Abstract
E-, P- and L-selectins critically function in lymphocyte recirculation and recruiting leukocytes to inflammatory sites. MECA-79 antibody inhibits L-selectin-mediated lymphocyte adhesion in several species and does not require sialic acid in its epitope. Many other antibodies, however, recognize human selectin ligands expressing N-acetylneuraminic acid but not mouse selectin ligands expressing N-glycolylneuraminic acid, suggesting that difference in sialic acid in sialyl Lewis X leads to differential reactivity. We found that HECA-452 and FH6 monoclonal antibodies bind Chinese hamster ovary (CHO) cells expressing N-acetylneuraminyl Lewis X oligosaccharide but not its N-glycolyl form. Moreover, synthetic N-acetylneuraminyl Lewis X oligosaccharide but not its N-glycolyl oligosaccharide inhibited HECA-452 and FH6 binding. By contrast, E-, P- and L-selectin bound to CHO cells regardless of whether they express N-acetyl or N-glycolyl form of sialyl Lewis X, showing that selectins have a broader recognition capacity than HECA-452 and FH-6 anti-sialyl Lewis x antibodies.
Keywords: Sialyl Lewis X, MECA-79, N-glycolylneuraminic acid, HECA-452 antibody, N-acetylneuraminic acid
Introduction
As a major activity in innate immunity, E- and P-selectin appear on their surface when pathogenic agents such as lipopolysaccharide and IL-2 activate endothelial cells [1-3]. The appearance of E- and P-selectin then recruits neutrophils and induces an inflammatory response, a response initiated by recognition of neutrophil cell surface carbohydrates by E- and P-selectin on endothelial cells. That recognition leads to tethering and rolling of neutrophils on activated endothelium, promoting chemokine-induced activation of integrins on neutrophils and eventually leading to extravasation and attack of pathogenic agents by neutrophils.
E- and P-selectin on activated endothelial cells recognize a specific carbohydrate, sialyl Lewis X, on neutrophils in a Ca2+-dependent manner. Sialyl Lewis X is formed as a capping structure of core 2 branched O-glycans present as sialic acid α2→3Galβ1→4(Fucα1→3)GlcNAcβ1→6 (±siaic acidα2→3Galβ1→3)GalNAcα1→Thr/Ser [4, 5] and formed by Core2β1,6-N-acetylglucosaminyltransferase 1 (Core2GlcNAcT-1) [6, 7]. While E-selectin binds to sialyl Lewis X on various counter-receptors, P-selectin recognition requires sulfated tyrosine residues and sialyl Lewis X on core 2 oligosaccharides close to the NH2-terminal domain of PSGL-1 [8-10]. In mice deficient in either PSGL-1 or P-selectin, neutrophil adhesion and the inflammatory response are impaired [11, 12].
A third selectin, L-selectin, plays a critical role in lymphocyte recirculation [13-17]. Lymphocyte recirculation through lymph nodes and Peyer's patches is important to detect foreign antigens and for an immune attack of pathogens. The process of lymphocyte recirculation requires entry of lymphocytes from the blood system into secondary lymphoid organs through specialized vasculature called high endothelial venules (HEV), a process known as lymphocyte homing. L-selectin on lymphocytes recognizes specific carbohydrate ligands, 6-sulfo sialyl Lewis X on HEV, whose structure is sialic acidα2→3Galβ1→4 [Fucα1→3 (SO3→6)]GlcNAcβ1→R[18-21].
6-sulfo sialyl Lewis X in L-selectin ligands forms as a capping structure on N-glycans [22] and either core 2 or extended core 1 structures on O-glycans. The latter structure, sialic acidα2→3Galβ1→4[Fucα1→3(SO3→6)] GlcNAcβ1→3Galβ1→3GalNAcα1→Thr/Ser, is also recognized by the MECA-79 antibody [21]. MECA-79 binds to 6-sulfo N-acetyllactosamine in extended core 1 O-glycans, regardless of whether sialic acid and fucose are attached [21]. MECA-79 inhibits lymphocyte homing in vivo and lymphocyte binding ex vivo [23, 24]. MECA-79 antibody binds to HEV of numerous animals including mice, rats, sheep, and humans [17].
Sialyl Lewis X on human neutrophils is detected by CSLEX1 and FH6 monoclonal antibodies [25, 26], which were raised against human cancer cells and human glycolipids, respectively. Similarly, HECA-452 antibody was raised against human tonsils in mice [27]. CSLEX1, FH6 and HECA-452 apparently do not require a particular underlying glycan and bind to sialyl Lewis X (for CSLEX1, FH6 and HECA-452) and 6-sulfo sialyl Lewis X (for HECA-452) on both O-glycans and N-glycans [28, 29]. In contrast to MECA-79, recognition by FH6, CSLEX1 and HECA-452 requires sialylation and fucosylation. FH6, CSLEX1 and HECA-452, however, were shown not to bind to mouse and other rodent neutrophils, thus raising the possibility that mouse selectin ligands may differ from those identified in humans [30]. On the other hand, it has been shown that both mouse and human selectin ligands include fucose and sialic acid as integral components [31-33]. A critical role of sulfation has also been shown for L-selectin ligands in humans and mice [17, 34, 35].
In contrast to FH6, CSLEX1 and HECA-452, MECA-79 binds to HEV in both humans and mice. Since MECA-79 does not recognize a sialic acid residue, these results suggest that different forms of sialic acid might account for differential recognition of CSLEX1, FH6 and HECA-452 toward mouse and human sialyl Lewis X.
Here, to test this hypothesis, we first show that FH6, CSLEX1 and HECA-452 bind to human but not to C57BL/ 6 mouse neutrophils. We then show that C57BL/6 mice contain almost exclusively the N-glycolylneuraminic (NeuGc) in contrast to the N-acetylneuraminic acid (NeuAc) found in humans. Chinese hamster ovary (CHO) cells, which almost exclusively express NeuAc, were converted to NeuGc-expressing cells by transfection with CMP-NeuAc hydroxylase-encoding cDNA [36]. Sialyl Lewis X on CHO cells expressing NeuGc was not recognized by HECA-452 or FH6 antibody. This observation was further supported by assaying inhibition of HECA-452 and FH6 antibody binding by N-glycolylneuraminyl or N-acetylneuraminyl Lewis X oligosaccharide. Using CHO cells we demonstrate that E-, P- and L-selectin bind to sialyl Lewis X and 6-sulfo sialyl Lewis X carrying either the N-acetyl or N-glycolyl form of sialic acid. These combined results indicate that sialyl Lewis X can be recognized by selectins irrespective of the different forms of the N-acyl group of sialic acid, while HECA-452 and FH6 antibodies bind only to the N-acetyl form of sialyl Lewis X.
