Abstract
Purpose
The purpose of this study was evaluate the potential of a second-generation implantable neuroprosthesis that provides improved control of hand grasp and elbow extension for individuals with cervical level spinal cord injury. The key feature of this system is that users control their stimulated function through electromyographic (EMG) signals.
Methods
The second-generation neuroprosthesis consists of 12 stimulating electrodes, 2 EMG signal recording electrodes, an implanted stimulator-telemeter device, an external control unit, and a transmit/receive coil. The system was implanted in a single surgical procedure. Functional outcomes for each subject were evaluated in the domains of body functions and structures, activity performance, and societal participation.
Results
Three individuals with C5/C6 spinal cord injury received system implantation with subsequent prospective evaluation for a minimum of 2 years. All 3 subjects demonstrated that EMG signals can be recorded from voluntary muscles in the presence of electrical stimulation of nearby muscles. Significantly increased pinch force and grasp function was achieved for each subject. Functional evaluation demonstrated improvement in at least 5 activities of daily living using the Activities of Daily Living Abilities Test. Each subject was able to use the device at home. There were no system failures. Two of 6 EMG electrodes required surgical revision because of suboptimal location of the recording electrodes.
Conclusions
These results indicate that a neuroprosthesis with implanted myoelectric control is an effective method for restoring hand function in midcervical level spinal cord injury.
Type of study/level of evidence
Therapeutic IV.
Keywords: functional electrical stimulation, neuroprosthesis, spinal cord injury, electromyography, activities of daily living
The Ability To Grasp And Manipulate objects is an important factor in determining if the tetraplegic individual can regain independence and return to a functional role in society. Implantable functional electrical stimulation systems, or neuroprostheses, provide hand and arm function that is both versatile and transparent. These systems have evolved from the feasibility stage through clinical evaluation in an outpatient population to a Food and Drug Administration (FDA)-approved medical product.1 Neuroprostheses provide the user with increased independence, thus improving the opportunity for a better quality of life.
A first-generation upper-extremity neuroprosthesis (NP) for hand control2 was first implanted in 19863 and became known as the Freehand System.4–12 The Freehand NP (NeuroControl Corp., Valley View, OH) used an 8-channel implanted receiver-stimulator with 8 epimysial or intramuscular forearm and hand electrodes, leads, and connectors. A radio-frequency inductive link provided the communication and power to the implant receiver-stimulator. The external components consisted of a control unit, a transmitting coil, and a shoulder position transducer.13 The control input was obtained from the contralateral shoulder position. Through preprogrammed activation patterns of the paralyzed muscles, 2 grasp patterns were provided for functional activities.14,15 The lateral pinch was used for holding small utensils such as a fork, spoon, or pencil. Palmar prehension was used for acquiring large objects, such as a glass or sandwich.
A multicenter clinical trial was performed to assess safety, effectiveness, and clinical utility for the C5 and C6 spinal cord injury level.1,9 This clinical trial demonstrated that with the implanted NP, individuals retained the ability to hold and maintain object grasp during arm movement through increased pinch force.9,16 The functional performance, as determined by an activities of daily living (ADL) test,17 demonstrated that all subjects were more independent in at least 1 task, and 78% were more independent in at least 3 tasks. All subjects preferred to use the NP for at least 1 task and 96% preferred to use the NP for at least 3 tasks tested. More than 90% of the subjects were satisfied with the NP, and regular use was documented.
Subsequent follow-up surveys indicate that usage patterns were maintained at least 4 years after implant.
Approximately 250 subjects have been implanted with the Freehand System at multiple sites in North America, Europe, Australia, and Asia.9 At the time of this writing, the longest time after implant placement is 20 years.3
This paper describes the first human implementation of a second-generation NP that enhances and extends the capabilities of the first-generation system (currently not marketed). The following second-generation system design objectives were determined from clinician and patient feedback:
Minimize use of external components; in particular, eliminate the need to wear external sensors for control.
Provide control that is “natural” and integrated within the user’s previous movement strategies. The control should be within the voluntary ipsilateral arm muscles, thus permitting bilateral implementation.
Include additional grasp patterns to the previous lateral and palmar grasp patterns.
Expand the available workspace through the provision of shoulder stability, elbow extension, and forearm pronation.
There were specific features of the first-generation system that were also essential to second-generation system development: safe implantation, simple external component donning, cosmetically acceptable, transportable, reliable, and clinically effective. Modularity was important as future component replacement and upgrades must be considered. Finally, widespread clinical deployment was desired in different hospital settings.
The specific system features necessary to achieve these functional goals were (1) an increased number of stimulus channels and (2) an implantable control source. An initial version of the second-generation NP used an implanted joint angle transducer as the control source.19 Because voluntary wrist extension was essential for use of the implanted joint angle transducer, the system was indicated for individuals with C6 spinal cord injury.
An alternative control method, described in this article, is the use of electromyographic (EMG) signals derived from muscles retaining voluntary control. Electromyographic control provides 3 important advantages relative to the design objectives. First, the control signal transducer (recording electrode) could be an implanted component. Second, the EMG electrode could be located in a wide variety of muscles, allowing the control source to be customized to the capabilities of each subject. Finally, EMG control signals can be obtained from weak or partially paralyzed muscles, thus different types of paralytic patterns can be addressed.
MATERIALS AND METHODS
Candidate Selection
Candidates considered for this advanced NP included those who had sustained a complete spinal cord injury at the C5, C6, or C7 motor level20 and were classified as group O/Cu:0 through O/Cu:2 using the International Classification for Surgery of the Hand in Tetraplegia.21 Further important criteria also included adequate proximal muscular control, minimal upper-extremity contractures, medical stability, electrically excitable muscles, and good social support. Surgical reconstruction with a NP is typically not considered until at least 6 months after injury.
Neuroprosthesis Design
The second-generation NP design provides control of grasp, forearm pronation, and elbow extension. Electromyographic signals, generated by voluntary musculature, are used to control the various functions of the NP. The system consists of an implanted stimulator-telemeter (IST-12), implanted electrodes for stimulation and recording, an external control unit, and a transcutaneous inductive link, as shown in Figure 1 13 (Trier et al presented at the 6th Annual Conference of the International Functional Electrical Stimulation Society, 2001). The EMG signal can be obtained from 2 independent muscles. These signals are recorded via implanted electrodes, thus reducing the need for external control components. Additionally, the IST-12 is capable of stimulating 12 different muscles.
