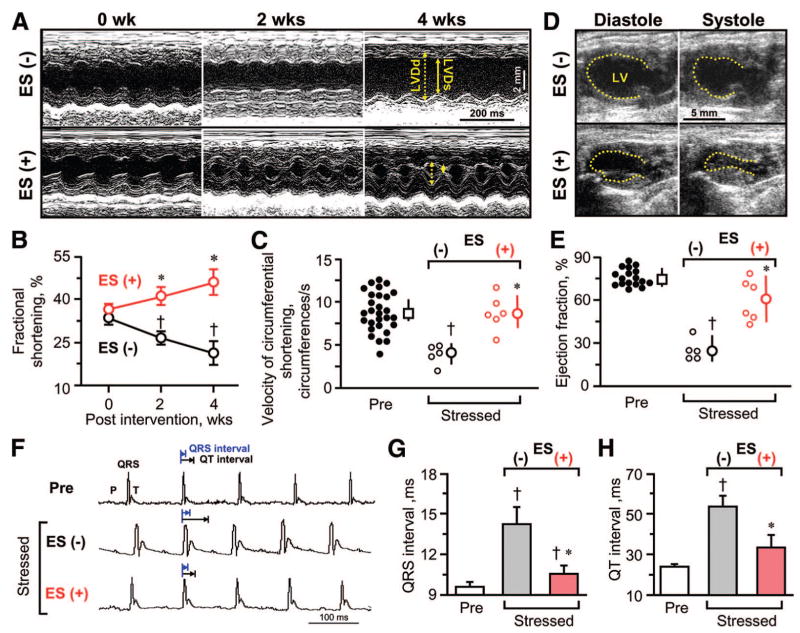
The Kir6.2-null mutant myocardium developed a pathological increase in the thickness of the interventricular and posterior walls within 2 weeks of transverse aortic constriction, that is, from 1.6 ± 0.1 mm prior to (n = 29) to 1.9 ± 0.1 mm following (n = 20) imposed stress load (p < .01). Despite equivalent fractional shortening at randomization, serial assessment of left ventricular function indicated significant disparity in the evolution of organ failure in untreated (Fig. 3A, 3B, ES(−)) versus embryonic stem cell-treated (Fig. 3A, 3B, ES(+)) cardiomyopathic hearts. In the untreated cohort, a progressive decline in systolic performance was observed, measured as reduction in fractional shortening, from 33.4% ± 2.3% (n = 14) to 21.1% ± 4.1% (n = 5; p < .05), calculated from LVDd and LVDs (Fig. 3B). In contrast, embryonic stem cell-treated hearts demonstrated steady recovery in fractional shortening during the 4 weeks of follow-up, that is, from 36.4% ± 2.0% preintervention to 45.9% ± 4.9% postintervention (n = 6; p < .01; Fig. 3B). Moreover, cell treatment significantly improved the velocity of circumferential shortening, a preload-independent measure of ventricular function, which deteriorated without treatment but essentially normalized following embryonic stem cell transplantation. On average, the median value was 4.3 circumferences per second (95% confidence interval, 2.6–5.5 circumferences per second; n = 5) versus 8.8 circumferences per second (95% confidence interval, 6.8–11.0 circumferences per second; n = 6) in untreated compared with treated groups, respectively (p < .05; Fig. 3C). This difference in outcome was further corroborated by enhanced cardiac cycle area shortening (Fig. 3D) and boosted ejection fraction (Fig. 3E) in stem cell-treated (n = 6) versus untreated (n = 5) hearts (p < .05). In addition to parameters indicating functional contractile recovery, stem cell treatment was also associated with prevention of electrical dispersion (Fig. 3F–3H). Specifically, ventricular conduction delay, measured as ventricular depolarization (QRS) and repolarization (QT) intervals, was significantly minimized in treated hearts approaching prestress values. The QRS interval was shortened from 14.2 ± 1.3 milliseconds in untreated (n = 10) to 10.6 ± 0.6 milliseconds in treated (n = 6) hearts (p < .05; Fig. 3G). Similarly, the QT interval was 53.5 ± 5.4 milliseconds in untreated hearts (n = 5) but retracted to 33.4 ± 6.0 milliseconds in treated (n = 6) hearts (p < .05; Fig. 3H). Thus, embryonic stem cell therapy reversed mechanoelectrical dysynchronization, providing contractile and electrical benefit to an otherwise refractory cardiomyopathy.
Figure 3.
