Abstract
BACKGROUND
Young female adult and adolescent cancer patients facing life-preserving but fertility-threatening chemo- or radiation-therapy are increasingly seeking options to protect their reproductive potential. Ovarian tissue cryopreservation with transplantation is a promising technique to safeguard fertility in cancer patients. However, this method may risk re-introduction of the original cancer to the survivor of the disease. Thus, developing a method for in vitro growth of immature follicles may provide a method for fertility restoration in the future.
METHODS
Human secondary follicles were isolated from ovarian tissues obtained from cancer patients and grown in vitro within a bio-engineered culture system for 30 days.
RESULTS
Human ovarian follicles became steroidogenically active, and developed from the early secondary to antral stage in vitro. The follicles contained healthy, growing oocytes that were connected by transzonal projections between the somatic cells and oocyte.
CONCLUSIONS
Our data support the notion that human follicle development can be achieved in vitro in a bio-engineered culture system. More studies are required to investigate whether the fully sized oocytes obtained from in vitro grown follicle are competent to resume meiosis and be fertilized.
Keywords: human, ovarian follicle, in vitro growth, oocyte
Introduction
Young women facing a new cancer diagnosis are left with difficult choices, not only in how to manage their cancer, but also to decide whether to take fertility-protective measures. Several chemotherapeutics used in anti-cancer regimens unintentionally threaten fertility by destroying the immature follicle pool. The elimination of the primordial follicle pool results in either acute ovarian failure or a chronic ovarian insufficiency, resulting in an early menopause and associated loss of reproductive capacity (Agarwal and Chang, 2007; Cvancarova et al., 2009; Jeruss and Woodruff, 2009; Schover, 2009). For female adolescents and young adults, the increasing probability of surviving a cancer diagnosis combined with the expectation and desire for reproductive options following cancer remission is driving the intense need for fertility sparing techniques.
Currently, there are several methods available to women hoping to preserve their fertility before cancer therapy. As it has been used most often, traditional hormone stimulation and in vitro fertilization (IVF) followed by embryo cryopreservation is the most successful approach to preserve fertility (Oktay et al., 2003, 2005; Rao et al., 2004; Juretzka et al., 2005; Lee et al., 2006). Recently, live births have also been achieved with cryopreserved oocytes harvested before cancer treatment (Yang et al., 2007; Porcu et al., 2008). However, both methods require a delay in cancer treatment and hormonal stimulation that are not prudent for some patients. In contrast, ovarian tissue cryopreservation followed by transplant is a promising fertility preservation approach that can usually be performed immediately without hormonal stimulation. Transplantation of ovarian cortical strips has resulted in viable offspring in sheep (from cryopreserved tissue) (Gosden et al., 1994; Salle et al., 2002) and monkeys (from fresh tissue) (Lee et al., 2004). In the human, ovarian function can be restored within 3 months after transplanting thawed tissue into a woman with ovarian failure (Oktay et al., 2004; Schmidt et al., 2005; Demeestere et al., 2006; Rosendahl et al., 2006). Furthermore, live births in humans have been achieved by transplanting fresh ovarian tissue (Silber et al., 2005, 2008; Silber and Gosden, 2007). Worldwide, five live births have been reported thus far as a result of auto-transplanting frozen/thawed ovarian tissues (Donnez et al., 2004; Meirow et al., 2005; Demeestere et al., 2007; Andersen et al., 2008). Despite these promising findings, transplantation of cryopreserved tissue carries the risk of re-introducing cancer cells into the patient (Shaw and Trounson, 1997; Meirow et al., 1998, 2008). Thus, the in vitro growth of immature follicles derived from ovaries collected prior to commencement of cancer treatment is an important direction for research.
Phenocopying the complex interplay of growth factors and hormone signals required for the coordinated developmental process of follicle development and oocyte maturation in vitro is challenging; yet follicle culture systems for mouse (Eppig and Schroeder, 1989; Spears et al., 1994; Cortvrindt et al., 1996; Eppig and O'Brien, 1996; O'Brien et al., 2003; Xu et al., 2006a, b), large animals (Newton et al., 1999; Gutierrez et al., 2000; Telfer et al., 2000; Wu et al., 2001; Picton et al., 2003; Thomas et al., 2007) and the human (Roy and Treacy, 1993; Abir et al., 1997, 1999, 2001, 2006; Hovatta et al., 1997; Wright et al., 1999; Scott et al., 2004a, b; Telfer et al., 2008, Amorim et al., 2009) have been developed in both 2-dimensional (2D) and 3-dimensional (3D) formats. These systems have been successful in supporting the development of mouse secondary follicles, but have not yet been adapted for routine use in large animals and the human. The purpose of the in vitro human follicle growth (IVFG) system is to mimic the in vivo process by providing follicles with appropriate growth factors and hormones, in the correct amount, at the right time, which allows growth of the follicle and oocyte whereas maintaining the essential connections between somatic cells and the oocyte called transzonal projections (TZPs) (Anderson and Albertini, 1976; Albertini and Barrett, 2003).
The aim of the present study was to investigate in vitro human secondary follicle growth and survival in a long-term 3D culture system. We examined the ability of these follicles to form an antrum, secrete hormones and support oocyte development.