Materials and methods
Antibodies and IgM chimeric proteins
Culture supernatants of hybridomas producing FH6, HECA-452 or CSLEX1 (American Type Culture Collection) were used for cell staining without purification. In some cases, HECA-452 antibody was purified by an ImmunoPure IgM Purification Kit (Pierce), followed by ultrafiltration with an Ultracel Amicon Ultrafiltration Disc YM-100 (Millipore, Billerica, MA).
Cloning of mouse CMP-N-acetylneuraminic acid hydroxylase (mCmah)
Mouse thymus total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA), and a mouse cDNA library was prepared from total RNA using Superscript reverse transcriptase (Invitrogen). Mouse CMP-N-acetylneuraminic acid hydroxylase [36] was amplified from the cDNA library using Expand high fidelity PCR system (Roche Applied Science). Oligonucleotide pairs used for the amplification were 5′-TCAAGCTTAAATACCCTGGAGCTGGCAG ATGA-3′ and 5′-TGTCTAGACAGGTCCAGACTAAT CACAGTGCA-3′ (HindIII and XbaI restriction sites denoted by underlines). The PCR product was inserted into the pCR2.1TOPO vector (Invitrogen) and sequenced. Inserts with the correct sequence were digested with XhoI-BamHI (New England Biolabs) and cloned into the same sites of pcDNA3.1(N-), which was created by digestion of pcDNA3.1/Zeo(+) with SphI (New England Biolabs) and BspLU11I (Roche Applied Science), followed by filling in and self-ligation to remove the Zeocin resistance gene and the f1 origin.
Cell culture and transfection
CHO and COS-1 cells were cultured in α-MEM and DMEM, respectively, supplemented with 10% fetal bovine serum (FBS). Transfection was performed with Lipofectamine and PLUS reagents (Invitrogen) as described [29]. CHO cells expressing PSGL-1, Core2GlcNAcT-1 (CHO/PSGL/C2/F7) and Fuc-TVII with or without GlcNAc6ST-2 were previously established [37].
To establish a line stably expressing mouse CMP-N-acetylneuraminic acid hydroxylase (mCmah) in CHO/ PSGL/C2/F7 cells, pcDNA3.1(N-)/mCmah was co-transfected with pCMV/Bsd (Invitrogen) and colonies were selected in 10 μg/ml Blasticidin S (Invitrogen) plus G418 (400 μg/ml) and hygromycin (400 μg/ml). Cells were stained with HECA-452 antibody and HECA-452-negative single cells, which should express N-glycolylneuraminic acid, were sorted into a 96-well cell culture plate (Corning Life Science, Acton, MA) with FACSDiVa (BD Biosciences, San Jose, CA). The resultant transformants were designated CHO/PSGL/C2/F7/Cmah. Two independent cell clones (5 and 11) were obtained from the same transfection.
To obtain selectin-IgM chimeric proteins, COS-1 cells were transiently transfected with pCDM8/human P-selectin-IgM [29], pCDM8/mouse E-selectin-IgM [29], pcDNA1/ human E-selectin-IgM [38], and pcDNA1/human L-selectin-IgM [38]. Transfected cells were cultured 3-4 days and conditioned media were collected. For human and mouse E-selectin-IgM, the conditioned media were concentrated ~10 times using Centriprep YM-30 (Millipore).
Identification of N-glycolylneuraminic acid and N-acetylneuraminic acid
Methods used to release sialic acid and undertake DMB fluorescence labeling were essentially as reported [39]. Briefly, samples were treated with 1 ml of 25 mM H2SO4 at 80°C for 1 h. After adding ammonium hydroxide to neutralize acid and centrifuged, the supernatant was dried under vacuum (SpeedVac concentrator). The dried material was derivatized with DMB (1,2-diamino-4,5-methylene-dioxybenzene). After the sample was placed on ice, the product was isolated after HPLC using an ODS-80T column (TOSO, 4.5 mm di×250 mm) equilibrated with water. After applying the product, the column was washed with water for 10 min and eluted with a linear gradient to 17% of 1% acetic acid in methanol for 10 min, followed by a linear gradient to 30% over an additional 40 min [40]. DMB derivatives of N-glycolylneuraminic acid and N-acetylneuraminic acid were eluted at 36 and 40 min, respectively. DMB fluorescence was detected by excitation at 373 nm and emission at 448 nm.
Mass spectrometric analysis of O-glycans from transfected CHO cells
Fifty million transfected CHO cells were pretreated as described [41, 42]. After digestion by N-glycanase, the remaining peptides/O-glycopeptides were separated from N-glycans by Sep-Pak purification. Subsequently, O-glycans were released by reductive β-elimination (1.0 M KBH4, 0.1 M KOH 45°C for 20 h) and, after desalting, were permethylated using the sodium hydroxide procedure, as described [42] MALDI-TOF MS and MALDI-TOF/TOF MS analysis was performed following published strategies [41, 42].
Assay of antibody binding using ELISA and transfected CHO cells
CHO/PSGL/C2/F7 and CHO/PSGL/C2/F7/ Cmah cells, were seeded onto 96-well culture plates. When confluent, cells were fixed with 4% formaldehyde for 15 min at room temperature. To quench endogenous peroxidase activity, cells were treated with 0.3% hydrogen peroxide in methanol for 30 min and washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and 1 mg/ml bovine serum albumin (BSA) (washing buffer). In the presence or absence of inhibitory oligosaccharides, cells were treated with 2 μg/ml HECA-452 antibody, 20×diluted FH6 culture supernatant, or CSLEX1 solution in washing buffer for 1 h. Cells were treated with washing buffer and then with 500×diluted horseradish peroxidase-conjugated goat anti-mouse IgM (Pierce) for 1 h. HECA-452 antibody, a rat IgM, can be detected using anti-mouse IgM due to cross-reactivity. After washing, 100 μl of 1-Step ABTS (Pierce) was applied to cells and absorbance at 405 nm was read using a microplate reader (Emax, Molecular Devices).
Flow cytometry
Cells were dissociated into mono-dispersed cells using an enzyme-free cell dissociation solution (Hanks' balanced saline solution-based) purchased from Chemicon. All the following procedures were performed at 4°C. For staining with HECA-452, CSLEX1 and FH6, mono-dispersed cells were incubated with culture supernatants of hybridoma cells followed by affinity-purified fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse or rat IgM antibodies (Pierce). Stained cells were subjected to FACS analysis using FACSort (BD Biosciences). Binding of selectin-IgM chimeric proteins was assayed using culture supernatants of COS-1 cells transiently transfected as described above. After incubation for 30 min, cells were washed with DMEM supplemented with 0.1 mg/ml BSA and 25 mM HEPES. FITC-conjugated goat anti-human IgM (Pierce) was used to detect the selectin-IgM chimeric proteins.
