Abstract
Objective
To compare the effect of two prebiotic/probiotic products on weight gain, stool microbiota, and stool short chain fatty acid content of premature infants.
Methods
This randomized, blinded, placebo-controlled trial included 90 premature infants treated with either a dietary supplement containing two lactobacillus species plus fructo-oligosaccharides (CUL, Culturelle®, ConAgra, Omaha, Nebraska, USA), a supplement containing several species of lactobacilli and bifidobacteria plus fructo-oligosaccharides (PBP, ProBioPlus DDS®, UAS Laboratories, Eden Prairie, Minnesota, USA), or placebo (a dilute preparation of Pregestamil formula) twice daily for 28 days or until discharge if earlier. The primary outcome was weight gain. Secondary outcomes were stool bacterial analysis by culture and 16S rDNA qPCR and stool short chain fatty acid content by high performance liquid chromatography.
Results
Both prebiotic/probiotic combinations contained more bacterial species than noted on the label. No significant effect on growth of either prebiotic/probiotic supplement was observed. By cultures, 64% of infants receiving PBP became colonized with bifidobacteria, compared to 18% of infants receiving CUL and 27% of infants receiving placebo (Chi Squared p=0.064). No differences were noted between groups in colonization rates for lactobacilli, Gram-negative enteric bacteria or staphylococci. By 16S rDNA PCR analysis, the bifidobacteria content in the stools of the infants receiving PBP was higher than in the infants receiving CUL or placebo (Kruskal-Wallis p=0.011). No significant differences in stool short chain fatty acid content were detected between groups. No adverse reactions were noted.
Conclusions
Infants receiving PBP were more likely to become colonized with bifidobacteria. No significant differences in weight gain or stool short chain fatty acid content were detected.
Keywords: Probiotic, prebiotic, synbiotic, premature infant, growth, microflora, bifidobacteria, lactobacilli, butyrate
Introduction
Growth is a major challenge to the small premature infant. The gastrointestinal barrier function, gut motility, mucosal immunity, and digestive/absorptive capacity are all significantly underdeveloped in the preterm infant and appear to increase the risk of poor growth as well as nosocomial infections and necrotizing enterocolitis (NEC).
Evidence suggests that the intestinal microbiota may be important to health and growth (1). In healthy breast-fed term infants, the predominant organisms of the intestinal microbiota are bifidobacteria from one week of age until the introduction of non-human milk or solid food (2, 3). Bifidobacteria often outnumber other bacteria in the intestine by more than 100-fold (4). Preterm infants cared for in modern neonatal intensive care units (NICUs) develop a very different intestinal microbiota (4-6). The predominant intestinal organisms in these infants are enterobacteriaciae, enterococci, clostridia, staphylococci, and yeasts. Bifidobacteria are significantly less common (7). This appears to be the case in both breast and formula-fed premature infants (5). These differences are likely due to decreased exposure to the maternal microbiota, increased exposure to organisms that colonize NICUs, multiple courses of systemic antibiotics, and delays in feeding.
Several investigators have attempted to change the intestinal microbiota of the premature infant so that this complex community of microorganisms more closely resembles that of the term breast-fed infant, in hopes of improving growth and development and decreasing episodes of nosocomial infection and NEC. The effect of dietary supplements containing live microorganisms (probiotics) on the premature intestinal microbiota has been variable, attributed to differences in both gestational age of the infants and products administered (8, 9). The effect of probiotics on weight gain has also been mixed. In term infants, formula supplemented with Lactobacillus rhamnosus GG led to increased weight gain (10), but formulas supplemented with Bifidobacterium longum (11), Bifidobacterium animalis subsp. lactis (12), and Lactobacillus reuteri (12) did not. In preterm infants direct administration of Bifidobacterium breve led to improved weight gain (13), but Saccharomyces boulardii did not (14). The mechanisms by which growth is affected are not yet clear.
A second approach to altering the intestinal microbiota is the addition of prebiotic oligosaccharides to the diet. Prebiotics are dietary supplements that modulate the composition and metabolic activity of the intestinal microbiota, enhancing survival of desirable microorganisms by providing the specific substrate that is fermentable by these microorganisms. In animal models, prebiotic oligosaccharides have been shown to have immunomodulatory effects (15, 16). In premature infants, these supplements have been shown to alter the microbiota (17-20). While formulas supplemented with prebiotic oligosaccharides have not been shown to improve weight gain in term infants (21-23), a decrease in both numbers of infections and numbers of infections requiring antibiotics has been demonstrated (24). The effect of these supplements on the growth of premature infants is unknown. The term “synbiotic” has been proposed to describe the combination of probiotic microorganisms and prebiotic oligosaccharides, the latter nourishing and protecting the former.
This double-blind, placebo-controlled, randomized study investigated the impact of two commercially available products, both of which contain a prebiotic oligosaccharide and two or more probiotic organisms on the weight gain, fecal microbiota, and fecal short-chain fatty acid (SCFA) content of premature infants.
Materials and Methods
Study Design
This study was approved and monitored by the Institutional Review Board at UC Davis and by an independent safety monitoring committee. The study was registered at ClinicalTrials.org (NCT00282113). Parents were approached regarding participation in the study if their infant met the following criteria: birth weight 750-2000 gm, gestational age at birth less than 35 completed weeks, born in or transferred to UC Davis Medical Center NICU, age less than 7 days, no gastrointestinal anomalies or cyanotic congenital heart disease. 90 premature infants were enrolled, after written informed consent was obtained from the parents, from Oct 2004 to Aug 2006. One patient was withdrawn from the study at the parents' request on day of life 5. The data from this patient were included with the assigned group (ProBioPlus®) on an intention to treat basis.
Sample Size
Calculation of study sample size was performed with nQuery software prior to the start of the study. Assuming a one-way analysis of variance (ANOVA) with three equal sized groups, an alpha of 0.05, and an effect size for differences in weight gain among the three groups of 0.2, 23 subjects in each group would provide 90% power to reject the hypothesis that the mean weight gain was the same in all three groups. In order to allow for a dropout rate of up to 20% (due to early discharges or transfers) we aimed to enroll a total of 90 patients.
