Abstract
Epidemiological and clinical studies report higher incidences of anxiety and increased emotional reactivity in individuals suffering from respiratory allergies. To evaluate if respiratory allergies are capable of promoting anxiety-like behavior in rodents, we used models of allergic rhinitis and behavioral evaluations followed by assessment of mRNA for cytokines in relevant brain regions. Mice and rats were sensitized to ovoalbumin or pollen respectively following standard sensitization and challenge protocols. After challenge, the animals were evaluated in the open field, elevated plus maze and resident intruder tests. Cytokines and corticotropin releasing factor expression were assessed in several brain regions by real-time RT-PCR and plasma corticostereone concentrations by radioimmunoassay. Mice and rats sensitized and exposed to allergen showed increased anxiety-like behavior and reduced social interaction without any overt behavioral signs of sickness. T-helper type 2 (TH2) cytokines were induced in both rats and mice in the olfactory bulbs and prefrontal cortex and remained unchanged in the temporal cortex and hypothalamus. The same results were found for CRF mRNA expression. No differences were observed in corticosterone concentrations one hour after the last behavioral test. These results show that sensitization and challenge with allergens induce anxiety across rodent species and that these effects were paralleled by an increased expression of TH2 cytokines and CRF in the prefrontal cortex. These studies provide experimental evidence that sensitized rodents experience neuroimmune-mediated anxiety and reduced social interaction associated with allergic rhinitis.
Keywords: Intranasal, Anxiety, Prefrontal Cortex, Allergies, Cytokines, CRH, Inflammation, Neuroimmune
1. Introduction
Allergic rhinitis affects about 20 to 30 % of adults and up to 40% of children (Meltzer, 1997; Sly, 1999; 2002; Berger, 2004; Nathan, 2007; Blaiss, 2007) and contributes to the development of allergic asthma (Meltzer et al., 2004; Meltzer, 2005). A hallmark of allergic disease is the expansion of the type 2 (TH2) subset of T cells and the generation of IgE antibodies to common allergens. In the sensitized individual following exposure to antigen, there is an early phase hypersensitivity response within 30 minutes, which may be followed by a late phase response 2-6 hours later (Galli et al., 2008). If there is ongoing exposure to antigen, allergic inflammation can persist for days or longer. The early phase primarily results from mast cell degranulation and release of pre-formed and newly synthesized mediators, like proteases, histamine and leukotrienes which effect vascular permeability, and mucus production. Release of chemokines and recruitment of inflammatory cells contribute to the late phase response which is characterized by an influx of eosinophils, and TH2 lymphocytes which produce TH2 cytokines such as IL-4 and IL-13 (Galli et al., 2008).
Allergic rhinitis is associated with congestion, nasal discharge, palatal itch and sneezing, however some patients report symptoms of fatigue and changes in mood. The most recognized behavioral complications of allergic rhinitis noted by clinical practitioners are the development of fatigue and sleep disturbances as a consequence of obstruction of the airways (Meltzer, 1997; Schoenwetter et al., 2004; Santos et al., 2006; Woods and Craig, 2006; Sundberg et al., 2007). However, psychiatric complications including anxiety and altered emotional reactivity are also acknowledged (Addolorato et al., 1999; Marshall et al., 2002; Burske-Kirschbaum et al., 2008; Chida et al., 2008; Blaiss, 2008). Nonetheless, the extent to which allergic rhinitis may contribute to mental problems is of relevance since the results of epidemiological and clinical studies consistently reports a higher incidence of anxiety and in some cases depression in individuals suffering from respiratory allergies (Hurwitz and Morgenstern, 1999; Addolorato et al., 1999; Goodwin, 2002; Timonen et al., 2003; Goodwin et al., 2004; Patten and Williams, 2007; Williams et al., 2007; Postolache et al., 2007; Barone et al., 2008; Burske-Kirschbaum et al., 2008; Goodwin and Buka, 2008). However, the possibility of a causal relationship between allergic rhinitis and pathological levels of anxiety is still a matter of controversy. For instance, it has been proposed that high levels of anxiety and further complications resulting in altered emotional reactivity develop as a process of suffering from a chronic condition (Chida et al., 2008). Moreover, it has been suggested that an exaggerated allergic inflammatory response in individuals with high anxiety is the result of hyperactivity of the nervous system and the hypothalamic-pituitary-adrenal (HPA) axis with subsequent stimulation of the immune system (Wright, 2005).
Recent human and rodent studies, however, suggest that there may be a direct relationship between antigen exposure and alteration in brain function that may precipitate high levels of anxiety and emotional reactivity (Rosenkranz et al., 2005; Costa-Pinto et al., 2005; 2006; 2007). Several mice studies showed that exposure to intranasal or aerosolized antigen in sensitized animals induces avoidance behavior and activation of limbic brain regions (Costa-Pinto et al., 2005; 2006; 2007). Moreover, increased anxiety determined by the open field and elevated maze tests was reported during the early phase of the allergic reaction in a rat model of asthma (Palermo-Neto and Guimaraes, 2000). Furthermore, increased anxiety in the open field test and activation of limbic brain regions was reported during the early phase in a mice model of food allergies (Basso et al, 2003; 2004). In humans, increased brain activity in the prefrontal cortex was observed using functional magnetic resonance during the late phase of an asthma episode (Rosenkranz et al., 2005). While these studies provide a foundation in identifying some brain regions and behavioral responses associated with allergen challenge, the notion that these allergies may initiate, perpetuate and exacerbate pathological anxiety remains controversial and largely unstudied. The scope of the problem may be of significance because allergies and allergic rhinitis in particular are a growing problem in developed countries (Sly, 1999; Schoenwetter, 2000; Galli et al., 2008) and may impose an important immune stressor in a considerable number of people.
In the present study we employed two models of allergic rhinitis to evaluate anxiety-like behavior, social interaction and aggression in mice and rats, behaviors that are indicative of altered emotional reactivity in rodents (Berton et al, 1997). Albumin from chicken egg (OVA) was used in inbred BALB/c mice (Gelfand et al., 2004; Kelly-Welch et al., 2004; Costa-Pinto et al., 2005; Zosky and Sly, 2007) and tree pollen, one of the most common aeroallergens was employed in inbred Brown Norway rats (Steerenberg et al., 1999). These models represent the most accepted approaches to study allergic rhinitis in rodents. We also used real-time RT-PCR to analyze the expression of TH2 cytokines in the olfactory bulbs, prefrontal cortex (PFC), temporal cortex and hypothalamus. These regions contains several structures shown to be responsive to several models of experimentally-induced allergies (Basso et al, 2003; Rosenkranz et al, 2005; Costa-Pinto et al., 2006). Because of evidence suggesting the involvement of corticotropin-releasing factor (CRF) in allergic processes (Silverman et al., 2004; Theoharides and Kalogeromitros, 2006; Theoharides and Konstantinidou, 2007) and its recognized role in the neurobiology of anxiety (Zoumakis et al., 2006; Ising and Holsboer, 2007; Holsboer and Ising, 2008), we also evaluated the expression of CRF in the same regions. Finally, plasma corticosterone concentrations were determined by radioimmunoassay.
2. Methods
2.1 Allergic Rhinitis to ovoalbumin (OVA)
Inbred BALB/c mice (n=40 for behavioral studies and n=12 for PCR determinations) were obtained from Taconic Farms. Because the only existing data on the effects of allergic inflammation on behavior were obtained using this strain (Costa-Pinto et al., 2005), the present studies were carried using these mice. This strain was also chosen for its consistent responses to experimentally induced allergies to OVA and its extensive use in the study of the biology of allergic responses (Gelfand, et al., 2004; Kelly-Welch et al., 2004; Zosky and Sly, 2007). Mice in general do not develop consistent responses to pollen and therefore, OVA is the most appropriate antigen. Allergic rhinitis to OVA was induced as previously described (KleinJan et al., 2006). Briefly, albumin from chicken egg (OVA) was purchased from Sigma (St Louis MO) and absorbed to aluminum hydroxide (Alum) (Pierce) in a 1:1 mixture for 30 minutes under constant shaking at room temperature. Mice were immunized by intraperitoneal injections of Alum alone or with 100 μg/mice OVA/Alum in a volume of 200 μl on day 1 and boosted with the same reagents on day 14. Mice were challenged on day 21, 22 by intranasal instillations with a 1% OVA solution in PBS (allergic mice) or PBS alone (control mice). For this procedure, mice were slightly anesthetized with isofluorane in induction chambers and at the moment of awakening 25 μl of OVA/PBS or PBS alone was delivered with a micropipette on each nostril. Mice were tested for social interaction at day 23 and in the elevated plus-maze test followed by the open field test at day 24 (Figure 1).