Results
HECA-452, FH6, and CSLEX1 do not bind neutrophils of C57BL/6 mice
To determine how sialyl Lewis X in mouse neutrophils differs from that expressed in human neutrophils, binding of CSLEX1, FH6 and HECA-452 antibodies were tested on the neutrophils. Previously it was shown that HECA-452 could bind both sialyl Lewis X and 6-sulfo sialyl Lewis X on both O- and N-glycans [28, 29]. However, it was not known if sialic acid has to contain N-acetyl group.
Figure 1 illustrates that CSLEX1, FH6, and HECA-452 bind to human neutrophils, a finding that is anticipated because N-acetylneuraminyl Lewis X has been shown to be present on human neutrophils [4]. By contrast, mouse neutrophils (Gr1+ cells) and lymphocytes (Gr1- cells) from C57BL/6 mice did not show positive staining for FH6, HECA-452 or CSLEX1 (Fig. 1). Similarly, HECA-452 did not stain HEV of C57BL/6 mouse (Fig. S1). These results indicate that FH6, HECA-452 and CSLEX1 distinguish sialyl Lewis X on human and mouse neutrophils. FH6 weakly binds human neutrophils since FH6 binds more strongly difucosyl sialyl Lewis X than monofucosyl sialyl Lewis X, and difucosyl sialyl Lewis X is barely present in human neutrophils [4, 43].
Fig 1.
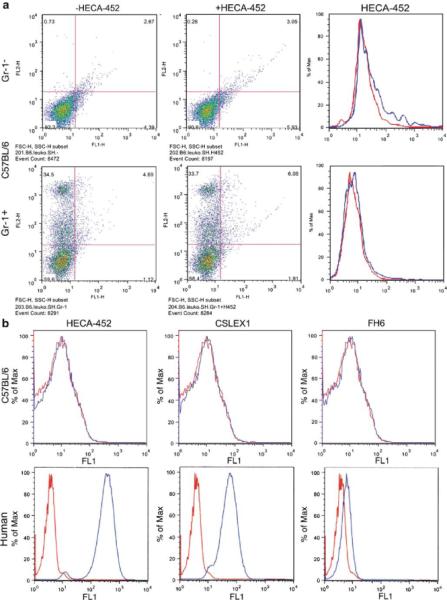
Differential expression of carbohydrate antigens detected by monoclonal antibodies on human and mouse neutrophils. a Leukocytes from C57BL/6 mice were stained with Gr-1 antibody and HECA-452, and Gr-1+ and Gr-1- cells were analyzed for HECA-452 expression. Right panels (upper and lower) were constructed from results shown in left and middle panel; staining by HECA-452 (blue) and control mouse IgM (red) is shown. Almost identical results were obtained using BALB/c mice. b Expression of HECA-452, CSLEX1, and FH6 antigen on C57BL/6 mouse and human neutrophils. C57BL/ 6 mice were negative for all of antibody staining of mouse neutrophils
C57BL/6 mice contain N-glycolylneuramic acid
To determine if C57BL/6 mice contain the N-glycolyl form of sialic acid, sialic acid was released by mild acid hydrolysis and derivatized with DMB. Since it is difficult to isolate sufficient amounts of mouse neutrophils, sialic acid was released from the whole lymph node. HPLC analysis of DMB derivatives of sialic acid showed that greater than 80% of C57BL/6 mouse sialic acid elutes at the same position that N-glycolylneuramic acid elutes and earlier than a DMB derivative of N-acetylneuraminic acid (Fig. 2). N-Acetylneuraminic acid and N-glycolylneuraminic acid are transferred, respectively, from CMP-NeuAc and CMPNeuGc by a sialyltransferase to a non-reducing terminal galactose. CMP-NeuGc is synthesized from CMP-NeuAc by CMP-NeuAc hydroxylase (Fig. 3), [36]. CMP-NeuAc hydroxylase is inactive in all humans due to a 92-bp deletion, producing an enzyme lacking the NH2-terminus [44]. C57BL/6 mice expressed CMP-NeuNAc hydroxylase transcripts, as did BALB/c and 129/SvJ mice, as expected since these mice express N-glycolylneuraminic acid (Fig. 4). These results demonstrate that all three mice express CMP-NeuAc hydroxylase and can thus form N-glycolylneuraminic acid. Consistent with these findings, recent studies show that lymph nodes from C57BL/6 mice exclusively contain N-glycolylneuraminic acid in sialyl Lewis X and 6-sulfo sialyl Lewis X [22]. These combined findings indicate that C57BL/6 mouse cells contain N-glycolylneuraminic acid while human cells contain N-acetylneuraminic acid.
Fig 2.
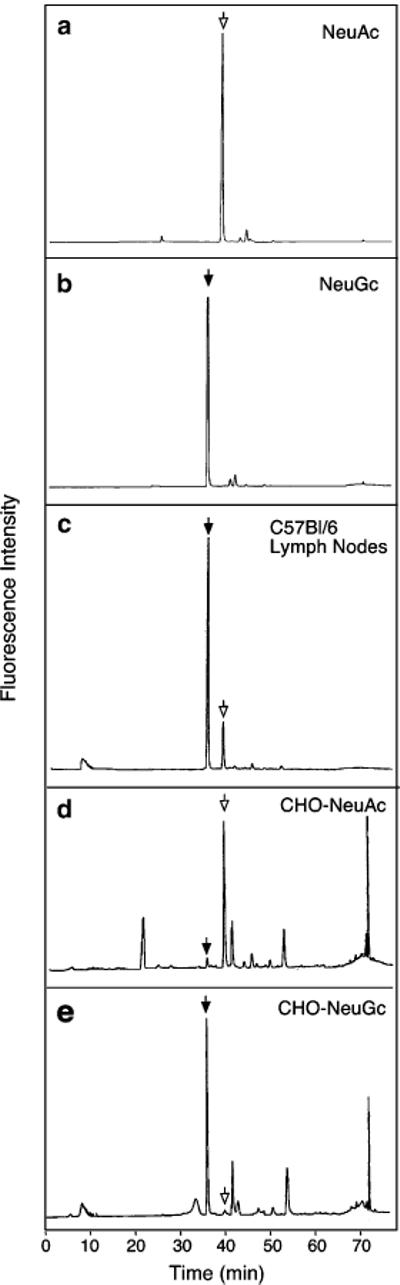
HPLC analysis of DMB-sialic acid. Sialic acid was released by mild acid hydrolysis, derivatized with DMB, and separated by HPLC using the elution condition described in “Materials and methods”. The elution positions of DMB-labeled standard NeuAc and NeuGc, and sialic acid derived from C57BL/6 lympnodes, parental CHO cells expressing NeuAc (CHO-NeuAc) and transfected CHO cells expressing NeuGc (CHO-NeuGc) are shown in a, b, c, d, and e, respectively. CHO cells expressing NeuAc and NeuGc are designated CHO/PSGL/ C2/F7 and CHO/PSGL/C2/F7/Cmah, respectively (Fig. 5)
Fig 3.

