Abstract
A tandem method for the synthesis of 2-hydrazolyl-4-thiazolidinones (5) from commercially available materials in a 3 component reaction has been developed. The reaction connects aldehydes, thiosemicarbazides and maleic anhydride, effectively assisted by microwave irradiation. The synthesis of a new type of compound, 2-hydrazolyl-5,5-diphenyl-4-thiazolidinone (7), obtained by treatment of thiosemicarbazone with benzil in basic media is also reported. HOMO/LUMO energies, orbital coefficients and charge distribution were used to explain the proposed reaction mechanism.
Keywords: 2-hydrazolyl-4-thiazolidinones, 2-hydrazolyl-5, 5-diphenyl-4-thiazolidinones, microwave, tandem reactions
4-Thiazolidinones are an important group of heterocycles found in numerous natural products and pharmaceuticals.1 In particular, 2-hydrazolyl-4-thiazolidinones (5) are a class of compounds that combine thiosemicarbazones with 4-thiazolidinones, two building blocks with interesting biological activities. For example, Trypanosoma cruzi,2 Plasmodium falciparum3 and antitumor4 activities have been described for thiosemicarbazones, and COX-2 inhibition,5 anti-HIV,6 and antibacterial7 effects as well as human chondrocyte antidegenerative8 properties have been found for 4-thiazolidinones. In addition, the combination of these two pharmacophores has been used to exhibit anti-Toxoplanma Gondii,9a antimicrobial,9b antiviral,10 and antifungal properties.11
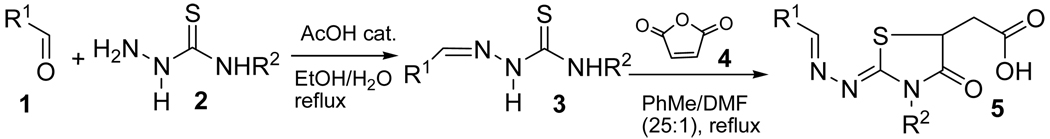
Among the reported methods for 2-hydrazolyl-4-thiazolidinone synthesis is a 2 step sequence: 1) a reaction between aldehydes (1) and thiosemicarbazides (2) to give thiosemicarbazones (3); 2) a thia-Michael addition of thiosemicarbazones (3) to maleic anhydride in dry PhMe and DMF at reflux to give the hydrazolyl-4-thiazolidinone (5) (Scheme 1).9
Scheme 1.
Stepwise 2-hydrazolyl-4-thiazolidinone synthesis under conventional conditions.
As a part of our search for new biologically active heterocyclic compounds, we focused on the possibility of optimizing this procedure by developing a tandem microwave-assisted reaction sequence.
Multi-step or cascade reactions can be defined as the combination of two or more reactions in a specific order that occur in one pot.12 They are very attractive due to their ease of setup. In traditional single-step processes, the reaction and product isolation are carried out independently and repeatedly to synthesize the target compounds. The former process allows a minimization of waste, and, compared to stepwise reactions, the amount of solvent, reagents, adsorbents, and energy is extensibly decreased.13
The use of microwave ovens to perform organic synthesis has received a great deal of attention over the last 10 years. Several publications have shown that microwave irradiation can circumvent the need for prolonged heating,14 and it is generally accepted that this source of energy minimizes side reactions and accelerates the rate of chemical reactions.15
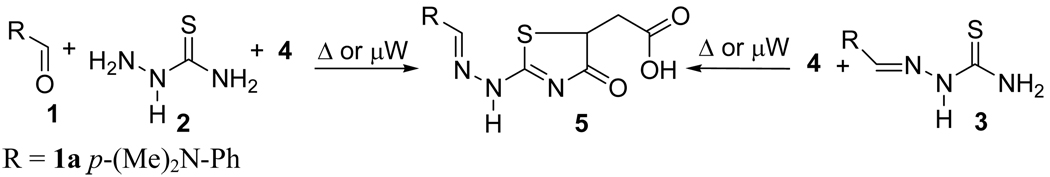
Herein, we wish to report an efficient tandem procedure for the synthesis of 2-hydrazolyl-4-thiazolidinones under microwave conditions. Different solvents and various reaction equivalents were explored until we obtained good isolated yields of thiazolidinones (Scheme 2, Table 1). Microwave heating for the synthesis of thiazolidinone 5a resulted in a significantly better yield compared to thermal conditions (75% vs 40%, entries 2 and 1, Table 1). Microwave irradiation also allowed for a faster conversion .