Figure 1.
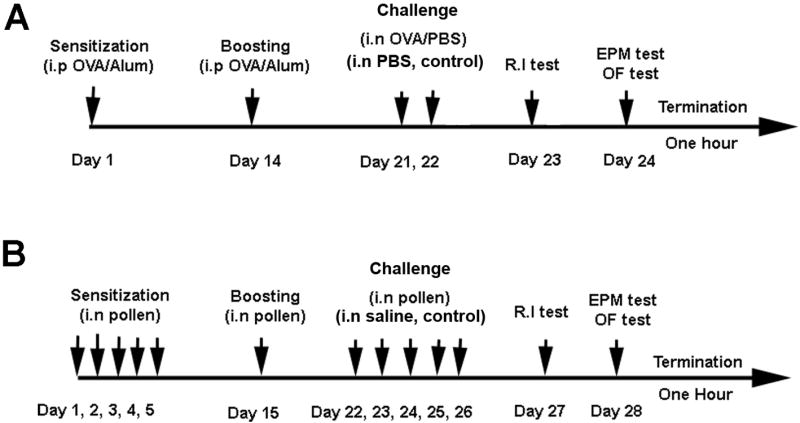
Representative diagram of the sensitization, challenge and behavioral testing schedule in BALB/c mice (A) and Brown Norway (NB/NHsd) rats (B). OVA: albumin from chicken egg; i.p: intraperitoneal; i.n: intranasal; R.I: resident intruder test; EPM: elevated plus maze test; OFT: open field test. Animals used for mRNA quantification were treated as shown and terminated at the same time but were not behaviorally tested.
2.2 Allergic Rhinitis to pollen
Inbred Brown Norway (BN/NHhsd) rats (n = 36 for behavioral studies and n = 16 for PCR determinations) were obtained from Harlan-Sprague Dawley (Indianapolis, IN). This strain was selected based on their susceptibility to develop allergic rhinitis after repeated exposure to pollen (Steerenberg et al., 1999; Motta et al., 2004). This strain has been proposed as a naturalistic model of respiratory allergies and therefore, provides a comparative model for the studies in mice. They were sensitized by repeated intranasal instillations with a mixture of equal parts of tree pollen (oak, cedar, maple and birch) (Greer Laboratories, Lenoir, NC) dissolved in saline (250 mg/ml pollen mixture) once a day for 5 consecutive days as previously described (Steerenberg et al., 1999). For this procedure, the animals were slightly anesthetized with isofluorane in induction chambers and at the moment of awakening were administered with 100 μl per nostril of saline solution or pollen mixture. The animals received an intranasal dose at day 15 after the last administration to boost the immune reaction. The rats were challenged at day 22, 23, 24, 25 and 26 for 5 consecutive days with the same solutions. Control animals were treated with saline under the same challenge schedule. Rats were tested on day 27 in the resident intruder test and on day 28 in the elevated plus maze test and open field test (Supplemental figure 1). Weight was monitored daily. Positive allergic reactions were evaluated by the passive cutaneous anaphylaxis technique (Supplemental figure 1).
2.3 Open Field Test
For mice studies, 49 cm × 49 cm arenas under constant illumination at 35 lux from the top were used (San Diego Instruments, San Diego, CA). The animals were brought to the room and acclimated for at least one hour before behavioral testing. The open field sessions were videotaped from the top of the arenas in 5 minutes session. Data was collected and analyzed with the use of the TopScan system from Cleversys (Reston, VA). The system automatically calculates in cm the distance traveled in the area corresponding to the periphery (75 % of the total area) as well as the percentage of time spent in the center area (25% of the total area) of the arena. For rat studies, experiments were carried out as described for the mice with the following modifications: ambulatory motor activity was monitored in similar 40.1 × 40.1 cm arenas maintained under constant illumination at 40 lux. The floor was divided in 16 equal squares (12 peripheral and 4 center squares representing 75 and 25 % respectively) and a digital video camera was placed at a top angle and recorded the entire 5 minutes of the sessions. Quantification was carried out by blind experimenters by reviewing the tapes and counting the number of times that an animal crossed a square (counts). Total activity was expressed as total number of counts and further expressed as percentage of activity in the periphery vs the center field. An additional parameter analyzed in both studies was number of fecal boli.
2.4 Elevated Plus-Maze Test
For mice studies, an elevated plus maze instrument with 2 open arms and 2 closed arms specific for mice was used (Stoelting, Wood Dale, IL). The animals were brought to the testing room and acclimated for at least one hour before starting with the sessions. Sessions of 5 minutes held under 5 lux of illumination were recorded with a video camera mounted on top of the maze and the time spent in open arms, total distance travelled and number of entries in open arms was analyzed with the use of the MazeScan system from Cleversys (Reston, VA). For rat studies, experiments were carried out as described for the mice with the following modifications: a fully automated plus-maze instrument (Acuscan, Columbus. OH) with 2 open and 2 closed arms specific for rats was used. Time spent in open arms and total number of crossings between arms was automatically quantified by this system in sessions of 5 minutes. Data was analyzed as a percentage of time spent in the open arms vs time spent in closed arms. Additional parameters analyzed for both rats and mice included number of entries on the open arms and fecal boli.
2.5 Resident Intruder Test
These studies were performed using standard procedures for the resident-intruder test; for mice studies, resident mice were male BALB/c, 9 weeks old at testing that had been housed individually for 4 weeks in our facility. Intruder mice were received one week prior to testing, were group housed, and were 8 weeks of age. Therefore, resident and intruder mice were of same age at the time of testing. All mice were weighed one day before testing and the groups were confirmed to be of comparable weight. Intruder animals were housed in groups of 5 and a total of 20 intruder mice were used and 2 cages were assigned as intruders for controls and 2 cages as intruders for allergic mice. One week prior to testing the resident mice had their cages changed, with no other cage changes prior to the test. Mice were allowed to acclimate to the testing room one hour prior to the onset of behavioral testing. For the procedure the resident’s (isolated mouse) home cage was used, with the food/water bin removed and lid on, and lighting was 30 lux. An unfamiliar group housed animal (intruder non treated mice) was placed in the cage of the isolated animal (resident allergic or control mice) for 10 minutes and behavior was videotaped. After each trial, group housed mice were placed into a new cage (no mouse was placed back into their original cage with test naïve mice). Scoring was done either live, or from video files, using CleverSys Inc Anostar behavioral annotation software. The observer manually enters keyboard strokes to register the incidence and length of events. Latency to initiate contact, duration of social contact (social investigation) and aggressive behavior (attacks and bites) were quantified solely from the resident towards the intruder.
Similarly for rats, intruder rats were of same age and similar weight than resident rats. Intruder rats were housed in groups of 3 and a total of 18 intruder rats were used and 3 cages assigned as intruders for controls and 3 cages assigned as intruders for allergic rats. Resident animals remained in its original home cage placed in the testing area for ten minutes prior to intruder entry. An unfamiliar intruder (untreated rat) was taken from a separate cage and placed in the resident (treated allergic or control rat) cage to begin the test. Tests were videotaped from a horizontal angle during ten minutes session. The same behavioral parameters as those for mice were scored by two observers both during the test, and re-scored on video after the test.
2.6 Corticosterone Determinations
Determinations were done in behaviorally tested animals 1 hour after the final behavioral test. At that time, mice were anesthetized with pentobarbital sodium (50mg/Kg) and blood collected by cardiac puncture. For rats, all animals were sacrificed by rapid decapitation without anesthesia and trunk blood collected in EDTA-containing tubes. Plasma corticosterone levels were determined by radioimmunoassay using a double antibody kit (ImmuChem, MP Biomedicals, Orangeburg, NY) according to manufacturer’s instruction. Intra assay variation was less than 8%.