Benefit of ES therapy on cardiac function. Serial echocardiography (A–E) demonstrated significant improvement in systolic contractile function in ES-treated (ES(+)) compared with untreated (ES(−)) cardiomyopathic Kir6.2-null mutants. Fractional shortening in M-mode (A, B), velocity of circumferential shortening (C), and LV ejection fraction (D, E) all demonstrated significant improvement following cell therapy. In (A), dotted line, LVDd; solid line, LVDs. (F–H): Ventricular conduction intervals in ES-treated cardiomyopathic hearts were shorter than those in untreated ones. Data are presented as means ± SEM (B, G, H) or medians with 95% confidence intervals (C, E). †, p < .05 versus Pre (0 weeks); *, p < .05 versus ES(−). Abbreviations: ES, embryonic stem cell; LV, left ventricular; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; ms, millisecond; Pre, prestress; s, second; wks, weeks.
Stem Cell Therapy Halts Maladaptive Remodeling and Decompensation
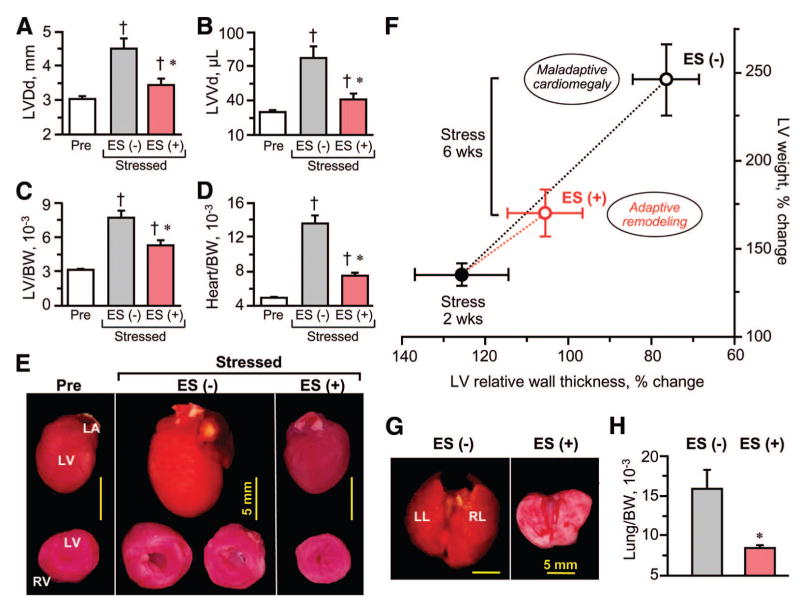
Embryonic stem cell therapy of cardiomyopathic hearts halted ventricular enlargement (Fig. 4A– 4F). Specifically, the end-diastolic dimension of the left ventricle increased from 3.2 ± 0.2 mm (n = 14) to 4.5 ± 0.3 mm (n = 7; p < .01) in the absence of cell therapy within 4 weeks of follow-up (Fig. 4A). During the same period, stem cell-treated hearts displayed stable diastolic dimensions at 3.2 ± 0.1 mm (n = 6) and 3.4 ± 0.2 mm (n = 6; p < .01 vs. untreated; Fig. 4A). Similarly, the pathologic expansion in end-diastolic left ventricular volume that characterized untreated hearts was reduced by stem cell therapy (Fig. 4B). In fact, the exaggerated increase in left ventricular mass (Fig. 4C) or overall heart weight (Fig. 4D) in untreated hearts (n = 7) was blunted by embryonic stem cell therapy (n = 6; p < .01). In the absence of cell therapy, continuous stress load induced cardiomegaly, which was prevented by cell therapy (Fig. 4E). During the natural course of the disease, the progressive enlargement of untreated hearts was associated with a massive wall thickening followed by fulminant ventricular dilatation, indicating maladaptive remodeling and ultimately de-compensation (Fig. 4F). The relative wall thickness/left ventricular mass relationship provides a predictive index of the severity of cardiomyopathy [38]. In contrast to untreated hearts (n = 7), 4 weeks of embryonic stem cell treatment (n = 6) protected heart geometry from gains in relative wall thickness and left ventricular weight (Fig. 4F). The benefit of cell therapy was validated on autopsy, with treated hearts avoiding not only gross cardiac dilation (Fig. 4E), but also retrograde pulmonary edema (Fig. 4G, 4H) observed in the untreated cohort. Thus, embryonic stem cell therapy stabilized the underlying ventricular structure in genetic cardiomyopathy, preventing end-stage disease progression.
