Abstract
This study examined the interaction between comparative cancer risk and efficacy perceptions on individuals’ adherence for colon, prostate, and breast cancer screenings, intentions to get these screenings in the future, and intent to adopt health lifestyle behaviors in the next year. A national probability sample of 2,226 adults aged 40–70 were surveyed. Overall, a positive interaction effect was found between comparative risk and efficacy on several outcomes. There was some methodological limitations worth noting but the findings do have implications for health campaigns, particularly the need to increase efficacy beliefs about reducing cancer risks within the general population.
In the United States, it is estimated that over 1.3 million new cancer cases will be diagnosed this year (American Cancer Society, 2004). Although this is a serious health concern, there is growing evidence that early screenings for different types of cancers may reduce mortality rates, and increase the likelihood that a cancer is detected in the early stages where it is curable (Hendrick, Smith, Rutledge, & Smart, 1997; Mandel, 1999). Additionally, a number of studies have found that a person’s cancer risk may be associated with certain lifestyle habits. For instance, physical inactivity, low consumption of fruits and vegetables, and smoking has all been found to be associated with increased risks for cardiovascular diseases and various cancers (see Schuit, van Loon, Tijhuis, & Ocke, 2002).
Despite the fact that following recommended guidelines for cancer screenings or adopting a healthy lifestyle helps reduce an individual’s cancer risk, adherence to cancer screening guidelines falls far short of recommended levels (Haitt and Rimer, 1999). A similar situation exists when it comes to adopting a healthy lifestyle. In response, various public health campaigns have been launched to try and persuade people to adhere to cancer screening guidelines, and adopt a healthy lifestyle. Many of these campaigns are designed to both increase people’s awareness of their cancer risks, as well as provide them with efficacy information for reducing those risks (e.g., getting screened for cancer, having a healthy diet, etc.). One goal of increasing people’s awareness of their cancer susceptibility may be to increase their perception of cancer risk, which in turn, should motivate them to enact protective behaviors against cancer.
Reviewing the health communication literature, it is evident that perceived risk is a central construct in a number of theories of health behavior (for reviews, see Van der Pilgt, 1998; Weinstein, 1993). Some examples where perceived risk is thought to be a significant predictor of self-protective behaviors include the Health Belief Model (Janz & Becker, 1984), the Precaution Adoption Model (Weinstein, 1988), the Self-regulation Model of Health Behavior (Leventhal & Cameron, 1987), and the Protection Motivation Theory (Rogers, 1975). Risk perception derives from threat appraisal, and the central premise is that individuals’ motivation to enact health protective or health promoting behaviors increases as a direct function of their belief that they are susceptible to a serious health threat (i.e., are at high risk) (Rimal, Flora, & Schooler, 1999). Given that perceived risk may be a strong motivator of health-related behaviors, it is important to understand the pattern of associations between perceived risk and specific health-related behaviors to develop effective risk communication messages.
Although a strong positive association between risk perception and health behavior change is expected, empirical findings are inconsistent in supporting this theoretical assumption. In a meta-analysis across studies on various health issues, it was found that increases in risk perceptions generally facilitated protective intentions and behaviors (Floyd, Prentice-Dunn, & Rogers, 2000). Numerous studies have found the hypothesized positive association between risk perception and the enactment of healthy behaviors (e.g., Aiken, West, Woodward, & Reno, 1994; Weinstein, 1982, 1983; Weinstein, Sandman, & Roberts, 1990). Yet, despite the fact that some studies have found the predicted positive relationship between perceived risk and behavior, there are a number of studies finding no relationship between perceived risk and behavior (e.g., Joseph et al., 1987; Svenson, Fischhoff, & McGregor, 1985) and in a few cases, a negative relationship was found between risk perception and behaviors (e.g., van der Velde, Hooijkaas, & van der Pilgt, 1991). Risk has been assessed with various methods and to date, most of the research on risk perception is based on absolute risk assessments.
Comparatively less work has been done to examine the effects of comparative risk assessments on behaviors. It is important to understand the effects of comparative risk perceptions on behaviors because there is good evidence to suggest that people rely on social comparisons to assess their standing on a variety of dimensions (see Suls & Wheeler, 2000), including their risk levels (Klein & Weinstein, 1997). Much work on risk perception shows that people are highly attentive to contrast, sometimes more so than they are to static risk (Klein, 2002). Across the limited number of studies that have been done to examine the relationship between comparative risk and affective (i.e., worry) and behavioral outcomes, the results are mixed. There is some evidence that comparative risk is a significant positive predictor of worry and behavioral intentions (e.g., Lipkus, Lyner, & Rimer, 2000; McCaul & O’Donnell, 1998). In some cases, comparative risk has been found to be a stronger predictor of future behavior than absolute risk (e.g., Blalock, DeVellis, Afifi, & Sandler, 1990; Lipkus et al., 2000). However, a few studies find comparative risk to be a poor predictor of future behaviors (e.g., van der Velde et al., 1991). This inconsistency in findings warrants further study.
Specifically related to cancer-related outcomes, perceived risk has often been used to explain cancer screening behaviors, and targeted as an important variable in interventions designed to promote cancer screenings (for a review, see Vernon, 1999). Although risk perception has been found to positively predict breast cancer screenings (McCaul, Branstetter, Schroeder, & Glasgow, 1996), colorectal cancer screenings (Mullens, McCaul, Erickson, & Sandgren, 2004), and use of supplements to improve prostate health (Beebe-Dimmer et al., 2004), numerous researchers have also failed to find the expected positive correlation between perceived risk and cancer screening behaviors (e.g., Eiser & Cole, 2002; Shiloh, Vinter, & Barak, 1997). In a review of the literature on colorectal cancer screening, Vernon (1997) reported that only 2 of 8 studies reviewed found a positive association, the other six studies found no relationship between perceived risk and colorectal cancer screening. Overall, the literature provides mixed support for the associations between risk perception and cancer prevention behaviors (e.g., Katapodi, Lee, Facione, & Dodd, 2004; Lerman & Schwartz, 1993). It is important to point out that much of this work relied on using absolute risk measures as opposed to comparative risk assessments. Hence, there is a need for more research to examine the effects of comparative cancer risk on cancer-related behaviors.
In the few studies that have used comparative risk, it has been found to positively relate with breast cancer worry, screening intentions, and screening behaviors (Lipkus et al., 2000; Klein, 2002; McCaul & O’Donnell, 1998). Experimentally, comparative risk seems to have a consistent influence on behavioral intentions, more so than absolute risk (Klein, 1997). Recently, in a longitudinal study of risk perception and cancer worry, Lipkus, Klein, Skinner, and Rimer (2005) found that perceived comparative risk, but not perceived absolute risk predicted subsequent worry about breast cancer. Together, there is some evidence to suggest that comparative cancer risk may be an important predictor of cancer-related behaviors independent of absolute cancer risk. Yet, because there is only limited evidence to support the positive association between comparative cancer risk and cancer-related behaviors, it is plausible that there are conditions under which comparative cancer risk may not be positively associated with cancer-related behaviors (e.g., low perceived efficacy to cope with cancer).