Figure 1.
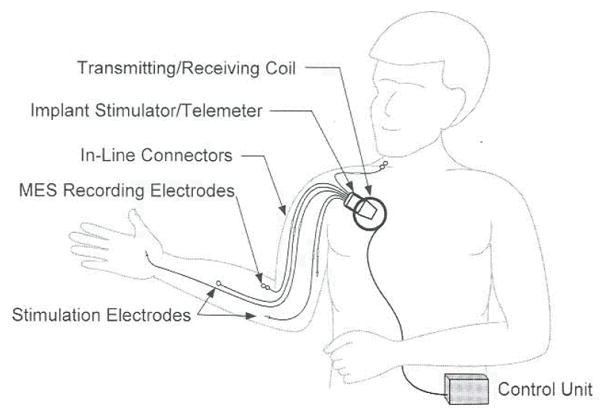
Drawing of the second-generation neuroprostheric system. The internal components include the IST-12, 12 stimulating electrodes (not all shown), and 2 recording electrodes. The external components include the control unit and the transmit/receive coil. This system provides grasp/release, forearm pronation, and elbow extension with myoelectric control for improved function in the tetraplegic spinal cord-injured individual, MHS, myoelectric signal.
An external power source and external processing to relate the control signal (input) to the stimulus commands (output) to each electrode are necessary. This design feature enables the size of the implanted components to remain small and provides the opportunity for customized control algorithms and stimulation patterns. The external control unit recovers the EMG data via the inductive transcutaneous link, processes this data using preprogrammed control algorithms, and transmits the necessary stimulus commands back to the internal device. Both power and bidirectional telemetry are provided through the same radio-frequency link so a single coil can be placed over the implanted device.
Electromyographic control algorithms
The NP functions controlled via the EMG signal include grasp pattern selection (typically 2 to 4 grasp patterns are provided), opening and closing of the hand in a proportional manner, turning the system on and off, turning elbow extension on and off, and the ability to “lock” and “unlock” the hand so that a grasp can be maintained in a fixed position without the need for continued control input. One EMG channel is used to control grasp opening and closing and is generally placed on the most distal upper-extremity muscle under voluntary control, typically the extensor carpi radialis longus (ECRL) or brachioradialis (Br). The second EMG channel is used to provide state or logic commands, such as system on/off and selection of the grasp pattern. This latter channel is placed on a more proximal muscle, such as trapezius or platysma. All control signals are derived from ipsilateral muscles, thus enabling bilateral function to be provided by implementing a second system in the contralateral limb.22,23
The selection of the 2 muscles for NP control is determined preoperatively with surface recording electrodes placed over the target muscles.24 Key criteria for muscle selection include the ability to generate graded signals from the muscle, the ability to generate signals during reaching, and the ability to isolate the signal from nearby voluntary muscles.
Stimulated grasp and arm movement patterns
The motor output stimulation for grasp and elbow extension is achieved from the implant generator through subcutaneous leads to the muscle electrode. The stimulating electrodes are placed within and on the fascia of the predetermined forearm and hand muscles. A template is established for each grasp pattern, which describes, the relationship between the input command signal and the activation of each muscle group.15 The stimulation patterns are fine-tuned for each subject to adjust for differences between the characteristics of each electrode.25
All subjects were provided at least 2 grasp patterns: a lateral grasp, in which the thumb contacts the lateral aspect of the index finger; and a palmar grasp, in which the thumb is abducted and the fingers contact the tip of the thumb. Subjects were provided with separate grasp patterns that were coupled with triceps activation for overhead reach. In addition, “specialty” grasp patterns were developed based on the functional goals of each subject.
Neuroprosthesis Implantation and Implementation
Muscle conditioning using surface stimulation was performed for at least 1 month prior to surgery. The implantation of the IST-12 device and electrodes was performed in a single surgical setting.3,17 Augmentative surgical procedures to the hand and arm were often performed in the same procedure.26 This included arthrodeses, tendon transfers of both voluntary and paralyzed muscles, and tendon synchronization using side-to-side repair.26 The objective was to provide improved hand function when the system was not being used while optimizing system use with electrically stimulated transfers.
In performing these procedures, conventional hand surgical and postsurgical management protocols were followed. An anterior chest exposure was necessary for the IST-12 implant. Medial and lateral brachial exposures were used as lead connector sites. Volar and dorsal midforearm incisions permitted visualization of the key finger flexors and extensors. The hand intrinsic electrode placement was performed through small incisions directly over these muscles.
The muscle electrode locations were mapped using a temporary epimysial or intramuscular electrode with direct stimulation of the muscle. Optimal position was estimated for each muscle as the electrode position on the muscle that provides the maximal force, maximal selectivity, minimal change in force with changes in muscle length, and modest recruitment gain.15 A reference electrode (anode) was placed in the chest wall pocket for this procedure.
The essential motions required for hand grasp were finger flexion through the flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP), finger extension with the extensor digitorum communis (EDC), thumb flexion and extension with the flexor pollicis longus (FPL) and extensor pollicis longus (EPL), thumb abduction and adduction through the abductor pollicis brevis (APB) and adductor pollicis (AdP), and finger metacarpal flexion and proximal interphalangeal extension with the dorsal and palmar interossei. Augmented wrist extension can be accomplished with the extensor carpi radialis longus/brevis (ECRL/B) stimulation or a stimulated extensor carpi ulnaris (ECU) transfer to the ECRB when the radial wrist extensors were denervated. Tendon transfers for elbow-extension (posterior deltoid to triceps) and thumb flexion (Br to FPL) have also been used.
Epimysial and intramuscular electrodes were used. Epimysial electrodes were secured to the muscle surface with nonabsorbable braided suture placed through the polyester reinforced silicone apron. The intramuscular electrodes were placed through a cannula inserted into the belly of the muscle. Typically, deep and small muscles were implanted with intramuscular electrodes.19 The electrode leads were subcutaneously tunneled to the upper arm approximately along the course of the median and radial nerves to the 2 connector sites.