Materials and Methods
Ovary collection
Ovarian tissues were obtained from 14 female cancer patients (Table I), ages 16–39 years (mean ± SD, 27.3 ± 8.1) following informed consent under an Institutional Review Board-approved protocol at Northwestern University. These patients were seeking fertility-sparing options in our Oncofertility program, which provided them with a full-range of fertility-sparing options. Ovaries were removed by laparoscopic and transported to the laboratory in <2 h in cold (4°C) MOPS-HTF Medium (CooperSurgical, Trumbull, CT, USA).
Table I.
Secondary follicle isolation
| Patient | Age | Diagnosis | FL-Mgel (n) | FL-ALG (n) | Total (n) |
|---|---|---|---|---|---|
| Pt1 | 36 | Breast Cancer | 2 | 0 | 2 |
| Pt2 | 39 | Breast Cancer | 0 | 4 | 4 |
| Pt3* | 36 | Breast Cancer | 0 | 0 | 0* |
| Pt4* | 36 | Breast Cancer | 0 | 0 | 0* |
| Pt5 | 29 | Cervical Cancer | 6 | 0 | 6 |
| Pt6 | 19 | Ewing's Sarcoma | 3 | 0 | 3 |
| Pt7* | 16 | Ewing's Sarcoma | 0 | 0 | 0* |
| Pt8* | 26 | Hodgkins Lymphoma | 2 | 3 | 5* |
| Pt9 | 16 | Non-Hodgkins Lymphoma | 4 | 0 | 4 |
| Pt10 | 33 | Ovarian Cancer | 0 | 0 | 0 |
| Pt11 | 27 | Ovarian Mass | 1 | 0 | 1 |
| Pt12 | 27 | Ovarian Mass | 0 | 0 | 0 |
| Pt13 | 16 | Penioblastoma | 2 | 4 | 6 |
| Pt14 | 26 | Rectal Cancer | 2 | 7 | 9 |
Note: FL-Mgel, follicle cultured in Matrigel; FL-ALG, follicle cultured in 0.5% alginate.
*The patients received chemo/radiations therapy before tissue donation.
Follicle isolation
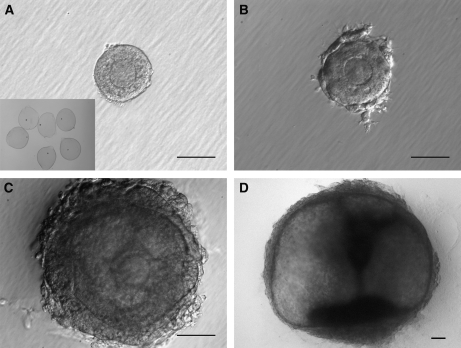
All follicles included in this study were isolated from fresh tissue using a modified method as described previously (Abir et al., 1999). Briefly, ovarian cortical strips were cut into ∼1 mm3 pieces in dissection media (Liebovitz L-15 media, Invitrogen, Carlsbad, CA, USA) supplemented with 1% Serum Protein Substitute (SPS, CooperSurgical, Trumbull, CT, USA), 100 IU/ml penicillin and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). The tissue was then enzymatically digested by transferring to incubation media, made by supplementing dissection media (above) with 0.2% collagenase and 0.02% DNase (Worthington Biochemical, Lakewood, NJ, USA), for 1.5 h at 37°C in a shaking water bath. In most cases, this short-term digestion was not enough to completely free follicles from the ovarian cortex because of the toughness of human cortex; however, the enzymatic digestion facilitated the following mechanical isolation. After rinsing cortex twice with fresh dissecting media, follicles were then mechanically isolated from the cortex using 25-gauge needles in dissection media, transferred to maintenance media (αMEM, (Invitrogen, Carlsbad, CA, ISA), supplemented with 1% SPS, 100 IU/ml penicillin and 100 µg/ml streptomycin), and placed in an incubator at 37°C and 5% CO2. The mechanical isolation was carried out for 2–6 h prior to encapsulation. Only immature follicles (Class 1 and 2) (Gougeon, 1996) that contained a clear, visible, centrally-located oocyte, healthy granulosa cells and no signs of antrum formation were encapsulated and cultured (Fig. 1A). A few small antral follicles were fixed in 4% paraformaldehyde (PFA) as a control for confocal analysis of follicle structure, oocyte-somatic cell interactions as well as germinal vesicle (GV) stage.
Figure 1.
Characteristics of in vitro growth follicles.
Secondary follicles (135 µm) (A) were isolated from the ovarian cortex and grown in a 3D environment (A, insert) for 30 days. Granulosa cells continuously proliferated. The follicles developed theca-like cells at day 5 (182 µm) (B), form an antrum and a central COC at day 15 (345 µm) (C), and maintained 3D structural integrity throughout the culture (1489 µm) (D). Bar = 100 µm.