Scheme 2.
Tandem and stepwise reactions for the synthesis of 2-hydrazolyl-4-thiazolidinone.
Table 1.
Comparison of stepwise and tandem reactions using regular thermal and microwave heating.
| Entry | Reagents | Conditions | Solvent | Additive | Product, Yield (%)a |
|---|---|---|---|---|---|
| 1 | 3a (1 eq.), 4 (1.2 eq.) | Δ, reflux, 12 h. | PhMe | p-TsOH (0.1 eq.) | 5c (40) |
| 2 | 3a (1 eq.), 4 (1.2 eq.) | µW, 120 °C, 6 min | PhMe | p-TsOH (0.1 eq.) | 5c (72) |
| 3 | 3a (1eq.), 4 (5 eq.) | µW,120 °C, 6 min. | PhMe/DMF | p-TsOH (0.1 eq.) | 5b (68) |
| 4 | 1a (1eq.), 2 (1.2eq.), 4 (5eq.) | µW,120 °C, 6 min. | PhMe/DMF | p-TsOH (0.1 eq.) | 5b (82) |
Isolated yields after purification.
For tandem reactions, the best yields were obtained when a solvent mixture of PhMe/DMF (1:1) was used. Thiazolidinone 5a was prepared in 68% yield (54% considering both reactions) using a stepwise sequence, and in 82% yield under tandem conditions (entries 3 and 4, Table 1). We found that a tandem sequence was more efficient than a stepwise conversion under microwave irradiation.
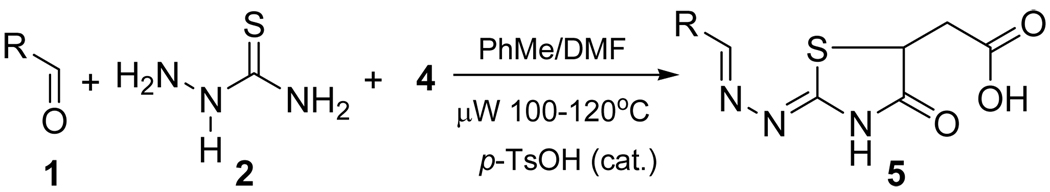
The optimal conditions for the microwave assisted tandem sequence were determined to be a mixture of PhMe/DMF (1:1) as a solvent, with catalytic p-TsOH and an excess of maleic anhydride (5 eq.) at 100–120 °C (Scheme 3).16
Scheme 3.
Optimized tandem reaction for 2-hydrazolyl-4-thiazolidinone synthesis.
Under optimized microwave conditions, a range of aromatic and some aliphatic aldehydes were converted to the desired heterocycles (Table 2). Aromatic aldehydes provided good yields from 45 to 82%, at 120 °C after a 6–12 min reaction time, except for 2-thiophenecarboxaldehyde where the yield dropped to 33%, probably due to the formation of polymeric materials derived from the starting material (entries 1 to 8, Table 2). The reaction seems to be independent of electron withdrawing or electron donating substitutions in the aldehydes (entries 1 and 3, Table 2).
Table 2.