The EMG recording electrodes were sutured on the muscle surface just distal to the motor point of the muscle, as identified through direct electrical stimulation using an epimysial mapping probe. For some muscles, such as the platysma, anatomic restrictions defined the placement of the recording electrode (eg, avoiding movement of the electrode over the clavicle).
The IST-12 was tested and then sutured in place to the underlying chest tissue with nonabsorbable braided suture through the stimulator apron. In female subjects, the IST-12 was located in the upper outer quadrant of the breast near the subclavicular area in the same location used for cardiac pacemakers. The IST-12 leads were subcutaneously tunneled to the 2 connector sites. Secured connection with the distal leads was then performed.
Postoperative radiographs were taken of the arm, shoulder, and chest to document implant position. A long cast was used for 3 weeks to ensure that there was encapsulation and stabilization of the electrodes and implant stimulator. The upper arm was immobilized in a soft Velpeau dressing. After a 3-week immobilization period, active muscle stimulation with unloaded, unresisted exercise was initiated to improve fatigue resistance. Functional use of the hand and the stimulation system began almost simultaneously, but intensive training and evaluation began 2 to 3 months after implantation.
The proper functioning of the implanted NP was assessed regularly during the first year after implantation. Proper stimulating electrode function was assessed by recording the electrode thresholds for each muscle.27 Electromyographic signals from each recording electrode were recorded during electrical stimulation through each channel to verify proper rejection of the stimulus artifact.
Operation of the Neuroprosthesis
Turning on the external control unit establishes the radio-frequency communication between the external control unit and the implant. Generation of 2 successive high-level bursts of muscle activity in the proximal EMG muscle is used to activate the stimulation to a hand grasp. The desired grasp pattern selection is accomplished using activity bursts from the same muscle.
Once the pattern is selected, the user has direct proportional control of the degree of hand opening and closing through the distal EMG activity level (typically from Br or ECRL). A strong muscle contraction results in hand closing, whereas relaxation of the muscle results in hand opening. If the user desires to hold an object for a long period of time, a “lock” command can be used. The lock command is initiated by holding the EMG above a high threshold for 2 seconds. Once the hand is locked, it will remain closed until an “unlock” command is given. The unlock command can consist of 2 quick bursts of activity from the forearm EMG muscle or a quick burst of activity from the proximal shoulder EMG muscle. The user can also independently activate other functions, such as elbow extension, by producing a specific pattern of EMG activity in the shoulder.
Outcomes Measurement and Analysis
The framework of the International Classification of Functioning. Disability and Health (ICF) developed by the World Health Organization was used to guide intervention and to structure the design and interpretation of the outcomes assessment.28,29 The domains of the ICF are (1) body functions and structures, (2) activities, and (3) participation. We have previously described the application of this framework as it directly relates to the measurement of outcomes after upper-extremity reconstruction in individuals with tetraplegia.30
Body functions and structures
The impact of the NP in this domain was determined by evaluating joint movement and force generation. Active and passive range of motion of the entire upper extremity31 and a manual muscle test of all upper-extremity muscles were preoperatively measured.32 All the extremity’s paralyzed muscles were additionally tested for electrical excitability with surface electrical stimulation. The maximum stimulated response for each muscle33 was used to determine which muscles could contribute to a stimulated grasp. Sensation in the hand was assessed using a 2-point discrimination method.34 Grasp and pinch force was measured using a modified pinch meter (B & L Engineering, Santa Fe Springs, CA) with metal bars to extend and enlarge the grasping surfaces of the meter.16
Activities
The ICE domain of activity performance was divided into 2 categories: hand function and ADL function. The hand function category addressed the ability to manipulate, hold, or move different objects. The ADL performance category encompassed the contribution of hand function in performing daily tasks in both its capacity and its actual performance. Hand function was evaluated using the Grasp and Release Test.16 Tins test has been previously used to measure hand function,6,10,35,36 and its psychometric properties have been established.37 This pick-and-place test requires the subject to unilaterally acquire, move, and release 6 objects varying in weight and size. The number of objects that can be successfully manipulated and the number of repetitions achieved in a 30-second trial are scored.
The ADL performance was measured using the Activities of Daily Living Abilities Test (ADLAT). This test was developed specifically to measure differences in activity performance with and without a hand NP.17,38,39 The test includes activities that are reasonable for individuals with tetraplegia to perform such as feeding, grooming, and writing, as well as other additional activities chosen by the subject. The activities are divided into phases, and each phase is scored for the amount of assistance required, including physical assistance, orthotic assistance, adaptive equipment, self-assistance, or no assistance (independent). Subjects were trained with and without the NP to achieve their highest capacity, or level of independence. A subjective assessment of performance and the therapist’s objective assessment of the activity quality are also included.
Participation
The impact of the NP on participation was determined using the Craig Handicap Assessment and Reporting Tool (CHART)40 and the NP Usage Survey.18 The CHART was administered prior to NP implantation and 1 year after implantation. The NP Usage Survey was administered at 1 to 2 years after implantation.
RESULTS
Demographics and System Description
Three IST-12 devices for hand-arm control were implanted in 3 spinal cord—injured subjects between 1 and 4 years after injury, as described in Table 1. Informed consent was obtained for study participation, which was conducted under an FDA investigational device exemption, with approval by the institutional review board. Subject A had group 0 motor function on the instrumented side and C4 motor function on the contralateral side. Subjects B and C had group 2 function bilaterally.
Table 1.
Subject Demographics
| Subject |
|||
|---|---|---|---|
| A | B | C | |
| Age at injury (years) | 24 | 43 | 34 |
| Time injury to implant (years) | 2 | 1 | 4 |
| Side implanted | Right | Left | Right |
| Injury level | |||
| Instrumented arm | C5 (O:0) | C6 (Cu:2) | C6 (O:2) |
| Opposite arm | C4 | C6 (O:2) | C6 (0:2) |
The selection of stimulating muscles was determined by the preoperative needs assessment and the muscle denervation pattern for each subject. Stimulating and recording electrodes were placed on the muscles shown in Table 2. All 3 subjects had tendon transfer procedures, as shown in Table 3. Proximal shoulder musculature power was achieved in subject A with stimulation of the pectoralis major and suprascapular nerve. This enabled improved cross-body reaching and shoulder stabilization. Elbow extension was augmented in subject B with triceps stimulation.