Conversion of CMP-NeuAc to CMP-NeuGc by CMP-NeuAc hydroxylase. CH3 is converted to CH2OH and depicted in bold. For this hydroxylation, NADH-dependent cytochrome b5 reductase and cytochrome b5 are also necessary for electron transport [36]
Fig 4.

Expression of CMP-NeuAc hydroxylase in mice. RNA was extracted from lymph nodes of BALB/c, 129/SvJ and C57BL/6 mice and reverse-transcribed to obtain cDNAs. RT-PCR was carried out for CMP-N-acetylneuraminic acid hydroxylase (Cmah) and glyceraldehyde 3-phosphate dehydrogenase (G3PDH) (plus sign). PCR products without template cDNAs serve as controls (minus sign)
FH6 and HECA-452 do not bind N-glycolylneuraminyl Lewis X
The above results suggest that both FH6 and HECA-452 do not bind to mouse neutrophils, most likely because mouse neutrophils express the N-glycolyl form of sialic acid. To determine if this is the case, we first prepared CHO cells expressing sialyl Lewis X on Core 2 branched O-glycans attached to PSGL-1.
CHO cells expressing PSGL-1, Core2GlcNAcT-1 and FucT-VII (CHO/PSGL/C2/F7) were further stably transfected with CMP-NeuAc hydroxylase (Cmah). Several colonies were isolated and assayed for HECA-452 antibody staining. As shown in Fig. 5, two clones did not express the HECA-452 antigen. HPLC analysis (Fig. 2) and mass spectrometric analysis of clone 5 showed that the cell line, which stably expresses CMP-NeuAc hydroxylase (CHO/ PSGL/C2/F7/Cmah-5), contained exclusively NeuGc, while parental CHO cells exclusively expressed NeuAc (Fig. 6). The same analysis showed that the mass spectra for lactosamine repeats were barely detected by those containing N-glycolylneuraminic acid (m/z=1,794 and 1,968). While N-glycolyl sialic acid may be more efficiently added to stop N-acetyllactosamine repeat, this difference may simply reflect the difference in the signal acquisition since the noise was higher in Fig. 6b than Fig. 6a. These parental (CHO/PSGL1/C2/F7) cells are highly positive for HECA-452 staining (Fig. 7).
Fig 5.

Binding of HECA-452 antibody to CHO/PSGL/C2/F7 cells before and after stable expression of CMP-NeuAc hydroxylase. CHO cells expressing PSGL-1, Core2GlcNAcT, FucT-VII were stably transfected with CMP-NeuAc hydroxylase. Resultant cell lines (clones 5 and 11) and the parental line were subjected to flow cytometry analysis using HECA-452 antibody. Binding of HECA-452 antibody was detected by FITC-conjugated secondary antibody (solid lines). Mouse IgM served as a control (dotted lines). Cell lines expressing N-glycolylneuraminic acid (clones 5 and 11) were negative for HECA-452 staining, while the parental cell line was positive
Fig 6.
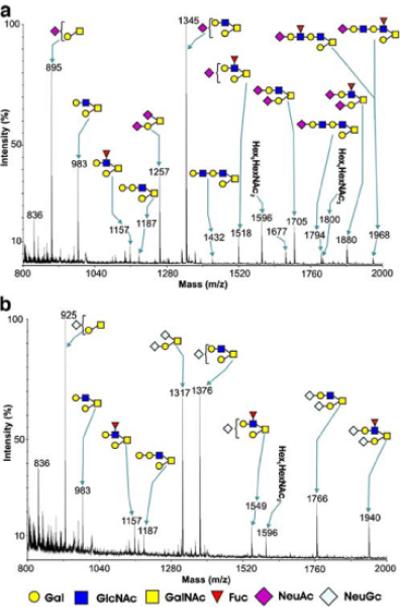
MALDI/TOF-MS profiles of O-glycans from CHO cells transfected with PSGL1, Core2GlcNAcT, FucTVII cDNAs with or without CMP-NeuAc hydroxylase cDNA. Shown are comparisons of MALDI-MS profiles of O-glycans from CHO cells transfected with cDNA-encoding PSGL-1, Core2GlcNAcT, FucT-VII with (b) or without (a) CMP-NeuAc hydroxylase. Major peaks representing core 1 and core 2 O-glycans are annotated with structures in symbol form. Both cell populations exhibit a predominance of core 2 sialylated structures, and some O-glycans are additionally fucosylated. Transfection with CMP-NeuAc hydroxylase results in exclusive expression of N-glycolyl neuraminic acid (b), whereas cells lacking this enzyme only express N-acetyl neuraminic acid (a). N-acetylneuraminic acid (NeuAc), N-glycolylneuraminic acid (NeuGc), galactose (Gal), N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), fucose (Fuc)
Fig 7.
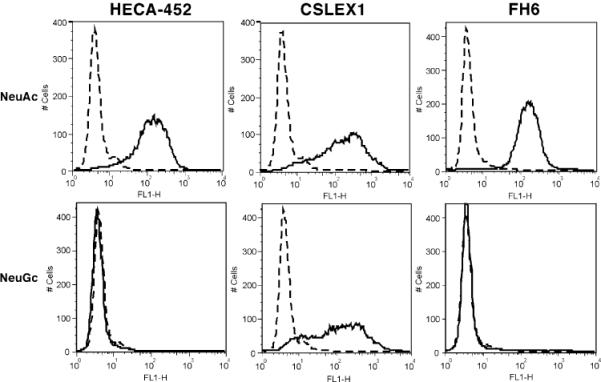
CHO/PSGL/C2/F7/Cmah cells expressing N-glycolylneuraminic acid are negative for HECA-452 and FH6 staining but positive for CSLEX1. CHO cells were generated to stably express PSGL-1, Core2GlcNAcT, and FucT-VII, thus expressing N-acetylneuraminyl sialyl Lewis X (NeuAc) on Core 2 O-glycans attached to PSGL-1. CHO-Cmah 5 cells were generated to stably express PSGL-1, Core2GlcNAcT, FucT-VII and CMP-NeuAc hydroxylase (NeuGc), which promotes expression of N-glycolylneuraminyl sialyl Lewis X on Core 2 branch O-glycans attached to PSGL-1. These two cell lines were subjected to flow cytometry using HECA-452, CSLEX1, and FH6 antibodies (solid lines). Control staining is shown in dotted lines. Staining was undertaken as described in Fig. 5
CHO/PSGL/C2/F7/GlcNAc6ST-2, which express 6-sulfo sialyl Lewis X, were then transiently transfected with a CMP-NeuAc hydroxylase expression vector and assayed for HECA-452 or FH6 binding. Transfected cells showed weaker HECA-452 binding than did untransfected cells (Fig. 7), indicating that 6-sulfo sialyl Lewis X containing N-glycolylneuraminic acid is not recognized by HECA-452.
