Abstract
Consumption of a high-fat diet (HFD) is associated with white adipose tissue (WAT) inflammation, which contributes to key components of the metabolic syndrome, including insulin resistance (IR) and hepatic steatosis (HS). To determine the differential effects of exercise training (EX), low-fat diet (LFD), and their combination on WAT inflammation, Balb/cByJ male mice (n=34) were fed an HFD for 12 wks before they were randomized into one of 4 intervention groups: HFD-EX, LFD-EX, HFD-sedentary (SED), or LFD-SED. EX mice performed 12 wks of exercise training on a motorized treadmill (1hr/d, 5d/wk, 12m/min, 5% grade, ∼65%VO2 max), while SED mice remained sedentary in their home cages. WAT gene expression of adipokines was assessed using rt-PCR. IR was measured using HOMA-IR, and HS via hepatic triglyceride content. EX significantly reduced (53%) WAT gene expression of MCP-1, and LFD significantly reduced (50%) WAT gene expression of the macrophage specific marker, F4/80 as well as the adipocytokine IL-1ra (25%). EX independently improved IR, while both EX and LFD improved HS. These findings suggest that both diet and exercise have unique beneficial effects on WAT inflammatory markers and the mechanism by which each treatment improves metabolic complications associated with chronic consumption of an HFD may be different.
Keywords: Inflammation, White adipose tissue, Obesity, Insulin resistance, Hepatic steatosis
1. Introduction
The Western diet, which is high in saturated fat, simple sugars, and cholesterol, is associated with several metabolic abnormalities, including insulin resistance (IR) and hepatic steatosis (HS) [1]. The high prevalence of factors such as less time at home to cook meals, higher consumption of convenience foods (i.e., fast food), and extremely low levels of physical activity contribute to obesity and metabolic disturbances [2]. The Western diet also has been independently associated with inflammation, the central initiating factor to the development of the metabolic syndrome [3].
The negative consequences of a Western-type high-fat diet (HFD) on disease susceptibility originate in the white adipose tissue (WAT), an endocrine organ responsible for coordinating the metabolic and immune systems [4]. Animals fed an HFD for extended periods of time have increased WAT inflammation, as characterized by macrophage infiltration and adipokine release [5]. Such WAT inflammation is causally related to both IR and HS[6]. Furthermore, mice with HS have greater WAT inflammation compared to equally obese mice with normal liver fat content [7], which supports the notion that an HFD which causes inflammation may initiate metabolic aberrations independent of obesity. This is important because it suggests that non-obese individuals consuming such HFDs may develop systemic inflammation, which places them at an increased risk of developing metabolic complications. This also may suggest that the inflammatory properties of such HFDs may precede the induction of obesity. There are few pharmacological remedies for inflammation that originates in the WAT. Thiazoladinediones (TZDs) target WAT and systemic inflammation while improving insulin sensitivity, but they have several unfavorable side effects including weight gain[8]. Thus, determining behavioral interventions that decrease WAT inflammation is a high public health priority.
Recent work has illustrated that exercise training has anti-inflammatory properties, although the mechanism(s) responsible for this effect is not known [9]. A weight-loss intervention in humans of combined diet and exercise resulted in a reduction in subcutaneous WAT inflammation, as measured by macrophage content and inflammatory cytokine gene expression, suggesting that exercise and/or dietary restriction may have anti-inflammatory effects on WAT [10]. Whether those effects were due to weight loss, exercise, or dietary restriction could not be confirmed, however. Gomez-Merino and colleagues published findings that seven weeks of exercise without dietary restriction reduced body weight and inflammatory cytokines in the WAT of non-obese, chow-fed rats [11], but similar to the human study cited above, the weight loss effect precluded the authors from being able to determine whether the result was due to exercise or weight loss per se. In another study, Bradley et al. (2008) investigated the effects of voluntary exercise on WAT expression of inflammatory cytokines on HFD-fed, obese C57/Bl6 mice. They found that fat mass, as well as WAT TNF-α and MCP-1 mRNA were reduced in exercising mice, even when the mice continued to consume an HFD. As obesity [12] and HFD [13] have both been shown to be inflammatory, exercise may mitigate the effects of obesity, HFD, or both. Use of a model that exhibits the inflammatory manifestations of HFD without strong fluctuations in weight with HFD and/or exercise would allow for the examination of the anti-inflammatory effect of exercise in isolation of it's obesity-reducing properties. The present study was done to reveal important information regarding the anti-inflammatory effects of exercise in isolation from its obesity-reducing properties. Since macrophage infiltration has recently been identified as a causative factor in WAT inflammation as well as its associated comorbidities [1, 14], F4/80 mRNA levels were measured as a surrogate marker of WAT macrophage content [15].