The present study extends on previous work by investigating the moderating role of efficacy beliefs on the relationship between comparative cancer risk and cancer prevention behaviors. Efficacy beliefs help to regulate human functioning and emotional well-being through a variety of processes (Turner, Rimal, Morrison, & Kim, 2006). When faced with adverse events, those who perceive themselves to be highly efficacious are more likely to persevere than those perceiving themselves to have low efficacy (Turner et al., 2006). Efficacy beliefs are comprised of both self-efficacy and response efficacy beliefs.
Bandura (1983, 1999) defined self-efficacy as an individual’s confidence in his or her ability to perform a given behavior. Those who have high self-efficacy are more likely to have fewer negative thoughts about themselves, and feel that they can exert greater control over a given event compared to those who have low self-efficacy (Ozer & Bandura, 1990). It is expected that unless people have high confidence in their abilities to perform the coping behavior in the face of difficulties, they will have little motivation to enact the given behavior. In addition to self-efficacy, overall efficacy beliefs also comprise of response efficacy. Bandura (1977) defines response efficacy as the belief that enacting a given behavior will result in the desired outcome (e.g., effectively averting a health threat). Research has generally found that individuals with high response efficacy beliefs are better able to translate knowledge into behavior (e.g., Rimal, 2000).
Interaction Between Risk and Efficacy
Several researchers have argued for an interaction effect between risk and efficacy on behaviors (e.g., Rimal, 2001, 2002; Witte, 1992, 1994; Witte & Allen, 2000). Specific predictions about how efficacy beliefs affect the association between risk and behaviors have been presented in various models (e.g., Extended Parallel Process Model, Witte, 1992; Risk Perception Attitude framework, Rimal & Real, 2003). The current study will be informed by Rimal and Real’s (2003) Risk Perception Attitude (RPA) in order to help make predictions about the interaction between comparative cancer risk perceptions and cancer-related behaviors. It is worth noting that this study does not provide a direct test of the RPA framework as the RPA focuses on the effects of absolute risk perceptions on behaviors.
Although the RPA was not originally conceived to address comparative risk perceptions, it nevertheless serves as an informative framework for understanding how the relationship between comparative risk and behaviors may be affected by efficacy beliefs. It may be argued that perceived comparative risk can be reconceptualized in terms of absolute risk. Specifically, individuals with low comparative risk (i.e., perceive themselves to be at lower risk compared to others) are likely to also have low absolute risk perceptions due to their optimistic bias about their own risk levels, and lower levels of worry. Conversely, individuals with high comparative risk (i.e., perceive themselves to be at higher risk compared to others) are likely to also possess high absolute risk perceptions due to their pessimistic bias about their own risk, and higher levels of worry. In a recent study, Lipkus et al. (2005) found that comparative risk for breast cancer at baseline positively predicted cancer worry, which in turn, positively predicted absolute risk for breast cancer at follow-up. Baseline absolute risk estimates however failed to significantly predict cancer worry. Moreover, it may also be argued that comparative risk estimates provide a better assessment of personal vulnerability (i.e., risk) than absolute risk estimates. There is some data to suggest that comparative rather than absolute risks are psychologically more meaningful to judge the extremity of risks (e.g., Klein, 1997). Also, several studies have found comparative risk to be associated with behavior, affect, health message processing, and other health outcomes even after controlling for absolute risk estimates (Klein, 2002; Lipkus et al., 2005; Radcliffe & Klein, 2002).
The RPA framework predicts both a main effect of efficacy on behaviors as well as an interaction between efficacy and risk on behaviors. It is argued that those who feel efficacious are likely to view potential risks as challenges to overcome, whereas those lacking in efficacy are likely to interpret potential risks more fatalistically, and as a result are less likely to enact risk-reducing behaviors (Rimal & Real, 2003). As for the interaction between efficacy and risk, when risk perception is low, it is expected that efficacy beliefs will have little impact on behaviors. When both perceived risk and efficacy are low (i.e., indifferent attitude), individuals are not motivated to consider the efficacy information (Rimal & Real, 2003; Turner et al., 2006) and thus no further action is taken. When efficacy perceptions are high, but perceived risk is low (i.e., proactive attitude), individuals have the confidence to enact protective behaviors but may lack the motivation to do so because of their low risk status (Rimal & Real, 2003). However, these individuals may also be motivated to enact protective behaviors because of their desire to remain disease-free (Rimal & Real, 2003).
On the other hand, when risk perception is high, it is expected that efficacy beliefs will have a greater effect on behaviors. Specifically, when both perceived risk and efficacy are high (i.e., responsive attitude), individuals are motivated to take some form of action (due to the fear they experience from being at high risk status), and feel confident in their abilities to effectively reduce their risk. As a result, these individuals are the most likely to enact risk-reducing behaviors (Rimal & Real, 2003). Conversely, when risk perception is high, but efficacy beliefs are low (i.e., avoidant attitude), individuals face conflicting motivations. While their high risk perception likely makes them concerned about their health status, their perceived inability to cope with their risks likely decreases their motivation. Hence, these individuals are expected to be the less likely to enact risk-reducing behaviors than those with responsive attitudes (Rimal & Real, 2003).
The interactive effect of risk and efficacy perceptions on behavioral intentions has been tested in a number of studies utilizing the RPA framework (e.g., Rimal & Real, 2003, Turner et al., 2006). Generally in these studies, four attitudinal groups are created based on the assessment of perceived absolute risk and efficacy beliefs. Rimal and Real (2003) used a median split procedure to form the four RPA groups in their study whereas Turner et al. (2006) used cluster analysis to classify individuals into the different RPA groups. Planned comparisons were made between the RPA groups to determine whether there was an interaction effect between risk and efficacy. Results yielded from these comparisons provide mixed support for the RPA framework. Consistent with the framework’s original set of predictions, behavioral intentions are higher among individuals with responsive than avoidant attitudes. However, contrary to the original predictions set of predictions, behavioral intentions are also higher among individuals with proactive than indifferent attitudes (see Rimal & Real, 2003). This pattern of findings from most to least positive outcomes: Responsive, proactive, avoidant, and indifferent has been found in two studies (Rimal & Real, 2003, study 2; Turner et al., 2006, study 1).
Most of the studies done so far using the RPA framework have relied on an experimental design, and a college sample (e.g., Rimal & Real, 2003; Turner et al., 2006). Few studies have tested the RPA-based predictions with a more representative population (e.g., national probability sample). Rimal and Real (2003, study 2) did test the RPA framework within a natural context (i.e., risk and efficacy were not manipulated) and found partial support for the framework. In predicting sunscreen use, individuals with high risk and efficacy perceptions reported more sunscreen use compared to those with high risk and low efficacy perceptions (as predicted), but those with low risk and high efficacy perceptions also reported greater sunscreen use than those with low risk and efficacy perceptions (contrary to predictions). Past RPA studies also typically use absolute risk measures to assess perceived risk. As discussed earlier, perceived risk may be measured as either absolute risk or comparative risk.
The purpose of this investigation was to use the RPA framework as a guide for testing the interaction effect between comparative cancer risk and efficacy perceptions on people’s adherence to prevention guidelines for cancer screening (colon, breast, and prostate) and intentions to enact various cancer prevention behaviors in the future (i.e., cancer screening tests, adoption of a healthy lifestyle). Based on the RPA framework, two sets of predictions are made. It is important to note that while the RPA framework bases its predictions on perceived absolute risk, the present study will use perceived comparative risk instead. Given the moderate positive correlation between comparative and absolute risk (Lipkus, Lyna, & Rimer, 2000), it is expected that the pattern of findings for the RPA framework predictions using comparative risk will be similar to those using absolute risk.