Matrix preparation
Sodium alginate (55–65% guluronic acid) was obtained from FMC BioPolymers (Philadelphia, PA, USA). A 1% (w/v) solution of sodium alginate was stripped using activated charcoal (0.5 g charcoal/g alginate) to remove organic impurities, sterile filtered using 0.22 µm filters, and lyophilized within Steriflip conical tubes (Millipore, Billerica, MA, USA). Sodium alginate was reconstituted to 0.5% (w/v) alginate in 1× phosphate buffered saline (PBS) for use (Xu et al., 2006a, b). Matrigel (BD Cat 354234, Bedford, MA, USA) was thawed on ice overnight, and diluted 1:3 with cold αMEM (Scott et al., 2004a, b).
Follicle encapsulation and culture
Selected follicles were encapsulated into either a 0.5% alginate bead (Xu et al., 2006a, b) or embedded into diluted Matrigel matrix as previously described (Scott et al., 2004a, b) with slight modification. Briefly, single follicles were transferred to individual droplets of alginate (∼5 µl) on a polypropylene mesh (0.1 mm opening, McMaster-Carr, Atlanta, GA, USA). The mesh was then inverted and gently tapped over a cross-linking solution (50 mM CaCl2 and 140 mM NaCl), causing the droplets to fall into the solution (Xu, et al., 2009). The beads were allowed to cross-link for two minutes, removed, and rinsed in maintenance media. Beads were then transferred to individual wells of a 96-well plate containing 100 µl of culture media (Xu et al., 2006a, b) [αMEM supplemented with 6% SPS (v/v), 1 mg/ml bovine fetuin (Sigma-Aldrich, St. Louis, MO, USA), 5 µg/ml insulin, 5 µg/ml transferrin, 5 ng/ml sodium selenite (Sigma-Aldrich, St. Louis, MO, USA) and 0.1 IU/ml FSH (gift from Organon, Roseland, NJ, USA)]. SPS was used here to replace the bovine serum albumin used for murine follicle culture. To embed a follicle into the Matrigel, the Millicell insert (Millipore, Billerica, MA, USA) was filled with 100 µl diluted Matrigel and permitted to pre-gel for 30 min. The individual follicle was then transferred into the middle of the Matrigel and allowed to gel in the incubator for an additional 30 min. The insert was then transferred to one well of a 24-well plate containing 400 µl of the culture media described above. Follicles, either encapsulated within 0.5% alginate or embedded within Matrigel were cultured at 37°C with 5% CO2 for up to 30 days. At different time points, some follicles were fixed or frozen for histological examination or gene expression analysis (unpublished data). Ten follicles from each treatment group were grown for 30 days. Every 2 days, half of the culture media was exchanged and stored at −80°C for hormonal measurements. Fresh culture media was prepared weekly.
Follicle and oocyte measurement
Photographs of each follicle (during the culture period) were captured using a Leica DM IL light microscope (Leica, Wetzlar, Germany) equipped with phase objectives, a heated stage, a Spot Insight 2 Megapixel Color Mosaic camera and Spot software (Spot Diagnostic Instruments, Sterling Heights, MI, USA). Follicle diameters were later measured using ImageJ software (National Institutes of Health, USA) as previously described (West et al., 2007; West-Farrell et al., 2009). Oocyte diameters were measured without zona pellucida.
Sectioning and staining
Follicles were fixed in 4% PFA for 30 min at 37°C and then overnight at 4°C. Fixed follicles were dehydrated in increasing concentrations of ethanol (10–100%) and embedded in paraffin using an automated tissue processor (Leica, Manheim, Germany). Serial 5 µm sections were cut and stained with hematoxylin and eosin.
3D confocal staining and imaging
Follicles for confocal microscopy studies were fixed in 4% PFA at 37°C for 1 h, followed by 1 h in wash buffer (Barrett and Albertini, 2007). They were then stained overnight at 4°C on a shaker with Rhodamine-Phalloidin (1:50 Molecular Probes, Invitrogen, Eugene, Oregon) that labels F-actin in TZPs, rabbit anti-connexin 43 (1:100 Zymed) that labels gap junctions or affinity purified polyclonal rabbit antisera that recognize the inhibin βA-subunit (1:100) or the inhibin βB-subunit (1:100). The inhibin subunit antisera were a gift from Dr W. Vale (The Salk Institute, La Jolla, CA, USA). The primary antibodies were followed by incubation with 1:800 goat anti-rabbit Alexa 568 for inhibin βA and connexin 43, 1:800 goat anti-rabbit Alexa 488 for inhibin βB (Molecular Probes) and 1 µg/ml Hoechst 33342 (Molecular Probes) for chromatin/DNA. Follicles were mounted in 5–10 µl of a 50% glycerol/PBS solution containing 25 µg/ml sodium azide. The coverglass was placed on glass shards, to prevent the compression of the follicle. Follicles were imaged on a Zeiss LSM 510 confocal microscope using a Neoflaur 40× oil objective. Overlapping 1–3 µm sections were taken throughout each follicle imaged.