Table 2.
Stimulating and Recording Electrode Placement
| Subject |
|||
|---|---|---|---|
| A | B | C | |
| Stimulating electrodes | |||
| Adductor pollicis (AdP) | I | I | I |
| Abductor pollicis brevis (AbPB) | I | I | I |
| Extensor pollicis longus (EPL) | I | I | I |
| Extensor digitorum communis (EDC) | I | E, E | E |
| Flexor digitorum superficialis (FDS) | I | I | I, I |
| Flexor digitorum profundus (FDP) | I | I | I, E |
| Flexor pollicis long (FPL) | I | ||
| Palmaris longus (PL) | I | ||
| Second dorsal interosseous (2DI) | I | I | I |
| Third dorsal interosseous (3DI) | I | I | I |
| Pronator quadratus (PQ) | I | ||
| Extensor carpi ulnaris (ECU) | I | I | |
| Extensor digiti quinti (EDQ) | I | ||
| Triceps | I | ||
| Pectoralis major | I | ||
| Suprascapular nerve | E | ||
| Recording electrodes | |||
| Brachioradialis (Br) | E | ||
| Extensor carpi radialis longus (ECRL) | E | E | |
| Trapezius | E | E | |
| Platysma | E | ||
I, intramuscular electrode; E, epimysial electrode.
Table 3.
Surgical Procedures Performed
| Subject |
|||
|---|---|---|---|
| A | B | C | |
| Voluntary muscle procedures | |||
| Posterior deltoid → triceps | • | • | |
| Br → FPL | • | • | |
| Paralyzed muscle procedures | |||
| FPL → EPL split | • | • | |
| ECU → ECRB | • | • | |
| Joint stabilization procedures | |||
| Small-finger PIP pinning | • | ||
| FDP index/long synchronization | • | ||
procedure performed; PIP, proxintal interphalangeal.
Recording electrodes were placed on the ECRL if it was under voluntary control (subjects B and C) or on the Br (subject A) if the ECRL was paralyzed. The second recording electrode was placed on the trapezius muscle on 2 subjects and the platysma muscle on the third.
Special grasps were programmed to facilitate performance of activities such as needlepoint and throwing. The grasp for needlepoint was similar to the palmar grasp, but the finger flexor stimulation was reduced so that hand closing was a result of intrinsic muscle stimulation. This allowed more of a finger pad to thumb pad contact, or an intrinsic plus posture of the hand, to hold the needle. The baseball-throwing grasp was also similar to the palmar grasp; however, finger flexion was reduced, and the EMG parameters were modified to permit faster and easier hand opening and ball release.
Control Algorithms
In all cases, usable EMG signals could be recorded from the implanted recording electrodes during stimulation of nearby muscles. The EMG signals have been stable throughout the follow-up period in this study, with no indication of loss of signal over time.
The control algorithm was customized for each subject, as shown in Table 4. Subject A had a recording electrode placed on the Br, but had difficulty separating Br from biceps activation. The trapezius EMG was therefore used to control grasp opening and closing through a gated ramp algorithm.41,42 When the trapezius EMG exceeded an upper threshold, the grasp dosed at a fixed rate until either the trapezius EMG dropped below a lower threshold or the grasp was fully closed. When the EMG threshold exceeded the upper threshold again, the grasp opened at a fixed rate. Subjects B and C both used a quick twitch of the neck or shoulder muscle to turn the stimulation on or off and to select the desired grasp pattern. Subject A used an existing sip/puff control to turn the stimulation on or off and to select grasp patterns.
Table 4.
Neuroprosthesis Control Algorithms
| Control Method |
|||
|---|---|---|---|
| Control Action | A | B | C |
| Turn stimulation on/off | Sip/puff | Platysma | Trapezius |
| Select grasp pattern | Sip/puff | Platysma | Trapezius |
| Control grasp opening and closing | Trapezius | ECRL | ECRL |
| Lock hand grasp | Trapezius | ECRL | ECRL |
| Unlock hand grasp | Trapezius | ECRL | Trapezius |
| Activate triceps stimulation | N/A | Platysma | N/A |
| Control triceps strength | N/A | ECRL | N/A |
N/A, not applicable; no triceps function provided.
The ECRL EMG was used for direct proportional control of grasp opening and closing for subjects B and C, as shown in Figure 2. The step-size–filtered EMG activity22 produced a smooth command signal, closing the hand in approximately 1.5 seconds. When the subject maintained a nigh level of activity for 2 seconds, the command was automatically locked (at the 6-second mark in Fig. 2). To generate an unlock command, subject B used 2 successive large twitches of the ECRL. Subject C preferred to use a quick twitch of the trapezius muscle to generate the unlock command. Subject B used the platysma muscle to turn triceps stimulation on or off. Subject B could control the level of triceps activation through activation of the ECRL muscle.
Figure 2.
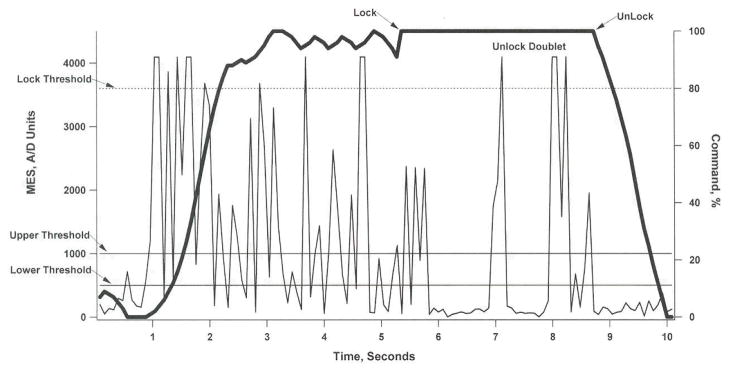
This graph shows the relationship between the recorded ECRL EMG (thin line, left axis) and stimulus command (thick line, right axis) as a subject closes their hand (1–5 seconds), locks their hand (6–7 seconds), unlocks their hand (7–9 seconds), and opens their hand (9–10 seconds). The recorded EMG is filtered by an adaptive step-size filter to produce the proportional command signal used to control grasp opening (0% command) and grasp closing (100% command). Between the lower and upper thresholds, the input to the adaptive filter is proportional to the level of the EMG. With each successive sample in which the change in signal is in the same direction as the previous sample, the allowable step size is increased. When the direction changes, the step size returns to a minimum value. When the command is above the “lock threshold” for 2.5 seconds, the command is locked at the 100% command level. To return to active control, the user generates 2 EMG twitches with a 1-second interval between them. When the unlock signal is generated, the command gradually returns to complete active control over a period of 1 second in order to avoid abrupt changes in command level. MES, myoelecctic signal; A/D, analog to digital.