Specifically, it was expected that individuals who are motivated and feel confident in their abilities to reduce cancer risks (via vigilant monitoring of risk) will be more likely to have adhered to cancer screening guidelines than: (a) those who are motivated but lack the confidence in their abilities/belief that screening reduces risks, and (b) those who are not motivated at all to take action in reducing their cancer risks. More formally stated, it is posited that:
-
H1Comparative risk and efficacy perceptions will interact on cancer screening adherence such that:
-
H1aWhen efficacy perceptions are low, cancer screening adherence will not differ as a function of comparative risk perceptions.
-
H1bWhen efficacy perceptions are high, cancer screening adherence will be more likely the higher the comparative risk perceptions.
-
H1a
Similarly, it is also expected that highly motivated individuals who feel confident in their abilities to avert cancer risks and believe that a given prevention behavior can reduce their cancer risks will report greater intentions to enact that behavior than: (a) those who are motivated but lack the confidence to perform the prevention behavior and/or belief that the behavior fails to reduce their risks, and (b) those who lack the motivation to enact risk-reducing behaviors. Formally stated, it is posited that:
-
H2There is a positive interaction effect between risk and efficacy perceptions on cancer screening and healthy lifestyle behavioral intentions such that:
-
H2aWhen efficacy beliefs are low, risk perception is not associated with cancer screening and lifestyle behavioral intentions.
-
H2bWhen efficacy beliefs are high, risk perception is positively associated with cancer screening and lifestyle behavioral intentions.
-
H2a
Method
Participants and Procedures
A total of 2,226 adults aged 40–70 were recruited to take part in this study by Knowledge Networks, a survey research company which has developed a nationally representative sample of adults in the United States. Data are gathered online and respondents are recruited through random digit dialing (RDD) procedures and provided internet access, if necessary. The current sample consisted of 1,087 males (48.8%) and 1,139 females (51.2%), with a majority of them having some college education or higher (59.7%). Approximately 76% of the participants were White, 11% African-American, 7.3% Hispanic, 2.9% Mixed, and 2.7% “other.” The average age of participants was 52.75 years (SD = 8.39).
Measures
The variables of interest were: (a) participant’s comparative risk perceptions for colon, prostate, and breast cancer, (b) their efficacy beliefs regarding cancer screening tests (colonoscopy, PSA test, and mammograms) and lifestyle behaviors (exercise, fruit and vegetable consumption, and dieting) in reducing cancer risks, (c) their intentions to engage in cancer screening and lifestyle behaviors, and (d) their adherence to cancer screening guidelines.
Risk perceptions
Two items assessed the extent to which individuals felt they were at risk for cancer compared to others their age. Specifically, participants were asked, “Compared to most others your age, what do you think your chances are of getting each of the following:” All participants were asked about their comparative risk perception for colon cancer. For males, they were also asked about their comparative risk perception for prostate cancer whereas females were asked about their comparative risk perception for breast cancer. The response options ranged from (1) a lot lower to (4) a lot higher. The three comparative cancer risk items (colon, prostate, and breast) each served as an independent predictor corresponding to a specific cancer-related outcome (e.g., colonoscopy intent). Additionally, overall comparative cancer risk was assessed by averaging across two comparative cancer risk measures (i.e., colon and prostate cancer risks for males, colon and breast cancer risks for females). Correlations for the two overall cancer risk measures are .70 (males) and .59 (females) respectively.
Efficacy perceptions
Several items assessed the extent to which individuals felt that they could perform various screening and lifestyle behaviors (i.e., self-efficacy) and how strongly they agreed that these prevention behaviors reduced their cancer risks (i.e., response efficacy). For self-efficacy, respondents were asked about their confidence in performing the different prevention behaviors (e.g., if you wanted to, how sure are you that you can get a colonoscopy in the next year/when it is next recommended). Similar items were asked for getting a PSA test, mammogram, exercise, fruits and vegetables consumption, and dieting to control weight. The response options ranged from (1) very unsure to (5) very sure.
For response efficacy, respondents were asked how likely they felt performing the prevention behavior reduces their risks for colon, breast, and prostate cancer (e.g., how likely is it that doing exercise at least three or more times in most weeks will reduce your risks of colon cancer). Similar items were asked for PSA testing, mammograms, fruit and vegetables consumption, and dieting to control weight. It is important to note that response efficacy for colonoscopy was not assessed in this study. The response options ranged from (1) very unlikely to (5) very likely.
Overall efficacy perceptions were calculated by averaging across self-efficacy and response efficacy perceptions. Correlations between self and response efficacy items for the cancer screening behaviors were as follows: PSA efficacy (r=.31) and mammogram efficacy (r=.25). For colonoscopy efficacy, only self-efficacy was assessed. Six items assessing self and response efficacy for three lifestyle behaviors (exercise, fruits and vegetables consumption, and dieting) were averaged into an index with an alpha of .70.
Behavioral intentions
Several items assessed participants’ intentions to enact cancer screening and lifestyle behaviors in the next year. Specifically, respondents were how likely they were to perform the different prevention behaviors in the next year (e.g., How likely is it that you will get a mammogram in the next year?). Similar items were also asked of intentions regarding colonoscopy, PSA testing, exercise, fruits and vegetables consumption, and dieting to control weight. Response options ranged from (1) very unlikely to (5) very likely.
Cancer screening adherence
Participants were also asked about their recent cancer screening behaviors to assess the extent to which they adhered to cancer screening guidelines. Given that guidelines are applicable to only certain age groups, for colon and prostate screening, only participants fifty years and above were included in the analyses for calculating adherence. For breast screenings, all participants were included given that the recommended age for mammograms is forty years old. For colonoscopy and PSA testing adherence, participants were first asked whether they had heard of the specific screening tests. It was assumed that all women had heard of mammograms. For all three cancer screening tests, participants were asked when they had their most recent screening (e.g., When did you have your most recent PSA test to check for prostate cancer). Response options varied depending on the specific test.
For colon screening adherence, participants fifty and over who had a colonoscopy within the past ten years were considered adherent, those that did not or had not heard of a colonoscopy were considered non-adherent. For prostate screening adherence, males fifty and over who had a PSA test within the past two years were considered adherent, those that did not or had not heard of the PSA test were considered non-adherent. For breast screening adherence, females forty and over who had a mammogram within the past two years were considered adherent, those that did not were considered non-adherent. Screening adherence was coded as ‘1’, whereas non-adherence was coded ‘0.’
Statistical Analyses
The first hypothesis was tested through sequential logistic regression with demographic variables entered into the first block, comparative risk and efficacy perceptions entered into the second block, and the interaction term (risk×efficacy) entered into the final block. Cancer screening adherence was the dependent variable in all analyses with ‘0’ coded as non-adherent and ‘1’ coded as adherent.