2.7 Real-time RT-PCR
These determinations were done in groups of animal that were treated as shown in Figure 1 but were not behaviorally tested (n = 12 for mice and n = 16 for rats). At the same day corresponding to the last behavioral test, non-behaviorally tested animals were anesthetized by an overdose of pentobarbital sodium and transcardially perfused with physiological saline solution to remove blood from the brain. Brains were then immediately removed and frozen in isopentane until further dissection. Frozen brains were allowed to reach ice-cold temperature and the olfactory bulbs and PFC (approximately Bregma 5.2 mm to 2.7 mm for rats and 3.08 mm to 1.94 for mice) were excised by single cuts. Two additional coronal cuts of the remaining brain portion were performed (approximately Bregma -1.8 mm to Bregma -3.6 mm for rats and -0.82 mm to -2.8 mm for mice) and the temporal cortex and hypothalamus were dissected from this portion. Dissected brain tissue was processed for mRNA extraction using the Trizol reagent (Invitrogen, USA) as described previously (Tonelli, et al., 2004). Total RNA was treated with DNAse (Invitrogen, USA) for 15 minutes at room temperature according to manufacturer’s instruction. Five hundred ng of total RNA per sample were reverse transcribed into cDNA in a 20 μl reaction using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to manufacturer’s instructions. Real-time RT-PCR was conducted using the iQ SYBR Green Supermix (Bio-Rad) in a 25 μl reaction using the set of primers listed in Table 1. The PCR products derived from all sets of primers were run on 0.9% agarose gel to confirm a single amplification product. The amplified products were directly cloned into the pCRII-Topo vector (Invitrogen, Paisley, Scotland, UK) and sequenced to confirm their identity. All the primer pairs were designed using the Accelrys Gene 2.0v software and based on the following criteria: a) primer size between 80 to 180 base pair b) annealing temperature of 55° C for primer vs template c) percentage of G + C content between 40 to 55 % d) unique binding sites.
Table 1.
Primer sequences used for real-time RT-PCR determinations. Accession: GeneBank accession number for sequences publicly available at http://www.ncbi.nlm.nih.gov/sites/entrez. IL-4: interleukin-4; IL-5: interleukin-5; IL-13; interleukin-13; CRF: corticotropin releasing factor; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ACTB: actin, beta isoform; 18S: ribosomal RNA subunit 18S; mus: mus musculus; rattus: rattus norvegicus
| Gene (specie) | Accession | Primer sequence | Region | Product length | |
|---|---|---|---|---|---|
| IL-4 (mus) | NM_021283 | Fwd | 5’AGAACACCACAGAGAGTGAG3’ | 266-417 | 152 |
| Rev | 5’TGAATCCAGGCATCGAAAAG3’ | ||||
| IL-13 (mus) | NM_008355 | Fwd | 5’TCTTGCTTGCCTTGGTGGTC3’ | 98-237 | 140 |
| Rev | 5’ATACCATGCTGCCGTTGCAC3’ | ||||
| IL-5 (mus/rattus) | NM_010558 (mus) | Fwd | 5’CATGAGCACAGTGGTGAAAG3’ | 115-252 (mus) | 138 |
| NM_021834 (rattus) | Rev | 5’AGCCCCTGAAAGATTTCTCC3’ | 69-206 (rattus) | ||
| CRF (mus/rattus) | NM_205769 (mus) | Fwd | 5’GGATCTCACCTTCCACCTTC3’ | 633-734 (mus) | 102 |
| NM_031019 (rattus) | Rev | 5’CCGATAATCTCCATCAGTTTCC3’ | 631-732 (rattus) | ||
| GAPDH (mus) | NM_008084 | Fwd | 5’CCACTCACGGCAAATTCAAC3’ | 196-338 | 142 |
| Rev | 5’AGACTCCACGACATACTCAG3’ | ||||
| ACTB (mus) | NM_007393 | Fwd | 5’TGAGACCTTCAACACCCCAG3’ | 452-591 | 140 |
| Rev | 5’GAGCATAGCCCTCGTAGATG3’ | ||||
| 18S (mus/rattus) | NR_003278 (mus) | Fwd | 5’CCAGTAAGTGCGGGTCATAAGC3’ | 1645-1727 (mus) | 83 |
| X01117 (rattus) | Rev | 5’CCATCCAATCGGTAGTAGCGAC3’ | 1650-1732 (rattus) | ||
| IL-4 (rattus) | NM_201270 | Fwd | 5’TTACGGCAACAAGGAACAC3’ | 197-336 | 140 |
| Rev | 5’ACAGAGTTTCCTCAGTTCACC3’ | ||||
| IL-13 (rattus) | NM_053828 | Fwd | 5’GCTGAGCAACATCACACAAG3’ | 117-279 | 162 |
| Rev | 5’GAGGCCATTCAATATCCTCTG3’ | ||||
| GAPDH (rattus) | NM_017008 | Fwd | 5’TACCCACGGCAAGTTCAACG3’ | 223-362 | 140 |
| Rev | 5’ACTCCACGACATACTCAGCAC3’ | ||||
| ACTB (rattus) | NM_031144 | Fwd | 5’TGAGACCTTCAACACCCCAG3’ | 453-592 | 140 |
| Rev | 5’GCGCGTAACCCTCATAGATG3’ | ||||
The real-time PCR reaction was run on a MyiQ instrument (Bio-Rad) with a three step cycling program as follows: an initial hot start for 5 min at 95°C followed by 40 cycles with a denaturation step of 15 sec at 95°C, an annealing step of 30 sec at 55°C, an extension step of 30 sec at 72°C with the optics on at this last step. In preparation of a melt curve, the samples were heated for 1 minute at 95°C then cooled for 1 minute at 55°C, and the melt curve was executed in 10 second increments of 0.5°C with the temperature increasing from 55°C to 95°C with the optics on. All the primers used were selected and tested for the described amplification conditions. Efficiency and consistency of the cDNA synthesis was determined by amplification of the 18S gene as a control. For each round of amplifications, only those samples that were within 1 cycle of difference respect to the mean for 18S were considered for further analysis. For each sample of a specific target gene, each cycle threshold was normalized with respect to the average of 3 control genes including 18S, GAPDH and Actin-β. Relative expression was determined using the 2-ΔΔCt method (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008)
2.8 Statistics
Behavioral studies and corticosterone concentrations were analyzed with unpaired t-test. Cytokine and CRF expression were analyzed with two way ANOVAs with treatment and brain regions as factors. Tukey’s tests were used for pair comparisons. Significance level was set at p<0.05. Data are expressed as means ± standard error mean (SEM).
3. Results
3.1 Challenge with allergen in sensitized rodents induces anxiety-like behavior as revealed by the open field test
In both mice and rats, no differences were detected in total motor horizontal activity (Figure 2 A, C) or fecal boli (data not shown) in sensitized animals challenged with allergen with respect to control saline. In mice, a significant difference in the time spent in the center field of the enclosures was detected (t=2.36, p< 0.03) with OVA-challenged animals showing reduced values with respect to control animals (Figure 2 B). In rats, a significant difference in the activity in the center field was detected in pollen challenged animals which showed a reduced number of counts with respect to saline treated animals (t=4.29; p<0.001) (Figure 2 D). Sensitized animals exposed to allergen in two different rodent species consistently displayed reduced activity in the center field of the arena indicating increased anxiety. Moreover, no effect in total motor horizontal activity indicates that these effects are independent of signs of sickness.
Figure 2.
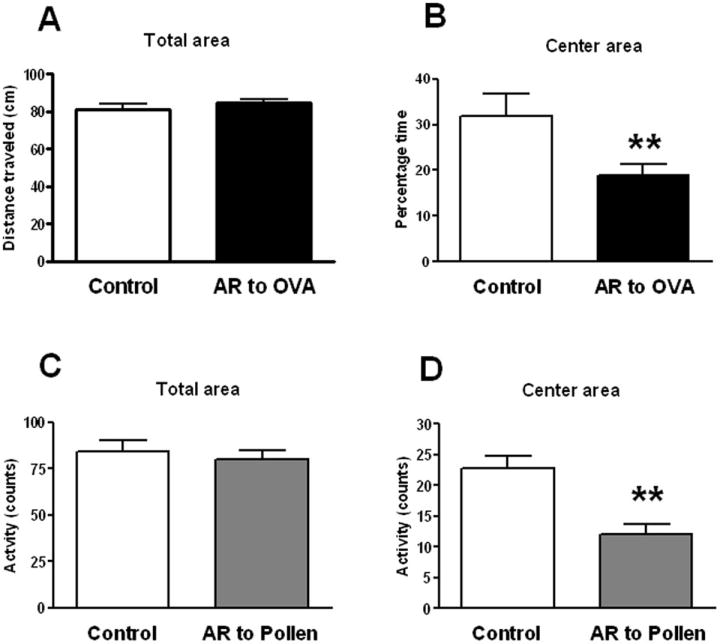
Activity in the open field test of mice (A; B) and rats (C; D) in control condition (sensitized but not challenged) or after intranasal challenge in sensitized animals with ovoalbumin (OVA) (A; B) or pollen (C; D) respectively. Activity is expressed as distance travelled (cm) in mice and crossing of squares (counts) in rats. Significant differences ** (p<0.001) were observed in the activity in the center area (B, D) and no differences were observed in the overall activity (A, C). Thus anxiety-like behavior is not the result of reduced motor activity which could be a component of sickness behavior.