In order to investigate the effect of chronic feeding of an HFD on metabolic aberrations in isolation from the adverse effects of obesity per se, we utilized a mouse model that has been shown to be less susceptible to diet-induced obesity when compared to other mouse strains [16]. Balb/c mice, when exposed to an HFD, gain more weight and body fat compared to their standard chow-fed littermates, but this weight gain is considered moderate and does not constitute morbid obesity [16].
The purpose of this study was to compare the effects of cardiovascular exercise training and LFD, on WAT inflammation (measured by epididymal WAT gene expression of the macrophage (Mϕ) marker, F4/80, as well as the adipocytokines MCP-1, IL-1ra, leptin and IL-6) in Balb/c mice chronically fed an HFD. A secondary aim was to evaluate the relationships between WAT inflammatory markers, HS and IR. We hypothesized that exercise, either with or without an LFD, would decrease WAT inflammation relative to sedentary controls, and that the combination of interventions would be better than either alone. Finally, we tested whether reductions in WAT inflammatory markers would be associated with improvements in HS and IR.
2. Materials and methods
2.1.Animals and Diets
Male Balb/cByJ mice (n=34) were bred on site. At 6 Wks of age, mice were individually caged and fed a standard chow diet (Harlan Teklad 8640, 5% fat). At 10 Wks of age, the animals were switched to a 45% fat diet (HFD) for 12 Wks and then were randomly assigned to: HFD-SED (HFD, no exercise), HFD-EX (HFD, exercise), LFD-SED (10% fat diet (LFD), no exercise), or LFD-EX (LFD, exercise) for 12 Wks. The HFD and LFD were purchased from Research Diets, inc. (New Brunswick, NJ); the composition of these diets can be found in Supplemental Table 1. All groups were allowed to eat ad libitum throughout the duration of the study. The animals were housed on a reverse light-dark cycle (lights on at 19:00 h and off at 07:00 h) at 25° C. NIH guidelines for the care and use of laboratory animals were strictly followed and all experiments were approved by the Animal Care and Use Committee at the University of Illinois at Urbana-Champaign. At the end of the study, the animals were sacrificed during their light cycle, 48 hours following the last exercise bout following a 12 hour fast.
2.2. Exercise Intervention
Animals were exercise trained (EX) during their dark cycle (i.e., during their active period) between 09:00 h and 12:00 h on a motorized treadmill for 1 hour/day at 12 m/min, 5% grade, 5 times per Wk, for 12 Wks; this intensity corresponds to 65-70% of maximal oxygen uptake [17]. Previous studies have found this duration to be effective in producing significant training effects in rodents [17, 18]. The duration and intensity were increased gradually, so that the animals were running at the prescribed level by Day 8. Plexiglass lanes and tops were fitted on top of a Jog-a-Dog® (Jog-a-Dog, Ottawa Lake, MI) treadmill to create 20 individual running lanes. A foam sponge was placed in the back of each lane to prevent the animal from being injured. Negative reinforcement (e.g. electrical shock) was not used to force the mice to run. In the event that a mouse stopped running during a bout, gentle prodding was used to encourage continued running.
2.3. Assessment of Insulin Resistance
Insulin resistance (IR) was estimated using the homeostasis model assessment method (HOMA) using post-intervention serum samples[19]. This method is routinely used for assessment of IR in rodents and is strongly correlated with glucose clamping (the gold standard for determination of insulin sensitivity) [20]. Briefly, two days after the last intervention exercise bout, all mice were fasted for 12 h and then sacrificed via CO2 inhalation. Blood was collected from the vena cava immediately after sacrifice and centrifuged for 15 minutes at 2400 rpm and 4° C. The serum was drawn and stored at −80° C until use. Post-intervention serum samples from 10 animals were insufficient in quantity for analysis, which resulted in an n of 24 instead of 34 for HOMA. Glucose was measured using an automated analyzer (YSI 2300, YSI Life Sciences, Yellow Springs, OH) via the glucose oxidase method. Insulin was measured using a commercial ELISA kit (R&D systems, Minneapolis, MN). According to the manufacturer's assay characteristics, this assay has a reported sensitivity of 0.2ng/mL, an inter-assay %CV of 6.03-17.9, and an intra-assay %CV of 0.92-8.35. All samples were run in duplicate.