However, CSLEX1 antibody, which binds strongly to human cells, bound to CHO/PSGL/C2/F7 cells irrespective of whether NeuAc or NeuGc was expressed (Fig. 7). These results indicate that HECA-452 and FH6 bind only to the form of sialyl Lewis X containing NeuAc, while CSLEX1 binds to forms of sialyl Lewis X containing either NeuAc or NeuGc.
To confirm this finding, CMP-NeuGc was chemically synthesized from CTP and NeuGc using CMP-sialic acid synthetase [45]. N-glycolylneuraminyl N-acetyllactosamine was then synthesized by incubation of CMP-NeuGc and octyl-N-acetyllactosamine using α2,3-sialyltransferase [46]. The resultant oligosaccharide N-glycolylneuraminyl N-acetyllactosamine was incubated with α1,3-fucosyltransferase to yield N-glycolylneuraminyl Lewis X oligosaccharide. NMR analysis supported the synthesis of those compounds (Figs. S2 and S3). Similarly, CMP-NeuAc was used in parallel to synthesize N-acetylneuraminyl Lewis X oligosaccharide.
Binding of HECA-452, CSLEX1 and FH6 antibodies to CHO/PSGL1/C2/F7 cells was then assayed in the presence of different amounts of N-acetylneuraminyl or N-glycolylneuraminyl Lewis X oligosaccharide. As shown in Fig. 8, binding of HECA-452 was inhibited by N-acetylneuraminyl Lewis X but not by N-glycolylneuraminyl Lewis X oligosaccharide. By contrast, CSLEX1 binding was inhibited by both oligosaccharides. The binding of FH6 was strongly and weakly inhibited by N-acetyl and N-glycolyl form of sialyl Lewis X, respectively. These results, combined with those shown in Fig. 7, demonstrate that HECA-452 and FH6 preferentially bind N-acetylneuraminyl Lewis X, while CSLEX1 binds both forms of sialyl Lewis X.
Fig 8.

Inhibition of CSLEX1, HECA-452, and FH6 binding by synthetic sialyl Lewis X oligosaccharides. CSLEX1, HECA-452 or FH6 antibody was incubated with CHO/PSGL/C2/F7 cells expressing N-acetylneuraminyl Lewis X in the absence or presence of sialyl Lewis X oligosaccharide. N-acetylneuraminyl Lewis X oligosaccharide (closed circles)or N-glycolylneuraminyl Lewis X oligosaccharide acid (squares) was used as an inhibitor. After washing, bound antibody was detected by horseradish peroxidase-conjugated goat anti-mouse IgM, and bound horseradish peroxidase was quantitated by color reaction. The results are shown at different concentrations of inhibitory oligosaccharides
Selectin equally recognizes N-glycolyl and N-acetyl forms of sialyl Lewis X
To determine whether selectins prefer one form of sialic acid over another, binding of different selectin-IgM chimeric proteins was tested on CHO/PSGL/C2/F7 and CHO/PSGL/C2/F7/Cmah cells, which exclusively express either the N-acetylneuraminyl and N-glycolylneuraminyl form of sialyl Lewis X, respectively.
As shown in Fig. 9, all E-, P-, and L-selectin-IgM chimeras bound well to CHO/PSGL/C2/F7 cells, regardless of whether they contained NeuAc or NeuGc. L-selectin-IgM chimeric protein bound slightly less to CHO cells expressing NeuGc than to those cells expressing NeuAc. However, binding of the mouse E-selectin-IgM chimera on CHO cells expressing N-glycolylneuraminyl Lewis X was slightly better compared to those expressing N-acetylneuraminyl Lewis X. The binding profile of human P-selectin, human L-selectin, and mouse E-selectin chimeras became more uniform toward cells expressing NeuGc, probably due to increased clonality of cells expressing N-glycolylneuramic acid. It is not clear why the human E-selectin-IgM chimera exhibited more heterogeneous binding than did other selectin-IgM chimeras. Two clones expressing N-glycolyl neuraminic acid provided almost identical results (Fig. 9). Together, these results demonstrate that all selectins bind to both N-acetylneuraminyl and N-glycolylneuraminyl forms of sialyl Lewis X.
Fig 9.

Binding of selectin·IgM chimeric proteins to sialyl Lewis X on CHO/PSGL/C2/F7 cells expressing N-acetylneuraminic acid or those cells expressing N-glycolylneuraminic acid. CHO/PSGL/C2/F7 and CHO/PSGL/C2/F7/Cmah cells express N-acetylneuraminyl Lewis X and N-glycolylneuraminyl Lewis X, respectively. Cells were dissociated with enzyme-free dissociation solution and incubated with human L-selectin·IgM (dark blue-hLM), human P-selectin·IgM (light blue-hPM), human E-selectin·IgM (green-hEM) or mouse E-selectin·IgM (orange-mEM) chimeric proteins and subjected to flow cytometry. The selectin·IgM chimeras were obtained from culture supernatants of COS-1 cells after transfection with respective cDNAs. Culture supernatants from COS-1 cells after mock transfection served as controls (red-COSCM)
Discussion
Our studies demonstrated that HECA-452 and FH6 do not bind to CHO cells expressing N-glycolylneuraminyl Lewis X, and that synthetic N-glycolylneuraminyl Lewis X oligosaccharide does not inhibit binding of HECA-452 or FH6 to CHO cells expressing sialyl Lewis X linked to core 2 branched O-glycans, while N-acetylneuraminyl Lewis X oligosaccharide does. As shown previously, C57BL/6 mouse lymph nodes express almost exclusively N-glycolylneuraminic acid [22], and HECA-452 does not stain mouse HEV. Our results indicate that HECA-452 and FH6 do not bind C57BL/6 neutrophils, lymphocytes, and HEV, since C57BL/6 mouse express N-glycolylneuraminic acid.
It has been suggested that rodent cells express selectin ligands different from those expressed by humans. This conclusion was, however, based on findings that antibodies, including FH6, do not bind to rodent neutrophils [30]. We showed that FH6 binds to N-acetylneuraminyl Lewis X but not to N-glycolylneuraminyl Lewis X. These results indicate that the apparent difference in rodent and human selectin ligands is mostly due to different forms of sialic acid in sialyl Lewis X and 6-sulfo sialyl Lewis X.
It has been shown that selectins bind to sialyl Lewis X and 6-sulfo sialyl Lewis X [47]. In contrast to HECA-452 and FH6 antibodies, E- and L-selectin bind both N-glycolyl and N-acetyl forms of sialyl Lewis X and 6-sulfo sialyl Lewis X. When co-expressed with PSGL-1, P-selectin also binds both forms of sialyl Lewis X. These results are consistent with the findings that removal of sialic acid by neuraminidase or genetic abrogation of α1,3-linked fucose inactivates selectin ligands in mice. In addition, L-selectin preferentially binds to 6-sulfated sialyl Lewis X in both humans and mice, and genetic abrogation of the 6-sulfated group results in significant loss of L-selectin-mediated lymphocyte trafficking in mouse.