Figure 4.
Stem cell intervention prevents maladaptive remodeling and cardiomegaly in genetic cardiomyopathy. Untreated (ES(−)) Kir6.2-knockout cardiomyopathic hearts were larger (A, B) and heavier on echocardiographic (C) and post-mortem (D, E) examination compared with ES-treated (ES(+)) counterparts. LV (C) and heart (D) weights were adjusted to BW (at day 0 of transverse aortic constriction). In (F), LV weight was plotted as a function of relative wall thickness, calculated as the sum of interventricular septum thickness and posterior wall thickness divided by LVDd. The evolution of heart geometry was monitored up to 6 wks following initiation of stress load imposed following randomization at 2 wks (closed circle) into treated (ES(+); red) and untreated (ES(−); black) groups. Data points represent an average of 5–24 Kir6.2-knockout mice. (G, H): Pulmonary congestion was reduced following cell therapy. †, p < .05 versus Pre; *, p < .05 versus ES(−). Scale bars = 5 mm. Abbreviations: BW, body weight; ES, embryonic stem cell; LA, left atrium; LL, left lobe; LV, left ventricular; LVDd, left ventricular end-diastolic dimension; LVVd, left ventricular end-diastolic volume; Pre, prestress; RL, right lobe; RV, right ventricle; wks, weeks.
Stem Cell-Based Repair
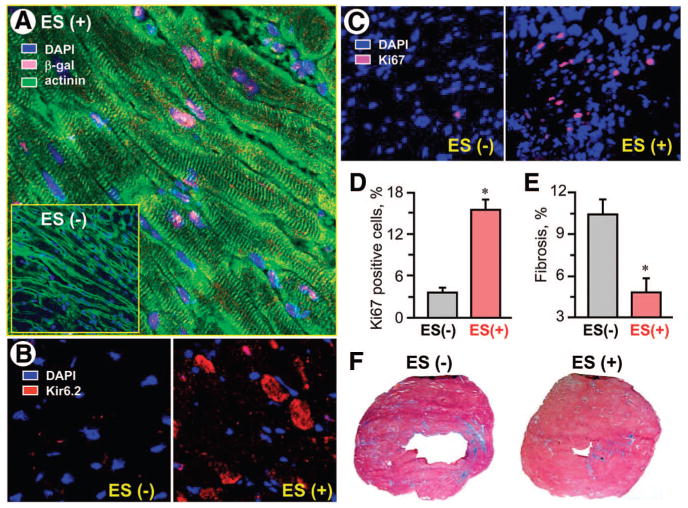
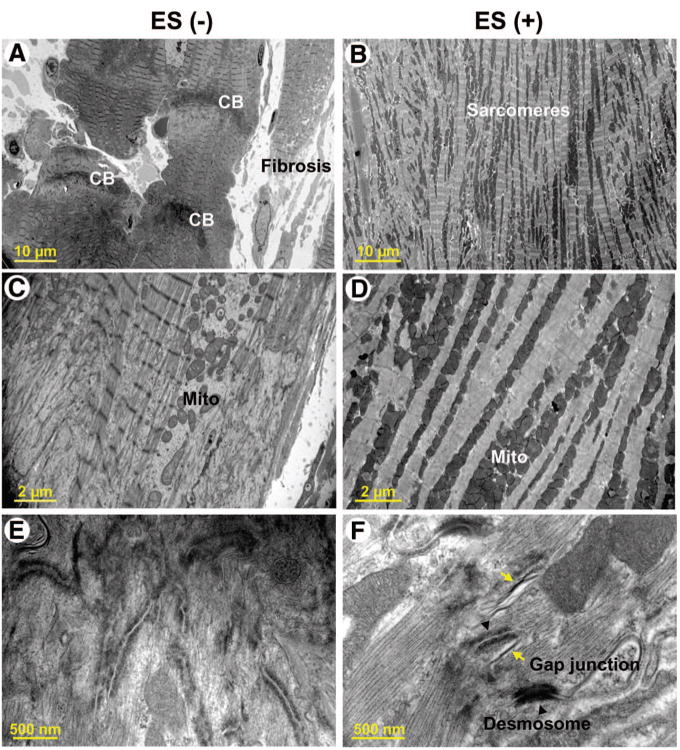
Transplanted embryonic stem cells, engineered to express the reporter lacZ transgene, engrafted into host myocardium and were detected by positive β-galactosidase expression (Fig. 5A). Cellular colocalization of β-galactosidase with cardiac-specific sarcomeric α-actinin indicated an aptitude for cardiomyogenic differentiation of the injected embryonic stem cell pool within cardiomyopathic hearts (Fig. 5A). Stem cell fate was confirmed by positive Kir6.2 immunofluorescence within an otherwise KATP channel-deficient myocardium (Fig. 5B). Beyond formation of new cardiac tissue, transplantation of embryonic stem cells induced cell cycle activation, detected by a significant increase in resident cells expressing Ki67, a marker of proliferation (Fig. 5C). The percentage of Ki67-positive cells over the total cell number was 3.7% ± 0.7% in untreated (n = 5) compared with 15.4% ± 1.4% in treated (n = 5) heart fields (p < .05; Fig. 5D). Moreover, whereas interstitial fibrosis was prominent in the untreated cardiomyopathic hearts, accounting for 10.2% ± 1.1% of overall muscle area (n = 5), stem cell therapy halved fibrotic zones to 4.7% ± 1.0% (n = 5) of the remuscularized hearts (p < .05; Fig. 5E, 5F). Electron microscopy verified, at the ultrastructural level, normalization of sarcomeric organization following stem cell therapy contrasting fibrosis and disarray with prominent contraction banding of the untreated cardiomyopathic hearts (Fig. 6A, 6B). Furthermore, untreated heart sections demonstrated disorganized mitochondria (Fig. 6C) and lacked tight junctions (Fig. 6E), in contrast to dense mitochondrial patterns and intact gap junction formations within the treated ventricular samples (Fig. 6D, 6F). Thus, core repair processes, set in motion by transplant-host interaction, contributed to stem cell-based therapy.