2.4.Real-time quantitative PCR
Epididymal white adipose tissue (WAT) was harvested immediately after sacrifice, flash frozen in liquid nitrogen, and stored at −80°C until assay. Total RNA was extracted from the whole adipose tissue using an RNeasy Mini Kit (Qiagen, Valencia, CA) and quantified using the Nanodrop® (Nanodrop Technologies, Wilmington, DE). RT-PCR was performed using the MX3000P™Real-Time PCR System using Brilliant® SYBR® Green Master Mix kits, 1-Step (Stratagene, La Jolla, CA). The thermal profiles consisted of 50° C for 30 min for generation of first strand synthesis of cDNA and 10 min at 95° C for denaturing, followed by 40 cycles of 95° C for 30 sec, annealing at 60° C for 1 min, and 72° C for 30 sec. GAPDH was used as the housekeeping gene and all data are represented relative to its expression (i.e., using the Δ-Ct method). All duplicate or triplicate Ct values were within 0.50 Ct units of each other. The primer sequences used can be found in Supplemental Table 2.
2.5.Assessment of hepatic triglyceride content
Liver tissue (50-200 mg) was homogenized for 7-10 min in 4 ml isopropanol with a polytron disrupter. The homogenate was centrifuged at 2,000 × g for 10 min and 5 μL of the resulting supernatant was used to determine triglyceride (TG) content using the microtiter procedure of the L-Type TG H test (Wako Diagnostis, Richmond, VA). Liver TG was expressed relative to initial sample wet weight.
2.6. Statistical Analysis
Body weight changes were analyzed using a general linear model analysis of variance (ANOVA) with repeated measures at 4-Wk intervals, with Post-Hoc Tukey's tests. Differences in mRNA expression, HS, HOMA-IR, and spleen weight were assessed using a general linear model univariate two-way ANOVA with treatment (EX or SED) and diet (HF or LF) as between-subjects factors. In any analysis, if significant interactions were observed, then Bonferoni-corrected post-hoc comparisons were run. Alpha level was set at P ≤ 0.05. Correlation values were determined using Pearson's bivariate correlation analysis. Where applicable, linear regression analyses were performed to determine the variance explained by individual variables on outcomes. All analyses were done using SPSS V15 (Chicago, IL) and data are presented as mean ± SEM.
3. Results
3.1. Body Weight and Food Intake
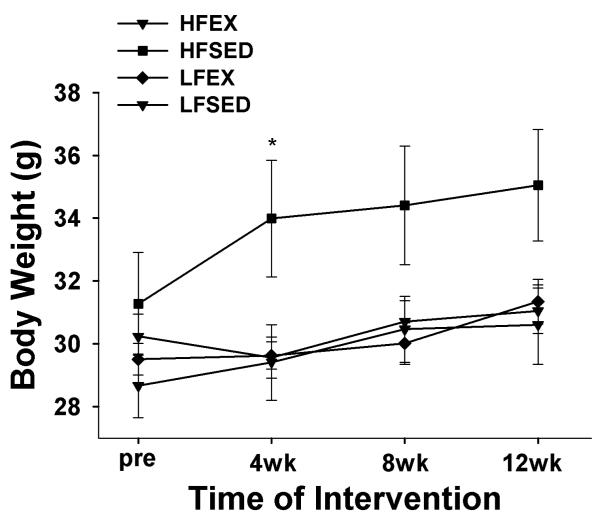
Figure 1 illustrates the changes in body weight that occurred during the intervention. After the HFD at the pre-intervention time point, we observed no significant differences between groups in initial body weights (31.3 ± 1.6, 28.7 ± 1.0, 30.2 ± 0.7, and 29.5 ± 0.5 g, for HFD-SED, LFD-SED, HFD-EX and LFD-EX respectively, F3,30 = 1.1; p = 0.35). Using repeated measures analysis of variance (ANOVA), there were significant time and time x group effects on body weight gain during the intervention. At individual time points, the HFD-SED group was heavier than LFSED and tended to be heavier than both EX groups (p < 0.06) at Wk 4 only; there were no other significant between-group differences. Interestingly, there were no group differences in the total percentage of weight gained at wk 12; however, at the end of the intervention, the HFD-SED group tended to weigh the most (35.05 ± 1.8, 30.61 ± 1.3, 31.05 ± 0.7, 31.34 ± 0.7 g, for HFD-SED, LFD-SED, HFD-EX and LFD-EX respectively, ANOVA: F3,30 = 2.9; p = 0.052). There were no differences in caloric intake between groups (ANOVA: F3,30 = 1.0; p = 0.3).
Figure 1. Body weight change at 4 wk intervals during the intervention.