Optimized tandem reaction for 2-hydrazolyl-4-thiazolidinone synthesis under microwave irradiation conditions.
| Entry | Compound RCHO | Temperature, time | Product (Yield)a | Mp °C |
|---|---|---|---|---|
| 1 | 1a p-N(Me)2-Ph | 120 °C, 6 min | 5a (82%) | 278–279 dec. |
| 2 | 1b p-OBut-Ph | 120 °C, 6 min | 5b (63%) | 249–250 |
| 3 | 1c p-NO2-Ph | 120 °C, 12 min | 5c (61%) | 270–271 dec. |
| 4 | 1d o-F-Ph | 120 °C, 12 min | 5d (57%) | 272–273 |
| 5 | 1e p-Cl-Ph | 120 °C, 12 min | 5e (70%) | 273–274 |
| 6 | 1f Ph | 120 °C, 6 min | 5f (45%) | 242–243 |
| 7 | 1g p-OMePh | 120 °C, 9 min | 5g (61%) | 262–263 |
| 8 | 1h 2-thiophenyl | 120 °C, 5 min | 5h (33%) | 255–256 |
| 9 | 1i CH(Me)2 | 100 °C, 12 min | 5i (34%) | 229–230 |
| 10 | 1j CH2CH2Ph | 100 °C, 6 min | 5j (64%) | 199–200 |
Isolated yields after purification.
For aliphatic aldehydes, we found that the optimum temperature was 100 °C; otherwise polymerization products were obtained. Compounds 5j and 5k were thus isolated in 34% and 64% yield, respectively (entries 9 and 10, Table 2).
The reactivity of thiosemicarbazone 3g with different Michael acceptors was also investigated. The reaction of thiosemicarbazone 3g with methyl acrylate17 or methyl cinnamate18 did not produce the expected 6-membered 1,3-thiazin-4-one.
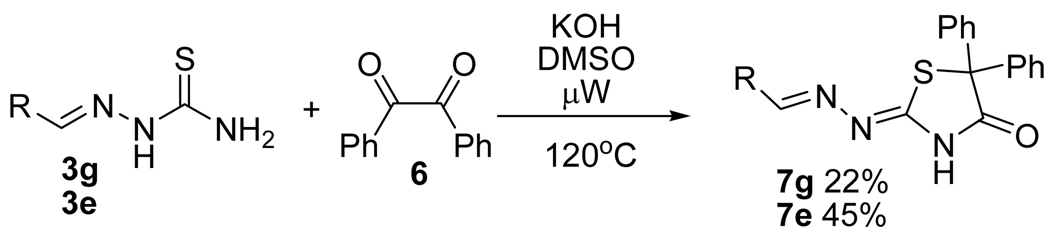
Furthermore, we explored the reaction of thiosemicarbazones 3e and 3g with benzil 9 as the electrophile. It is well known that ureas and thioureas react with benzil 9 to give 4-imidazolidinones through a benzilic acid rearrengenment.19 Barbainte and coworkers reported recently the reaction of thiosemicarbazide (2) with benzil to give 1,2,4-triazin-3-thione. The formation of the 6-membered ring can be explained by the nucleophilic attack of both N1 and N4 in compound 2 to the benzil carbonyl groups.20 Our results indicate that the reaction of thiosemicarbazone 3 with benzil 6 in KOH/DMSO under microwave conditions led to 2-hydrazolyl-5,5-diphenyl-4-thiazolidinones 10e, and 10g in 45% and 22% yield, respectively (Scheme 4).21 This heterocycle was previously unknown and was fully characterized. The HMBC experiment revealed a cross-peak between the N-H proton and the carbonyl carbon at C4, thus confirming the regiochemistry of the reaction.
Scheme 4.
Synthesis of 2-hydrazolyl-5,5-diphenyl-4-thiazolidinone.
With the goal of rationalize how this reaction proceeded, we undertook a frontier orbital analysis using the semi-empirical parametrization PM3.22 Table 3 shows HOMO/LUMO energies, coefficients and charge distributions calculated for model compounds. The HOMO/LUMO energy gap is small and it would seem that the reaction with thiosemicarbazone and benzil is kinetically favored and frontier orbital control, and not charge control, should govern the process.
Table 3.