Using variance estimates based on the results of a previous study (13), an effect size of 0.2 would be realized, for example, by a configuration of group means in which each of two intervention groups had a mean weight gain of 60 gm/week greater than the control group, i.e. this study was powered to detect substantial increases in weight gain due to treatment.
To assess appropriate sample size for fecal SCFA analysis, a pilot analysis of 18 samples was performed. A prospectively performed sample size calculation using STATA software, assuming an alpha of 0.05 and an effect difference similar to that seen in the pilot analysis, showed that a total analysis of 33 specimens would provide an estimated power of 80%.
Study Protocol
The infants were randomly assigned by the UC Davis Investigational Pharmacy based on a computer generated list to one of three groups. One group received Culturelle® (CUL, ConAgra Foods, Omaha, Nebraska, USA); ConAgra states that one capsule contains 1010 viable Lb. rhamnosus GG as well as inulin, a fructo-oligosaccharide prebiotic. The product was prepared in the pharmacy by mixing the contents of one capsule in 20 mL saline and shaking vigorously. Single unit doses of this mixture (5 × 108 organisms) were delivered to the NICU. The second group received ProBioPlus DDS® (PBP, UAS labs, Minneapolis, Minnesota, USA); UAS labs states that each capsule contains 1010 each of viable Lb. acidophilus, B. longum, B. bifidum, and B. infantis as well as inulin. The dose was prepared in the same fashion as the CUL (5 × 108 of each organism dissolved in saline). The third group received a placebo consisting of a 1:30 dilution of an elemental formula, Pregestamil. The placebo had no prebiotic or probiotic activity; its caloric value was minimal (0.023 kcal per dose). The three products were similar in appearance and had no discernible scent. The unit dose volume of each product was 1 mL. All products were given twice daily by mouth or gavage tube for 28 days or until hospital discharge, whichever came first. The parents, nurses, dietitians, and physicians were not informed of group assignment. Measurements of infant weight, length, and head circumference were recorded each week. A fresh stool specimen was obtained each Monday morning that the patients received a study product.
Product analysis
Terminal restriction fragment length polymorphism (T-RFLP) analysis of duplicate capsules of each prebiotic/probiotic combination product was performed following a standard protocol, details available on request (25,26). Bacterial species identification of the T-RFLP patterns was done by comparison to the T-RFLP Analysis Program (TAP) database (27). As expected, minor differences were observed between the predicted and the observed T-RFLP lengths (28). Each DNA sample was tested twice by T-RFLP.
Stool Bacterial Analysis
For the first 33 patients enrolled in the study, approximately 1 gram of stool, as estimated by the health care provider, was placed in a test-tube containing dilute L-cysteine and the air evacuated to create an anaerobic chamber. The remaining stool specimen was placed in a sterile container, and both were kept at 4° C until transport to the laboratory. For these initial 33 patients, three serial 100-fold dilutions of the aerobic and anaerobic specimens were analyzed each week. The aerobic specimens were plated on MacConkey and mannitol salt agars (for detection of Gram-negative enteric organisms and staphylococci respectively), and the anaerobic specimens were plated on deMan, Rogosa, Sharpe (MRS) agar and MRS agar with added nalidixic acid, neomycin sulfate, lithium chloride, and paromomycin sulfate (NNLP) (29) for detection of lactobacilli and bifidobacteria respectively. The anaerobic specimens were incubated in an anaerobic chamber for 72 hours. Colonization was defined as > 102 colonies on the 104 fold dilution plate. The prebiotic/probiotic combination PBP was cultured each week as a control and demonstrated consistent growth on both MRS agar and MRS agar with added NNLP, and no growth on MacConkey and mannitol salt agars.
For the remaining 57 patients, a fresh stool specimen was obtained each week of study participation, placed in a sterile container, and kept at 4° C until transport to the laboratory where the specimen was frozen at -80° C for later analyses (DNA extraction and SCFA analysis). For the last 18 of these patients who remained in the NICU for 4 weeks, DNA was extracted and the microbiota analyzed by qPCR (details regarding technique and primers available on request).
Short Chain Fatty Acid Analysis by High Performance Liquid Chromatography
For analysis of fecal SCFA content, 100 mg of frozen stool was homogenized in 1 mL 0.15mmol/L H2SO4, centrifuged and filtered following an established protocol (30). 100 μL of the filtered supernatant was placed in each of two tubes. 100 nmol of butyrate was added to one tube as an internal control. Fatty acid derivatization of both samples was accomplished following a published protocol (31). The samples were then mixed with 0.03 M phosphate buffer (pH6.4)-0.5 M hydrochloric acid (3.8:0.4 v/v), extracted with hexane to remove long chain fatty acid hydrazides, and then extracted with di-ethyl ether to isolate the short chain fatty acid derivatives. After overnight evaporation of solvent, the residue was dissolved in methanol and analyzed by reverse phase high performance liquid chromatography on a C18 column and a gradient of acetonitrile (5%-100%). The absorbance of the eluate was analyzed at 230 nm.
Data Analysis
Data were analyzed using STATA and SAS/STAT (version 9.1 of the SAS system) software. Bivariate and multifactorial methods were used to assess differences between groups with respect to potential confounders and to identify important covariates to use to reduce the effects of selection bias arising from subjects being lost to follow-up due to early discharge from the NICU. Mixed-effects regression models with weekly follow-up gain scores (from birth) as the unit of analysis were used to adjust for the impact of potential confounders and to increase the sensitivity of the analysis to detect meaningful baseline-adjusted between-group differences. Separate models were used for each of the growth outcomes (weight, length, and head circumference).