Functional Outcomes
The functional outcomes demonstrated an increased ability for object grasp and release and increased independence in ADL performance. All 3 subjects are able to use their NP to perform activities that they could not perform prior to implantation, Total postimplant follow-up time ranges from 2 years to 4 years. The study results to date indicate that every subject has shown improvement in all ICF domains.
Body structures and functions
Every subject improved in their pinch force strength, as shown in Figure 3. The postoperative pinch force with the NP was significantly greater than without the NP (paired-sample t-test, p = .038). Preoperatively, pinch force was achieved by passive finger and thumb tone augmented with wrist extension. The augmentative surgical procedures changed the passive properties of the wrist and hand, thus affecting the postoperative pinch measurements. For example, subject B demonstrated an increase in pinch force from 4 N before surgery to 12 N after surgery with stimulation turned off due to a tendon transfer for thumb pinch (Table 3). With stimulation turned on, the pinch force further increased to 19 N. The slight postoperative decrease in pinch force for subject C when the NP was turned off was probably the result of reduced tendon tightness in the thumb due to the stimulated hand opening achieved with the NP.
Figure 3.
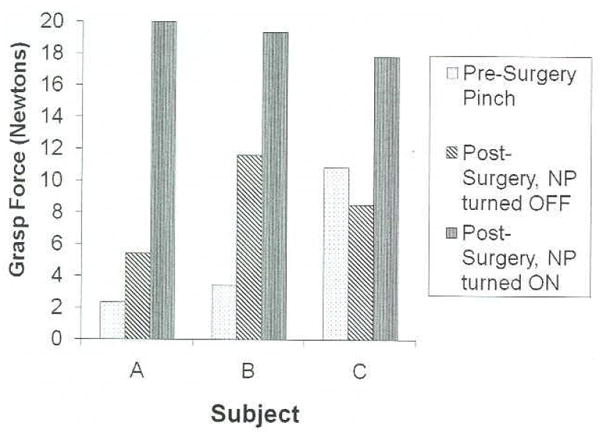
Grasp force results for all 3 subjects. Grasp force obtained before surgery is due to passive tenodesis function (no active thumb flexion). Changes in grasp force after surgery with the NP turned off are due to augmentative surgical procedures, such as tendon transfers, and changes in passive properties of the hand. The increased grasp force with the NP on is due to the force provided by AdP and FPL stimulation. AdP, adduct or pollicis.
Activities
Every subject was able to double the number of objects manipulated in the grasp release test with the NP, as shown in Table 5. Two subjects were able to successfully complete all 6 tasks when using the NP, and the third could complete 5 of the 6 tasks. After surgery, subject B successfully completed 1 additional object (video tape) with the NP off, probably due to the voluntary tendon transfer performed for thumb pinch. Subject C was able to successfully complete 3 objects with the NP turned off. Notably, preoperatively, the can object could be manipulated, but not after surgery. This result demonstrates the changes in hand posture that can occur as a result of electrically stimulated exercise even in the presence of a voluntary tendon transfer for thumb pinch.
Table 5.
Grasp Release Test Performance
| After Surgery |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Surgery |
NP Off |
NP On |
|||||||
| Subject | |||||||||
| A | B | C | A | B | C | A | B | C | |
| Peg | * | † | † | * | † | † | † | † | † |
| Block | * | † | † | * | † | † | † | † | † |
| Can | * | † | † | * | † | * | † | † | † |
| Video tape | * | * | * | * | † | † | * | † | † |
| Weight | * | * | * | * | * | * | † | † | † |
| Fork | * | * | * | * | * | * | † | † | † |
, unable to complete task;
, able to complete task.
The ability to perform ADL was assessed in each subject using the ADLAT, as shown in Table 6. All 3 subjects demonstrated improvement in at least 5 activities, with 1 subject demonstrating improvement in all 9 activities tested. Improvement in these activities generally indicates that the subject can complete the task more independently with the NP than when the NP is turned off, although this measure can also indicate improvements in the quality of performance, ease of performance, and/or time to complete the task. All 3 subjects showed improved function in 3 tasks: eating with a fork, drinking from a glass, and writing with a pen. These tasks have been shown to be some of the most common tasks for which subjects use their NP to accomplish in the home environment.18 An example of a subject using the NP to perform embroidery is shown in Figure 4.
Table 6.
Activities of Daily Living Performance changes
| Subject |
|||
|---|---|---|---|
| Activity | A | B | C |
| Eating with a fork | • | • | • |
| Drinking from a glass | • | • | • |
| Writing | • | • | • |
| Using a phone | X | • | • |
| Brushing teeth | • | • | X |
| Eating finger foods | • | – | – |
| Applying lip gloss | – | • | – |
| Applying Chapstick | – | • | – |
| Brushing hair | – | • | – |
| Wiping nose | – | • | – |
| Drinking from a mug | – | – | • |
| Total tasks improved | 5 | 9 | 5 |
improved; x, not improved; –, not tested.
Figure 4.