Figure 5.
Engraftment of transplanted stem cells. KATP channel-deficient cardiomyopathic hearts treated with ES, engineered to express the lacZ reporter transgene (ES(+)) demonstrated β-gal ([A], pink) nuclear expression with cellular coexpression of actinin ([A], green), along with Kir6.2 expression ([B], red) within otherwise KATP channel-deficient cardiomyocytes. Treated hearts had a significant increase in cells positive for Ki67 ([C, D], pink), a cellular marker for proliferation, and a decrease in interstitial fibrosis ([E]; [F], blue), compared with untreated hearts (ES(−)). In (A–C), blue indicates DAPI. *, p < .05 versus ES(−). Abbreviations: actinin, sarcomeric α-actinin; DAPI, 4′,6′-diamidino-2-phenylindole; ES, embryonic stem cell; β-gal, β-galactosidase.
Figure 6.
Ultrastructure in untreated versus treated cardiomyopathic hearts. Untreated Kir6.2-knockout cardiomyopathic hearts (ES(−)) displayed significant fibrosis and CBs (A), disorganized Mito (C), and a paucity of tight junctions (E). (B, D, F): In contrast, stem cell-treated hearts (ES(+)) demonstrated proper sarcomeric ultrastructure (B), dense mitochondrial clusters (D), and desmosome with tight junction formation ([F], arrowheads and arrows). Abbreviations: CB, contraction band; ES, embryonic stem cell; Mito, mitochondria.
Favorable Outcome of Stem Cell Therapy on Heart Failure Syndrome
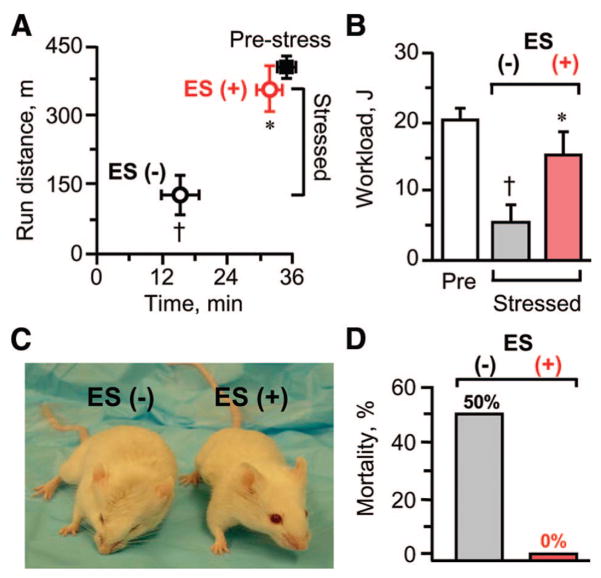
Systemically, genetic cardiomyopathy is characterized by an overt congestive heart failure symptomatology. This includes the triad of clinically relevant endpoints, namely a poor exercise tolerance (Fig. 7A, 7B), severe cachexia (Fig. 7C), and pronounced mortality (Fig. 7D), all of which are recapitulated in the KATP channel knockout model under stress. Despite the continuous imposition of transverse aortic constriction, 1 month following initiation of embryonic stem cell therapy, cardiomyopathic Kir6.2-null mutants (n = 6) demonstrated an apparent normalization of exercise capacity. This was documented on the treadmill by a longer running distance (Fig. 7A) and a higher achieved workload (Fig. 7B), compared with the underperformance of the untreated counterparts (n = 7; p < .05), and approaching target levels of the prestress controls (n = 16). Improvement in systemic function induced by stem cell therapy translated into an amelioration of overall stamina and absence of anasarca (Fig. 7C), with significant survival benefit and no mortality observed in the treated group by the end of the follow-up period (Fig. 7D). Thus, the therapeutic impact afforded by stem cell delivery in genetic nonischemic cardiomyopathy encompasses, beyond the heart itself, rescue from the systemic, malignant consequences of end-stage heart failure.