Infant vectors of follow-up gain scores were modeled with both fixed and between-infant random effects for intercepts and linear slopes. Slope effects correspond to postnatal age, which was measured in completed weeks and centered at 3. Additional fixed effects included gestational age at birth (measured in weeks and centered at 30), a quadratic effect of postnatal age, and the interaction of centered gestational age with slope. These terms were added to reduce biases arising from confounding and attrition. To be able to assess the effects of each experimental treatment versus placebo, additional fixed effect binary indicators were specified so that the fixed effects for growth (intercept, linear trend) were specific to each experimental group. Treatment versus placebo contrasts at each of the latter 3 follow-up weeks (weeks 3, 4, and 5) were used to estimate adjusted treatment effects.
Exploratory subgroup analyses were performed to compare treatment versus control group contrasts in weight gains from the start of enteral feeding. These analyses were restricted to the subgroup of infants who were first fed enterally within the first week of life and who were followed for at least 3 weeks. In these analyses an alternative method was used to adjust the weight measurement for infant age (gestational age at birth plus postnatal age). Weekly weight measurements were converted to weight-for-age Z (WAZ) scores using Cole's LMS method (32) as applied to Fenton's growth chart for preterm infants (33,34). WAZ scores are particularly useful in comparing growth of premature infants as they include empirically based adjustments for gestational age (34). At each of the last 3 follow-up measurement occasions (Weeks 3, 4 and 5), within-infant gains in WAZ scores from Week 1 were computed and between-group differences in mean WAZ gain scores were assessed using oneway analysis of variance and Scheffe's method for posthoc comparisons.
Results
Prebiotic/Probiotic combination product characterization
Numerous studies have demonstrated significant differences between the label claims on probiotic products and the actual microbial composition (35-38). Cultures confirmed the presence of live lactobacilli in both products and live bifidobacteria in PBP. To characterize the CUL (lot #0135A7) and PBP (lot #228032F) products we employed T-RFLP (25, 27). While the CUL product label indicates only a single bacterial species, Lb. rhamnosus GG, T-RFLP indicated the presence of an additional species, Lb. casei. The PBP product label indicates four bacterial species, Lb. acidophilus, B. longum (B. longum bv. Longum), B. infantis (B. longum bv. Infantis) and B. bifidum, however, the T-RFLP analysis indicates several additional Lactobacillus and Bifidobacterium species (details available on request). The T-RFLP analyses were performed in duplicate with identical results. It should be noted, however, that T-RFLP is not a quantitative method and therefore no information as to the quantity of specific bacterial constituents can be reported. No potentially pathogenic organisms were detected in either product.
Study outcomes
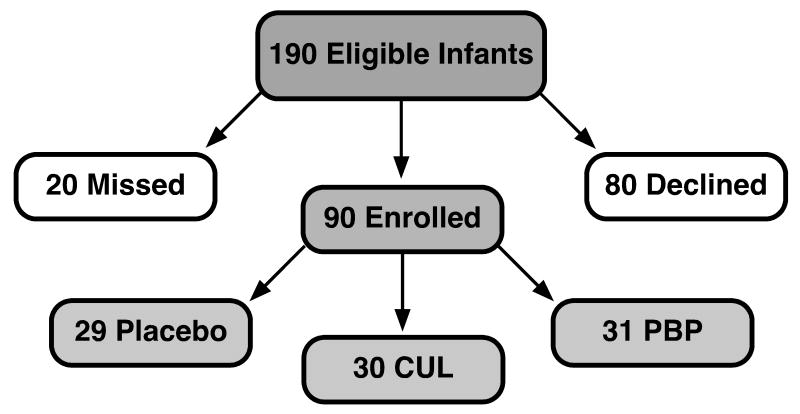
Figure 1 is a flow chart of patient enrollment for the 90 infants in the study. Infants were enrolled in the study and started on the assigned study product as soon as informed consent was obtained from the parents (prior to 7 days of age in all cases). Table 1 summarizes the baseline characteristics, outcomes, and prevalence of potential confounders in each group. There were no statistical differences between groups for any of these variables. Four infants were transferred to another NICU prior to completion of two weeks in the study, and an additional 15 infants were transferred to another NICU or discharged home prior to completion of three weeks in the study. Numbers of infants remaining in each group at three, four, and five weeks are summarized in Table 2 stratified by birth weight.
Figure 1.
Flow diagram of enrollment and randomization.
Table 1.
Patient characteristics, outcomes, and potential confounders
| Placebo [n=29] |
CUL [n=30] |
PBP [n=31] |
|
|---|---|---|---|
| Gestational Age (completed weeks) a | 29.3 (2.6) | 29.5 (2.6) | 30.2 (2.4) |
| Birth weight (gm) a | 1393 (363) | 1394 (356) | 1461 (372) |
| Birth length (cm) a | 38.9 (3.6) | 39.5 (3.8) | 39.7 (3.2) |
| Birth head circumference (cm) a | 27.7 (2.2) | 27.6 (2.3) | 28.0 (2.3) |
| Males b | 19 (66) | 21 (70) | 19 (61) |
| 1 minute Apgar c | 6 (4,8) | 7 (6,8) | 7 (5,8) |
| 5 minute Apgar c | 8 (7,9) | 8 (8,9) | 8 (7,9) |
| C-section b | 23 (79) | 17 (57) | 23 (74) |
| Prenatal Betamethasone b | 18 of 26 (69) | 22 (73) | 26 (84) |
| Prenatal antibiotics b | 9 of 26 (35) | 13 (43) | 11 (35) |
| Umbilical catheter b | 21 (72) | 17 (57) | 21 (68) |
| Total days post-natal antibiotics c | 5 (2, 10) | 4 (2, 8) | 3 (2, 7) |
| Day of first enteral feeding c | 2 (1,6) | 2 (1,6) | 3 (1,6) |
| Day of full enteral feeds c | 10 (7,15) [n=27] |
12 (7, 16) [n=28] |
9 (7, 14) [n=30] |
| Feeding: breast milk only b | 11 (38) | 9 (30) | 10 (32) |
| Feeding: formula only b d | 7 (24) | 4 (13) | 5 (16) |
| Chronic lung disease b e | 5 (17) | 3 (10) | 2 (6) |
| Necrotizing enterocolitis-stage 1/2/3 f | 1/1/0 | 3/1/0 | 0/1/0 |
| Documented infection < 4 days of life b | 1 (3.4) | 2 (6.7) | 0 (0) |
| Documented infection > 3 days of life b | 4 (14) | 4 (13) | 2 (6) |
mean (S.D.),
number (%),
median (25th and 75th percentiles),
Similac Special Care,
Oxygen requirement at 36 weeks post-conceptual age,
NEC at any time during hospitalization by modified Bell criteria.