Electronic parameters for selected model compounds and intermediates.
| Entry | Compound | Energy (eV) | Coefficients (%)a | Charge | ||
|---|---|---|---|---|---|---|
| HOMO | LUMO | HOMO | LUMO | |||
| 1 | 3g | −8.77 | −1.05 | S 44.3 | - | S −0.32 |
| N 13.6 | - | N 0.09 | ||||
| 2 | 6 | −10.01 | −0.61 | C1 25.5 | C1 0.29 | |
| C2 25.5 | C2 0.29 | |||||
| 3 | 9 | −8.69 | −0.38 | C2 17.0 | C2 0.09 | |
| C1 0.2 | C1 0.12 | |||||
Coefficients were calculated as (Σ ci2)× 100.
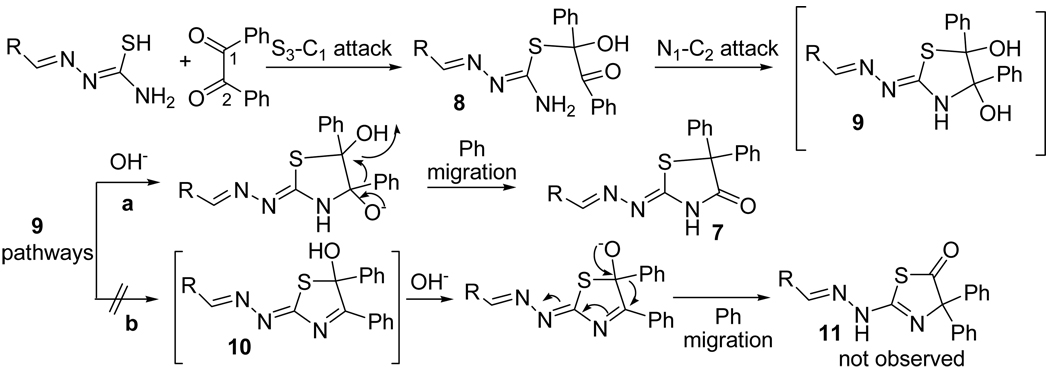
The proposed mechanism for the formation of 2-hydrazolyl-5,5-diphenyl-4-thiazolidinone 7 is depicted in Scheme 5. Based on HOMO/LUMO energies and orbital coefficients, the first step should be the nucleophilic attack of S to C1, one of the two carbonyl groups present in benzil, to form the tetrahedral intermediate 8. This intermediate proceeds by nucleophilic attack of N to C2 to give intermediate 9, in similar fashion to imidazoline formation.23
Scheme 5.
Proposed mechanism for 2-hydrazolyl-5,5-diphenyl-4-thiazolidinone (10) formation.
According to our results, the diol 9 undergoes a phenyl group migration, from C1 to C2, where the largest LUMO coefficient is located (entry 3, Table 3), which is in agreement with the observed regiochemistry, pathway a. Even though intermediate 10 was proposed by Butler and coworkers as the most favorable for phenyl group migration in the synthesis of imidazolines, in our case intermediate 10 would led to 5-thiazolidinone 11, which was never isolated.
In summary, in this investigation we explored the microwave-mediated tandem reactions of aldehydes, thiosemicarbazones and maleic anhydrides to produce 2-hydrazolyl-4-thiazolidinones, with yields ranging from 33 to 82%. The advantages in the use of this methodology are shorter time reactions, higher yields, and a minimization of synthetic operations, solvent use, and waste generation.
When we investigated the scope of the tandem synthesis for hydrazolyl-4-thiazolidinones 5, we were able to demonstrate that the process is general for aromatic and aliphatic aldehydes; however, the use of different types of Michael acceptors has not yet been accomplished. As an important part of this work, we also present the synthesis of 2-hydrazoyl-5,5-diphenyl-4-thiazolidinone 7, a new class of 4-thiazolidinones. We propose a mechanism for the heterocycle formation based on a benzilic acid rearrangement promoted by thiosemicarbazone.