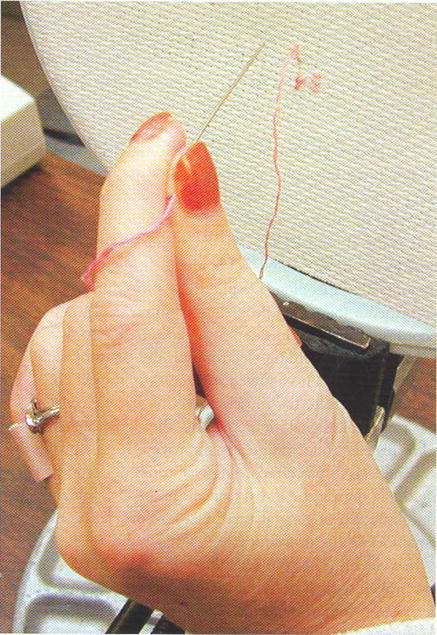
Subject performing embroidery using the NP. A palmar grasp pattern, providing tip pinch between the thumb and index finger, is used to hold the needle.
Participation
Table 7 shows the scores before and after implantation for 4 of the 6 CHART subscales. The scores for economic self-sufficiency were not included because of subject refusal. All 3 subjects demonstrated increased scores for the physical independence subscale. Subject C improved in the mobility subscale, and subject A improved in the social integration subscale. Subject A showed a decrease in the occupation subscale after implantation. This subject reported a decrease in the number of hours spent working toward his degree (15 instead of 25 hours per week) and reported an increase in volunteer, recreational, and self-improvement activities (6 instead of 3 hours per week). The remaining subscales show small changes after implantation that are not clinically important. Subjects B and C achieve the maximum score of 100 before and after implantation for the social integration subscale.
Table 7.
Results of CHART Subscales Before and after Implantation
| Subject |
||||||
|---|---|---|---|---|---|---|
| A |
B |
C |
||||
| Subscale | Before | After | Before | After | Before | After |
| Physical independence | 69.75 | 81.7 | 1.3 | 26.4 | 44 | 54 |
| Mobility | 100 | 97 | 88 | 81 | 53.5 | 74 |
| Occupation | 53 | 36 | 22.5 | 16 | 100 | 100 |
| Social integration | 89 | 100 | 100 | 100 | 100 | 100 |
Device Usage
Subjects B and C report daily usage of the NP, and subject A reports usage approximately 50% of the time. Subjects reported the following activities for which they commonly use the NP: using a fork, brushing hair, answering the phone, moving a book, eating finger foods, and drinking.
Incidents
The technological performance of the NP was successful during the course of this study. To date, there have been no device or electrode failures. There were no cases of infection or other system-related medical incidents in these subjects. This is consistent with our previous experience with implantable devices and electrodes.27
All 3 subjects experienced poor signal quality in 1 of their 2 EMG electrodes and required another operation. In subject A, the Br recording electrode produced a small signal that could only be generated with marked elbow flexion. This electrode was subsequently moved to a better location on the muscle in a follow-up procedure, and the output signal improved by a factor of 4. The procedure was performed with the subject awake so that voluntary signals could be generated, allowing better determination of the optimum electrode location. Subject B exhibited an inconsistent signal from the platysma recording electrode. The connector for this electrode was exposed during a follow-up procedure. Although there were no obvious defects in the connector, replacement of the connector spring and sleeve permitted the electrode to function properly. The position of the trapezius electrode for subject C was too anterior for activation. After a more posterior (5 cm) relocation, more consistent signals could be obtained.
DISCUSSION
This article describes the results from the first 3 individuals to receive a myoelectrically controlled implantable NP, the IST-12, for hand and arm function. The results show that the NP provides significantly increased pinch force and grasp function. All 3 subjects demonstrated increased independence and improved function in ADL that cannot be achieved by any other means. All 3 subjects use the device at home on a regular basis. These results demonstrate the potential of implanted, myoelectrically controlled NPs to improve the functional abilities of individuals with cervical level spinal cord injury.
The IST-12 system provides some important improvements over the first-generation Freehand system. The implanted myoelectric control provides subjects with multiple control options and eliminates the need to wear a joystick on the shoulder. The latter feature simplifies the donning of the device and may lead to higher long-term usage. The IST-12 system also permits stimulation to 12 different muscles compared with 8 for the Freehand system. For the 3 subjects studied, these additional electrodes provided improved hand function through activation of the finger intrinsic muscles, improved wrist extension through activation of the wrist extensors, improved reach through activation of the triceps, and improved shoulder stability through activation of the shoulder musculature.
The results of subjects B and C show the benefits of combining surgical augmentation and NP. In particular, subject B demonstrated improved thumb pinch from the Br → FPL tendon transfer when compared with presurgery pinch. Additional thumb pinch was obtained by stimulation of both the FPL and AdP muscles in the NP and produced nearly twice the pinch force when compared with the tendon transfer alone. By using an end-to-side transfer of the Br to the FPL tendon, we were able to use the FPL as a stimulated motor as well as a tendon transfer recipient. The results from subjects B and C indicate that this type of parallel muscle activation is functionally beneficial. We would recommend maximizing function for each subject through synergistic combinations of tendon transfers and electrical stimulation.
Use of myoelectric control in NPs allows considerable flexibility in the control algorithms. Performance is improved by customizing the control scheme for each subject. Whenever possible, muscles that were synergistic to the described function would be selected as the control muscle. All control signals are derived from ipsilateral muscles, thus enabling bilateral function to be provided in the future by implementing the system in the contralateral limb.
Use of an implanted control source greatly reduces the time required for system donning and doffing. By reducing the external cabling to a single coil, we have reduced the number and type of external failures that subjects experience during home use. Backup coils are provided to each subject, allowing quick replacement if needed. External control unit failures have also been rare and have primarily consisted of battery life limitations. An extra battery pack has been added to this system to extend battery life.
The physical independence subscale from the CHART shows a positive change after NP implantation. As anticipated, the remaining subscales show variability across subjects. Subscales such as mobility and cognitive independence are unlikely to show an impact as a result of increased upper-extremity function from a NP. Other-factors, such as the subject’s motivation, living situation, and attendant care availability, can have a notable effect on each subscale. It will be necessary to analyze these results in a larger cohort to identify significant effects.
All 3 subjects reported regular home use. However, subject A had difficulty maintaining upper arm motion for long periods of time due to partial paralysis in the shoulder and elbow muscles. This subject reported a usage rate about half of that of the other 2 subjects. This underscores the fact that proximal arm strength is a critical factor in subject selection and should be addressed prior to implementation of the NP. Despite this factor, an improvement in physical independence and social integration was demonstrated in this subject.