Table 2.
Number of patients remaining in the study (not discharged or transferred) at weeks 3, 4, and 5, stratified by birth weight.
| 3 weeks | 4 weeks | 5 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BW (gm) | Placebo | CUL | PBP | Placebo | CUL | PBP | Placebo | CUL | PBP |
| 750-1000 | 6 | 3 | 5 | 6 | 3 | 5 | 6 | 3 | 5 |
| 1001-1500 | 8 | 13 | 7 | 8 | 12 | 6 | 8 | 11 | 6 |
| 1501-2000 | 9 | 8 | 12 | 8 | 4 | 9 | 5 | 1 | 6 |
Treatment was well tolerated. There were 38 sets of blood cultures performed on the enrolled infants after the first 72 hours of life (aerobic and anaerobic cultures) of which 9 were positive (4 coagulase-negative staphylococci, 2 E. coli, 2 S. aureus, and 1 Group B Streptococcus). None of the anaerobic blood cultures was positive. We defined feeding intolerance as emesis, gastric distention, or excessive gastric residuals. All infants in the study were evaluated by the nursing staff prior to each feeding. Any infant with a distended abdomen, an increase in abdominal girth of more than 2 cm, or a gastric residual more than a few mL was then assessed by a physician or neonatal nurse practitioner. The decision to hold or decrease feedings was at the discretion of the physician or neonatal nurse practitioner based upon the clinical exam. In four infants, feedings were held due to feeding intolerance, but no changes suggestive of NEC were noted on X-ray (these infants are reported in Table 1 as NEC stage 1 based on modified Bell criteria (39)). In these infants, the study product (placebo or prebiotic/probiotic combination) was continued even though the infant was not being fed. In each of these cases the feeding intolerance resolved in spite of continued administration of the study product and did not recur with re-initiation of feedings, suggesting that it was not the study product causing the feeding intolerance.
Growth
Regression-adjusted treatment versus placebo contrasts in weight gains between each intervention group and the control group showed no significant differences as summarized in Table 3. The regression-adjusted cumulative weight gain for infants receiving PBP after 5 weeks was 30.7 g greater than that of infants receiving the placebo (95% CI -60.5, 121.9) p=0.50 and for infants receiving CUL was 12.5 g greater than that of infants receiving the placebo (95% CI -41.7, 71.1) p=0.79. The regression results indicate that weekly weight gains are significantly influenced by gestational age at birth and are subject to significant between-infant heterogeneity even after adjusting for gestational age. Differences between groups for length and head circumference were also not significant. Details of the mixed-effects regression model for the primary outcome, weekly weight gain scores, are available on request.
Table 3.
Regression-adjusteda treatment vs. placebo contrasts in cumulative weight gains (in grams) at weeks 3, 4, and 5.
| Label | Estimate | 95% CI Lower Limit | 95% CI Upper Limit | p-value |
|---|---|---|---|---|
| CUL vs. Placebo, week 3 | 3.9 | -52.4 | 60.2 | 0.890 |
| CUL vs. Placebo, week 4 | 8.2 | -64.0 | 80.5 | 0.821 |
| CUL vs. Placebo, week 5 | 12.5 | -78.4 | 103.5 | 0.785 |
| PBP vs. Placebo, week 3 | 14.7 | -41.7 | 71.1 | 0.605 |
| PBP vs. Placebo, week 4 | 22.7 | -49.7 | 95.1 | 0.534 |
| PBP vs. Placebo, week 5 | 30.7 | -60.5 | 121.9 | 0.504 |
Mixed-effects regression of longitudinal weekly infant weight gains (from birth to week specified) with a fixed-effect adjustment for gestational age at birth and infant-specific mixed-effects adjustments for levels and rates of change.
Subgroup analyses based on gestational age and age at first enteral feeding indicate the presence of significant between-group differences in weight-for-age Z (WAZ) score gains when gains are measured from follow-up week 1 and when the analysis is restricted to the subgroup of infants who received their first enteral feeding prior to 7 days of life (Table 4).
Table 4.
Summary of differences in gain in weight-for-age Z-score (WAZ score) in subgroup of infants starting enteral feeds prior to the 7th day of life and still in the NICU at 3, 4, and 5 weeks.
| n | p value for comparison of gain in WAZ score (all 3 groups)a | Improvement in WAZ score and 95% CI's (comparing PBP to placebo) b | |||
|---|---|---|---|---|---|
| Placebo | CUL | PBP | |||
| Week 1 to week 3 | 20 | 19 | 21 | 0.023 | 0.22 (0.02-0.41) |
| Week 1 to week 4 | 19 | 14 | 17 | 0.018 | 0.26 (0.04-0.48) |
| Week 1 to week 5 | 16 | 10 | 14 | 0.052 | 0.26 (-0.02-0.54) |
Oneway ANOVA.
Scheffe's method with alpha 0.05.
Fecal Microbiota
Standard aerobic and anaerobic culture results and changes in colonization from week 1 to week 4 in the initial 33 patients are summarized in Table 5. Colonization was defined as > 102 colonies on the 104 fold dilution plate. The PBP group started with low percentages of colonization with both bifidobacteria and lactobacilli, but showed significant increases in colonization with these organisms. We hypothesized that treatment with CUL would lead to a significant increase in colonization with lactobacilli and that treatment with either of the prebiotic/probiotic combinations would lead to a decrease in colonization with Gram-negative enteric organisms and staphylococci, but neither of these hypotheses were supported by the data.
Table 5.