Recent crystallographic studies show that E- or P-selectin forms complexes with the sialyl Lewis X oligosaccharide through Ca2+ ligation to the 3- and 4-hydroxyl groups of fucose and through hydrogen bonding to the carboxyl group of sialic acid [48, 49]. In the P-selectin complex with an amino terminal glycopeptide of PSGL-1 having tyrosine sulfates and sialyl Lewis X core 2 oligosaccharide, ionic interaction of P-selectin with tyrosine sulfates in PSGL-1 is stronger than the interaction with sialic acid of core 2 O-glycan-bound sialyl Lewis X in PSGL-1. This finding suggests that L-selectin interacts with the 6-sulfate group in 6-sulfo sialyl Lewis X, allowing a high affinity interaction [49]. The findings also indicate that the N-acyl group of sialic acid is positioned far from the binding site of selectins, and the difference in the acyl group should not influence the binding to selectins [49], consistent with our present findings.
In contrast to E-, P- and L-selectin, HECA-452 and FH6 recognizes the N-acetyl group of sialic acid, and replacement of N-acetyl group with the N-glycolyl group hinders its binding to these antibodies. On the other hand, CSLEX1 can also bind N-glycolyl neuraminyl Lewis X, but it cannot bind mouse neutrophils. Rodents are known to express the O-acetylated form of sialic acid [50, 51]. These O-acetyl groups are attached to C-4, C-7, C-8, and C-9 positions of sialic acid. During methylation analysis, these O-acetyl groups are removed, thus escaping detection. It is likely that O-acetylated sialic acid is not recognized by CSLEX1, although it is difficult to demonstrate this since O-acetyltransferase has not been cloned. In addition, CSLEX1 does not bind 6-sulfated sialyl Lewis X (Fig. 10)[28, 29]. These results suggest that CSLEX1 binding sites likely differ from those recognized by HECA-452 and FH6. It is also possible that mouse cells express sialyl Lewis X on restricted number of counterreceptors and this may be the reason why mouse cells have limited binding to these anti-sialyl Lewis X antibodies [52]. Further studies are important to determine how this difference influences FH6 and HECA-452 binding.
Fig 10.
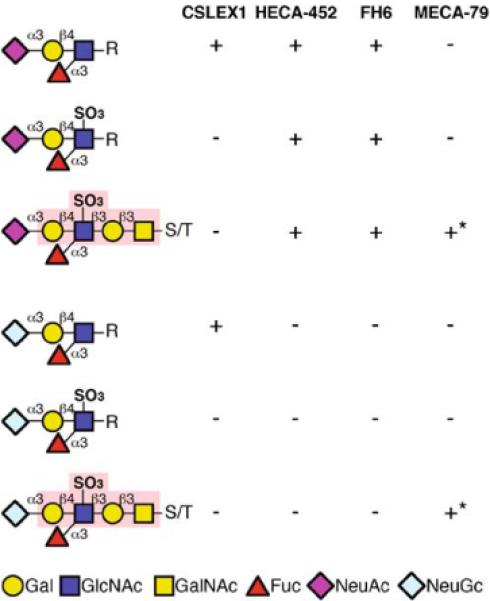
Binding specificity of CSLEX1, FH6, HECA-452 and MECA-79. CSLEX1 binds sialyl Lewis X containing either N-acetyl or N-glycolyl form of sialic acid but not 6-sulfo sialyl Lewis X. HECA-452 and FH6 binds both sialyl Lewis X and 6-sulfo sialyl Lewis X, which contain N-acetyl form of sialic acid but do not bind their N-glycolyl form. See the text for the evidence of these conclusions. MECA-79 binds to 6-sulfated N-acetyllactosamine regardless of the form of sialic acid. The MECA-79 epitope is indicated by light red [21]. S/T serine/threonine
Subtle differences in binding specificity of anti-sialyl Lewis X antibodies may be useful in distinguishing different cell types. Indeed, it was reported recently that human lymphocytes, which were positive for HECA-452 but not CSLEX1 or MECA-79 staining, correspond to cutaneous lymphocyte-associated antigen CLA-positive T-cells [53]. FH6 does not bind 6-sulfo sialyl Lewis X, and MECA-79 requires extended core 1 O-glycans as underlying glycans. These results suggest that 6-sulfo sialyl Lewis Xon N-glycans, as reported for L-selectin ligands on HEV [22], may function to recruit skin-homing T-lymphocytes. Similarly, human dendritic cells can be classified as positive or negative for HECA-452 antigen, and HECA-452-positive dendritic cells roll along non-inflamed dermal endothelium [54]. L-selectin ligands on these vessels have not been identified, but they may also play a role in CCL21-mediated adhesion to non-lymphoid tissues [54]. G-CSF was shown to induce E-selectin ligand in human peripheral blood leukocytes, and the E-selectin ligand on CD44, which may be attached to N-glycans, was detected by HECA-452 antibody [55]. It will be of interest to determine if some of these selectin ligands are recognized by FH6 or MECA-79.
In mouse inguinal lymph nodes, HEV can be divided into orders I-V. Orders III-IV are in sub- and paracortex, and order V is formed by merging capillaries, while orders I and II are located mainly in the medulla. Orders III-V are highly positive for MECA-79 antigen, whereas orders I and II do not express MECA-79 antigen and lack a specific carbohydrate marker [56]. In mouse thymocyte development, P-selectin on thymus epithelial cells interacts with PSGL-1 on lymphoid progenitor cells, an interaction likely critical for thymocyte development [57]. L-selectin-mediated fast rolling of lymphoid cells was observed on non-HEV vessels in mouse [58]. However, the carbohydrate ligands in these interactions have not yet been identified due to lack of specific antibodies.
In conclusion, we show that different antibodies apparently bind different portions of sialyl Lewis X and 6-sulfo sialyl Lewis X, thus exhibiting different profiles of selectin ligands in humans and rodents. By contrast, E-, P-, and L-selectin can bind N-glycolyl and N-acetylneuraminyl forms of sialyl Lewis X and 6-sulfo sialyl Lewis X, and possibly an O-acetylated form of sialic acid. Development of antibodies specific to 6-sulfo sialyl Lewis X on N-glycans, and to the N-glycolyl form of sialyl Lewis X and 6-sulfo sialyl Lewis X would be very useful in determining the functions of these selectin ligands in both human and rodents.
Supplementary Material
Acknowledgement
We thank Yoav Altman and Anette Flesman for technical assistance, Dr. Elise Lamar for critical reading of the manuscript, and Aleli Morse for organizing the manuscript.
This work was supported by NIH grants (CA48737 to M.F., CA71932 to M.F. and P.H.S., and HL85607 to R.O.C.), the Biotechnology and Biological Sciences Research Council BBSRC (to A.D. and H.R.M.), and NIH grant GM62116 for Consortium for Functional Glycomics. A.D. is a BBSRC Professor Fellow.