The second hypothesis was tested through sequential multiple regression analyses, with demographic variables (age, gender, education) entered into the first block, risk and efficacy perceptions entered into the second block, and the interaction term (risk×efficacy) entered in the final block. To control for the potential problem of collinearity, all predictor variables were centered. Intentions to engage in cancer screening and lifestyle behaviors were the dependent variables. It is important to note that for cancer risk perceptions, scores were based on responses only from participants of appropriate age (e.g., for colon cancer risk, it is for everyone aged 50 and over). Additionally, an overall cancer risk perception score was calculated for the entire sample by averaging across the two cancer risk perception scores (i.e., for colon and prostate/breast cancer).
Results
Descriptive Statistics
The means and standard deviations for the key predictor and dependent variables are presented in Table 1. Cancer screening adherence was relatively high within the appropriate age and gender groups for all three cancer screening tests: colonoscopy adherence (57.1%), PSA test adherence (52.4%), and mammogram adherence (70.4%). It is worth noting that for prostate and breast screening, participants who reported having a PSA or a mammogram in the last 2 years were considered adherent rather than in the last year, which would have been a more stringent criteria.
Table 1.
Means and Standard Deviations for Key Study Variables
| Variables | M | S.D. |
|---|---|---|
| colon cancer risk perception (those 50 and over) | 2.10 | .74 |
| Prostate cancer risk perception (males 50 and over) | 2.13 | .74 |
| breast cancer risk perception (females 40 and over) | 2.14 | .81 |
| overall cancer risk perception | 2.11 | .68 |
| colonoscopy self-efficacy | 4.11 | 1.26 |
| PSA efficacy beliefs | 4.25 | .81 |
| mammogram efficacy beliefs | 4.36 | .80 |
| exercise efficacy beliefs | 3.61 | .85 |
| fruits and vegetables consumption efficacy beliefs | 3.76 | .84 |
| dieting efficacy beliefs | 3.83 | .80 |
| colonoscopy intention | 3.03 | 1.61 |
| PSA testing intention | 3.61 | 1.41 |
| mammogram intention | 4.06 | 1.38 |
| moderate exercise intention (3+ times a week most weeks) | 3.27 | 1.28 |
| fruits and vegetables consumption intention (5+ servings most weeks) | 3.62 | 1.23 |
| dieting intention | 3.42 | 1.24 |
Effects on Cancer Screening Adherence
It was predicted that risk and efficacy perceptions would interact such that when risk perceptions were low, efficacy beliefs should have little impact on cancer screening adherence. Efficacy beliefs were expected to have an impact on likelihood of adherence only when risk perceptions were high. Sequential logistic regression analyses were performed to assess prediction of cancer screening adherence (adherent, non-adherent), first on the basis of demographic predictors (age, sex, education), then the addition of comparative risk and efficacy perceptions (low, high) as independent predictors, and finally the interaction between comparative risk and efficacy.
Colonoscopy adherence
In predicting colonoscopy screening adherence, there was a good model fit for the entire model based on the Hosmer and Lemeshow test, χ2(8, N=1171) = 10.53, p=.23. A significant interaction effect was found between risk and efficacy perceptions in predicting colonoscopy adherence, odd ratio=1.25 (C.I. for 95% interval=1.06, 1.47), p<.01. To tease out the interaction effect, separate sequential logistic regressions were performed for those with low efficacy and high efficacy perceptions. For those with low colonoscopy efficacy beliefs, risk perception was not a significant predictor of colon screening adherence, odds ratio=0.72 (C.I. for 95% interval=.45, 1.15), p=.17. Specifically, among individuals reporting low self-efficacy for getting a colonoscopy, risk perception had no impact on colonoscopy adherence.
For those with high colonoscopy self-efficacy, risk perception was a significant predictor of colonoscopy adherence, odds ratio=1.24 (C.I. for 95% interval=1.03, 1.49). Specifically, among individuals reporting high self-efficacy for getting a colonoscopy, the higher their level of perceived comparative colon cancer risk, the more likely they were to be adherent for colonoscopy screening, controlling for age, sex, and education.
PSA test adherence
There was no significant interaction effect found between risk and efficacy perceptions on PSA test adherence, odds ratio=1.27 (C.I. for 95% interval=.90, 1.79), p=.18. Removing the interaction term, there was a somewhat poor fit for the overall model, χ2(8, N=518) =19.29, p<.05. Comparative prostate cancer risk was not significant predictor of PSA test adherence, odds ratio=.89 (C.I. for 95% interval=.67, 1.16), p=.38. PSA test efficacy was a significant predictor of PSA test adherence, odds ratio=2.90 (C.I. for 95% interval=2.14, 3.94), p<.001. The higher a man’s efficacy perceptions were regarding the PSA test, the more likely he was to be adherent for PSA testing, controlling for age and education.
Mammogram adherence
No significant interaction effect was found between risk and efficacy perception on mammogram adherence, odds ratio=.98 (C.I. for 95% interval=.78, 1.25), p=.89. Removing the interaction term, the overall model for predicting mammogram adherence yielded a good fit, χ2(8, N=1119) = 10.94, p=.21. Both risk and efficacy perceptions were significant independent predictors of mammogram adherence. For risk perception, the higher women perceived themselves to be at risk for breast cancer compared to others their age, the more likely they were to be adherent for mammogram screening, controlling for age and education, odds ratio=1.37 (C.I. for 95% interval=1.14, 1.64), p<.01. As for efficacy perception, the higher a woman’s efficacy perceptions were about mammograms, the more likely she was to be adherent for mammogram screenings, odds ratio=2.52 (C.I. for 95% interval=2.09, 3.04), p<.001, controlling for age and education.
Overall, the results provide only limited support for the first hypothesis. Of the three cancer screening tests examined, an interaction effect between comparative cancer risk and efficacy perceptions on cancer screening adherence was found only in the case of colonoscopy screening. For PSA test adherence, the most significant predictor was the participant’s efficacy perceptions about the PSA test (i.e., self-efficacy in getting a PSA test and response efficacy of the PSA test).
Effects on Behavioral Intentions
An interaction effect between risk and efficacy perceptions on behavioral intentions was also predicted such that when efficacy beliefs were low, perceived risk would not be associated with intentions to enact cancer screening tests or healthy lifestyle behaviors in the future. Alternatively, when efficacy beliefs were high, perceived risk would positively predict individuals’ intentions to engage in both cancer screening and healthy lifestyle behaviors. For both cancer screening and lifestyle behavioral intentions, the overall model consisted of age, sex, and education, cancer risk and efficacy perceptions as main effects, and the interaction term (risk×efficacy) as predictors.