Repeated measures analysis of variance (ANOVA) revealed significant time and time × group effects for body weight gain. Post-hoc analyses at individual time points revealed that the HFSED group was significantly heavier than LFSED (p=0.04) and tended to be heavier than HFEX and LFEX (p<0.06) at 4 wks, but there were no between-group differences at any other time point., * p < 0.05 compared to LFSED
3.2. Insulin Resistance and Hepatic Steatosis
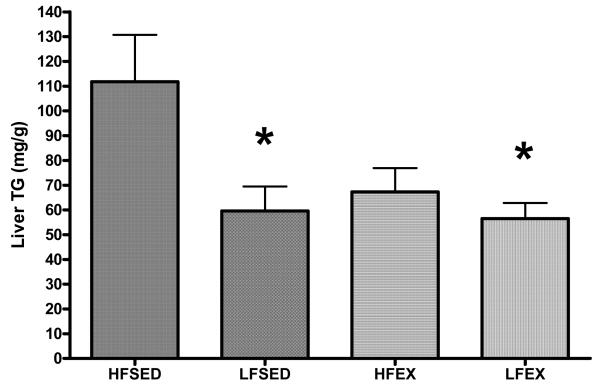
Fasting glucose and insulin values can be found in Table 1. We found a significant exercise x diet interaction (F1,20 = 4.9; p = 0.04) on HOMA-IR (Table 1), an indicator of insulin sensitivity [19]. Post-hoc analyses revealed that the HFD-EX group had a significantly lower HOMA-IR when compared to HFD-SED and there was a tendency (p = 0.06) for a difference between LFD-EX and HFD-SED. There were also significant treatment effects of both LFD (F1,30 = 7.18; p = 0.01) and EX (F1,30 = 4.1; p = 0.05) on liver TG content such that LFD-SED and LFD-EX were both significantly lower than HFD-SED, while there was a tendency for HFD-EX to be lower than HFD-SED (p = 0.09) (Figure 2).
Table 1.
Fasting Blood Metabolites, HOMA-IR, Final Body Weight, and Energy Intake.1
| Group | HOMA | Glucose (mg/dl) |
Insulin (ng/mL) |
Final Body Weight (g) |
Energy Intake (total kcals) |
|---|---|---|---|---|---|
| HFSED | 49.35 ± 22.42b | 294.38 ± 28.90 | 2.61 ± 1.01a | 35.05 ± 1.8 | 1245 ± 49 |
| HFEX | 9.00 ± 1.53a | 238.81 ± 27.03 | 0.63 ± 0.07b | 31.05 ± 0.7 | 1290 ± 69 |
| LFSED | 19.56 ± 4.55b | 258.08 ± 41.67 | 1.38 ± 0.40a | 30.61 ± 1.3 | 1202 ± 147 |
| LFEX | 14.70 ± 3.80a | 236.00 ± 28.50 | 0.87 ± 0.20b | 31.34 ± 0.7 | 1299 ± 161 |
Values are mean ± SEM; 4-8 mice per group. Different letters represent significant differences between groups (p<0.05) based on post hoc Tukey's tests.
Figure 2. Effects of LFD, EX, and LFD + EX on hepatic steatosis (HS).
Using a GLM-ANOVA with exercise (EX or SED) and diet (HFD or LFD) as between-subjects factors, there were significant exercise and diet effects, and a tendency toward a significant EX × LFD interaction. * p < 0.05 compared to HFSED.
3.3. Adipose Tissue Gene Expression
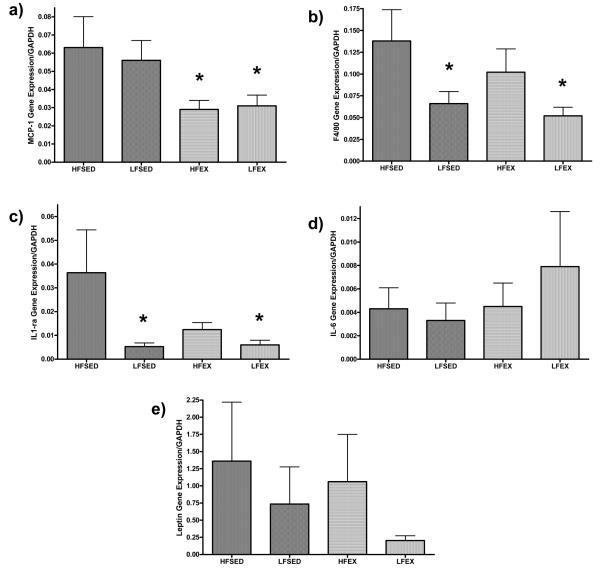
In epididymal fat pads, we found a significant EX main effect (F1,30 = 7.2; p = 0.01) but no LFD or EX × LFD interaction indicating that EX, and not LFD, reduced the Mϕ chemokine, MCP-1 (Figure 3a). In contrast, there was a diet main effect (F1,30 = 6.9, p = 0.01) but no exercise or LFD × EX interaction effect in F4/80 gene expression (a specific marker for Mϕ) in epididymal adipose tissue, indicating that while EX reduced the signal for recruiting Mϕ to adipose, LFD reduced Mϕ content in adipose (Figure 3b). Likewise, we found an LFD main effect (F1,30 = 4.5, p = 0.04) but no EX or interaction effect for IL-1ra gene expression in epididymal adipose (Figure 4c). There was no effect of either LFD or EX intervention or the combination on adipose tissue mRNA levels of IL-6 or leptin (Figure 3d and 3e). Using the percentage of weight gained throughout the intervention as a covariate did not alter the significance of the relationships between the treatments and WAT cytokine expression (data not shown) suggesting that the treatment effects were not fully explained by differences in intervention-induced changes in body weight.