Figure 7.
ES therapy produces favorable systemic outcome on morbidity and mortality. Exercise running capacity and workload evaluated by treadmill (A, B), overall fitness (C), and mortality (D) were all favorably affected by a 1-month-long treatment with ES (ES(+)) in KATP channel-null mutants mice despite the stress of continuous transverse aortic constriction. †, p < .05 versus Pre; *, p < .05 versus ES(−). Abbreviations: ES, embryonic stem cell; min, minutes; Pre, prestress.
Discussion
Cardiomyopathies result from complex interactions between the genetic make-up of an individual and the imposed environmental stress [39]. Although decoding of disease-causing mechanisms and cardioprotective molecular circuits has provided a real opportunity for development of predictive strategies using emerging genomic information [40], management of heritable cardiomyopathies remains largely palliative in scope, limited to treatment of symptoms. Despite advances in pharmacotherapy and introduction of cardiac resynchronization in the treatment of congestive heart failure, prognosis is poor overall, mandating consideration of new modalities capable of targeting the root cause of disease [41].
The prevalence of ongoing cell death in the chronic failing heart and the limited capacity for innate regeneration impede effective repair [42–45]. Resident stem cell compartments are endowed with the aptitude to acquire the distinct cell lineages of the myocardium and actively contribute to heart homeostasis, yet functional exhaustion and aging of progenitor cells in patients with heart failure limit the available autologous stem cell pools, contributing to disease progression [46, 47]. Moreover, diseases with a genetic origin potentially preclude the full effectiveness of an autologous reparative approach [18], warranting consideration of nonself sources as alternative therapeutic platforms devoid of the inheritance of disease-causing mutations. The present study conducted in the Kir6.2 knockout, a surrogate model that recapitulates features of the human life-threatening cardiomyopathic CMD10 syndrome associated with mutations in cardioprotective KATP channels [5], provides the first evidence that transplantation of allogeneic embryonic stem cells achieves functional and structural repair in stress-precipitated, nonischemic genetic cardiomyopathy.
Hearts lacking KATP channels are characterized by loss of action potential control of calcium influx, creating under stress a severe susceptibility to contractile dysfunction, heart failure, and ultimately death [7, 8, 11]. The malignant cascade of exaggerated cardiac remodeling associated with ventricular dilatation was here demonstrated in the Kir6.2-null mutant under the chronic stress of transverse aortic constriction, precipitating a moribund state that led to serious survival disadvantage. Epicardial transplantation of 200,000 embryonic stem cells into cardiomyopathic hearts restored systolic performance, over the course of 1 month, and prevented progression of dysynchronization and structural maladaptive deterioration, which are known predictors of poor outcome [1, 39, 40]. Therapeutic benefit was demonstrated in situ, by echocardiography and electrocardiography, and verified ex vivo at the macroscopic, microscopic, and ultrastructural levels, in line with the robust capacity of embryonic stem cells for cardiomyogenic differentiation both in vivo and in vitro [21, 31, 48]. The innate cardiogenic potential of embryonic stem cells facilitates electrical and mechanical coupling of derived cardiomyocytes with the native myocardium [26, 27, 49]. It was first linked to de novo remuscularization in models of ischemic heart disease [29, 30, 31], and here extended to nonischemic cardiomyopathy.