Hormone assays
17β-estradiol (E2) and Progesterone (P4) concentrations in culture media were measured by specific electrochemiluminescent assay using a Roche Elecsys 2010 Analyzer (Roche, Indianapolis, IN, USA). The inter-assay variations were 6.1% for 17β-estradiol and 5.4% for progesterone. The limits of sensitivity were 5 pg/ml for 17β-estradiol and 0.03 ng/ml for progesterone. Inhibin A, inhibin B and anti-Müllerian hormone (AMH) were measured using ELISA kits (DSL-10-28100, DSL-10-84100 and DSL-10-14400, Diagnostic Systems Laboratories, Webster, TX, USA) following manufacturer instructions. The intra-assay variations were 8.7% for inhibin A, 3.2% for inhibin B and 3.8% for AMH. The limits of sensitivity for inhibin A, inhibin B and AMH were 10, 10 and 20 pg/ml, respectively.
Results
Isolation and growth of human secondary follicles
In order to assess the ability of human secondary follicles to grow in 3D matrices, we isolated follicles from ovarian tissues donated from 14 different Oncofertility patients (Table I). The number of secondary follicles isolated, from each patient sample ranged from 0 to 9, which varied based on tissue amount, patient age, cancer type and whether the patient received prior chemo/radiation therapy.
Follicles were cultured for up to 30 days by embedding in matrigel (n = 18) or encapsulation in alginate (n = 22). The average size of un-cultured isolated follicle was similar between culture groups, matrigel = 170.8 µm (±51.1) and alginate = 178.4 µm (±69.2). Matrigel embedded secondary follicles (Fig. 1A) or alginate encapsulated follicles (Fig. 1A inset) displayed at least two cell layers of granulosa cells. After 5 days of culture both groups of follicles displayed theca-like cells surrounding the follicle basement membrane (Fig. 1B). By day 15 of culture, follicles observed by brightfield microscopy contained a fluid filled antrum and distinct mural granulosa and cumulus granulosa cell types (Fig. 1C). The follicle also displayed a thickening of the theca-like cell layer. By day 30 of culture, the cumulus-oocyte complex was barely detectable by light microscopy due to the size of the follicle (Fig. 1D).
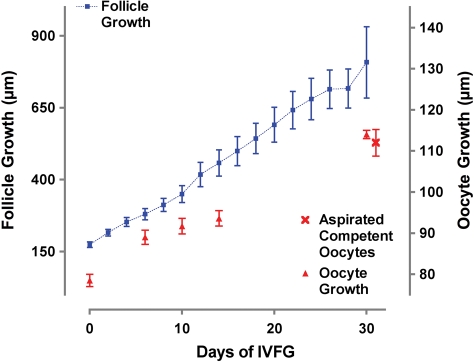
Because both matrigel and alginate environments supported follicle growth without significant differences (data not shown), the growth data were combined (Fig. 2). From day 0 to day 30 follicles linearly grew in size from 175 ± 10 to 715 ± 68 µm. Moreover, the oocyte size also increased in culture as the follicle grew three-dimensionally. After day 15 of culture, the oocyte could no longer be observed for measurement until the follicle was fixed for analysis. All secondary follicles that were grown for 30 days survived through the culture, and 75% of follicles developed visible antrum that commenced on approximately day 12 (±3.9) of culture in follicles with an average diameter of 393.6 µm (±93.6).
Figure 2.
In vitro follicle and oocyte growth.
From day 0 to day 30, the average follicle size of the entire follicle population increased from 175 µm (±10) to 715 µm (±68), and the average oocyte size increased from 78.5 µm (±1.5) to 114.0 µm (±1.1). The terminal size of the oocytes grown in vitro was indistinguishable from oocytes aspirated from in vivo matured follicles during IVM. Data are represented as mean ± SEM.
In vitro grown follicles secrete steroid and peptide hormones
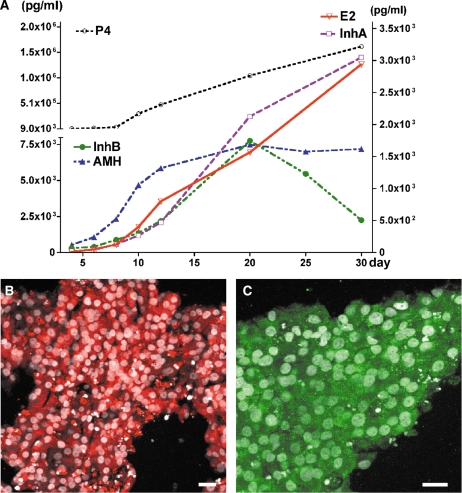
To determine whether IVFG was similar to in vivo follicles in their capacity to secrete hormones, we analyzed growth media from individual follicles every other day of culture. Steroid hormones were not detected in the condition media before day 5. Increasing levels of steroid hormones, 17β-estradiol and progesterone, were measured in conditioned media collected from individual follicle cultures on day 5 through day 30 (Fig. 3A and B). The ratio of 17β-estradiol to progesterone decreased over the culture period (Fig. 3C). The peptide hormone inhibin A continually increased as follicles were cultured to 30 days whereas inhibin B increased from day 4 to day 16 but decreased from day 16 to day 30. AMH was also measured in cultured follicles and followed the secretion of inhibin B (Fig. 3). When comparing all hormones (Fig. 4A) over time in one single follicle (seen in Fig. 1), it was found that secondary follicles grown in vitro did not secrete steroid nor peptide hormones at detectable levels during the initial days of culture (days 0–4), however, all hormones were measurable by day 8. All hormones examined appeared to increase steadily until day 20 of culture after which levels of AMH began to drop slowly and inhibin B decreased rapidly. Both inhibin β-subunits (βA and βB) were localized to the mural granulosa cells of the same follicle at 30-days of culture (Fig. 4B and C).