The proper placement of the recording electrodes is accomplished through a combination of presurgical mapping using surface recordings and intraoperative mapping using an epimysial stimulating electrode. For longitudinal muscles, such as the Br and ECRL, the recording electrode is placed distal to the motor point, as identified through intraoperative mapping. Despite these procedures, we have performed a follow-up procedure to move 2 of the 6 recording electrodes in these 3 subjects. In 1 case, we repositioned a recording electrode on the Br using only local anesthesia. This allowed us to ask the subject to voluntarily activate the muscle to verify that we could obtain a usable signal. Subsequently, the signal obtained from this electrode was greatly improved. It may prove to be beneficial to place recording electrodes under local anesthesia during the initial implant procedure prior to administration of general anesthesia for completion of the remainder of the procedure.
In a second case, the recording electrode on the trapezius was moved to a more posterior placement. Although this electrode recorded trapezius activity in its original placement, the shoulder protraction necessary to generate a consistent signal was difficult for the subject to perform. The anterior placement of this electrode had been selected based on easier surgical access when the subject was supine during the implantation procedure. Subsequently, we have modified the procedure for implantation of the trapezius recording electrode by placing the subject in a beach-chair position at the beginning of the procedure. This allows direct access to the posterior portion of the upper trapezius. Once the electrode is placed and tunneled, the subject’s position is changed to supine for the remainder of the procedure. No redraping is required.
The placement of some recording electrodes is determined by the anatomic constraints of the muscle. For example, the platysma recording electrode is placed so that the electrode is not over the top of the clavicle. It must be sutured to tissue underneath because the muscle is too thin to retain sutures. We have found intraoperative stimulation mapping of this muscle to be of little benefit because it is such a broad, flat muscle with a weak contraction response across a large surface area. Therefore, we rely on preoperative skin surface recordings for locating this electrode.
There have been no cases of infection or device or lead failures in this series to date. One subject had an apparent poor connection at the connector site, which resolved with replacement of the connector spring and sleeve, although no obvious defect could be identified. The durability of the implanted components in tills study is comparable with the long-term results from the first–generation NP, where the incidence of failure for any reason was less than 2%.27
Based on the successful results from these 3 subjects, we anticipate that myoelectric control will become a standard component of the control scheme for future neuroprosthetic systems. The ability to use any voluntary muscle for control allows the IST–12 system to be applicable to individuals with high tetraplegia whose control signals need to be obtained from the head and neck musculature.43 We also expect to begin the bilateral implementation of these systems in spinal cord–injured subjects. We expect that this will provide subjects with further improvement in function and will allow them to complete bimanual tasks that are currently difficult with 1 hand only, such as cutting food and removing jar lids.
Acknowledgments
The authors acknowledge the significant contributions of Fred W. Montague, Carol J. Sams, and Niloy Bhadra to this research and to this manuscript. This research is supported by the National Institutes of Health-National Institute of Neurological Disorders and Stroke (R01-NS-29549), the Food and Drug Administration Orphan Products (FD-R-002389), the National Institutes of Health General Clinical Research Center at MetroHealth Medical Center (M01-RR00080), the Rehabilitation Research and Development Service of the Department of Veterans Affairs (Merit Review #A3707R), and the Cleveland Department of Veterans Affairs Functional Electrical Stimulation Center (#C3819C).
References
- 1.Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–360. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- 2.Smith B, Buckett JR, Peckham PH, Keith MW, Roscoe DD. An externally powered, multichannel, implantable stimulator for versatile control of paralyzed muscle. IEEE Trans Biomed Eng. 1987;34:499–508. doi: 10.1109/tbme.1987.325979. [DOI] [PubMed] [Google Scholar]
- 3.Keith MW, Peckham PH, Thrope GB, Stroh KC, Smith B, Buckett JR, et al. Implantable functional neuromuscular stimulation in the tetraplegic hand. J Hand Surg. 1989;14A:524–530. doi: 10.1016/s0363-5023(89)80017-6. [DOI] [PubMed] [Google Scholar]
- 4.Davis SE, Mulcahey MJ, Betz RR, Smith BT, Weiss AA. Outcomes of upper-extremity tendon transfers and functional electrical stimulation in an adolescent with C–5 tetraplegia. Am J Occup Ther. 1997;51:307–312. doi: 10.5014/ajot.51.4.307. [DOI] [PubMed] [Google Scholar]
- 5.Davis SE, Mulcahey MJ, Smith BT, Betz RR. Self-reported use of an implanted FES hand system by adolescents with tetraplegia. J Spinal Cord Med. 1998;21:220–226. doi: 10.1080/10790268.1998.11719530. [DOI] [PubMed] [Google Scholar]
- 6.Carroll S, Cooper C, Brown D, Sormann C, Flood S, Denison M. Australian experience with the Freehand system for restoring grasp in quadriplegia. Aust N Z J Surg. 2000;70:563–568. doi: 10.1046/j.1440-1622.2000.01899.x. [DOI] [PubMed] [Google Scholar]
- 7.Biering-Sotensen F, Gregersen H, Hagen E, Haugland M, Keith M, Larsen CF, et al. Improved function of the hand in persons with tetraplegia using electric stimulation via implanted electrodes. Ugeskr Laeger. 2000;162:2195–2198. [PubMed] [Google Scholar]