Changes in number of infants colonized over time by group.
| Placebo n=11 | CUL n=11 | PBP n=11 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wk 1 | Wk 4 | p* | Wk 1 | Wk 4 | p* | Wk 1 | Wk 4 | p* | |
| Lactobacilli | 3 | 4 | 0.34 | 3 | 5 | 0.44 | 1 | 5 | 0.038 |
| Bifidobacteria | 3 | 3 | 1.0 | 3 | 2 | 0.59 | 1 | 7 | 0.006 |
| Gm neg enterics | 3 | 8 | 0.053 | 1 | 7 | 0.006 | 7 | 10 | 0.082 |
| Staphylococci | 3 | 4 | 0.34 | 3 | 3 | 1.0 | 2 | 4 | 0.17 |
paired t-test
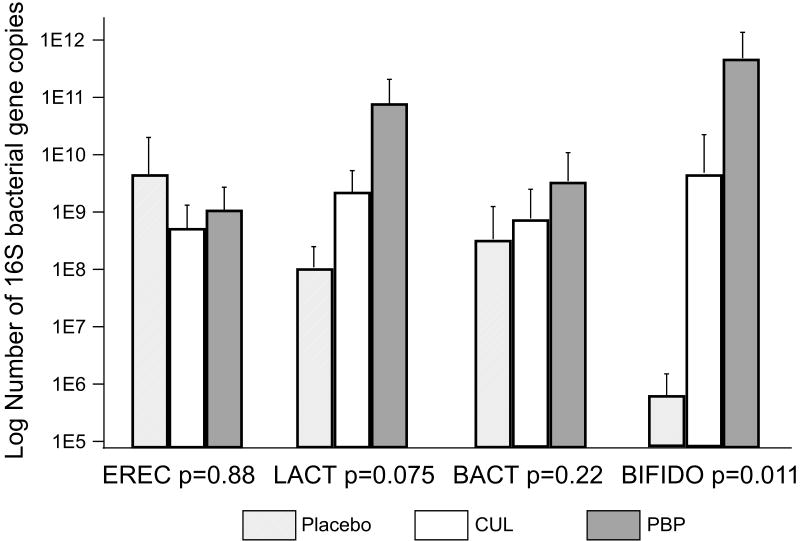
Composition of the stool bacteria was analyzed by qPCR on samples obtained at 4 weeks (Figure 2, note that the y axis is a logarithmic scale). One of the 18 patients whose stool was analyzed by qPCR had a difficult clinical course and was treated with antibiotics for 22 of the first 28 days of life. Stool specimens from this patient consistently showed minimal or no evidence of the bacterial groups analyzed. Therefore, this patient's results were removed from the final analysis. The largest differences between treatment groups were seen for bifidobacteria. The placebo group had very low mean bifidobacteria numbers as is typical of premature infants; the PBP group had bifidobacterial numbers more like those seen in term breast-fed infants. The difference between the two groups was >100,000 fold.
Figure 2.
PCR analysis of stool bacterial groups at week four. EREC = Eubacterium rectale/Clostridium coccoides, LACT = lactobacilli, BACT = bacteroides, and BIFIDO = bifidobacteria. N=6 placebo, 6 CUL, and 5 PBP. Statistical comparison: Kruskal-Wallis.
Fecal SCFA content
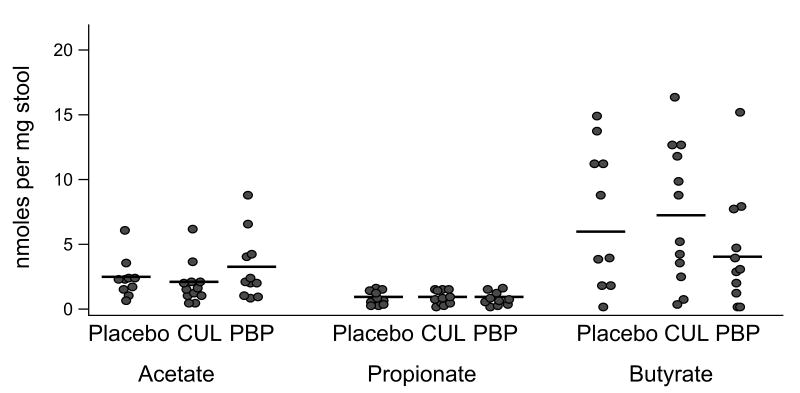
The initial analysis of 18 specimens suggested an increase in acetic acid content among infants receiving PBP compared to those receiving CUL or placebo, with no clear difference among groups for propionic acid or butyric acid. A power analysis on this limited pool suggested that an additional 15 samples would be adequate to test this hypothesis. When the additional 15 specimens were analyzed, however, the observed difference was not significant. The data from all 33 analyses are summarized in Figure 3.
Figure 3.
Fecal SCFA content from 33 infants at week four. N=10 placebo, 12 CUL, 11 PBP. ANOVA p=0.32 acetate, 0.99 propionate, 0.32 butyrate.
Discussion
The study of a potential role of probiotic and prebiotic products in the care of premature infants remains in a very early stage. While reports of improved growth and decreases in NEC with probiotic use are enticing (13, 40), the mechanisms by which probiotics impact the intestinal biota and its interactions with the intestinal mucosa, the preferred probiotic organism or combinations of organisms, the advantage of viable versus nonviable probiotics, the impact of added prebiotics, and the purity, stability, variability between lots, and viability of probiotic products remain unclear. Our T-RFLP analysis demonstrating differences between advertised and actual probiotic composition underscores the importance of including molecular analysis in future probiotic studies, including dose ranging studies and clinical trials of probiotics for prevention of NEC and allergic diseases as well as treatment of diarrhea and inflammatory bowel disease.
To our knowledge, this is the first clinical trial in premature infants to compare two over-the-counter U.S. prebiotic/probiotic combination products to each other and to placebo. We sought to compare the impact of the two products on the growth, fecal microbiota, and fecal SCFA content of premature infants.