Abbreviations
- NeuGc
N-glycolylneuraminic acid
- NeuAc
N-acetylneuraminic acid
- HEV
high endothelial venule
- DMB
1,2-diamino-4.5-methylene-dioxybenzene
- Cmah
CMP-NeuAc hydroxylase
References
- 1.McEver RP, Cummings RD. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Invest. 1997;100:485–491. doi: 10.1172/JCI119556. doi:10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe JB. Glycosylation in the control of selectin counter-receptor structure and function. Immunol. Rev. 2002;186:19–36. doi: 10.1034/j.1600-065x.2002.18603.x. doi:10.1034/j.1600-065X.2002.18603.x. [DOI] [PubMed] [Google Scholar]
- 3.Ley K, Kansas GS. Selectins in T-cell recruitment to nonlymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4:325–335. doi: 10.1038/nri1351. doi:10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M, Carlsson SR, Klock JC, Dell A. Structures of O-linked oligosaccharides isolated from normal granulocytes, chronic myelogenous leukemia cells, and acute myelogenous leukemia cells. J. Biol. Chem. 1986;261:12796–12806. [PubMed] [Google Scholar]
- 5.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J. Biol. Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. doi:10.1074/jbc.271.6.3255. [DOI] [PubMed] [Google Scholar]
- 6.Bierhuizen MF, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal β 1-3-GalNAc-R (GlcNAc to Gal-NAc) β 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc. Natl. Acad. Sci. U. S.A. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. doi:10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. doi:10.1016/S1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 8.Leppanen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereo-specific contributions of individual tyrosine sulfate and sugar residues. J. Biol. Chem. 2000;275:39569–39578. doi: 10.1074/jbc.M005005200. doi:10.1074/ jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- 9.Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. doi:10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 10.Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. doi:10.1016/0092-8674(95) 90174-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, Flaumenhaft R, Furie BC, Furie B. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J. Exp. Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. doi:10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. doi:10.1016/0092-8674(93)80055-J. [DOI] [PubMed] [Google Scholar]
- 13.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. doi:10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 14.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. doi:10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 15.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. doi:10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 16.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. doi:10.1038/ nri1222. [DOI] [PubMed] [Google Scholar]
- 17.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. doi:10.1146/ annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 18.Hemmerich S, Leffler H, Rosen SD. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J. Biol. Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. doi:10.1074/ jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- 19.Mitsuoka C, Sawada-Kasugai M, Ando-Furui K, Izawa M, Nakanishi H, Nakamura S, Ishida H, Kiso M, Kannagi R. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis X. J. Biol. Chem. 1998;273:11225–11233. doi: 10.1074/jbc.273.18.11225. doi:10.1074/jbc.273.18.11225. [DOI] [PubMed] [Google Scholar]
- 20.Hiraoka N, Kawashima H, Petryniak B, Nakayama J, Mitoma J, Marth JD, Lowe JB, Fukuda M. Core 2 branching beta1,6-N-acetylglucosaminyltransferase and high endothelial venule-restricted sulfotransferase collaboratively control lymphocyte homing. J. Biol. Chem. 2004;279:3058–3067. doi: 10.1074/jbc.M311150200. doi:10.1074/jbc.M311150200. [DOI] [PubMed] [Google Scholar]
- 21.Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. doi:10.1016/S0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 22.Mitoma J, Bao X, Petryanik B, Schaerli P, Gauguet JM, Yu SY, Kawashima H, Saito H, Ohtsubo K, Marth JD, Khoo KH, von Andrian UH, Lowe JB, Fukuda M. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat. Immunol. 2007;8:409–418. doi: 10.1038/ni1442. doi:10.1038/ni1442. [DOI] [PubMed] [Google Scholar]
- 23.Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. doi:10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen SD, Tsay D, Singer MS, Hemmerich S, Abraham WM. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am. J. Pathol. 2005;166:935–944. doi: 10.1016/S0002-9440(10)62313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukushi Y, Nudelman E, Levery SB, Hakomori S, Rauvala H. Novel fucolipids accumulating in human adenocarcinoma. III. A hybridoma antibody (FH6) defining a human cancer-associated difucoganglioside (VI3NeuAcV3III3Fuc2nLc6) J. Biol. Chem. 1984;259:10511–10517. [PubMed] [Google Scholar]
- 26.Fukushima K, Hirota M, Terasaki PI, Wakisaka A, Togashi H, Chia D, Suyama N, Fukushi Y, Nudelman E, Hakomori S. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Res. 1984;44:5279–5285. [PubMed] [Google Scholar]
- 27.Duijvestijn AM, Horst E, Pals ST, Rouse BN, Steere AC, Picker LJ, Meijer CJ, Butcher EC. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am. J. Pathol. 1988;130:147–155. [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsuoka C, Kawakami-Kimura N, Kasugai-Sawada M, Hiraiwa N, Toda K, Ishida H, Kiso M, Hasegawa A, Kannagi R. Sulfated sialyl Lewis X, the putative L-selectin ligand, detected on endothelial cells of high endothelial venules by a distinct set of anti-sialyl Lewis X antibodies. Biochem. Biophys. Res. Commun. 1997;230:546–551. doi: 10.1006/bbrc.1996.6012. doi:10.1006/bbrc.1996.6012. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi M, Mitoma J, Nakamura N, Katsuyama T, Nakayama J, Fukuda M. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17807–17812. doi: 10.1073/pnas.0407503101. doi:10.1073/pnas.0407503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito K, Handa K, Hakomori S. Species-specific expression of sialosyl-Le(x) on polymorphonuclear leukocytes (PMN), in relation to selectin-dependent PMN responses. Glycoconj. J. 1994;11:232–237. doi: 10.1007/BF00731223. doi:10.1007/BF00731223. [DOI] [PubMed] [Google Scholar]