3.2 Challenge with allergen in sensitized rodents induces anxiety-like behavior as revealed by the elevated plus maze test
In mice, a significant effect was obtained for percentage of time spent in the open arms (t=3.2; p < 0.01) with reduced time in OVA challenged animals with respect to control treated mice (Figure 3 B). No differences were observed in locomotor activity measured by the distance traveled within the plus-maze in both open and closed arms indicating that potentially altered motor functions were not driving the main effect (Figure 3 A). No differences were observed in the number of entries in the open arms and in the number of fecal boli. Similarly, rats sensitized and challenged with pollen showed reduced percentage of time in open arms with respect to saline treated rats (t= 2.150; p<0.05) (Figure 3 D). No differences were observed in activity measured by the total number of crossings between the 4 segments of the plus-maze suggesting normal motor function (Figure 3 C). No differences were observed in the total number of entries in the open arms and fecal boli. In allergic mice and rats, the reduced time spent in the open arms was the opposite to the sum of the time spent in the closed arms and the center of the maze. These results are consistent across two different rodent species in two different allergens used indicating that sensitization and intranasal challenge with allergen induces anxiety-like behavior as indicated by reduced time spent in the open arms of an elevated plus maze, an effect independent of reduced total activity indicative of sickness behavior.
Figure 3.
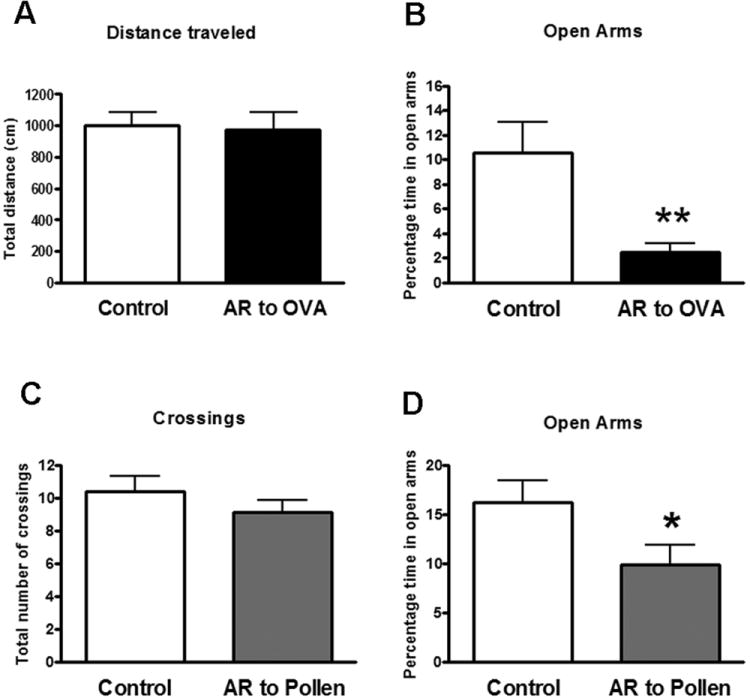
Activity in the elevated plus maze test of mice (A; B) and rats (C; D) in control conditions (sensitized but not challenged) or after intranasal challenge in sensitized animals with ovoalbumin (OVA) (A; B) or pollen (C; D) respectively. Overall activity is expressed as total distance traveled in the closed and open arms for mice and number of crossings between the segments of the plus-maze in rats. Activity in the open arms is expressed as the percentage of time spent in the open arms. Significant differences * (p<0.05); ** (p<0.001)
3.3 Challenge with allergen in sensitized rodents reduces social interaction
These behaviors correspond only to resident animals representing allergic (sensitized and challenged) and control (sensitized not-challenged) treated mice and rats. No data from intruder animals are presented. In mice, a significant effect was detected for latency to initiate social behavior (t=2.37, p<0.04) (Figure 4 A) and duration of social behavior (t=2.4, p<0.03) (Figure 4 B) in OVA-challenged animals with respect to control. No differences were observed on the frequency of social contact. No significant differences were observed on measures of aggressive behavior (Supplemental Figure 2). Similarly, in rats, a significant effect was observed for the latency to initiate social contact (t=2.596; p< 0.03) (Figure 4 C) and in the duration of the contact (t=3.2; p< 0.05) (Figure 4 D) in pollen-challenged animals with respect to control. No differences in aggressive behaviors were observed between control and pollen treated rats.
Figure 4.
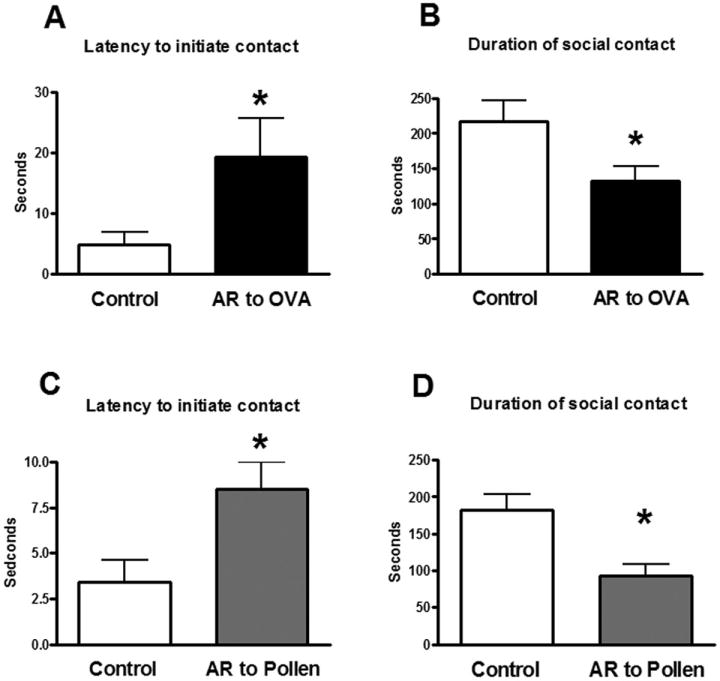
Latency to initiate contact (A, C) and duration of social contact (non-aggressive) (B, D) of mice (A; B) and rats (C; D) in control condition (sensitized but not challenged) or after intranasal challenge in sensitized animals with ovoalbumin (OVA) (A; B) or pollen (C; D) respectively. Significant differences * (p<0.05)
3.4 Plasma corticosterone levels and body weight
No significant differences were observed in plasma corticosterone levels between control and sensitized rodents exposed to the allergen 1 hour after the last behavioral tests. Values were 118.4 ± 23.4 ng/ml for control and 133.5 ± 23.7 ng/ml for OVA challenged mice. For rats values were 171.3 ± 19.8 ng/ml in control and 151.3 ± 87.39 ng/ml in pollen challenged animals. Similarly, no significant changes in body weight between control and sensitized rodents exposed to antigen were observed (Supplemental Figure 3).