Our initial intent was to compare a single organism product to a multiple organism product based on the apparent discrepancies between the studies available at the time of study initiation (41, 42). In a recent meta-analysis (40), the trials to date showing a pronounced decrease in NEC used multi-organism products (43, 44), while the trials using a single organism showed a lesser decrease (8, 14, 42, 45). Analysis of each product by T-RFLP revealed more species in both products than noted on the labels, a common observation among commercial probiotic products (25).
In this study, PBP caused a significant increase in the stool content of bifidobacteria compared to placebo, both by standard enrichment and qPCR. While this increase is not unexpected, it may be particularly consequential for the premature infant. Recent evidence of the selective ability of the oligosaccharides in human milk to stimulate growth of the specific bifidobacteria strains found in the infant (46) and the anti-inflammatory properties of some strains of bifidobacteria (47, 48) suggest that these microorganisms, which dominate the microbiota of the term infant, may be ideally suited to prevent NEC in the preterm infant. The infants receiving CUL also had increases in lactobacilli and bifidobacteria compared to controls, but these differences did not reach statistical significance. This may be related to the dose administered or to a lesser prebiotic effect of oligosaccharides on lactobacilli than on bifidobacteria. In each of the 3 groups, there was an increase in Gram-negative enteric organisms by standard culture when comparing week 1 to week 4. This is consistent with Gewolb's study of extremely premature infants wherein the numbers of infants with K. pneumoniae, E. cloacae, P. aeruginosa, E. coli, and Citrobacter spp all increased from 10 to 20 to 30 days of life (5). Treatment with prebiotic/probiotic products did not affect colonization with either Gram-negative organisms or staphylococci. Caution in interpreting studies of stool microbiota is justified as the fecal microbes may not accurately represent the mucosa-associated microbiota (49), and prolonged storage of stool specimens, even at -70° C, may lead to changes in composition (50).
We chose infant growth as our primary outcome variable. Premature infants are at very high risk for poor growth after birth due to high insensible losses, immaturity of the gastrointestinal tract, and high metabolic needs. The formidable task of overcoming these obstacles to growth has been emphasized (51). The impact of the composition of the intestinal microbiota on weight gain is an area of active interest (52, 53). In premature infants, possible mechanisms by which prebiotic/probiotic combination products might improve growth include improved feeding tolerance, deconstruction of indigestible carbohydrates by probiotic organisms (resulting in monosaccharides which are then available as substrate for the infant), and production of vitamins and short chain fatty acids by probiotic organisms.
In the present study we found no significant differences between groups for gains in weight, length, or head circumference. It is possible that we were unable to detect a difference in weight gain due to errors in the study design and/or the sample size calculations (Type 2 errors). For instance, since the infants were only included in the study until discharge from the NICU, the infants who grew more quickly were likely to be discharged sooner and were therefore no longer in the pool of patients measured. This type of error could have been avoided by treating and measuring all enrolled patients for a given time period (continuing treatment after discharge). In addition, the study (13) used for the initial power calculation adopted a different study design in that the comparison was between infants who were colonized with the administered organism (B. breve) and infants who were not colonized (rather than between infants receiving product vs. placebo as in our study). The effect size generated from this different study design may have been inadequate for the current study, i.e. our calculated sample size may have been too small. It is noteworthy that analysis of the subset of infants who started enteral feedings within the first week of life (75 of 90 patients, presumably the healthiest) did show significant differences in gain in WAZ score between groups (ANOVA p=0.023 at 3 weeks and 0.018 at 4 weeks) with WAZ score gains in the PBP group significantly greater than placebo (Table 4, final column). Subgroup analyses can be misleading as they are not usually powered to answer a specific question; their utility is in generating new hypotheses (54). This subgroup analysis is not powered to conclude that there is improved weight gain, however it does suggest the hypothesis that the prebiotic/probiotic PBP improves weight gain among those premature infants well enough to start enteral feeds in the first week of life. This hypothesis would require testing in future studies. Finally, the drop out rate (due to discharges) was such that our study was underpowered at the most important time points. The difference in weight gain in the reference study (13) became most prominent at weeks 5-7. These problems represent a major limitation to the study and preclude us from confidently concluding that there was no difference in weight gain between groups.
SCFAs are organic carboxylic acids with 1 to 6 carbon atoms that appear to promote colonic homeostasis (55). Fermentation of saccharides, proteins, and peptides by commensal intestinal bacteria yield energy for the bacteria. The end products of this fermentation are SCFAs, gases, and heat. The production of SCFAs by probiotic microorganisms may benefit the host by regulating colonic pH, ion transport, cellular proliferation and gene expression (56). Addition of galacto-oligosaccharides and fructo-oligosaccharides to the diet of formula-fed infants produced a stool SCFA content similar to breast fed infants (57). Conversely, some investigators have proposed that increased production of butyrate by intestinal clostridia may actually be harmful, contributing to the pathogenesis of NEC and that bifidobacteria exert a protective effect by displacing clostridia thus decreasing butyrate production (58-60). Wang et al noted lower levels of fecal propionic acid and butyric acid in premature infants supplemented for 4 weeks with B. breve compared to controls, but no changes in fecal lactic acid, acetic acid, or total SCFAs (58). While our method of analysis differs from that used in the study by Wang, the range of SCFA levels is similar. We found no significant differences in stool SCFA content between those infants receiving either of the two products and those receiving placebo. Unlike the pattern in adults, which is dominated by acetate (61), butyrate was the dominant SCFA in most infants studied. The efficiency of SCFA absorption in the colon of the premature infant is unknown, therefore it is unclear how well stool SCFA content reflects SCFA production by the intestinal microbiota.
A major motivation for investigating probiotic use in premature infants is the hope of preventing systemic infections and NEC. Given the low incidence of NEC and nosocomial infection in many institutions, large multi-center trials will be needed before recommendations can be made regarding widespread use of probiotics in premature infants (62, 63). Our study showed no differences between groups in NEC, documented infections, or adverse outcomes but it was not powered to detect such differences.