Acknowledgment
This work was supported by the National Institutes of Health-FIRCA (R03TW007772) and UdelaR (CSIC-No 349). We would like to thank Prof. O. Ventura and Dr. P. Saenz in the Computational Chemistry and Biology Group for assistance in theoretical calculations, and Dr. A. Rodríguez for HRMS ESI TOF-MS analyses (Polo Tecnólogico-FQ; Proyecto Enlaces-UE URY-2003-5906).
References
- 1.Verma A, Saraf SK. Eur. J. Med. Chem. 2008;43:897–905. doi: 10.1016/j.ejmech.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 2.a) Du X, Guo C, Hansell E, Doyle P, Caffrey C, Holler T, McKerrow J, Cohen F. J. Med. Chem. 2002;45:2695–2703. doi: 10.1021/jm010459j. [DOI] [PubMed] [Google Scholar]; b) Cohen FE, Du X, Guo C, McKerrow JH. 6 897 240, 2004 U.S. Patent.
- 3.Walcourt A, Loyevsky M, Lovejoy DB, Gordeuk VR, Richardson DR. Biochem. Cell Biol. 2004;36:401–407. doi: 10.1016/s1357-2725(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 4.Senaratne D, Kalinowski DS, Islam M, Bernhardt PV. J. Med. Chem. 2006;49:6510–6521. doi: 10.1021/jm0606342. [DOI] [PubMed] [Google Scholar]
- 5.Vigorita MG, Ottanà R, Monforte F, Maccari R, Monforte MT, Trovato A, Taviano MF, Miceli N, De Luca G, Alcaro S, Ortuso F. Bioorg. Med. Chem. 2003;11:999–1006. doi: 10.1016/s0968-0896(02)00518-7. [DOI] [PubMed] [Google Scholar]
- 6.Rawal RK, Tripathi R, Katti SB, Pannecouque C, De Clerq E. Bioorg. Med. Chem. 2007;15:1725–1731. doi: 10.1016/j.bmc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Bonde CG, Gaikwad NJ. Bioorg. Med. Chem. 2004;12:2151–2161. doi: 10.1016/j.bmc.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Ottanà R, Maccari R, Ciurleo R, Vigorita MG, Panico AM, Cardile V, Garufi F, Ronsisvalle S. Bioorg. Med. Chem. 2007;15:7618–7625. doi: 10.1016/j.bmc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.a) Tenorio R, Carvalho C, Pessanha C, de Lima J, de Faria A, Alves A, de Melob EJ, Goes A. Bioorg. Med. Chem. Lett. 2005;15:2575–2578. doi: 10.1016/j.bmcl.2005.03.048. [DOI] [PubMed] [Google Scholar]; b) Aquino T, Liesen A, Silva R, Lima V, Carvalho C, Faria A, Araujo JM, de, Lima JG, Alves AJ, de Melob EJT, Goesa AJS. Bioorg. Med. Chem. 2008;16:446–456. doi: 10.1016/j.bmc.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Plut M, Pollak A, Tisler M, Likar M, Schauer PS. J. Med Chem. 1966;9:430–431. doi: 10.1021/jm00321a047. [DOI] [PubMed] [Google Scholar]
- 11.Krbavcic A. [Google Scholar]; a) Badawy MA, Abdel-Hady SA, Kadry AM, Ibrahim YA. Sulfur Lett. 1989;9:149–157. [Google Scholar]
- 12.Ho T. Tandem Organic Reactions. New York: Wiley-Interscience; 1992. [Google Scholar]
- 13.a) Tietze LF, Beifuss U. Angew. Chem. 1993;105:137. [Google Scholar]; b) Tietze LF. Chem. Rev. 1996;96:115. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]
- 14.Wipf P, Fletcher JM, Scarone L. Tetrahedron Lett. 2005;46:5463–5466. [Google Scholar]