Figure 3.
Hormone levels in human follicle cultures.
17β-Estradiol (A), progesterone (B), E2/P4 ratio (C), inhibin A (D), inhibin B (E) and AMH (F) were measured in individual follicles. Hormone levels on an individual follicle basis change throughout the culture period and reflect the overall maturation status of that individual follicle.
Figure 4.
Steroid and peptide hormone profile in a growing secondary follicle.
Hormone data obtained from one follicle growth for 30 days in vitro (A). The inhibin subunits βA (B) and βB (C) were expressed in the human mural granulosa cells on paraffin sections. Bar = 20 µm in B and C.
In vitro follicle growth supports oocyte growth
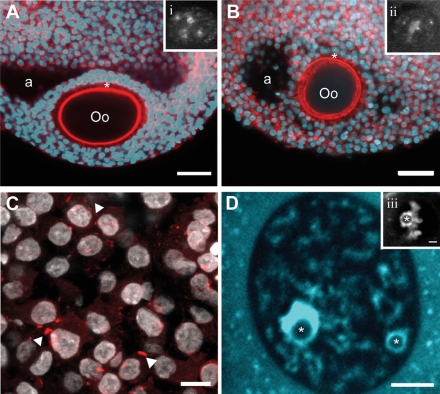
Since the connection between somatic cells and the oocyte has never before been studied in human follicles, we used confocal reconstructions to observe the F-actin based connections (TZPs) in freshly isolated, early-antral follicles (Fig. 5A). We examined 14-day cultured follicles for the presence of TZPs and found that they maintain a dense network of TZPs emanating from the surrounding granulosa cells (Fig. 5B). We also reconstructed these cultured follicles and found them to be similar to the in vivo benchmark follicles (http://oncofertility.northwestern.edu/research/human-follicle-maturation-in-vitro/shared-files). This follicle also showed the formation of an early antrum and the differentiation of a mural granulosa and cumulus granulosa. Connexin 43, a gap junction protein, was also examined in cultured follicles and was shown to be present at the cumulus granulosa cell junctions (Fig. 5C). Oocyte growth increased with time in culture from 75 µm to a terminal size of 110 µm (Fig. 2). This size was indistinguishable from competent oocytes aspirated from in vivo antral human follicles that went on to mature to MII stage in culture (Fig. 2). Healthy and intact GVs were seen during early stages of culture. Stage II GVs were observed in both fresh-isolated follicles (Fig. 5A, inset) as well as cultured follicles (14 days) (Fig. 5B inset) and progressed to stage IV by the end of culture (Fig. 5D).
Figure 5.
In vitro growth follicles resemble in vivo follicles.
(A) A fresh-fixed human follicle, and (B) an isolated human follicle grown in alginate for 14 days show similar characteristics including an antral cavity (a) as well as the development of the cumulus-oocyte complex (Oo =oocyte). TZPs (*) are maintained between the oocyte and surrounding granulosa cells (shown by F-actin in red). In both follicles, the oocytes are incompetent to resume meiosis as shown by the GV stage II (insets i and ii, chromatin/DNA). Scale represents 50 µm. (C) Localization of connexin-43 (arrowheads) to the gap junctions between granulosa cells in in vitro growth follicles show the cell–cell interactions that are important for bidirectional transport of small molecules between cells and that are essential for follicle growth and cell differentiation. Scale represents 20 µm. (D) An oocyte from an in vitro growth follicle contains a stage IV GV (chromatin/DNA-blue) represented by a chromatin-surrounded nucleolus (*). Scale represents 5 µm. This can be compared with a fresh-isolated human cumulus-oocyte complex that is competent to undergo meiotic resumption as depicted by the stage IV GV (inset iii, scale represents 5 µm) containing a chromatin surrounded nucleolus (*).
Discussion
In vitro follicle growth provides the opportunity to expand research initiatives in follicle development, as well as to potentially provide expanded options to young cancer patients. Various groups (Abir et al., 1997; Hovatta et al., 1997; Wright et al., 1999; Telfer et al., 2008), including our own are working toward robust, reliable in vitro systems that will provide the next generation of research discoveries as well as 1 day aiding in fertility sparing plans for cancer patients. To achieve these two goals, we have adapted two 3D systems to human follicles. To the best of our knowledge, this is the first report in the humans showing the in vitro growth of large, antral follicles, which contains full size oocytes with stage IV GVs, from secondary stage in vitro. Importantly, the systems reported here can be further engineered to continue improving the in vitro environment in which these follicles grow. We suspect that the combination of engineered ECM (Kreeger et al., 2006; Xu et al., 2007), rigidity (Xu et al., 2006a, b; West et al., 2007) and the ability to temporally provide hormones (Kreeger et al., 2005) to individual follicles will create the next generation of improvement in a system that has already demonstrated significant success in the murine model.