Figure 3. Epididymal adipose tissue gene expression in response to LFD, EX, and LFD + EX.
mRNA levels are expressed as arbitrary units (AU) using delta-Ct values relative to GAPDH (which was not significant between the groups), the reference housekeeping gene. Using a GLM-ANOVA with exercise (EX or SED) and diet (HFD or LFD) as between-subjects factors, there was a significant main effect of EX on MCP-1 (A), a significant LFD main effect on F4/80 and IL-1ra (B and C, respectively), and no intervention-induced differences on adipose IL-6 or leptin gene expression (D and E, respectively).
3.4. Relationships between Intervention-induced Changes in Adipose Gene Expression and Changes Obesity-related Co-morbidities
To determine whether intervention-induced changes in WAT gene expression were related to improvements in IR and HS, we performed Pearson bivariate correlations and regression analyses. HOMA-IR was significantly correlated with hepatic TG content, body weight at sacrifice, and epididymal mRNA content of IL-1ra and leptin (Table 2). Hepatic TG content was also correlated with leptin and IL-1ra adipose tissue mRNA, and end body weight. Among the adipose tissue inflammatory markers, correlations were observed between MCP-1 and the Mϕ marker, F4/80 and between leptin and IL-1ra (Table 2). No significant negative correlations were found. Regression analyses revealed that IL1-ra was the most significant predictor of HOMA-IR (Model R2 = 0.94, F8,24 = 14.1, p<0.01; IL-1ra: β = 0.81, t = 4.48, p<0.01) and HS (Model R2 = 0.72, F8,33= 7.9, p<0.01; IL-1ra: β = 0.35, t = 2.14, p = 0.04), when % weight gain, WAT leptin, diet (LFD or HFD), exercise (EX or SED), WAT F4/80, WAT IL-6, and WAT MCP-1 were put into the model as independent variables.
Table 2.
Relationships among adipocytokines, HOMA, HS, and Body Mass Variables1
| F4/80 | IL-6 | MCP-1 | Leptin | IL1-ra | HOMA | %BW inc |
BW (g) FINAL |
|
|---|---|---|---|---|---|---|---|---|
| Liver TG | .152 | .056 | −.003 | .509* | .650* | .728* | .242 | .679* |
| F4/80 | −.172 | .458* | .071 | .240 | .054 | .060 | .160 | |
| IL-6 | −.101 | −.015 | .081 | −.086 | −.225 | −.314 | ||
| MCP-1 | −.003 | .048 | .070 | .147 | .085 | |||
| Leptin | .690* | .675* | .295 | .366* | ||||
| IL1-ra | .843* | .293 | .501* | |||||
| HOMA | .578* | .703* |
Pearson's bivariate correlations (r values). All correlations were calculated using all 34 mice, except for HOMA (n=24).
P<0.05
4. Discussion
White adipose tissue (WAT) inflammation has been shown to be causally-related to metabolic aberrations such as insulin resistance (IR) and hepatic steatosis (HS) [21, 22] that often accompany chronic consumption of a high-fat diet (HFD) and/or obesity [23]. Interventions designed to reduce chemokine expression in WAT may limit the accumulation and inflammatory activation of Mϕ, and thus improve such metabolic conditions [6]. The most novel finding of this study was that exercise training, with or without low-fat diet (LFD), decreased epididymal WAT gene expression of the Mϕ - attracting chemokine, monocyte chemoattractant protein-1 (MCP-1) in non-obese, HFD-fed mice. In contrast, an LFD, with or without exercise training, decreased total Mϕ content (measured by the Mϕ surface marker, F4/80) and the insulin de-sensitizing adipocytokine, Interleukin-1 receptor antagonist (IL-1ra) [24] in WAT.