Traced using a sensitive reporter transgene [29], transplanted stem cells engrafted into the KATP channel-deficient host myocardium, contributing to formation of new cardiac tissue, along with cell cycle activation. Remuscularization of cardiomyopathic hearts was associated with the emergence of Kir6.2 immunofluroscence, consistent with restitution of the originally deleted KATP channel subunit. Moreover, cell transplantation eliminated contraction banding and reduced noncontracting fibrotic regions characteristic of a failing heart, normalizing sarcomeric and junctional organization. It has been established in this regard that the host myocardium secretes cardiogenic growth factors implicated in cardiac differentiation of stem cells, promoting expression of cardiac contractile and gap junction proteins [31]. In addition, other cardiovascular cell types could arise directly from injected stem cells or through local recruitment leading to neovascularization, augmenting the metabolic support to the healing myocardium [30]. Convergence of multifactorial stem cell-based repair processes contributes to a synergistic reparative mechanism [13, 19, 21], suspending disease progression. Indeed, improved cardiac condition following embryonic stem cell therapy translated into a systemic benefit associated with prevention of end-stage disease, improved global performance, and increased survival.
The observed absence of deleterious arrhythmia or dysregulated growth 1 month following transplantation of stem cells is consistent with electromechanical integration within host tissue [50]. The mitogenic potential associated with pluripotency may, however, hinder therapeutic translation, because of the risk of uncontrolled growth once stem cells are transplanted outside the native embryonic niche [22, 28, 51], as here observed when the dose regimen exceed the threshold for guidance by the host environment. Accordingly, the therapeutic index for allogeneic stem cell application is narrow [21, 28, 32, 52], necessitating careful dose titration to avoid teratoma formation while maintaining engraftment and therapeutic efficacy. It has recently been demonstrated that successfully engrafted embryonic cells do not generate immunological memory, exerting local immunosuppression and enabling engraftment across allogeneic barriers [53–56].
Summary
The present study provides initial evidence supporting an expanded indication for embryonic stem cell-based therapy to include nonischemic genetic cardiomyopathy, beyond the previously established treatment of myocardial infarction [26–31]. In view of the multiple etiologies causing cardiomyopathic phenotypes [2], as well as the increased implication of KATP channelopathies in cardiac disease [5, 57, 58], embryonic stem cell therapy needs to be further validated before its broader value can be established. Optimization of the safety and efficacy profile of embryonic stem cells [59–62] is now a critical step in fully harnessing their multifaceted reparative capacity. In this regard, lineage-specified cardiogenic precursors, rather than undifferentiated embryonic stem cells, deserve particular consideration, as approaches to cardiopoietic programming and biomarker-based selection are increasingly available [32, 63]. Moreover, issues of stem cell origin also need to be addressed. Patient-specific approaches are attractive but may be limited to monogenic disease etiologies, where genetic repair by homologous recombination could complement pluripotent reprogramming of an adult source [49]. Furthermore, a close evaluation of genetic variations among individuals to identify optimal disease targets and customize stem cell cytotypes for therapy [64–66] opens a new era of personalized regenerative medicine.
Acknowledgments
We thank Jonathan J. Nesbitt and Lois A. Rowe for excellent technical support throughout the study, and we acknowledge Jessica Johnston for assistance with the treadmill exercise test. This work was supported by grants from NIH, American Heart Association, Marriott Heart Disease Research Program, Marriott Foundation, Ted Nash Long Life Foundation, Ralph Wilson Medical Research Foundation, Mayo Clinic Clinician-Investigator Program, and Japanese Ministry of Education, Science, Sports, Culture and Technology. S.Y. is the recipient of the Young Investigator Award from the American Society for Clinical Pharmacology and Therapeutics. A.T. holds the Marriott Family Professorship in Cardiovascular Research at Mayo Clinic.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