Follicle growth
Previous studies have shown human follicle development in vitro (Roy and Treacy, 1993; Abir et al., 1997; Telfer et al., 2008). Antrum formation was previously achieved using cultured secondary follicles (Roy and Treacy, 1993; Abir et al., 1997); this development was shown to be dependent on FSH (Abir et al., 1997). Theca-like cells were observed when unilaminar follicles developed to secondary follicles in a collagen gel culture system (Abir et al., 1999, 2001). More recently, primordial/primary follicles were shown to enter the growing pool of follicles and develop to early antral stage (∼150 µm) in 10 days using a two-step serum-free culture system in the presence of activin A (Telfer et al., 2008). In another study, primordial/primary follicles (34–51 µm) isolated from cryopreserved tissue survived for 7 days and increased in size up to 44–70 µm in alginate matrix culture system (Amorim et al., 2009). We used 3D culture systems to culture small secondary follicles (Class 1 and 2 as described by Gougeon, 1996). In our study, individual non-antral, secondary follicle grew substantially over 30 days, and reached an average size of 800 µm. Our studies have also shown that 30 days of culture was enough time to grow follicles to 1 mm and produce oocytes with nuclear competence (stage IV GVs). The growth of the oocyte correlated with an increase in follicle size; the fact that oocyte growth and development was supported in conjunction with follicle growth was a significant achievement. Though we have not yet explored whether these oocytes also have cytoplasmic competence after 30 days in culture, it has been shown that a follicle size of 1.6 mm can be aspirated for cumulus-oocyte complexes and matured in vitro (Chian et al., 2004), therefore 30 days of IVFG may be enough time for the secondary follicle to reach this step.
The final expansion of the follicle is largely attributed to the size of the antrum, which is associated with a prodigious increase in hormones (principally estrogen) necessary to drive an ovulatory signal (LH) from the pituitary gland. Moreover, the follicle must grow outward from the perimedullary zone to permit contact between the follicle surface and the ovarian surface in anticipation of ovulation. In vitro systems do not have to phenocopy the properties of the follicle that relate to maintaining a menstrual cycle and extruding an oocyte—they simply have to accommodate the oocyte to the point where nuclear and cytoplasmic maturation can occur. Thus, our goal for the human follicle growth project is not to generate a 20 mm follicle, rather, to determine what length of culture and what antrum size is necessary to produce an oocyte competent to resume meiosis in vitro. Based on the data presented here, our culture conditions support follicle growth to an appropriate in vitro terminal size.
Hormones
We next investigated whether the human ovarian follicles grown in vitro were hormonally active. The pattern of hormone secretion from the growing human follicles was similar to the pattern observed during the human menstrual cycle (Welt et al., 1999; Klein et al., 2004). The earliest detectable hormone released (above baseline) from the follicle was AMH. AMH secretion was followed by estradiol and the inhibins. Inhibin A and 17β-estradiol were positively correlated throughout follicle development, although inhibin B levels fell as the follicles achieve ‘dominant’ status. Average AMH decreased as the inhibin A increased and inhibin B fell (Fig. 3). AMH remains elevated in the individual follicle depicted in Fig. 4. Understanding the relationship between AMH and follicle/oocyte maturity is a future goal of this work. Baseline levels of progesterone from newly isolated follicles were unexpectedly higher than one might predict, however, progesterone levels have never been measured in a secondary human follicle. The effects of the progesterone on follicle development were unknown, but did not impact follicle growth or oocyte development. Like the other hormones, progesterone secretion was maintained at basal level for the first week of culture and did not increase until estrogen and the peptide hormones levels rose. Importantly, the ratio of estradiol to progesterone was consistent during the last 2 weeks culture after the antrum has formed, which indicated no significant luteinization has occurred in the cultured follicle. We can confirm the relative health of granulosa cells by observing the low cytoplasm to nucleus ratio in the confocal images of freshly isolated and in vitro cultured follicles. Moreover, the inhibin βB subunit was known to be expressed in healthy granulosa cells and not in luteinized human tissue (Groome et al., 1996; Klein et al., 2004). Thus, the ovarian follicles grown in vitro were capable of sustained hormone production, which phenocopied the patterns of the hormones secreted by follicles in vivo.
Oocyte status
In addition to overall follicle size and growth patterns, the development and maturation of the oocytes described here mirrors that of in vivo matured follicles, or freshly isolated in vitro matured follicles. The key anatomical structures that provide communication routes between the somatic cells and the oocyte are the TZPs (Albertini and Barrett, 2003). Although these actin-based cytoplasmic processes are known to be present in mature human cumulus-oocyte complexes, their existence in immature human follicles, although expected, has not previously been established. We isolated fresh, immature human follicles that were fixed, and then imaged by confocal microscopy. Importantly, we found that cultured immature human follicles did indeed maintain a dense network of TZPs. Because we were able to maintain these structures with our 3D matrices, oocyte growth increased with time in culture from 75 µm to a terminal size of 110 µm. This size was indistinguishable from meiotically competent oocytes aspirated from small antral human follicles in vivo (Cavilla et al., 2008). During the early stages of culture, freshly isolated follicles and cultured follicles (14 days) had displayed healthy, appropriately staged, intact GVs (nucleoli stage II), which progressed to stage IV by the end of culture. It was documented that oocytes with GV stage IV developed nuclear competence and that, depending on cytoplasmic competence, these oocytes should be able to resume meiosis in preparation for fertilization (Combelles et al., 2002). Thus, the in vitro 3D culture system supported coordinated follicle and oocyte growth creating an environment in which the major steps of human follicle development were maintained.