Several studies have shown that exercise training combined with an LFD is associated with reductions in circulating inflammatory markers [25-27]. Bruun et al (2006) demonstrated that the combination of exercise training and a dietary restriction intervention for 15 weeks resulted in reduced expression of several inflammatory genes and Mϕ infiltration in subcutaneous abdominal WAT of morbidly obese humans, but whether this effect was due to the exercise training or dietary restriction could not be determined [10]. Moreover, that study did not assess inflammation in the visceral WAT, which is the major source of inflammatory molecules that enter the circulation in obesity and the depot most related to co-morbidities [28]. At least two other animal studies have investigated the effect of exercise training on WAT inflammation [11, 29]. One study done with rats showed reductions in IL-1ra, IL-1β and IL-12 in the WAT of standard chow-fed rats that were exercise trained for 7 weeks, but MCP-1 levels and/or macrophage infiltration were not reported [11]. Another study recently showed that voluntary wheel running reduced WAT inflammatory gene expression and insulin resistance in HFD-fed obese mice [29]. In both of those studies, the animals lost weight on the intervention; thus, whether the effects were due to exercise or weight loss could not be determined.
Unlike other studies that have used HFD, sedentary mice fed a HFD in this study did not weigh significantly more than the 3 intervention groups at the end of the intervention. This is likely due to the excellent energy regulation ability in this mouse strain [30]. That the exercising mice did not consume more calories than the sedentary mice was an unexpected finding, given that the energy expenditure increase with exercise should have been compensated by an increase in food intake. Other work [31] has shown that exercise may actually reduce caloric intake by interfering with hypothalamic receptor signaling. Although this is speculatory, this mechanism may have played a role here. Interestingly, while LFD led to significant improvements in HS, the combination of LFD and EX did not have a greater effect than the LFD intervention. This was surprising, given the evidence that EX improves HS even during HFD consumption [32]. It is thought that IR contributes to HS. In this study, EX reduced IR, which was correlated to HS, but did not significantly reduce HS. Exercise tended to reduce HS, however. Perhaps there is a “threshold” hepatic TG level and behavioral interventions cannot lead to reductions beyond that point. As this is the first study to assess hepatic lipid content in Balb/c mice after HFD feeding and in response to LFD and exercise, more studies are necessary to determine the mechanisms behind these effects in this particular strain.
Recent studies show a causal link between adipose tissue MCP-1 and IR [33] and suggest that MCP-1 be used as a therapeutic target given that it represents a common link between obesity, WAT inflammation and type 2 diabetes. Work by Kanda et al. has shown that mice engineered to lack MCP-1 are protected against IR, HS, and Mϕ infiltration into WAT, even when placed on an HFD [6]. Reciprocally, when mice are engineered to over-express MCP-1 in their WAT they experience IR, increased WAT Mϕ accumulation, and increased HS [6]. Similarly, in this study, WAT MCP-1 was correlated with F4/80. However, in contrast to other studies [34], we found no apparent relationship between WAT MCP-1 gene expression and IR or HS. A possible explanation for the lack of association between MCP-1 and/or F4/80 and the metabolic aberrations assessed here may be that relationships between these inflammatory indicators and metabolic outcomes are more evident in cases of extreme obesity. It may also suggest that HFD-induced comorbidities occur by mechanisms besides an increase in WAT inflammation. Possibly, WAT inflammation exacerbates, but is not the only cause of, these metabolic disturbances.
Studies have shown that IL-1ra levels increase with obesity [35] and are associated with IR [24]. We also report positive associations between IL-1ra and body weight and leptin gene expression, as well as with IR. Our finding that WAT gene expression of IL-1ra is related to HS is interesting because no previous studies have investigated the relationship between IL-1ra and HS. Somewhat paradoxical, one study using IL-1ra knock out mice reported that those mice experienced severe fatty liver and inflammation when placed on an HFD[36]. Those findings were not surprising, given the role of IL-1ra as an anti-inflammatory cytokine. However, recent reports implicate IL-1ra as an obesity-associated adipocytokine that exacerbates IR [24]. Indeed, using regression analysis, we found IL-1ra to independently predict IR. Combined with our data that implicate IL-1ra in exacerbating HS (based on the regression analysis revealing IL-1ra to also be an independent predictor of HS), this may suggest that it is rather immunomodulatory, depending on the level at which it is expressed. Although these are associative and not causative data, the robustness of the predictive ability of IL-1ra to explain variance in the metabolic perturbations evaluated in this study (e.g., IR and HS) suggests that its expression may be independently related to obesity-related complications. Further investigation into the mechanisms responsible for these relationships is warranted.