3.5 Intranasal challenge with allergen induced TH2 cytokine expression in the prefrontal cortex and olfactory bulbs of sensitized rodents
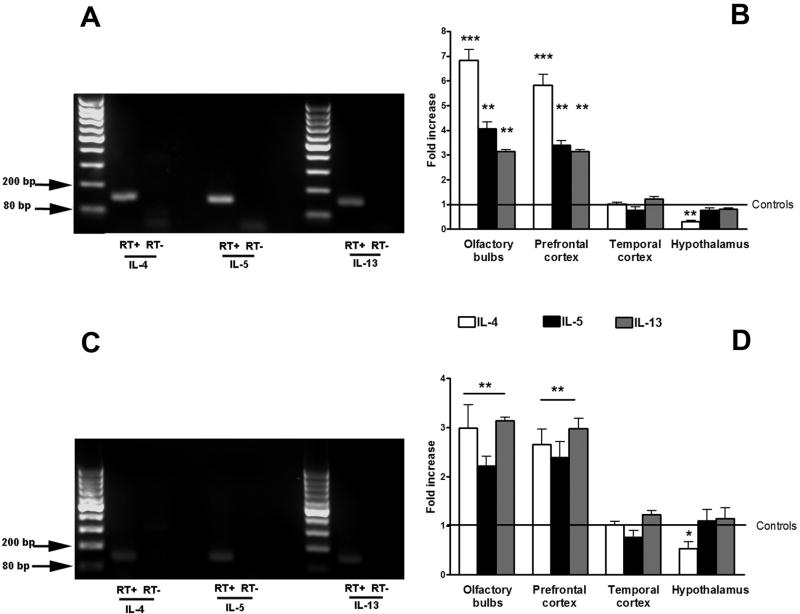
Single melting curves peaks were obtained for all the genes analyzed in mRNA originated from brain tissue. Amplified products for TH2 cytokines were further analyzed by gel electrophoresis (Figure 5 A, C). The figure shows final amplified product for TH2 cytokines in the PFC of OVA-challenged mice (Figure 5 A) and pollen-challenged rats (Figure 5 C). No product was detected when the reverse transcription step was omitted (RT-) confirming that the amplification was specific for mRNA that originated from the prefrontal cortex. The same was obtained for the olfactory bulbs (data not shown). ANOVA analysis detected significant differences in IL-4 expression in mice [F (1, 24) = 92.59; p< 0.0001]. Post hoc analysis indicated significant increases for OVA treated mice with respect to control in the olfactory bulbs (p<0.001) and PFC (p<0.001) (Figure 5 B). No differences were observed in the temporal cortex and hypothalamus. Similarly, significant differences were also observed for IL-5 [F (1, 24) = 80.33; p<0.0001] and IL-13 [F (1, 24) = 168.6; p<0.001]. As it was observed for IL-4, post hoc comparisons showed significant increases in IL-5 and IL-13 transcription in the olfactory bulbs and PFC (Figure 5 A). No significant changes in IL-5 and IL-13 were detected in the temporal cortex and hypothalamus.
Figure 5.
Final products for TH2 cytokines after 40 cycles of real-time RT-PCR performed from mRNA obtained from the area corresponding to the prefrontal cortex of mice (A) and rats (C) after intranasal challenge in sensitized animals with ovoalbumin (OVA) or pollen respectively. No product was detected when the reverse-transcriptase enzyme step was omitted (RT-). Final products were observed between the 80 and 200 base pair standards as expected (see Table 1). Relative expression of TH2 cytokines (IL-4, IL-5 and IL-13) as determined by real-time RT-PCR (B, D) in different brain regions after the induction of allergic rhinitis to ovoalbumin (OVA) (B) or pollen (D). Values are fold increase with respect to the average value of control mice and rats represented by the number 1. Significant differences with respect to control values: ** (p<0.001); *** (p<0.0001).
In rats, significant differences were also detected for IL-4 [F (1, 20) = 17.3; p<0.0001]; IL-5 [F (1, 20) = 12.6; p<0.0001] and IL-13 [F (1, 20) = 48.8; p<0.0001] (Figure 5 D). Post hoc analysis showed significant increases in the olfactory bulbs and PFC of pollen treated rats with respect to controls for the 3 cytokines analyzed (Figure 5 D). No significant differences were observed in the temporal cortex and hypothalamus for any cytokine analyzed.
3.6 Intranasal challenge with allergen increased CRF transcription in the prefrontal cortex and olfactory bulbs of sensitized rodents
Analysis of mRNA expression for CRF in the PFC, temporal cortex and hypothalamus showed a significant effect in mice [F (3, 24) = 49.75; p<0.0001] and rats [F (3, 21) = 16.02; p<0.001]. Post hoc analysis revealed significant increases in CRF expression in OVA treated mice with respect to controls in the PFC and no changes in the temporal cortex and hypothalamus (Figure 6). Similarly, pollen challenged rats showed elevated CRF transcription in the PFC and no changes in the temporal cortex and hypothalamus.
Figure 6.
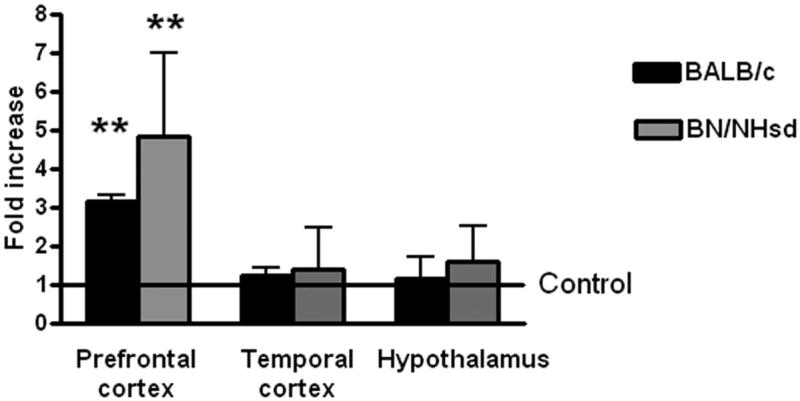
Relative expression of CRF as determined by real-time RT-PCR in the prefrontal cortex, temporal cortex and hypothalamus after the induction of allergic rhinitis to ovoalbumin (OVA) in BALB/c mice or pollen in Brown Norway (BN/NHsd) rats. Values are fold increase with respect to the average value of controls treated mice or rats represented by the number 1. Significant differences with respect to control values: ** (p<0.001)
4. Discussion
The present study is the first to report behavioral responses of anxiety and reduce social interaction during experimentally-induced allergic rhinitis and show that these effects are consistent across two rodent species. Moreover, these effects were achieved using two different types of antigens indicating that the host immune response is likely to mediate these effects. In addition, the present results suggest that these effects may be related with the induction of TH2 cytokines and CRF at least in the PFC and olfactory bulbs. Behavioral responses of anxiety in mice and rats using OVA as an antigen has been described previously during the early phase in experimental models of asthma (Costa-Pinto et al, 2005; Palermo-Neto and Guimaraes, 2000). Together with the present results, these studies show that anxiety is a feature of respiratory allergic processes that occurs during the differences phases and when different antigens are involved. These findings strengthen the idea that the cellular and molecular mediators of allergic inflammatory processes activate common neuroimmune mechanisms that involve emotionally relevant circuits and the induction of anxiety.
The association of allergies with anxiety disorders and increased emotional reactivity has long been recognized and known to be complex. It has been proposed that the distress caused by chronic inflammatory diseases such as allergic rhinitis or allergic asthma could induce pathological anxiety as the result of psychological anticipatory processes of recurrent chronic episodes (Chida et al., 2008). Also, psychological stress and emotion in allergic individuals have been proposed to affect stress responsiveness resulting in exaggerated adrenergic and cortisol responses that may in turn result in aggravation of allergic symptoms (Wright, 2005). In addition, it has been proposed that co morbidity of allergies with anxiety disorders are related with genetic factors that segregate together and influence each other (Wamboldt et al., 1998; 2000). While the relationship between allergic rhinitis and anxiety has been shown to be bidirectional and likely to share genetic and neuroimmune mechanisms, these mechanisms remains undefined. Indeed, the idea that allergen exposure in sensitized individuals can elicit responses of anxiety has received little attention. Two relatively recent reports by the same group using a modified version of the passive avoidance test showed for the first time that sensitized mice display avoidance behavior towards an allergen-associated compartment and that these effects were IgE dependent (Costa-Pinto et al, 2005; 2007). The authors also showed that the avoidance effect occurred during the early phase of the allergic response. In the present study, we used 2 independent tests to evaluate anxiety behaviors during the late phase of the allergic reaction and observed consistent responses of anxiety. In this regard, the elevated plus maze (EPM) test is one of the most validated tests to evaluate anxiety in rodents (Carobrez and Bertoglio, 2005). It is ethologically relevant to the animal involving the natural spontaneous exploration of environments by rodents in the absence of reward (Rodgers et al., 1997; Carobrez and Bertoglio, 2005). The open field test (OFT) is also an ethologically relevant test of anxiety-like behavior (Prut and Belzung, 2003). It is also based on the innate behavior of rodents to explore in novel spaces that is opposed to the fear of open spaces in the absence of reward or aversive stimulus. The open field test also analyzes total motor horizontal activity which is an important measure in immunologically relevant behavioral studies because they indicate the possibility of effects related to sickness. In the present study, no differences in motor activity were detected in the EPM or OF tests suggesting that sickness did not influenced behavioral responses.