In summary, commercial probiotic products may not contain the precise organisms advertised; future studies of probiotics should include analysis of the products administered. Premature infants supplemented with PBP (which contains bifidobacteria and lactobacilli) showed a significant increase in the stool content of bifidobacteria and a lesser increase in the stool content of lactobacilli. No significant differences in somatic growth or stool SCFA content were seen between groups.
Acknowledgments
The authors express gratitude to the nursing staff in the UC Davis Medical Center NICU for their excellent care of the patients and their assistance with sample collection. We also thank Kathleen Shifley of the Department of Pediatrics at the Medical College of Wisconsin for her assistance with the qPCR analysis.
This research was supported in part by grants from the NIH UC Davis K30 Program (#UL1RR024146, MAU) and the Children's Miracle network (CLB). AM was supported by the Ministry of Education and Science of Spain. The authors have indicated they have no financial relationships relevant to this article to declare.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Roberts AK. Prospects for further approximation of infant formulas to human milk. Hum Nutr Appl Nutr. 1986;40 1:27–37. [PubMed] [Google Scholar]
- 3.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 4.Sakata H, Yoshioka H, Fujita K. Development of the intestinal flora in very low birth weight infants compared to normal full-term newborns. Eur J Pediatr. 1985;144:186–90. doi: 10.1007/BF00451911. [DOI] [PubMed] [Google Scholar]
- 5.Gewolb IH, Schwalbe RS, Taciak VL, et al. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F167–73. doi: 10.1136/fn.80.3.f167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millar M, Wilks M, Costeloe K. Probiotics for preterm infants? Arch Dis Child Fetal Neonatal Ed. 2003;88:F354–8. doi: 10.1136/fn.88.5.F354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westerbeek EA, van den Berg A, Lafeber HN, et al. The intestinal bacterial colonisation in preterm infants: a review of the literature. Clin Nutr. 2006;25:361–8. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Mohan R, Koebnick C, Schildt J, et al. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study. J Clin Microbiol. 2006;44:4025–31. doi: 10.1128/JCM.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal R, Sharma N, Chaudhry R, et al. Effects of oral Lactobacillus GG on enteric microflora in low-birth-weight neonates. J Pediatr Gastroenterol Nutr. 2003;36:397–402. doi: 10.1097/00005176-200303000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Vendt N, Grunberg H, Tuure T, et al. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: double-blind, randomized trial. J Hum Nutr Diet. 2006;19:51–8. doi: 10.1111/j.1365-277X.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 11.Puccio G, Cajozzo C, Meli F, et al. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum BL999 and prebiotics. Nutrition. 2007;23:1–8. doi: 10.1016/j.nut.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Weizman Z, Alsheikh A. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: a pilot study. J Am Coll Nutr. 2006;25:415–9. doi: 10.1080/07315724.2006.10719554. [DOI] [PubMed] [Google Scholar]
- 13.Kitajima H, Sumida Y, Tanaka R, et al. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 1997;76:F101–7. doi: 10.1136/fn.76.2.f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costalos C, Skouteri V, Gounaris A, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev. 2003;74:89–96. doi: 10.1016/s0378-3782(03)00090-2. [DOI] [PubMed] [Google Scholar]
- 15.Tako E, Glahn RP, Welch RM, et al. Dietary inulin affects the expression of intestinal enterocyte iron transporters, receptors and storage protein and alters the microbiota in the pig intestine. Br J Nutr. 2007:1–9. doi: 10.1017/S0007114507825128. [DOI] [PubMed] [Google Scholar]
- 16.Hoentjen F, Welling GW, Harmsen HJ, et al. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11:977–85. doi: 10.1097/01.mib.0000183421.02316.d5. [DOI] [PubMed] [Google Scholar]
- 17.Boehm G, Lidestri M, Casetta P, et al. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002;86:F178–81. doi: 10.1136/fn.86.3.F178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lidestri M, Agosti M, Marini A, et al. Oligosaccharides might stimulate calcium absorption in formula-fed preterm infants. Acta Paediatr Suppl. 2003;91:91–2. doi: 10.1111/j.1651-2227.2003.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 19.Fanaro S, Boehm G, Garssen J, et al. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatr Suppl. 2005;94:22–6. doi: 10.1111/j.1651-2227.2005.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 20.Marini A, Negretti F, Boehm G, et al. Pro- and pre-biotics administration in preterm infants: colonization and influence on faecal flora. Acta Paediatr Suppl. 2003;91:80–1. doi: 10.1111/j.1651-2227.2003.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 21.Fanaro S, Jelinek J, Stahl B, et al. Acidic oligosaccharides from pectin hydrolysate as new component for infant formulae: effect on intestinal flora, stool characteristics, and pH. J Pediatr Gastroenterol Nutr. 2005;41:186–90. doi: 10.1097/01.mpg.0000172747.64103.d7. [DOI] [PubMed] [Google Scholar]
- 22.Schmelzle H, Wirth S, Skopnik H, et al. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr. 2003;36:343–51. doi: 10.1097/00005176-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler E, Vanderhoof JA, Petschow B, et al. Term infants fed formula supplemented with selected blends of prebiotics grow normally and have soft stools similar to those reported for breast-fed infants. J Pediatr Gastroenterol Nutr. 2007;44:359–64. doi: 10.1097/MPG.0b013e31802fca8c. [DOI] [PubMed] [Google Scholar]
- 24.Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–4. doi: 10.1093/jn/137.11.2420. [DOI] [PubMed] [Google Scholar]