- 8.Fromm B, Rupp R, Gerner HJ. The Freehand system: an Implantable neuroprosthesis for functional electrical stimulation of the upper extremity. Handchir Mikrochir Plast Chir. 2001;33:149–152. doi: 10.1055/s-2001-15129. [DOI] [PubMed] [Google Scholar]
- 9.Peckham PH, Keith MW, Kilgore KL, Grill JH, Wuolle KS, Thrope GB, et al. Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch Phys Med Rehabil. 2001;82:1380–1388. doi: 10.1053/apmr.2001.25910. [DOI] [PubMed] [Google Scholar]
- 10.Taylor P, Esnouf J, Hobby J. The functional impact of the Freehand system on tetraplegic hand function, clinical results. Spinal Cord. 2002;40:560–566. doi: 10.1038/sj.sc.3101373. [DOI] [PubMed] [Google Scholar]
- 11.Mulcahey MJ, Bee RR, Kozin SH, Smith BT, Hutchinson D, Lutz C. Implantation of the Freehand System during initial rehabilitation using minimally invasive techniques. Spinal Cord. 2004;42:146–155. doi: 10.1038/sj.sc.3101573. [DOI] [PubMed] [Google Scholar]
- 12.Cornwall R, Hausman MR. Implanted neuroprostheses for restoration of hand function in tetraplegic patients. J Am Acad Orthop Surg. 2004;12:72–79. doi: 10.5435/00124635-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Buckett JR, Peckham PH, Thrope GB, Braswell SD, Keith MW. A flexible, portable system for neuromuscular stimulation in the paralyzed upper extremity. IEEE Trans Biomed Eng. 1988;35:897–904. doi: 10.1109/10.8669. [DOI] [PubMed] [Google Scholar]
- 14.Peckham PH, Thrope GB, Buckett JR, Freehafer AA, Keith MW. Coordinated two mode grasp in the quadriplegic initiated by functional neuromuscular stimulation. In: Campbell RM, editor. IFAC control aspects of prosthetics and orthotics. Oxford: Pergamon Press; 1983. pp. 29–32. [Google Scholar]
- 15.Kilgore KL, Peckham PH, Thrope GB, Keith MW, Stone KA. Synthesis of hand movement using functional neuromuscular stimulation. IEEE Trans Biomed Eng. 1989;36:761–770. doi: 10.1109/10.32109. [DOI] [PubMed] [Google Scholar]
- 16.Wuolle KS, Van Doren CL, Thrope GB, Keith MW, Peckham PH. Development of a quantitative hand grasp and release test for patients with tetraplegia using a hand neuroprosthesis. J Hand Surg. 1994;19A:209–218. doi: 10.1016/0363-5023(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 17.Kilgore KL, Peckham PH, Keith MW, Thrope GB, Wuolle KS, Bryden AM, Hart RL. An implanted upper extremity neuroprosthesis: a five patient follow-up. J Bone Joint Surg. 1997;79A:533–541. doi: 10.2106/00004623-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Wuolle KS, Van Doren CL, Bryden AM, Peckham PH, Keith MW, Kilgore KL. Patient satisfaction and usage of a hand neuroprosthesis. Arch PM&R. 1998;80:206–213. doi: 10.1016/s0003-9993(99)90123-5. [DOI] [PubMed] [Google Scholar]
- 19.Peckham PH, Kilgore KL, Keith MW, Bryden AM, Bhadra N, Montague FW. An advanced neuroprosthesis for restoration of hand and upper arm control employing an implantable controller. J Hand Surg. 2002;27A:265–276. doi: 10.1053/jhsu.2002.30919. [DOI] [PubMed] [Google Scholar]
- 20.American Spinal Injury Association. International standards for neurological classification of spinal cord injury. Chicago: American Spinal Injury Association; 2002. pp. 1–23. [Google Scholar]
- 21.McDowell CL, Moberg EA, Graham-Smith A. International conference on surgical rehabilitation of the tipper extremity in tetraplegia. J Hand Surg. 1979;4:387–390. doi: 10.1016/s0363-5023(79)80083-0. [DOI] [PubMed] [Google Scholar]
- 22.Hart RL, Kilgore KL, Peckham PH. A comparison between control methods for implanted FES hand grasp systems. IEEE Trans Rehab Eng. 1998;6:1–11. doi: 10.1109/86.681187. [DOI] [PubMed] [Google Scholar]
- 23.Scott TRD, Peckham PH, Kilgore KL. Tri-state myoelectric control of bilateral upper extremity neuroprostheses for tetraplegic individuals. IEEE Trans Rehab Eng. 1996;4:251–263. doi: 10.1109/86.547925. [DOI] [PubMed] [Google Scholar]
- 24.Knutson JS, Hoyen HA, Kilgore KL, Peckham PH. Simulated neuroprosthesis state activation and hand position control using myoelectric signals from wrist muscles. J Rehab Res Dev. 2004;41:461–472. doi: 10.1682/jrrd.2003.04.0053. [DOI] [PubMed] [Google Scholar]
- 25.Kilgore KL. Synthesis of hand grasp. In: Winters JM, Crago PE, editors. Biomechanics and neural control of posture and movement. New York: Springer–Verlag; 2000. pp. 605–616. [Google Scholar]
- 26.Keith MW, Kilgore KL, Peckham PH, Wuolle KS, Creasey G, Lemay M. Tendon transfers and functional electrical stimulation for restoration of hand function in spinal cord injury. J Hand Surg. 1996;21A:89–99. doi: 10.1016/s0363-5023(96)80160-2. [DOI] [PubMed] [Google Scholar]
- 27.Kilgore KL, Peckham PH, Keith MW, Montague FW, Hart RL, Gazdik MM, et al. The durability of implanted electrodes and leads in upper extremity neuroprostheses. J Rehab Res Dev. 2003;40:457–468. doi: 10.1682/jrrd.2003.11.0457. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. International classification of functioning, disability and health (ICF) Geneva, Switzerland: WHO; 2001. pp. 1–299. [Google Scholar]
- 29.Sinnott KA, Dunn JA, Rothwell AG. The use of the ICF for evaluation of outcome measures used in 10 year re-review following reconstructive hand surgery. Int J Spinal Cord. 2004;42:396–400. doi: 10.1038/sj.sc.3101610. [DOI] [PubMed] [Google Scholar]
- 30.Bryden AM, Sinnott KA, Mulcahey MJ. Innovative strategies for improving upper extremity function in tetraplegia and considerations in measuring functional outcomes. Topics Spinal Cord Injury Rehabil. 2005;10:75–93. [Google Scholar]
- 31.Trombly CA, Scott AD. Evaluation. In: Trembly CA, editor. Occupational therapy for physical dysfunction. 3. Baltimore: Williams & Wilkins; 1989. pp. 184–286. [Google Scholar]
- 32.Kendall FP, McCreary EK. Muscles: testing and function. 3. Baltimore: Williams & Wilkins; 1983. pp. 59–128. [Google Scholar]