In addition to anxiety disorders, the burden of allergic rhinitis on quality of life has been documented by numerous studies across cultures (Meltzer, 1997; 2001; Nathan, 2007; Blaiss, 2007). The most common reported impairments are sleep disturbances, poor cognitive functioning, absenteeism from work and school and in children difficulties integrating with peers (Marshall et al., 2000; 2002; Berger, 2004; Baiardini, et al., 2006; Santos et al., 2006; Sundberg, et al., 2007; Blaiss, 2007; 2008). While many of the social impairments caused by allergic rhinitis are likely to be related with the symptoms of the disease, the question if allergic rhinitis may induce altered social interactions in an experimental setting was never tested before. We used the resident intruder test that is one of the most commonly used tests to elicit and measure aggression and also analyze social interactions among conspecifics of rodent species. This test is based on agonistic behaviors displayed during encounters between unfamiliar rodents (Mitchell, 2005). After introduction of an intruder rodent into the home cage of a resident rodent, the resident rodent invariably shows investigative behavior and postures that are measures of social behavior or interactions. These behaviors may lead to the expression of aggressive behavior. Although aggressive behaviors were rare in the present experiments, a consistent reduction in social interaction was seen in mice and rats with experimentally induced allergic rhinitis. This indicates that other behavioral components suggestive of altered emotional reactivity are also affected by the inflammatory process of allergic rhinitis. However, it is also possible that reduced social interaction was related or produced by increased social anxiety (Nicolas and Prinssen, 2006). If reduced social interaction in the present study is the resultant of increased anxiety remains to be determined. An important consideration of the present experiments is that the animals were subjected to all of the different behavioral testing. While a minimal effect is expected, it can not be excluded the possibility of an influence of a particular test on any further behavioral testing. In any case, together with the studies of Costa-Pinto et al and Palermo-Neto and Guimaraes showing anxiety-like behaviors during the early phase in a model of allergic asthma, the present study provides additional evidence that similar behavioral alterations reported in humans suffering from respiratory allergies can be mimicked in rodents by experimentally inducing allergic rhinitis or asthma.
Another important consideration to the present studies is that the corticosterone determinations were done in behaviorally tested animals one hour after the last behavioral test and thus comparisons between groups do no represent HPA-axis reactivity. Instead, the reported values are likely to reflect certain degree of recovery following acute activation of the HPA-axis. Transient increases in cortisol levels short after nasal provocation has been reported in allergic patients (Kalogeromitros et al., 2007). Conversely, another study showed blunted cortisol responses to stress in atopic adolescents (Wamboldt et al., 2003). Theses studies show that the responsiveness of the HPA-axis to allergic processes depends on several parameters that may include the magnitude of the allergic reaction, the timing after the contact with the allergen and also psychological stressors (Palermo-Neto and Guimaraes, 2000). It is notably however, the limited research addressing these issues. Another important consideration of the present study is that allergies have been also associated with increased incidence in depressive disorders (Hurwitz and Morgenstern, 1999; Wamboldt et al., 2000; Timonen, et al., 2003; Goodwin and Buka, 2008), while we have performed studies in rodents using the forced swim test, the results have been so far inconclusive.
The neural circuitry involved in the expression of anxiety and the brain regions compromised in pathological anxiety has been extensively studied (Drevets, 2000; 2003; Bremner, 2004). Studies in humans, non-human primates and rodents implicate the PFC as an important structure that provides modulatory function in the manifestation of anxiety. In addition, functional imaging studies strongly implicates the PFC in providing inhibitory functions for anxiety and depression (Drevets, 2000; 2003; Bremner, 2004). The present study showed that this region responded with increases in the transcriptional activity of TH2 cytokines and CRF during an allergic inflammatory process in the upper respiratory tract. These results are relevant in the context of the finding reported by Rozenkranz et al (Rosenkranz et al., 2005) showing increased blood flow in the anterior cingulate cortex during the late phase of an allergic reaction when allergic individuals are exposed to antigen. Thus, this region appears to be involved in the neural responses elicited during the late phase of respiratory allergic reactions. Previous studies reported that other regions including the amygdala and hypothalamus are also responsive to respiratory allergies as measured by the expression of c-fos (Costa-Pinto et al., 2005; 2006; 2007). These regions are also involved in the neurocircuitry of anxiety and anxiety disorders. The present study did not reveal any changes in the transcriptional activity of the genes for cytokines and CRF in these regions. This may be related to the approach employed, because the PCR determinations in the temporal cortex and hypothalamus were performed from portions of tissue that contains several distinct neuronal populations of CRF cells, differences in CRF expression may have occurred and were not quantified using our dissection approach. Moreover, if the determinations are made during the early or the late phase of the allergic reaction may also play an important role. Finally, the use of anesthesia prior dissection of the brain regions may have influenced the sensitivity for detection of mRNA changes.
The present study is also the first to report on TH2 cytokine expression in the brain in response to a peripheral immune challenge. While the expression of IL-4, IL-5 and IL-13 follows the pattern of a TH2 cell-dependent allergic reaction, the meaning and function of this transcriptional increase in the PFC is unknown at this time. A tempting speculation is that increases in these cytokines may be related at least in part to CRF transcription and that CRF may provide the link between TH2 cytokine expression in the brain and behavioral changes. The involvement of T-cell dependent processes in influencing behavioral responses has been shown by studies using bacterial superantigens (Rossi-George et al., 2004; Rossi-George et al., 2005; Urbach-Ross et al., 2008). The study of Rossi-George et al., 2004 showed that anxiety-like behavior after activation of the TH1 subset of T-cells and expression of pro-inflammatory cytokines was reduced in the elevated plus maze test or no evident in other test of anxiety. In this regard is possible that TH2 immune processes may provide negative regulatory function over a set of pro-inflammatory cytokines and that the behavioral outcomes of allergic inflammation may be the resultant of these interactions rather than the effect of single cytokines. In this regard, decreased IL-2 mRNA in the prefrontal cortex was found associated with increased anxiety in the EPM (Pawlak et al, 2005) further supporting this notion. These effects may be also influenced by the genetic background as well as the specific nature of the immune stressor (Bartolomucci et al, 2003; Lu et al., 1999; Anisman et al., 2008; Dantzer et al., 2008).
In sum, experimentally induced allergic rhinitis in naïve rodents elicits responses of anxiety-like behavior and reduced social interaction on ethologically relevant tests, an effect that is paralleled by increased TH2 cytokine and CRF transcription in the PFC. These studies indicate that allergic rhinitis is a condition capable of influencing behavioral responses and warrants further studies to deepen the understanding of the complex relationship between atopy, emotional reactivity and possibly mental disorders.
Supplementary Material
Passive Cutaneous Anaphylaxis (PCA)
Positive allergic reactions were determined by the passive cutaneous anaphylaxis reaction. Receiving (non-allergic) control animals were shaved with Remington clippers on either side of the midline of the back. The animals were injected intravenously with 5% solution of Evans Blue (catalog #23860 Fisher Scientific) mixed with allergen diluted in saline via the tail vein approximately 15 minutes before intradermal injections. After intravenous injection of dye, the animals were given different intradermal injections on either side of the back, 1.5 cm from midline and 1 cm from each other. The injections consisted of the plasma of allergen treated animals in one side (A, B) and saline treated animals on the opposite side (C, D). Animals were then placed under a heat lamp in a small box and monitored for signs of a reaction. Positive allergic reaction consisted of a blue stain caused by extravasation of Evans blue at the site of intradermal plasma injection. The extravasation is the response to the cross-linking of IgE with the Fc receptor and local activation and degranulation of mast cells.
Frequency (A) and duration (B) of defensive and aggressive postures of mice in control condition (sensitized but not challenged) or after intranasal challenge with ovoalbumin (OVA) in sensitized animals. No significant differences were detected; (t=1.7, p<0.10) for A and (t=1.6, p<0.11) for B. Only 3 events involving attacks and bites were recorded from OVA treated mice against untreated intruders and no attacks were recorded from the control sensitized but not challenged animals against intruders.
Weight in grams of mice (A) and rats (B) sensitized and challenged with OVA (A) or pollen (B) respectively. Control animals were handled and treated under the same schedule but administered with vehicle solution. No significant differences were detected.