Our studies revealed that early secondary follicles, once removed from the restricted environment of the ovary, had the capability of surviving and growing in culture. By using a 3D supportive matrix, follicles were able to maintain bidirectional communication between somatic cells and the germ cell creating an environment conducive for oocyte growth and proper steroid production. The next frontier will be maturation of in vitro grown oocytes to MII, fertilization and embryo development, and the adaption of these techniques to primordial follicles and cryopreserved tissues.
Author's Role
M.X. design, acquisition of data, analysis, interpretation. S.L.B. acquisition of data, interpretation and critical review. E.W.-F., L.A.K. and S.E.K. acquisition of data, analysis. L.D.S. interpretation, critical review. T.K.W. design, interpretation, critical review, final approval.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Funding
This research was supported by grants from the National Institutes of Health to the Oncofertility Consortium, grant numbers: NIH RL1HD058295 and PL1EB008542. The content is solely the responsibilities of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Acknowledgements
The authors thank Jill Trainer and Jen Jozefik for their assistance in the preparation of this manuscript; Tyler Wellington and the Northwestern University Reproductive Science Histology Core for tissue processing and sectioning; and members of the Oncofertility Consortium for helpful discussions including: Steve Rosen, M.D., Jacqueline Jeruss, M.D., Ph.D., Richard Stouffer, Ph.D., Mary Zelinski, Ph.D., Jeff Chang, M.D., Christos Coutifaris, M.D., Ph.D. Marybeth Gerrity, Ph.D. and Bob Brannigan, M.D.
References
- Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil steril. 1997;68:682–688. doi: 10.1016/s0015-0282(97)00264-1. [DOI] [PubMed] [Google Scholar]
- Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod. 1999;14:1299–1301. doi: 10.1093/humrep/14.5.1299. [DOI] [PubMed] [Google Scholar]
- Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75:141–146. doi: 10.1016/s0015-0282(00)01668-x. [DOI] [PubMed] [Google Scholar]
- Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–898. doi: 10.14670/HH-21.887. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Chang RJ. Fertility management for women with cancer. Cancer Treat Res. 2007;138:15–27. doi: 10.1007/978-0-387-72293-1_2. [DOI] [PubMed] [Google Scholar]
- Albertini DF, Barrett SL. Oocyte-somatic cell communication. Reprod Suppl. 2003;61:49–54. [PubMed] [Google Scholar]
- Amorim CA, Van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–99. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol. 1976;71:680–686. doi: 10.1083/jcb.71.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–957. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- Cavilla JL, Kennedy CR, Byskov AG, Hartshorne GM. Human immature oocytes grow during culture for IVM. Hum Reprod. 2008;23:37–45. doi: 10.1093/humrep/dem178. [DOI] [PubMed] [Google Scholar]
- Chian RC, Buckett WM, Abdul Jalil AK, Son WY, Sylvestre C, Rao D, Tan SL. Natural-cycle in vitro fertilization combined with in vitro maturation of immature oocytes is a potential approach in infertility treatment. Fertil Steril. 2004;82:1675–1678. doi: 10.1016/j.fertnstert.2004.04.060. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11:2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- Cvancarova M, Samuelsen SO, Magelssen H, Fosså SD. Reproduction rates after cancer treatment: experience from the Norwegian radium hospital. J Clin Oncol. 2009;27:334–343. doi: 10.1200/JCO.2007.15.3130. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Buxant F, Robin V, Fernandez SA, Centner J, Delbaere A, Englert Y. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21:2010–2014. doi: 10.1093/humrep/del092. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–276. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Groome NP, Illingworth PJ, O'Brien M, Pai R, Rodger FE, Mather JP, McNeilly AS. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81:1401–1405. doi: 10.1210/jcem.81.4.8636341. [DOI] [PubMed] [Google Scholar]
- Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62:1322–1328. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK. Preservation of Fertility in Patients with Cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juretzka MM, O'Hanlan KA, Katz SL, El-Danasouri I, Westphal LM. Embryo cryopreservation after diagnosis of stage IIB endometrial cancer and subsequent pregnancy in a gestational carrier. Fertil Steril. 2005;83:1041. doi: 10.1016/j.fertnstert.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Klein NA, Houmard BS, Hansen KR, Woodruff TK, Sluss PM, Bremner WJ, Soules MR. Age-related analysis of inhibin A, inhibin B, and activin a relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J Clin Endocrinol Metab. 2004;89:2977–2981. doi: 10.1210/jc.2003-031515. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–950. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, Wolf DP. Live birth after ovarian tissue transplant. Nature. 2004;428:137–138. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- Meirow D, Ben Yehuda D, Prus D, Poliack A, Schenker JG, Rachmilewitz EA, Lewin A. Ovarian tissue banking in patients with Hodgkin's disease: is it safe? Fertil Steril. 1998;69:996–998. doi: 10.1016/s0015-0282(98)00093-4. [DOI] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra'anani H, Slyusarevsky E, Amariglio N, Schiff E, Rechavi G, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–1013. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- Newton H, Picton H, Gosden RG. In vitro growth of oocyte-granulosa cell complexes isolated from cryopreserved ovine tissue. J Reprod Fertil. 1999;115:141–150. doi: 10.1530/jrf.0.1150141. [DOI] [PubMed] [Google Scholar]