Author contributions: S.Y. and T.J.N.: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; R.J.C.-D. and X.-K.L.: provision of study material, collection and assembly of data, final approval of manuscript; C.P.-T.: provision of study material, collection and assembly of data, data analysis and interpretation, final approval of manuscript; T.M. and S.S.: provision of study material, final approval of manuscript; A.B.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; A.T.: conception and design, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- 1.Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- 3.Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 4.Ashrafian H, Watkins H. Cardiomyopathies: Therapeutics based on molecular phenotype. J Am Coll Cardiol. 2007;49:1251–1264. doi: 10.1016/j.jacc.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 5.Bienengraeber M, Olson TM, Selivanov VA, et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane GC, Behfar A, Dyer RB, et al. KCNJ11 gene knockout of the Kir62 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 7.Yamada S, Kane GC, Behfar A, et al. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zingman LV, Hodgson DM, Bast PH, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zingman LV, Hodgson DM, Alekseev AE, et al. Stress without distress: Homeostatic role for KATP channels. Mol Psychiatry. 2003;8:253–254. doi: 10.1038/sj.mp.4001323. [DOI] [PubMed] [Google Scholar]
- 10.Kane GC, Behfar A, Yamada S, et al. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53(suppl 3):S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 11.Kane GC, Liu XK, Yamada S, et al. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong X, Porter LM, Liu G, et al. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol Heart Circ Physiol. 2006;291:H543–H551. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: The scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–1099. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- 15.Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez P, San Román JA, Villa A, et al. Contemplating the bright future of stem cell therapy for cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3(suppl 1):S138–S151. doi: 10.1038/ncpcardio0456. [DOI] [PubMed] [Google Scholar]
- 17.Bartunek J, Vanderheyden M, Wijns W, et al. Bone-marrow-derived cells for cardiac stem cell therapy: Safe or still under scrutiny? Nat Clin Pract Cardiovasc Med. 2007;4(suppl 1):S100–S105. doi: 10.1038/ncpcardio0744. [DOI] [PubMed] [Google Scholar]
- 18.Pouly J, Hagège AA, Vilquin JT, et al. Does the functional efficacy of skeletal myoblast transplantation extend to nonischemic cardiomyopathy? Circulation. 2004;110:1626–1631. doi: 10.1161/01.CIR.0000142861.55862.15. [DOI] [PubMed] [Google Scholar]
- 19.Van Laake LW, Van Hoof D, Mummery CL. Cardiomyocytes derived from stem cells. Ann Med. 2005;37:499–512. doi: 10.1080/07853890500327843. [DOI] [PubMed] [Google Scholar]
- 20.Menasché P. The potential of embryonic stem cells to treat heart disease. Curr Opin Mol Ther. 2005;7:293–299. [PubMed] [Google Scholar]
- 21.Kolossov E, Bostani T, Roell W, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solter D. From teratocarcinomas to embryonic stem cells and beyond: A history of embryonic stem cell research. Nat Rev Genet. 2006;7:319–327. doi: 10.1038/nrg1827. [DOI] [PubMed] [Google Scholar]
- 23.He JQ, Ma Y, Lee Y, et al. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 24.Chung S, Dzeja PP, Faustino RS, et al. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4(suppl 1):S60–S67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Terzic C, Faustino RS, Boorsma BJ, et al. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat Clin Pract Cardiovasc Med. 2007;4(suppl 1):S68–S76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson DM, Behfar A, Zingman LV, et al. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H471–H479. doi: 10.1152/ajpheart.01247.2003. [DOI] [PubMed] [Google Scholar]
- 27.Ménard C, Hagège AA, Agbulut O, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: A preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 28.Behfar A, Hodgson DM, Zingman LV, et al. Administration of allogenic stem cells dosed to secure cardiogenesis and sustained infarct repair. Ann NY Acad Sci. 2005;1049:189–198. doi: 10.1196/annals.1334.018. [DOI] [PubMed] [Google Scholar]
- 29.Nelson TJ, Ge ZD, Van Orman J, et al. Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1216–1224. doi: 10.1002/ar.a.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singla DK, Hacker TA, Ma L, et al. Transplantation of embryonic stem cells into the infarcted mouse heart: Formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Behfar A, Zingman LV, Hodgson DM, et al. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 32.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Laake LW, Hassink R, Doevendans PA, et al. Heart repair and stem cells. J Physiol. 2006;577:467–478. doi: 10.1113/jphysiol.2006.115816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraidenraich D, Benezra R. Embryonic stem cells prevent developmental cardiac defects in mice. Nat Clin Pract Cardiovasc Med. 2006;3(suppl 1):S14–S17. doi: 10.1038/ncpcardio0402. [DOI] [PubMed] [Google Scholar]