- 15.Hayes BL. Microwave Synthesis, Chemistry at the Speed of Light. North Carolina: CEM Publishing: Matthews; 2002. p. 16. Chapter 1. [Google Scholar]
- 16.Typical procedure for thiazolidinone preparation (5c): To a stirred solution of p-N,N-dimethylamine benzaldehyde (300 mg, 2.0 mmol) in toluene (1 mL) and DMF (1 mL) were added thiosemicarbazide (220 mg, 2.4 mmol), p-toluene sulfonic acid (30 mg, 0,2 mmol,) and maleic anhydride (987 mg, 10.0 mmol). The reaction mixture was heated in a stirred microwave vial for 9 min at 120 °C (200 W), poured into water (30 mL) and extracted with ethyl acetate (3 ×30 mL). The combined organic layers were dried (Na2SO4) filtered and concentrated. The residue was finally recrystallized from methanol to give 5c (527 mg, 82 % yield) as a yellow solid: mp 278–279 °C: 1H NMR (400 MHz, D2O/K2CO3) δ̣ 2.42 (dd, J2 = 11.7, J3 = 16.2 Hz, 1 H), 2.93 (s, 6 H), 3.06 (dd, J2 = 4.1, J3= 16.2 Hz, 1 H), 4.22 (dd, J1 = 4.1, J2 = 11.7 Hz, 1 H), 6.88 (d, J = 9.0 Hz, 2 H), 7.61 (d, J = 9.0 Hz, 2 H), 8.20 (s, 1 H); 13C NMR (100MHz, D2O/K2CO3) δ̣ 40.02, 41.90 (Cendo), 41.99 (Cexo), 49.90, 113.44, 122.80, 129.22, 152.83, 155.88, 161.05, 178.95 (Cexo), 179.37 (Cendo), 190.77 (COOH); HRMS calculated for C14H16N4O3S [M+-H]: 319.0865, found: 319.0859.
- 17.Under the following reaction conditions: methyl acrylate acid, 3h, EtOH, µW, K2CO3, 120 °C, 15 min or EtOH, KOH, µW 120 °C, 15 min, no product was observed.
- 18.Under the following reaction conditions: methyl cinnamate, 3h, PhMe, µW 120 °C, p-TosOH 19 min, no product was observed.
- 19.Muccioli GG, Poupaert JH, Wouters J, Norberg B, Poppitz W, Scriba G, Lambert DM. Tetrahedron. 2003;59:1301–1307. [Google Scholar]
- 20.Braibante M, Braibante H, Uliana MP, Costa CC, Spenazzatto M. J. Braz. Chem. Soc. 2008;19:909–913. [Google Scholar]
- 21.To a stirred solution of 4-methoxybenzylidene thiosemicarbazone 3g (177 mg, 0.91 mmol) in DMSO (1.5 mL) were added benzil (150 mg, 0.60 mmol) and KOH 1.2 M (0.3 mL, 0.36 mmol). The reaction mixture was heated in a microwave for 10 min at 120 °C (200 W) with stirring, poured into water (30 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were dried (Na2SO4) filtered and concentrated. The residue was finally purified by chromatography on SiO2 (AcOEt/Hexanes, 1:4) to give compound 10g (80 mg, 22%) as a white solid: mp 192.5–193.1 °C; 1H NMR (400MHz, CDCl3) δ 3.85 (s, 3 H), 6.94 (d, J = 8.8 Hz, 2 H), 7.36–7.42 (m, 10 H), 7.81 (d, J = 8.8 Hz, 2 H), 9.08 (s, 1 H), 9.10 (s, 1 HNH). 13C NMR (100 MHz, CDCl3) 55.41, 71.36, 114.25, 125.29, 127.01, 127.56, 128.69, 129.01, 130.64, 137.60, 162.91, 169.73, 180.14; HRMS calculated for C14H16N4O3S [M++Na]: 424.1081, found: 424.1090.
- 22.Available in the commercial computer program package HyperChem Pro Molecular.
- 23.Butler AR, Leitch E. J. Chem. Soc. Perkin Trans.2. 1977;14:1972–1976. [Google Scholar]