Colonoscopy intention
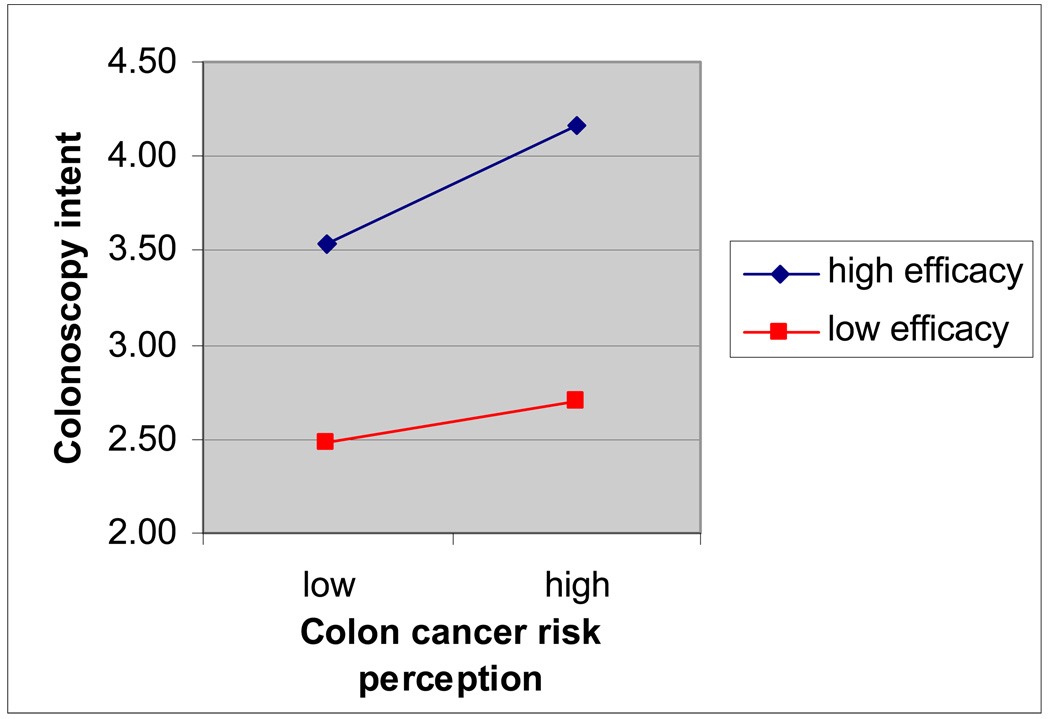
The overall model predicting colonoscopy intent was significant, F(6, 1165)=45.18, R2 = .19, p<.001. A significant interaction effect was found between risk and efficacy perceptions on intent to get a colonoscopy in the next year or when next recommended, controlling for main effects and demographic factors (age, sex, and education), β=.06, t=2.35, p<.05, partial r=.07 (see Figure 1 for plot of interaction effect). As predicted, when perceived colonoscopy efficacy was low, comparative colon cancer risk perception was not associated with colonoscopy intent controlling for demographic factors, β=.04, t=.54, p=.59. Also as predicted, when perceived colonoscopy efficacy was high, comparative colon cancer risk perception positively related to colonoscopy intent controlling for demographic factors, β=.17, t=5.39, p<.001, partial r=.17.
Figure 1.
Interaction Effect of Risk and Efficacy on Colonoscopy Intent
PSA testing intention
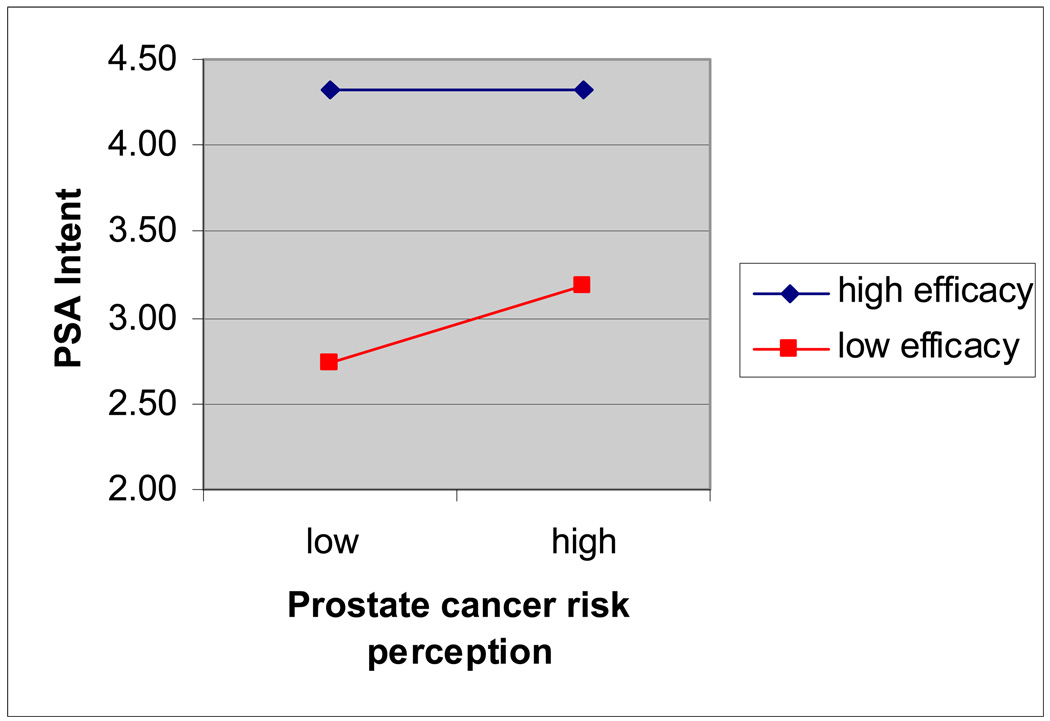
In predicting PSA testing intention, the overall model was significant, F(5, 518)=27.11, R2=.21, p<.001. No significant interaction effects were found between risk and efficacy perceptions on intent to get a PSA test in the next year, controlling for main effects and demographic factors (age and education), β=−.02, t=−.37, p=.71 (see Figure 2 for plot of interaction effect). Significant effects were found for comparative prostate cancer risk on PSA test intent, β=.15, t=3.86, p<.001.and PSA efficacy on PSA test intent, β=.39, t=9.93, p<.001. PSA efficacy had a significantly stronger impact in predicting PSA test intent than comparative prostate cancer risk, t(521)=4.25, p<.001.
Figure 2.
Interaction Effect of Risk and Efficacy on PSA Test Intent
Mammogram intention
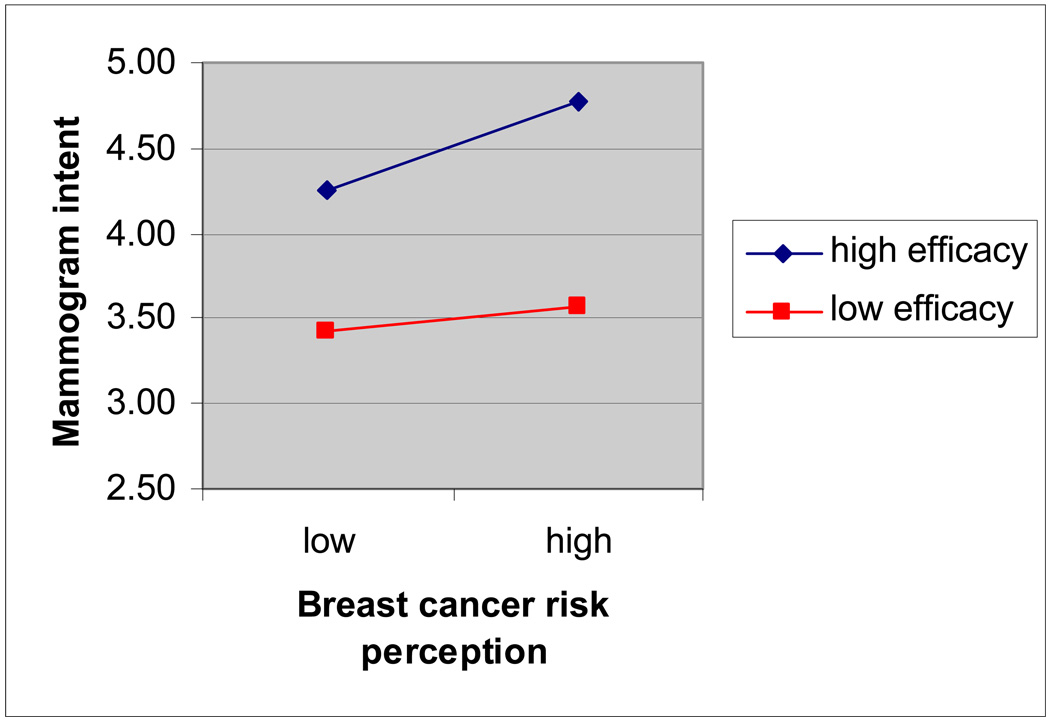
The overall model predicting mammogram intention was significant, F(5, 1118)=47.89, R2=.18, p<.001. A significant interaction effect was found between perceived risk and efficacy on intent to get a mammogram in the next year, controlling for main effects and demographic factors, β=.08, t=2.97, p<.05, partial r=.09 (see Figure 3 for plot of interaction effect). Consistent with predictions, when mammogram efficacy perceptions were low, comparative breast cancer risk perception was not associated with mammogram intent, controlling for age and education, β=−.04, t=−.47, p=.64. When mammogram efficacy was high, comparative breast cancer risk perception was positively related to mammogram intentions, controlling for age and education, β=.18, t=5.93, p<.001, partial r=.18.