- 31.Rosen SD, Singer MS, Yednock TA, Stoolman LM. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985;228:1005–1007. doi: 10.1126/science.4001928. doi:10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- 32.Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB. The α(1,3) fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. doi:10.1016/S0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 33.Homeister JW, Thall AD, Petryniak B, Maly P, Rogers CE, Smith PL, Kelly RJ, Gersten KM, Askari SW, Cheng G, Smithson G, Marks RM, Misra AK, Hindsgaul O, von Andrian UH, Lowe JB. The α (1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. doi:10.1016/S1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 34.Kawashima H, Petryniak B, Hiraoka N, Mitoma J, Huckaby V, Nakayama J, Uchimura K, Kadomatsu K, Muramatsu T, Lowe JB, Fukuda M. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 2005;6:1096–1104. doi: 10.1038/ni1259. doi:10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- 35.Uchimura K, Gauguet JM, Singer MS, Tsay D, Kannagi R, Muramatsu T, von Andrian UH, Rosen SD. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat. Immunol. 2005;6:1105–1113. doi: 10.1038/ni1258. doi:10.1038/ni1258. [DOI] [PubMed] [Google Scholar]
- 36.Kawano T, Koyama S, Takematsu H, Kozutsumi Y, Kawasaki H, Kawashima S, Kawasaki T, Suzuki A. Molecular cloning of cytidine monophospho-N-acetylneuraminic acid hydroxylase. Regulation of species- and tissue-specific expression of N-glycolylneuraminic acid. J. Biol. Chem. 1995;270:16458–16463. doi: 10.1074/jbc.270.27.16458. doi:10.1074/jbc.270.27.16458. [DOI] [PubMed] [Google Scholar]
- 37.Mitoma J, Fukuda M. Expression of specific carbohydrates by transfection with carbohydrate modifying enzymes. Methods Enzymol. 2006;416:293–304. doi: 10.1016/S0076-6879(06)16019-X. doi:10.1016/S0076-6879(06)16019-X. [DOI] [PubMed] [Google Scholar]
- 38.Mitoma J, Petryniak B, Hiraoka N, Yeh JC, Lowe JB, Fukuda M. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis X-type L-selectin ligand activity. J. Biol. Chem. 2003;278:9953–9961. doi: 10.1074/jbc.M212756200. doi:10.1074/jbc. M212756200. [DOI] [PubMed] [Google Scholar]
- 39.Hikita T, Tadano-Aritomi K, Iida-Tanaka N, Toyoda H, Suzuki A, Toida T, Imanari T, Abe T, Yanagawa Y, Ishizuka I. Determination of N-acetyl- and N-glycolylneuraminic acids in gangliosides by combination of neuraminidase hydrolysis and fluorometric high-performance liquid chromatography using a GM3 derivative as an internal standard. Anal. Biochem. 2000;281:193–201. doi: 10.1006/abio.2000.4561. doi:10.1006/abio.2000.4561. [DOI] [PubMed] [Google Scholar]
- 40.Sato C, Inoue S, Matsuda T, Kitajima K. Development of a highly sensitive chemical method for detecting α2,8 linked oligo/ polysialic acid residues in glycoproteins blotted on the membrane. Anal. Biochem. 1998;261:191–197. doi: 10.1006/abio.1998.2718. doi:10.1006/abio. 1998.2718. [DOI] [PubMed] [Google Scholar]
- 41.Sutton-Smith M, Morris HR, Dell A. A rapid mass spectrometric strategy suitable for the investigation of glycan alterations in knockout mice. Tetrahedron: Asymmetry. 2000;11:363–369. doi:10.1016/S0957-4166(99)00581-9. [Google Scholar]
- 42.Sutton-Smith M, Dell A. Analysis of carbohydrates/glycoproteins by mass spectrometry. In: Celis J, editor. A laboratory handbook. 3rd edn. Academic; San Diego: 2006. pp. 415–425. [Google Scholar]
- 43.Fukuda M, Spooncer E, Oates JE, Dell A, Klock JC. Structure of sialylated fucosyl lactosaminoglycan isolated from human granulocytes. J. Biol. Chem. 1984;259:10925–10935. [PubMed] [Google Scholar]
- 44.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J. Biol. Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. doi:10.1074/ jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 45.Higa HH, Paulson JC. Sialylation of glycoprotein oligosaccharides with N-acetyl-, N-glycolyl-, and N-O-diacetylneuraminic acids. J. Biol. Chem. 1985;260:8838–8849. [PubMed] [Google Scholar]
- 46.Ding Y, Fukuda M, Hindsgaul O. Efficient synthesis of 3′-glycosylated LacNAc-based oligosaccharides. Bioorg. Med. Chem. Lett. 1998;8:1903–1908. doi: 10.1016/s0960-894x(98)00332-1. doi:10.1016/S0960-894X(98)00332-1. [DOI] [PubMed] [Google Scholar]
- 47.Kanamori A, Kojima N, Uchimura K, Muramatsu T, Tamatani T, Berndt MC, Kansas GS, Kannagi R. Distinct sulfation requirements of selectins disclosed using cells that support rolling mediated by all three selectins under shear flow. L-selectin prefers carbohydrate 6-sulfation totyrosine sulfation, whereas p-selectin does not. J. Biol. Chem. 2002;277:32578–32586. doi: 10.1074/jbc.M204400200. doi:10.1074/jbc.M204400200. [DOI] [PubMed] [Google Scholar]
- 48.Graves BJ, Crowther RL, Chandran C, Rumberger JM, Li S, Huang KS, Presky DH, Familletti PC, Wolitzky BA, Burns DK. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature. 1994;367:532–538. doi: 10.1038/367532a0. doi:10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- 49.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. doi:10.1016/S0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 50.Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. doi:10.1016/S0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- 51.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2:25–40. doi: 10.1093/glycob/2.1.25. doi:10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawar ZS, Johnson TK, Natunen S, Lowe JB, Cummings RD. PSGL-1 from the murine leukocytic cell line WEHI-3 is enriched for core 2-based O-glycans with sialyl Lewis X antigen. Glycobiology. 2008;18:441–446. doi: 10.1093/glycob/cwn020. doi:10.1093/glycob/cwn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohmori K, Fukui F, Kiso M, Imai T, Yoshie O, Hasegawa H, Matsushima K, Kannagi R. Identification of cutaneous lymphocyte-associated antigen as sialyl 6-sulfo Lewis X, a selectin ligand expressed on a subset of skin-homing helper memory T cells. Blood. 2006;107:3197–3204. doi: 10.1182/blood-2005-05-2185. doi:10.1182/ blood-2005-05-2185. [DOI] [PubMed] [Google Scholar]
- 54.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J. Immunol. 2003;170:4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 55.Dagia NM, Gadhoum SZ, Knoblauch CA, Spencer JA, Zamiri P, Lin CP, Sackstein R. G-CSF induces E-selectin ligand expression on human myeloid cells. Nat. Med. 2006;12:1185–1190. doi: 10.1038/nm1470. doi:10.1038/nm1470. [DOI] [PubMed] [Google Scholar]
- 56.M'Rini C, Cheng G, Schweitzer C, Cavanagh LL, Palframan RT, Mempel TR, Warnock RA, Lowe JB, Quacken-bush EJ, von Andrian UH. A novel endothelial L-selectin ligand activity in lymph node medulla that is regulated by alpha (1,3)-fucosyltransferase-IV. J. Exp. Med. 2003;198:1301–1312. doi: 10.1084/jem.20030182. doi:10.1084/jem.20030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossi FM, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, So L, Yi L, Ziltener HJ. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat. Immunol. 2005;6:626–634. doi: 10.1038/ni1203. doi:10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 58.Ley K, Tedder TF, Kansas GS. L-selectin can mediate leukocyte rolling in untreated mesenteric venules in vivo independent of E- or P-selectin. Blood. 1993;82:1632–1638. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