- 35.Miki T, Nagashima K, Tashiro F, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu XK, Yamada S, Kane GC, et al. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53(suppl 3):S165–S168. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- 37.Kane GC, Lam CF, O’Cochlain F, et al. Gene knockout of the KCNJ8-encoded Kir6.1 KATP channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006;20:2271–2280. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- 38.Chien KR. Genomic circuits and the integrative biology of cardiac diseases. Nature. 2000;407:227–232. doi: 10.1038/35025196. [DOI] [PubMed] [Google Scholar]
- 39.Opie LH, Commerford PJ, Gersh BJ. Controversies in ventricular remodeling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 40.Bell J. Predicting disease using genomics. Nature. 2004;429:453–456. doi: 10.1038/nature02624. [DOI] [PubMed] [Google Scholar]
- 41.Gersh BJ, Simari RD. Cardiac cell-repair therapy: Clinical issues. Nat Clin Pract Cardiovasc Med. 2006;3(suppl 1):S105–S109. doi: 10.1038/ncpcardio0400. [DOI] [PubMed] [Google Scholar]
- 42.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 43.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 44.Ott HC, Matthiesen TS, Brechtken J, et al. The adult human heart as a source for stem cells: Repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4(suppl 1):S27–S39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 45.Ellison GM, Torella D, Karakikes I, et al. Myocyte death and renewal: Modern concepts of cardiac cellular homeostasis. Nat Clin Pract Cardiovasc Med. 2007;4(suppl 1):S52–S59. doi: 10.1038/ncpcardio0773. [DOI] [PubMed] [Google Scholar]
- 46.Kissel CK, Lehmann R, Assmus B, et al. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 47.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Terzic C, Behfar A, Méry A, et al. Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circ Res. 2003;92:444–452. doi: 10.1161/01.RES.0000059415.25070.54. [DOI] [PubMed] [Google Scholar]
- 49.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 50.Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;10:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 51.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Erdö F, Bührle C, Blunk J, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 53.Drukker M, Katz G, Urvach A, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fändrich F, Lin X, Chai GX, et al. Preimplantation-stage stem cells induce long-term allogenic graft acceptance without supplementary host conditioning. Nat Med. 2002;8:171–178. doi: 10.1038/nm0202-171. [DOI] [PubMed] [Google Scholar]
- 55.Lila N, Amrein C, Guillemain R, et al. Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation. 2002;105:1949–1954. doi: 10.1161/01.cir.0000015075.89984.46. [DOI] [PubMed] [Google Scholar]
- 56.Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26:89–98. doi: 10.1634/stemcells.2007-0151. [DOI] [PubMed] [Google Scholar]
- 57.Olson TM, Alekseev AE, Moreau C, et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reyes S, Terzic A, Mahoney DW, et al. KATP channel polymorphism is associated with left ventricular size in hypertensive individuals: A large-scale community-based study. Hum Genet. 2008;123:665–667. doi: 10.1007/s00439-008-0519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arrell DK, Niederländer NJ, Perez-Terzic C, et al. Pharmacoproteomics: Advancing the efficacy and safety of regenerative therapeutics. Clin Pharmacol Ther. 2007;82:316–319. doi: 10.1038/sj.clpt.6100310. [DOI] [PubMed] [Google Scholar]
- 60.Behfar A, Terzic A. Cardioprotective repair through stem cell-based cardiopoiesis. J Appl Physiol. 2007;103:1438–1440. doi: 10.1152/japplphysiol.00713.2007. [DOI] [PubMed] [Google Scholar]
- 61.Arrell DK, Niederländer NJ, Faustino RS, et al. Cardioinductive network guiding stem cell differentiation revealed by proteomic cartography of TNFα-primed endodermal secretome. Stem Cells. 2008;26:387–400. doi: 10.1634/stemcells.2007-0599. [DOI] [PubMed] [Google Scholar]
- 62.Faustino RS, Behfar A, Perez-Terzic C, et al. Genomic chart guiding embryonic stem cell cardiopoiesis. Genome Biol. 2008;9:R6. doi: 10.1186/gb-2008-9-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson TJ, Faustino RS, Chiriac A, et al. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells. 2008;26:1464–1473. doi: 10.1634/stemcells.2007-0808. [DOI] [PubMed] [Google Scholar]
- 64.Chien KR. Regenerative medicine and human models of human disease. Nature. 2008;452:302–305. doi: 10.1038/nature07037. [DOI] [PubMed] [Google Scholar]
- 65.Waldman SA, Terzic A. Individualized medicine and the imperative of global health. Clin Pharmacol Ther. 2007;82:479–483. doi: 10.1038/sj.clpt.6100398. [DOI] [PubMed] [Google Scholar]
- 66.Waldman SA, Terzic A. Therapeutic targeting: A crucible for individualized medicine. Clin Pharmacol Ther. 2008;83:651–654. doi: 10.1038/clpt.2008.65. [DOI] [PubMed] [Google Scholar]