Figure 3.
Interaction Effect of Risk and Efficacy on Mammogram Intent
Exercise intention
No significant interaction effect was found between overall cancer risk and cancer efficacy beliefs related to exercise on exercise intent, β=−.01, t=−.49, p=.63. Removing the interaction term, the overall model in predicting respondents’ intentions to engage in moderate exercise (i.e., 3 or more times a week most weeks) in the next year was significant, F(5, 2200)=109.19, R2=.20, p<.001. For the main effects model, only efficacy beliefs related to exercise was significantly associated with exercise intent, controlling for demographics and perceived comparative cancer risk, β=.42, t=21.83, p<.001, partial r=.42. Overall comparative cancer risk perception was not related to exercise intent and in the wrong direction (i.e., β=−.03, p=.12).
Fruit and vegetable consumption intention
There was no significant interaction effect found between overall cancer risk perception and cancer efficacy beliefs related to fruits and vegetables on intent to consume fruits and vegetables in the next year, β=−.01, t=−.36, p=.72. The overall main effects model was significant, F(5, 2199)=199.69, R2=.31, p<.001. Efficacy beliefs related to fruits and vegetables consumption was related to fruits and vegetables consumption intent, controlling for demographics and perceived risk, β=.52, t=28.79, p<.001, partial r=.52. Overall cancer risk perception was also associated with intentions to consume fruits and vegetables in the future, but in the opposite direction to what was expected, β=−.06, t=−3.25, p<.01, partial r=−.07.
Dieting intention
Similar to the other two lifestyle behaviors, no interaction effect was found between cancer risk perceptions and cancer efficacy beliefs related to dieting on dieting intent, β=−.01, t=−.53, p=.60. The main effects model was overall significant, F(5, 2192)=156.49, R2=.26, p<.001. Only cancer efficacy beliefs regarding dieting was found to be positively related to dieting intentions, controlling for cancer risk and demographic factors, β=.47, t=25.28, p<.001, partial r=.48. Overall cancer risk perception was not associated with dieting intent, and the relationship was in a direction contrary to what was expected (i.e., β=−.01, p=.55).
Discussion
This study tested the interaction effect between perceived comparative cancer risk and efficacy on cancer adherence and intentions to enact cancer prevention behaviors (screening and lifestyle) in the next year. Predictions were derived from Rimal and Real’s (2003) RPA framework. Specifically, it was hypothesized that only when efficacy perceptions were high would risk perception have an impact on behaviors. Risk perception was expected to have little effect on behaviors if efficacy beliefs were low because of the inability to cope with high risk or lack of motivation to cope with low risk.
Overall, the study found limited support for the RPA framework’s predictions (Rimal & Real, 2003; Turner et al., 2006). Significant interaction effects between risk and efficacy on behavioral outcomes was only found for 3 of the 9 outcomes examined: colonoscopy adherence, colonoscopy intentions, and mammogram intentions. In all three cases, the patterns of the interaction effects were consistent with RPA framework predictions. Specifically, when efficacy beliefs were high, colonoscopy adherence and intent were significantly higher for those with high comparative colon cancer risk than those with low comparative colon cancer risk. When efficacy beliefs were low, colonoscopy adherence and intent were low regardless of risk perception. Similar results were found for mammogram intent. For the other outcomes, main effects were generally found for both perceived comparative risk (except PSA adherence and lifestyle behaviors intention) and efficacy. The findings clearly show that efficacy beliefs were the most important predictor of both cancer screening adherence and intent to engage in future cancer prevention behaviors.
One potential explanation for the lack of support found for the RPA framework predictions may be due to how perceived risk was measured in this study. In previous RPA-based studies, perceived risk was conceptualized as including both susceptibility and severity. In the present investigation, perceived risk was conceptualized as a comparative assessment of susceptibility. Obviously, this is a clear departure from how the original RPA framework defines risk and may explain the lack of support found for the model’s predictions. Although severity was not directly assessed, it may be safe to assume that most individuals would perceive cancer to be a severe health condition and so variations in the level of cancer risk perceived ought to be largely accounted for by how vulnerable people feel toward getting cancer. As discussed earlier in this paper, the use of a comparative rather than absolute risk measure should not affect results, but nevertheless this is also another limitation to the study.
Another potential explanation for the results may be due to the type of cancer examined. The expected positive interaction effect was found for both colonoscopy adherence and intentions, but was not found for the PSA test (both adherence and intent) or mammogram adherence. Both the PSA test and mammograms are routinely performed as part of annual health exams, and so perceptions of risk may not be as relevant as efficacy beliefs in influencing either adherence or intent. The decision to get a PSA test or a mammogram may primarily be based on how confident the person is about getting the screening test (which is likely to be high), or more likely the case, how effective the person feels the test is at detecting cancer early. As for colonoscopy screening, this test is not performed annually and requires greater effort on the part of the individual to get it done. Also, the experience of getting a colonoscopy is likely more unpleasant compared to getting a PSA test (i.e., a blood test) or even a mammogram. Therefore, individuals may only be motivated to be adherent and/or have high intent to get a colonoscopy when they perceive themselves to be a high risk and have high efficacy beliefs.