We found that LFD and exercise training had differential effects on F4/80 and MCP-1, such that exercise reduced MCP-1 but not F4/80, while LFD reduced F4/80 but not MCP-1. The role that MCP-1 plays in recruiting Mϕ into WAT of obese subjects is unclear. Some [1], but not all [37], studies report that MCP-1 knock out mice exhibit reductions in WAT Mϕ content when fed a HFD, implying that other factors may be responsible for recruiting Mϕ into WAT of obese subjects [38]. Interestingly, Mϕ's exhibit heterogeneity in their activation states, which render them either classically (inflammatory) or alternatively activated (anti-inflammatory) [39]. HFD-induced obese mice exhibit a shift in the activation state of WAT tissue Mϕ, such that the majority are inflammatory (producing molecules such as TNF-α, iNOS, IL-1β, IL-6, MCP-1), as opposed to alternatively activated (producing molecules such as IL-10 and arginase), as is the case with the majority of WAT Mϕ present in WAT of lean animals [40]. MCP-1 also activates Mϕ toward an inflammatory phenotype [41] and is produced by inflammatory Mϕ. Regular exercise may reduce the pro-inflammatory phenotype of visceral WAT Mϕ without altering the content. Future studies will need to address this hypothesis.
These differential treatment effects suggest that EX and LFD may work to improve WAT inflammation by different mechanisms. It has been shown that diets high in saturated fats directly cause WAT inflammation and the mechanism is thought to involve toll-like receptor (TLR) signaling [42] and/or Mϕ phenotype switching toward an inflammatory profile [43]; which may explain the independent effect of LFD on the F4/80 gene expression. That it, replacing the HFD with LFD was more successful in reducing Mϕ content than exercising on HFD. It is possible that EX altered the Mϕ phenotype in the WAT without significantly changing the total Mϕ content. The fact that EX significantly reduced MCP-1, which is produced by inflammatory Mϕ, supports this hypothesis. Other possible mechanisms by which EX reduces WAT inflammation may involve improvements in the “health” of the WAT, including reduced adipocyte size, increased blood flow, increased mitochondrial function and facilitated fatty acid oxidation, decreased cellular stress, and/or improved resistance to cell stress.
The findings presented here should be considered along with this study's limitations. The mode of EX used in this study was treadmill running, which has the disadvantage of the stress associated with its forced nature (although our control animals were yoked). Treadmill training has significant advantages over voluntary wheel running (which could be considered an observational intervention), because the intensity and duration of the running can be accurately dosed. When given a running wheel, some mice partake in extreme physical activity (>10km/day) and the variability in the amount run between mice is high and difficult to control. Thus, the public health generalizability of such doses (e.g. 10km) of exercise could be questioned. We feel that treadmill running is an appropriate model to examine the influence of exercise. Although we assessed body weight changes from baseline and throughout the intervention, another limitation of our study was that we did not directly measure body fat. Trϕseid and colleagues (2004) found 3 months of exercise training reduced circulating levels of MCP-1 in obese humans, and this reduction was associated loss of visceral WAT [44]. It is possible that the exercise-induced reduction in MCP-1 in WAT in our study was due to a loss of visceral WAT. Similarly, the effects of LFD on F4/80 and IL-1ra may have been related to alterations in body composition. However, in comparing the LFD-SED and HFD-EX groups, there were no significant differences in body weight gain during the intervention or final body weight. In addition, using body weight as a covariate in the analyses of WAT mRNA levels did not alter the results. Since Balb/c mice tend to be better able to regulate their energy balance when compared to other mouse strains [30] they respond only moderately to HFD-feeding, in terms of the amount of weight gained when exposed to this diet. Thus, it is likely that the weight (and fat) loss attributed to our individual interventions were similar. Nonetheless, whether the differences between treatment groups in the inflammatory variables measured were due to differences in body fat content or distribution is an important distinction that future studies should address.
Our finding that exercise training decreases MCP-1 gene expression in WAT lends support to the recent body of literature suggesting that exercise training may have unique anti-inflammatory properties [9]. Public health efforts to prevent or attenuate complications associated with poor diet and obesity are currently a major priority in the United States. Given that WAT inflammation is at the heart of many metabolic complications, determining ways of decreasing this inflammation is of great interest. The evidence that exercise training may have this potential supports the current public health initiative to encourage the public to adopt and maintain a physically active lifestyle.