Acknowledgments
Supported by grants R21MH075905, R21MH075891 and R01MH074891. The authors wish to thanks Dr. James I. Koenig, PhD for his help with the corticosterone determinations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addolorato G, Ancona C, Capristo E, Graziosetto R, Di Rienzo L, Maurizi M, Gasbarrini G. State and trait anxiety in women affected by allergic and vasomotor rhinitis. J Psychosom Res. 1999;46:283–9. doi: 10.1016/s0022-3999(98)00109-3. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Baiardini I, Braido F, Brandi S, Canonica GW. Allergic diseases and their impact on quality of life. Ann Allergy Asthma Immunol. 2006;97:419–28. doi: 10.1016/S1081-1206(10)60928-3. quiz 429-30, 476. [DOI] [PubMed] [Google Scholar]
- Barone S, Bacon SL, Campbell TS, Labrecque M, Ditto B, Lavoie KL. The association between anxiety sensitivity and atopy in adult asthmatics. J Behav Med. 2008;4:331–9. doi: 10.1007/s10865-008-9164-5. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Parmigiani S, Pederzani T, Merlot E, Neveu PJ, Dantzer R. Chronic psychosocial stress down-regulates central cytokines mRNA. Brain Res Bull. 2003;62(3):173–8. doi: 10.1016/j.brainresbull.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233–50. doi: 10.2165/00148581-200406040-00003. [DOI] [PubMed] [Google Scholar]
- Berton O, Ramos A, Chaouloff F, Mormde P. Behavioral reactivity to social and nonsocial stimulations: a multivariate analysis of six inbred rat strains. Behav Genet Mar. 1997;27(2):155–66. doi: 10.1023/a:1025641509809. [DOI] [PubMed] [Google Scholar]
- Blaiss MS. Allergic rhinoconjunctivitis: burden of disease. Allergy Asthma Proc. 2007;28:393–7. doi: 10.2500/aap.2007.28.3013. [DOI] [PubMed] [Google Scholar]
- Blaiss MS. Pediatric allergic rhinitis: physical and mental complications. Allergy Asthma Proc. 2008;29:1–6. doi: 10.2500/aap2008.29.3072. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Brain imaging in anxiety disorders. Expert Rev Neurother. 2004;4:275–84. doi: 10.1586/14737175.4.2.275. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Ebrecht M, Kern S, Gierens A, Hellhammer DH. Personality characteristics in chronic and non-chronic allergic conditions. Brain Behav Immun. 2008;22:762–8. doi: 10.1016/j.bbi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008;70:102–16. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- Costa-Pinto FA, Basso AS, Britto LR, Malucelli BE, Russo M. Avoidance behavior and neural correlates of allergen exposure in a murine model of asthma. Brain Behav Immun. 2005;19:52–60. doi: 10.1016/j.bbi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Costa-Pinto FA, Basso AS, De Sa-Rocha LC, Britto LR, Russo M, Palermo-Neto J. Neural correlates of IgE-mediated allergy. Ann N Y Acad Sci. 2006;1088:116–31. doi: 10.1196/annals.1366.028. [DOI] [PubMed] [Google Scholar]
- Costa-Pinto FA, Basso AS, Russo M. Role of mast cell degranulation in the neural correlates of the immediate allergic reaction in a murine model of asthma. Brain Behav Immun. 2007;21:783–90. doi: 10.1016/j.bbi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–44. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Burgess PW, Blakemore SJ. Development of rostral prefrontal cortex and cognitive and behavioural disorders. Dev Med Child Neurol. 2008;50:168–81. doi: 10.1111/j.1469-8749.2008.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–54. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand EW, Joetham A, Cui ZH, Balhorn A, Takeda K, Taube C, Dakhama A. Induction and maintenance of airway responsiveness to allergen challenge are determined at the age of initial sensitization. J Immunol. 2004;173:1298–306. doi: 10.4049/jimmunol.173.2.1298. [DOI] [PubMed] [Google Scholar]
- Goodwin RD. Self-reported hay fever and panic attacks in the community. Ann Allergy Asthma Immunol. 2002;88(6):556–9. doi: 10.1016/S1081-1206(10)61885-6. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Lewinsohn PM, Seeley JR. Respiratory symptoms and mental disorders among youth: results from a prospective, longitudinal study. Psychosom Med. 2004;66:943–9. doi: 10.1097/01.psy.0000138123.70740.92. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Buka SL. Childhood respiratory disease and the risk of anxiety disorder and major depression in adulthood. Arch Pediatr Adolesc Med. 2008;162:774–80. doi: 10.1001/archpedi.162.8.774. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–7. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Hurwitz EL, Morgenstern H. Cross-sectional associations of asthma, hay fever, and other allergies with major depression and low-back pain among adults aged 20-39 years in the United States. Am J Epidemiol. 1999;150:1107–16. doi: 10.1093/oxfordjournals.aje.a009936. [DOI] [PubMed] [Google Scholar]
- Ising M, Holsboer F. CRH-sub-1 receptor antagonists for the treatment of depression and anxiety. Exp Clin Psychopharmacol. 2007;15:519–28. doi: 10.1037/1064-1297.15.6.519. [DOI] [PubMed] [Google Scholar]
- Kalogeromitros D, Syrigou EK, Makris M, Kempuraj D, Stavrianeas NG, Vasiadi M, Theoharides TC. Nasal provocation of patients with allergic rhinitis and the hypothalamic- pituitary-adrenal axis. Ann Allergy Asthma Immunol. 2007;98:269–73. doi: 10.1016/S1081-1206(10)60717-X. [DOI] [PubMed] [Google Scholar]
- Kelly-Welch AE, Melo ME, Smith E, Ford AQ, Haudenschild C, Noben-Trauth N, Keegan AD. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol. 2004;172:4545–55. doi: 10.4049/jimmunol.172.7.4545. [DOI] [PubMed] [Google Scholar]
- KleinJan A, Willart M, van Rijt LS, Braunstahl GJ, Leman K, Jung S, Hoogsteden HC, Lambrecht BN. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118:1117–25. doi: 10.1016/j.jaci.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu ZW, Hayley S, Ravindran AV, Merali Z, Anisman H. Influence of psychosocial, psychogenic and neurogenic stressors on several aspects of immune functioning in mice. Stress. 1999;3:55–70. doi: 10.3109/10253899909001112. [DOI] [PubMed] [Google Scholar]
- Marshall PS, O’Hara C, Steinberg P. Effects of seasonal allergic rhinitis on selected cognitive abilities. Ann Allergy Asthma Immunol. 2000;84:403–10. doi: 10.1016/S1081-1206(10)62273-9. [DOI] [PubMed] [Google Scholar]
- Marshall PS, O’Hara C, Steinberg P. Effects of seasonal allergic rhinitis on fatigue levels and mood. Psychosom Med. 2002;64:684–91. doi: 10.1097/01.psy.0000021944.35402.44. [DOI] [PubMed] [Google Scholar]
- Meltzer EO. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997;99:S805–28. [PubMed] [Google Scholar]
- Meltzer EO. Quality of life in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2001;108:S45–53. doi: 10.1067/mai.2001.115566. [DOI] [PubMed] [Google Scholar]
- Meltzer EO. The relationships of rhinitis and asthma. Allergy Asthma Proc. 2005;26:336–40. [PubMed] [Google Scholar]
- Meltzer EO, Szwarcberg J, Pill MW. Allergic rhinitis, asthma, and rhinosinusitis: diseases of the integrated airway. J Manag Care Pharm. 2004;10:310–7. doi: 10.18553/jmcp.2004.10.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PJ. Antidepressant treatment and rodent aggressive behaviour. Eur J Pharmacol. 2005;526:147–62. doi: 10.1016/j.ejphar.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Motta A, Peltre G, Dormans JA, Withagen CE, Lacroix G, Bois F, Steerenberg PA. Phleum pratense pollen starch granules induce humoral and cell-mediated immune responses in a rat model of allergy. Clin Exp Allergy. 2004;34:310–4. doi: 10.1111/j.1365-2222.2004.01872.x. [DOI] [PubMed] [Google Scholar]
- Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9. doi: 10.2500/aap.2007.28.2934. [DOI] [PubMed] [Google Scholar]
- Nicolas LB, Prinssen EP. Social approach-avoidance behavior of a high-anxiety strain of rats: effects of benzodiazepine receptor ligands. Psychopharmacology (Berl) 2006;184:65–74. doi: 10.1007/s00213-005-0233-y. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J, Guimarães RK. Pavlovian conditioning of lung anaphylactic response in rats. Life Sci. 2000 Dec 29;68(6):611–23. doi: 10.1016/s0024-3205(00)00966-8. [DOI] [PubMed] [Google Scholar]