Consistent with previous studies on risk perception (e.g., Clark, Lovegrove, Williams, & Machperson, 2000) the current sample showed signs of an optimistic bias in their estimates of colon, prostate, and breast cancer risks in comparison with others their age. Specifically, for all three cancer types explored in this study, the mean risk perception score was below the mid-point (2.5) on the 4-point scales used to measure comparative cancer risks. Similar biases have been reported in studies assessing perceived cancer worry, a closely related construct to perceived cancer risks. In a recent review of the cancer worry literature, Hay, Buckley, and Ostroff (2005) report that cancer worry among the general population for colon, prostate, and breast cancer are predominantly low, citing studies that find about 83–87% reporting little or no worry about colon cancer, 71% reporting no worry about prostate cancer, and 62–67% reporting no worry about breast cancer. Because efficacy beliefs may have a stronger impact on behavioral intentions and behaviors when risk perceptions are high rather than low (as was the case for several outcomes in this study), research efforts to identify effective strategies to reduce optimistic biases about cancer risks is of great importance. Moreover, there is a growing awareness in the field that people’s beliefs about their comparative risks are predictive of health cognitions and behaviors such as cancer screening (Blalock, et al., 1990), and processing of health information (Radcliffe & Klein, 2002). Studies have also begun to show that the inclusion of social comparison information in risk communication messages may work to reduce optimistic biases about cancer risk perceptions (e.g., Lipkus & Klein, 2006).
The present findings have some important implications for health communication campaigns promoting individuals to get cancer screenings and to adopt healthier lifestyles. In the case of breast cancer and in particular colon cancer prevention, it may be important that campaign messages target both comparative risk and efficacy beliefs, whereas for prostate cancer prevention, the primary focus should be on targeting efficacy beliefs. Given that many current communication campaigns aimed at cancer prevention may already focus on providing risk information to motivate individuals to get screened, more attention may be needed to foster greater efficacy beliefs (both self and response efficacy) related to coping with cancer risks. Individuals need to feel confident in their abilities to perform the screening behaviors, and perhaps more importantly be better informed about how effective early detection is for making cancer more treatable. More research is needed to identify the best strategies for framing cancer efficacy information.
As for campaigns promoting healthier lifestyle behaviors, the results of this study provide clear evidence that the focus should be on increasing efficacy beliefs related to different lifestyle behaviors (e.g., exercise, fruits and vegetables consumption, dieting). Overall cancer risk perception failed to significantly predict people’s intent to engage in healthy lifestyle behaviors in the next year. One potential explanation for this finding may be that individuals who see themselves at high risk for cancer may not necessarily attribute that risk to their lifestyle behaviors. They could attribute their high cancer risks to other factors such as family history and/or genetics, and environmental factors. If this is the case, then it may not be surprising to find the lack of a relationship between cancer risk perception and intent to adopt a healthier lifestyle. Given the positive association between cancer efficacy beliefs related to lifestyle behaviors and intentions to adopt a healthier lifestyle, it may be more effective to use positive message framing (e.g., emphasize the benefits of exercise for reducing cancer risks) than negative message framing (e.g., emphasize the health risks associated with physical inactivity) to motivate people to adopt a healthier lifestyle.
There were several limitations to this study. First, although the probability sample recruited for this study is nationally representative, the responses gathered is based on self-reports. Second, as discussed earlier, the use of a comparative risk measure (as opposed to an absolute risk measure) may be problematic for testing the RPA framework, which is primarily based on predicting the effects of absolute risk. Future work should measure both comparative and absolute risk along with severity to fully capture the idea of perceived risk (see Rimal & Real, 2003; Turner et al., 2006). It is likely that the same pattern of results will be obtained by including absolute cancer risk estimates as part of the perceived risk measure. Zajac, Klein, and McCaul (2006) compared absolute and comparative risk perceptions as predictors of cancer worry, and found both to be equally effective predictors. This suggests that adding absolute cancer risk estimates to the cancer risk perception measure would only strengthen the effects found. Nevertheless, it would be useful to know whether or not one type of risk perception estimate is more predictive of health behaviors than another so that the appropriate approach is taken in designing risk communication messages. Lastly, because this study assessed only cross-sectional data, inferences about causal direction between risk and efficacy perceptions and health behaviors cannot be made with certainty.
Acknowledgements
The author wishes to acknowledge the funding support of the National Cancer Institute’s Center of Excellence in Cancer Communication (CECCR) located at the Annenberg School for Communication, University of Pennsylvania (P50-CA095856-05). The views expressed are those of the author’s alone. The author is especially grateful to Robert Hornik, Bridget Kelly, Stacy Gray, and Aaron Smith-McLallen for their contributions to the development of measures used in this paper.
References
- Aiken LS, West SG, Woodward CK, Reno RR. Health beliefs and compliance with mammography screening recommendations in asymptotic women. Health Psychology. 1994;13:122–129. doi: 10.1037//0278-6133.13.2.122. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts and figures. New York, NY: American Cancer Society Publication; 2004. pp. 1–48. [Google Scholar]
- Bandura A. Social learning theory. Prentice-Hall: Englewood Cliffs, NJ; 1977. [Google Scholar]
- Bandura A. Self-efficacy determinants of anticipated fears and calamities. Journal of Personality and Social Psychology. 1983;45:464–469. [Google Scholar]
- Bandura A. A social cognitive theory of personality. In: Pervin K, John O, editors. Handbook of personality. 2nd ed. New York: Guilford; 1999. pp. 154–196. [Google Scholar]
- Beebe-Dimmer JL, Wood DP, Gruber SB, Chilson DM, Zuhlke KA, Claeys GB, et al. Risk perception and concern among brothers of men with prostate cancer. Cancer. 2004;100(7):1537–1544. doi: 10.1002/cncr.20121. [DOI] [PubMed] [Google Scholar]
- Blalock SJ, DeVellis BM, Afifi RA, Sandler RS. Risk perceptions and participation in colorectal cancer screening. Health Psychology. 1990;9(6):792–806. doi: 10.1037//0278-6133.9.6.792. [DOI] [PubMed] [Google Scholar]
- Clark VA, Lovegrove H, Williams A, Machperson M. Unrealistic optimism and the health belief model. Journal of Behavioral Medicine. 2000;23(4):367–376. doi: 10.1023/a:1005500917875. [DOI] [PubMed] [Google Scholar]
- Eiser JR, Cole N. Participation in cervical screening as a function of perceived risk, barriers, and need for cognitive closure. Journal of Health Psychology. 2002;7:99–105. doi: 10.1177/1359105302007001657. [DOI] [PubMed] [Google Scholar]
- Floyd DL, Prentice-Dunn S, Rogers RW. A meta-analysis of research on protection motivation theory. Journal of Applied Social Psychology. 2000;30:407–429. [Google Scholar]
- Hay JL, Buckley TR, Ostroff JS. The role of cancer worry in cancer screening: A theoretical and empirical review of the literature. Psycho-Oncology. 2005;14:517–534. doi: 10.1002/pon.864. [DOI] [PubMed] [Google Scholar]