Supplementary Material
Acknowledgements
This research was funded by an American College of Sports Medicine student grant to VJ Vieira, who was also supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (NIH T32 DK59802).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boeckner LS, Pullen CH, Walker SN, Hageman PA. Differences in eating and activity behaviors, health history, and biomarkers among normal-weight, overweight, and obese rural midwestern hispanic women. J Am Diet Assoc. 2006;106(11):1870–4. doi: 10.1016/j.jada.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Bullo M, Casas-Agustench P, Amigo-Correig P, Aranceta J, Salas-Salvado J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. 2007;10(10A):1164–72. doi: 10.1017/S1368980007000663. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56(8):1960–8. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 8.Boden G, Zhang M. Recent findings concerning thiazolidinediones in the treatment of diabetes. Expert Opin Investig Drugs. 2006;15(3):243–50. doi: 10.1517/13543784.15.3.243. [DOI] [PubMed] [Google Scholar]
- 9.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 10.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290(5):E961–7. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Merino D, Drogou C, Guezennec CY, Chennaoui M. Effects of chronic exercise on cytokine production in white adipose tissue and skeletal muscle of rats. Cytokine. 2007 doi: 10.1016/j.cyto.2007.07.188. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante AW., Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262(4):408–14. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 13.Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52(2):318–28. doi: 10.1007/s00125-008-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11(10):805–15. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp Anim. 2007;56(4):263–72. doi: 10.1538/expanim.56.263. [DOI] [PubMed] [Google Scholar]
- 17.Lowder T, Padgett DA, Woods JA. Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun. 2005;19(5):377–80. doi: 10.1016/j.bbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Lee KY, Kim SJ, Cha YS, et al. Effect of exercise on hepatic gene expression in an obese mouse model using cDNA microarrays. Obesity (Silver Spring) 2006;14(8):1294–302. doi: 10.1038/oby.2006.147. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Muniyappa R, Yan X, et al. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab. 2008;294(2):E261–70. doi: 10.1152/ajpendo.00676.2007. [DOI] [PubMed] [Google Scholar]
- 21.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144(9):3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13(26):3540–53. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouloumie A, Curat CA, Sengenes C, Lolmede K, Miranville A, Busse R. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care. 2005;8(4):347–54. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 24.Somm E, Cettour-Rose P, Asensio C, et al. Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia. 2006;49(2):387–93. doi: 10.1007/s00125-005-0046-x. [DOI] [PubMed] [Google Scholar]
- 25.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52(7):1098–104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 26.Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100(1):93–9. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 27.Kohut ML, McCann DA, Russell DW, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20(3):201–9. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Despres JP. Health consequences of visceral obesity. Ann Med. 2001;33(8):534–41. doi: 10.3109/07853890108995963. [DOI] [PubMed] [Google Scholar]
- 29.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary Exercise Improves Insulin Sensitivity and Adipose Tissue Inflammation in Diet-Induced Obese Mice. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262(6 Pt 2):R1025–32. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 31.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R290–301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 32.Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol. 2003;94(6):2127–34. doi: 10.1152/japplphysiol.01164.2002. [DOI] [PubMed] [Google Scholar]
- 33.Sell H, Eckel J. Monocyte chemotactic protein-1 and its role in insulin resistance. Curr Opin Lipidol. 2007;18(3):258–62. doi: 10.1097/MOL.0b013e3281338546. [DOI] [PubMed] [Google Scholar]
- 34.Di Gregorio GB, Yao-Borengasser A, Rasouli N, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54(8):2305–13. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 35.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab. 2002;87(3):1184–8. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- 36.Isoda K, Sawada S, Ayaori M, et al. Deficiency of interleukin-1 receptor antagonist deteriorates fatty liver and cholesterol metabolism in hypercholesterolemic mice. J Biol Chem. 2005;280(8):7002–9. doi: 10.1074/jbc.M412220200. [DOI] [PubMed] [Google Scholar]
- 37.Inouye KE, Shi H, Howard JK, et al. Absence of CC Chemokine Ligand 2 Does Not Limit Obesity-Associated Infiltration of Macrophages into Adipose Tissue. Diabetes. 2007 doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 38.Kiefer FW, Zeyda M, Todoric J, et al. Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008;149(3):1350–7. doi: 10.1210/en.2007-1312. [DOI] [PubMed] [Google Scholar]
- 39.Goerdt S, Politz O, Schledzewski K, et al. Alternative versus classical activation of macrophages. Pathobiology. 1999;67(56):222–6. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- 40.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 41.Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006;14(8):1353–62. doi: 10.1038/oby.2006.153. [DOI] [PubMed] [Google Scholar]
- 42.Vitseva OI, Tanriverdi K, Tchkonia TT, et al. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16(5):932–7. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Troseid M, Lappegard KT, Claudi T, et al. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J. 2004;25(4):349–55. doi: 10.1016/j.ehj.2003.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.