- O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18:90–95. doi: 10.1093/humrep/deg045. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Picton HM, Danfour MA, Harris SE, Chambers EL, Huntriss J. Growth and maturation of oocytes in vitro. Reprod Suppl. 2003;61:445–462. [PubMed] [Google Scholar]
- Porcu E, Venturoli S, Damiano G, Ciotti PM, Notarangelo L, Paradisi R, Moscarini M, Ambrosini G. Healthy twins delivered after oocyte cryopreservation and bilateral ovariectomy for ovarian cancer. Reprod Biomed Online. 2008;17:265–267. doi: 10.1016/s1472-6483(10)60204-0. [DOI] [PubMed] [Google Scholar]
- Rao GD, Chian RC, Son WS, Gilbert L, Tan SL. Fertility preservation in women undergoing cancer treatment. Lancet. 2004;363:1829–1830. doi: 10.1016/S0140-6736(04)16320-4. [DOI] [PubMed] [Google Scholar]
- Rosendahl M, Loft A, Byskov AG, Ziebe S, Schmidt KT, Andersen AN, Ottosen C, Andersen CY. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Hum Reprod. 2006;21:2006–2009. doi: 10.1093/humrep/del140. [DOI] [PubMed] [Google Scholar]
- Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59:783–790. [PubMed] [Google Scholar]
- Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J. Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertil Steril. 2002;77:403–408. doi: 10.1016/s0015-0282(01)02960-0. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–3546. doi: 10.1093/humrep/dei250. [DOI] [PubMed] [Google Scholar]
- Schover LR. Rates of postcancer parenthood. J Clin Oncol. 2009;27:321–322. doi: 10.1200/JCO.2008.19.7749. [DOI] [PubMed] [Google Scholar]
- Scott JE, Carlsson IB, Bavister BD, Hovatta O. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod Biomed Online. 2004;a 9:287–293. doi: 10.1016/s1472-6483(10)62143-8. [DOI] [PubMed] [Google Scholar]
- Scott JE, Zhang P, Hovatta O. Benefits of 8-bromo-guanosine 3′,5′-cyclic monophosphate (8-br-cGMP) in human ovarian cortical tissue culture. Reprod Biomed Online. 2004;b 8:319–324. doi: 10.1016/s1472-6483(10)60912-1. [DOI] [PubMed] [Google Scholar]
- Shaw J, Trounson A. Oncological implications in the replacement of ovarian tissue. Hum Reprod. 1997;12:403–405. [PubMed] [Google Scholar]
- Silber SJ, Gosden RG. Ovarian transplantation in a series of monozygotic twins discordant for ovarian failure. N Engl J Med. 2007;356:1382–1384. doi: 10.1056/NEJMc066574. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, Crawford EC, Gosden RG. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359:2617–2618. doi: 10.1056/NEJMc0804321. [DOI] [PubMed] [Google Scholar]
- Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod. 1994;9:527–532. doi: 10.1093/oxfordjournals.humrep.a138539. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Binnie JP, McCaffery FH, Campbell BK. In vitro development of oocytes from porcine and bovine primary follicles. Mol Cell Endocrinol. 2000;163:117–123. doi: 10.1016/s0303-7207(00)00216-1. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Campbell BK, Armstrong DG, Telfer EE. Effects of IGF-I bioavailability on bovine preantral follicular development in vitro. Reproduction. 2007;133:1121–1128. doi: 10.1530/REP-06-0382. [DOI] [PubMed] [Google Scholar]
- Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105–111. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80:432–439. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S, Hardy K. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14:1555–1562. doi: 10.1093/humrep/14.6.1555. [DOI] [PubMed] [Google Scholar]
- Wu J, Emery BR, Carrell DT. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol Reprod. 2001;64:375–381. doi: 10.1095/biolreprod64.1.375. [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;a 12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;b 75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Xu M, Woodruff TK, Shea LD. Bioengineering and the ovarian follicle. Cancer treatment and research. 2007;138:75–82. doi: 10.1007/978-0-387-72293-1_6. [DOI] [PubMed] [Google Scholar]
- Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009 doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Brown SE, Nguyen K, Reddy V, Brubaker C, Winslow KL. Live birth after the transfer of human embryos developed from cryopreserved oocytes harvested before cancer treatment. Fertil Steril. 2007;87:1469e1–4. doi: 10.1016/j.fertnstert.2006.07.1546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.