- Haitt RA, Rimer BK. A new strategy for cancer control research. Cancer Epidemiology Biomarkers Prevention. 1999;8:957–964. [PubMed] [Google Scholar]
- Hendrick RE, Smith RA, Rutledge JH, Smart CR. Benefits of screening mammography in women aged 40–49: A new meta-analysis of randomized controlled trials. Journal of the National Cancer Institute Monograph. 1997;22:87–92. doi: 10.1093/jncimono/1997.22.87. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The health belief model: A decade later. Health Education Quarterly. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Joseph JG, Montgomery SB, Emmons CA, Kirscht JP, Kessler RC, Ostrow DG, et al. Perceived risk of AIDS: Assessing the behavioral and psychosocial consequences in a cohort of gay men. Journal of Applied Social Psychology. 1987;17:231–250. [Google Scholar]
- Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: A meta-analytic review. Preventive Medicine. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Klein WM. Comparative risk estimates relative to the average peer predict behavioral intentions and concern about absolute risk. Risk Decision and Policy. 2002;7:193–202. [Google Scholar]
- Klein WM, Weinstein ND. Social comparison and unrealistic optimism about personal risk. In: Buunk BP, Gibbons FX, editors. Health, coping, and well-being: Perspectives from social comparison theory. Hillsdale, NJ: Lawrence Erlbaum; 1997. pp. 25–61. [Google Scholar]
- Lerman C, Schwartz M. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Research and Treatment. 1993;28(2):144–155. doi: 10.1007/BF00666427. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Cameron L. Behavioral theories and the problem of compliance. Patient Education and Counseling. 1987;10:117–138. [Google Scholar]
- Lipkus IM, Klein WMP. Effects of social comparison information on colorectal cancer risk perceptions. Journal of Health Communication. 2006;11(5):391–407. doi: 10.1080/10810730600671870. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Klein WMP, Skinner CS, Rimer BK. Breast cancer risk perceptions and breast cancer worry: What predicts what? Journal of Risk Research. 2005;8(5):439–452. [Google Scholar]
- Lipkus IM, Lyna PR, Rimer BK. Colorectal cancer risk perceptions and screening intentions in a minority population. Journal of the National Medical Association. 2000;92:492–500. [PMC free article] [PubMed] [Google Scholar]
- Mandel JS. Reducing mortality from colorectal cancer by screening for fecal occult blood. Journal of the National Cancer Institute Monograph. 1999;91:434–437. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychology. 1996;15:423–429. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- McCaul KD, O’Donnell SM. Naïve beliefs about breast cancer risk. Women’s Health. 1998;4:93–101. [PubMed] [Google Scholar]
- Mullens AB, McCaul KD, Erickson SC, Sandgren AK. Coping after cancer: Risk perceptions, worry, and health behaviors among colorectal cancer survivors. Psycho-Oncology. 2004;13:367–376. doi: 10.1002/pon.751. [DOI] [PubMed] [Google Scholar]
- Ozer EM, Bandura A. Mechanisms governing empowerment effects: A self-efficacy analysis. Journal of Personality and Social Psychology. 1990;58:472–486. doi: 10.1037//0022-3514.58.3.472. [DOI] [PubMed] [Google Scholar]
- Radcliffe NM, Klein WMP. Dispositional, unrealistic and comparative optimism: Differential relations with the knowledge and processing of risk information and beliefs about personal risk. Personality & Social Psychology Bulletin. 2002;28(6):836–846. [Google Scholar]
- Rimal RN. Closing the knowledge-behavior gap in health promotion: The mediating role of self-efficacy. Health Communication. 2000;12:219–237. doi: 10.1207/S15327027HC1203_01. [DOI] [PubMed] [Google Scholar]
- Rimal RN. Longitudinal influences of knowledge and self-efficacy on exercise and behavior: Tests of a mutual reinforcement model. Journal of Health Psychology. 2001;6:31–46. doi: 10.1177/135910530100600103. [DOI] [PubMed] [Google Scholar]
- Rimal RN. Perceived risk and self-efficacy as motivators: Understanding individuals’ long-term use of health information. Journal of Communication. 2002;51:633–654. [Google Scholar]
- Rimal RN, Flora JA, Schooler C. Achieving improvements in overall health orientation: Effects of campaign exposure, information seeking, and health media use. Communication Research. 1999;26:322–348. [Google Scholar]
- Rimal RN, Real K. Perceived risk and efficacy beliefs as motivators of change: Use of the risk perception attitude (RPA) framework to understand health behaviors. Human Communication Research. 2003;29(3):370–399. [Google Scholar]
- Rogers RW. A protection motivation theory of fear appeals and attitude change. Journal of Psychology. 1975;91:93–114. doi: 10.1080/00223980.1975.9915803. [DOI] [PubMed] [Google Scholar]
- Schuit AJ, van Loon JM, Tijhuis M, Ocke MC. Clustering of lifestyle risk factors in a general adult population. Preventive Medicine. 2002;35:219–224. doi: 10.1006/pmed.2002.1064. [DOI] [PubMed] [Google Scholar]
- Shiloh S, Vinter M, Barak A. Correlates of health screening utilization: The roles of health beliefs and self-regulation motivation. Psychology and Health. 1997;12:301–317. [Google Scholar]
- Suls J, Wheeler L. Handbook of social comparison: Theory and research. New York: Kluwer Academic/Plenum Publishers; 2000. [Google Scholar]
- Svenson O, Fischhoff B, McGregor D. Perceived driving safety and seatbelt usage. Accident Analysis and Prevention. 1985;17:119–133. doi: 10.1016/0001-4575(85)90015-6. [DOI] [PubMed] [Google Scholar]
- Turner MM, Rimal RN, Morrison D, Kim H. The role of anxiety in seeking and retaining risk information: Testing the risk perception attitude framework in two studies. Human Communication Research. 2006;32:130–156. [Google Scholar]
- van der Pilgt J. Perceived risk and vulnerability as predictors of precautionary behavior. British Journal of Health Psychology. 1998;3:1–14. [Google Scholar]
- van der Velde FW, Hooijkaas C, van der Pilgt J. Risk perception and behavior: Pessimism, realism, and optimism about AIDS-related health behavior. Psychology and Health. 1991;6:23–28. [Google Scholar]
- Vernon SW. Participation in colorectal cancer screening: A review. Journal of the National Cancer Institute Monographs. 1997;89:1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- Vernon SW. Risk perception and risk communications for cancer screening behaviors: A review. Journal of the National Cancer Institute Monographs. 1999;25:101–119. doi: 10.1093/oxfordjournals.jncimonographs.a024184. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Unrealistic optimism about susceptibility to health problems. Journal of Behavioral Medicine. 1982;5:441–460. doi: 10.1007/BF00845372. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Reducing unrealistic optimism about illness susceptibility. Health Psychology. 1983;2:11–20. [Google Scholar]
- Weinstein ND. The precaution adoption process. Health Psychology. 1988;7:355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Testing four competing theories of health protective behavior. Health Psychology. 1993;12:324–333. doi: 10.1037//0278-6133.12.4.324. [DOI] [PubMed] [Google Scholar]
- Weinstein ND, Sandman PM, Roberts NE. Determinants of self-protective behavior: Home radon testing. Journal of Applied Social Psychology. 1990;20:783–801. [Google Scholar]
- Witte K. Putting the fear back into fear appeals: The extended parallel process model. Communication Monographs. 1992;59:225–249. [Google Scholar]
- Witte K. Fear control and danger control: A test of the extended parallel process model (EPPM) Communication Monographs. 1994;61:113–134. [Google Scholar]
- Witte K, Allen M. A meta-analysis of fear appeals: Implications for effective public health campaigns. Health and Education Behavior. 2000;27:591–615. doi: 10.1177/109019810002700506. [DOI] [PubMed] [Google Scholar]
- Zajac LE, Klein WMP, McCaul KD. Absolute and comparative risk perceptions as predictors of cancer worry: Moderating effects of gender and psychological distress. Journal of Health Communication. 2006;11:37–49. doi: 10.1080/10810730600637301. [DOI] [PubMed] [Google Scholar]