- Patten SB, Williams JV. Self-reported allergies and their relationship to several Axis I disorders in a community sample. Int J Psychiatry Med. 2007;37:11–22. doi: 10.2190/L811-0738-10NG-7157. [DOI] [PubMed] [Google Scholar]
- Pawlak CR, Schwarting RK, Bauhofer A. Cytokine mRNA levels in brain and peripheral tissues of the rat: relationships with plus-maze behavior. Brain Res Mol Brain Res. 2005;137(12):159–65. doi: 10.1016/j.molbrainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Postolache TT, Lapidus M, Sander ER, Langenberg P, Hamilton RG, Soriano JJ, McDonald JS, Furst N, Bai J, Scrandis DA, Cabassa JA, Stiller JW, Balis T, Guzman A, Togias A, Tonelli LH. Changes in allergy symptoms and depression scores are positively correlated in patients with recurrent mood disorders exposed to seasonal peaks in aeroallergens. ScientificWorldJournal. 2007;7:1968–77. doi: 10.1100/tsw.2007.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Braz J Med Biol Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Busse WW, Johnstone T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci U S A. 2005;102:13319–24. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, LeBlanc F, Kaneta T, Urbach D, Kusnecov AW. Effects of bacterial superantigens on behavior of mice in the elevated plus maze and light-dark box. Brain Behav Immun. 2004;18:46–54. doi: 10.1016/s0889-1591(03)00087-4. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov AW. Neuronal, endocrine, and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: dependence on tumor necrosis factor-alpha. J Neurosci. 2005;25:5314–22. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CB, Pratt EL, Hanks C, McCann J, Craig TJ. Allergic rhinitis and its effect on sleep, fatigue, and daytime somnolence. Ann Allergy Asthma Immunol. 2006;97:579–86. doi: 10.1016/S1081-1206(10)61084-8. quiz 586-9, 671. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schoenwetter WF. Allergic rhinitis: epidemiology and natural history. Allergy Asthma Proc. 2000;21:1–6. doi: 10.2500/108854100778248971. [DOI] [PubMed] [Google Scholar]
- Schoenwetter WF, Dupclay L, Jr, Appajosyula S, Botteman MF, Pashos CL. Economic impact and quality-of-life burden of allergic rhinitis. Curr Med Res Opin. 2004;20:305–17. doi: 10.1185/030079903125003053. [DOI] [PubMed] [Google Scholar]
- Silverman ES, Breault DT, Vallone J, Subramanian S, Yilmaz AD, Mathew S, Subramaniam V, Tantisira K, Pacak K, Weiss ST, Majzoub JA. Corticotropin-releasing hormone deficiency increases allergen-induced airway inflammation in a mouse model of asthma. J Allergy Clin Immunol. 2004;114:747–54. doi: 10.1016/j.jaci.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Sly RM. Changing prevalence of allergic rhinitis and asthma. Ann Allergy Asthma Immunol. 1999;82:233–48. doi: 10.1016/S1081-1206(10)62603-8. quiz 248-52. [DOI] [PubMed] [Google Scholar]
- Sly RM. Epidemiology of allergic rhinitis. Clin Rev Allergy Immunol. 2002;22:67–103. doi: 10.1007/s12016-002-0007-9. [DOI] [PubMed] [Google Scholar]
- Steerenberg PA, Dormans JA, van Doorn CC, Middendorp S, Vos JG, van Loveren H. A pollen model in the rat for testing adjuvant activity of air pollution components. Inhal Toxicol. 1999;11:1109–22. doi: 10.1080/089583799196619. [DOI] [PubMed] [Google Scholar]
- Sundberg R, Toren K, Hoglund D, Aberg N, Brisman J. Nasal symptoms are associated with school performance in adolescents. J Adolesc Health. 2007;40:581–3. doi: 10.1016/j.jadohealth.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann N Y Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Konstantinidou AD. Corticotropin-releasing hormone and the blood-brain-barrier. Front Biosci. 2007;12:1615–28. doi: 10.2741/2174. [DOI] [PubMed] [Google Scholar]
- Timonen M, Jokelainen J, Hakko H, Silvennoinen-Kassinen S, Meyer-Rochow VB, Herva A, Rasanen P. Atopy and depression: results from the Northern Finland 1966 Birth Cohort Study. Mol Psychiatry. 2003;8:738–44. doi: 10.1038/sj.mp.4001274. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Gunsolly CA, Belyavskaya E, Atwood AR, Sternberg EM. Increased pro-thyrotropin-releasing hormone transcription in hypophysiotropic neurons of Lewis rats. J Neuroimmunol. 2004;153:143–9. doi: 10.1016/j.jneuroim.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Urbach-Ross D, Crowell B, Kusnecov AW. Relationship of varying patterns of cytokine production to the anorexic and neuroendocrine effects of repeated Staphylococcal enterotoxin A exposure. J Neuroimmunol. 2008;196:49–59. doi: 10.1016/j.jneuroim.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamboldt MZ, Hewitt JK, Schmitz S, Wamboldt FS, Rasanen M, Koskenvuo M, Romanov K, Varjonen J, Kaprio J. Familial association between allergic disorders and depression in adult Finnish twins. Am J Med Genet. 2000;96:146–53. doi: 10.1002/(sici)1096-8628(20000403)96:2<146::aid-ajmg4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wamboldt MZ, Laudenslager M, Wamboldt FS, Kelsay K, Hewitt J. Adolescents with atopic disorders have an attenuated cortisol response to laboratory stress. J Allergy Clin Immunol. 2003;111:509–14. doi: 10.1067/mai.2003.140. [DOI] [PubMed] [Google Scholar]
- Wamboldt MZ, Schmitz S, Mrazek D. Genetic association between atopy and behavioral symptoms in middle childhood. J Child Psychol Psychiatry. 1998;39:1007–16. [PubMed] [Google Scholar]
- Williams WR, Richards JP, Ameen JR, Davies J. Recurrent brief depression and personality traits in allergy, anxiety and premenstrual syndrome patients: a general practice survey. Med Sci Monit. 2007;13:CR118–24. [PubMed] [Google Scholar]
- Woods L, Craig TJ. The importance of rhinitis on sleep, daytime somnolence, productivity and fatigue. Curr Opin Pulm Med. 2006;12:390–6. doi: 10.1097/01.mcp.0000245710.43891.5f. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Stress and atopic disorders. J Allergy Clin Immunol. 2005;116:1301–6. doi: 10.1016/j.jaci.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Zosky GR, Sly PD. Animal models of asthma. Clin Exp Allergy. 2007;37:973–88. doi: 10.1111/j.1365-2222.2007.02740.x. [DOI] [PubMed] [Google Scholar]
- Zoumakis E, Rice KC, Gold PW, Chrousos GP. Potential uses of corticotropin-releasing hormone antagonists. Ann N Y Acad Sci. 2006;1083:239–51. doi: 10.1196/annals.1367.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Passive Cutaneous Anaphylaxis (PCA)
Positive allergic reactions were determined by the passive cutaneous anaphylaxis reaction. Receiving (non-allergic) control animals were shaved with Remington clippers on either side of the midline of the back. The animals were injected intravenously with 5% solution of Evans Blue (catalog #23860 Fisher Scientific) mixed with allergen diluted in saline via the tail vein approximately 15 minutes before intradermal injections. After intravenous injection of dye, the animals were given different intradermal injections on either side of the back, 1.5 cm from midline and 1 cm from each other. The injections consisted of the plasma of allergen treated animals in one side (A, B) and saline treated animals on the opposite side (C, D). Animals were then placed under a heat lamp in a small box and monitored for signs of a reaction. Positive allergic reaction consisted of a blue stain caused by extravasation of Evans blue at the site of intradermal plasma injection. The extravasation is the response to the cross-linking of IgE with the Fc receptor and local activation and degranulation of mast cells.
Frequency (A) and duration (B) of defensive and aggressive postures of mice in control condition (sensitized but not challenged) or after intranasal challenge with ovoalbumin (OVA) in sensitized animals. No significant differences were detected; (t=1.7, p<0.10) for A and (t=1.6, p<0.11) for B. Only 3 events involving attacks and bites were recorded from OVA treated mice against untreated intruders and no attacks were recorded from the control sensitized but not challenged animals against intruders.
Weight in grams of mice (A) and rats (B) sensitized and challenged with OVA (A) or pollen (B) respectively. Control animals were handled and treated under the same schedule but administered with vehicle solution. No significant differences were detected.