- 25.Marcobal A, Underwood MA, Mills DA. Rapid determination of the bacterial composition of commercial probiotic products by terminal restriction fragment length polymorphism analysis. J Pediatr Gastroenterol Nutr. 2008 doi: 10.1097/MPG.0b013e3181660694. In press. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto M, Hayashi H, Benno Y. Terminal restriction fragment length polymorphism analysis for human fecal microbiota and its application for analysis of complex bifidobacterial communities. Microbiol Immunol. 2003;47:133–42. doi: 10.1111/j.1348-0421.2003.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 27.Marsh TL, Saxman P, Cole J, et al. Terminal restriction fragment length polymorphism analysis program, a web-based research tool for microbial community analysis. Appl Environ Microbiol. 2000;66:3616–20. doi: 10.1128/aem.66.8.3616-3620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu WT, Marsh TL, Cheng H, et al. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997;63:4516–22. doi: 10.1128/aem.63.11.4516-4522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laroia S, Martin J. Methods for Enumerating and Propagating Bifidobacteria. Cultured Dairy Products Journal. 1991;26:32–33. [Google Scholar]
- 30.Chen HM, Lifschitz CH. Preparation of fecal samples for assay of volatile fatty acids by gas-liquid chromatography and high-performance liquid chromatography. Clin Chem. 1989;35:74–6. [PubMed] [Google Scholar]
- 31.Miwa H, Yamamoto M. High-performance liquid chromatographic analysis of serum short-chain fatty acids by direct derivatization. J Chromatogr. 1987;421:33–41. doi: 10.1016/0378-4347(87)80376-6. [DOI] [PubMed] [Google Scholar]
- 32.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 33.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenton TR, Sauve RS. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr. 2007;61:1380–5. doi: 10.1038/sj.ejcn.1602667. [DOI] [PubMed] [Google Scholar]
- 35.Canganella F, Paganini S, Ovidi M, et al. A microbiology investigation on probiotic pharmaceutical products used for human health. Microbiol Res. 1997;152:171–9. doi: 10.1016/s0944-5013(97)80009-2. [DOI] [PubMed] [Google Scholar]
- 36.Fasoli S, Marzotto M, Rizzotti L, et al. Bacterial composition of commercial probiotic products as evaluated by PCR-DGGE analysis. Int J Food Microbiol. 2003;82:59–70. doi: 10.1016/s0168-1605(02)00259-3. [DOI] [PubMed] [Google Scholar]
- 37.Szajewska H, Fordymacka A, Bardowski J, et al. Microbiological and genetic analysis of probiotic products licensed for medicinal purposes. Med Sci Monit. 2004;10:BR346–50. [PubMed] [Google Scholar]
- 38.Masco L, Huys G, De Brandt E, et al. Culture-dependent and culture-independent qualitative analysis of probiotic products claimed to contain bifidobacteria. Int J Food Microbiol. 2005;102:221–30. doi: 10.1016/j.ijfoodmicro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet. 2007;369:1614–20. doi: 10.1016/S0140-6736(07)60748-X. [DOI] [PubMed] [Google Scholar]
- 41.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. doi: 10.1016/s1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 42.Dani C, Biadaioli R, Bertini G, et al. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate. 2002;82:103–8. doi: 10.1159/000063096. [DOI] [PubMed] [Google Scholar]
- 43.Lin HC, Su BH, Chen AC, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 44.Bin-Nun A, Bromiker R, Wilschanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–6. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 45.Manzoni P, Mostert M, Leonessa ML, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis. 2006;42:1735–42. doi: 10.1086/504324. [DOI] [PubMed] [Google Scholar]
- 46.Ward RE, Ninonuevo M, Mills DA, et al. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl Environ Microbiol. 2006;72:4497–9. doi: 10.1128/AEM.02515-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riedel CU, Foata F, Philippe D, et al. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-kappaB activation. World J Gastroenterol. 2006;12:3729–35. doi: 10.3748/wjg.v12.i23.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He F, Morita H, Ouwehand AC, et al. Stimulation of the secretion of pro-inflammatory cytokines by Bifidobacterium strains. Microbiol Immunol. 2002;46:781–5. doi: 10.1111/j.1348-0421.2002.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 49.Ott SJ, Musfeldt M, Timmis KN, et al. In vitro alterations of intestinal bacterial microbiota in fecal samples during storage. Diagn Microbiol Infect Dis. 2004;50:237–45. doi: 10.1016/j.diagmicrobio.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Rochet V, Rigottier-Gois L, Rabot S, et al. Validation of fluorescent in situ hybridization combined with flow cytometry for assessing interindividual variation in the composition of human fecal microflora during long-term storage of samples. J Microbiol Methods. 2004;59:263–70. doi: 10.1016/j.mimet.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: a universal problem in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F428–30. doi: 10.1136/adc.2001.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 54.Brookes ST, Whitley E, Peters TJ, et al. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess. 2001;5:1–56. doi: 10.3310/hta5330. [DOI] [PubMed] [Google Scholar]
- 55.Wong JM, de Souza R, Kendall CW, et al. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–43. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 56.Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12:499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 57.Boehm G, Jelinek J, Stahl B, et al. Prebiotics in infant formulas. J Clin Gastroenterol. 2004;38:S76–9. doi: 10.1097/01.mcg.0000128927.91414.93. [DOI] [PubMed] [Google Scholar]
- 58.Wang C, Shoji H, Sato H, et al. Effects of oral administration of bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44:252–7. doi: 10.1097/01.mpg.0000252184.89922.5f. [DOI] [PubMed] [Google Scholar]
- 59.Waligora-Dupriet AJ, Dugay A, Auzeil N, et al. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr Res. 2005;58:629–35. doi: 10.1203/01.PDR.0000180538.13142.84. [DOI] [PubMed] [Google Scholar]
- 60.Pender SL, Quinn JJ, Sanderson IR, et al. Butyrate upregulates stromelysin-1 production by intestinal mesenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G918–24. doi: 10.1152/ajpgi.2000.279.5.G918. [DOI] [PubMed] [Google Scholar]
- 61.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–79. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kliegman RM, Willoughby RE. Prevention of necrotizing enterocolitis with probiotics. Pediatrics. 2005;115:171–2. doi: 10.1542/peds.2004-2271. [DOI] [PubMed] [Google Scholar]
- 63.Bell EF. Preventing necrotizing enterocolitis: what works and how safe? Pediatrics. 2005;115:173–4. doi: 10.1542/peds.2004-2360. [DOI] [PubMed] [Google Scholar]