Abstract
The need for techniques to facilitate the regeneration of failing or destroyed tissues remains great with the aging of the worldwide population and the continued incidence of trauma and diseases such as cancer. A 16-year history in biomaterial scaffold development and tissue engineering is examined, beginning with the synthesis of novel materials and fabrication of 3D porous scaffolds. Exploring cell-scaffold interactions and subsequently cellular delivery using biomaterial carriers, we have developed a variety of techniques for bone and cartilage engineering. In addition to delivering cells, we have utilized growth factors, DNA, and peptides to improve the in vitro and in vivo regeneration of tissues. This review covers important developments and discoveries within our laboratory, and the increasing breadth in the scope of our work within the expanding field of tissue engineering is presented.
Keywords: biomaterials, tissue engineering, drug delivery, polymer scaffolds, hydrogels, biomimetics, bioreactors
Introduction
The field of tissue engineering, loosely defined as the use of engineering principles and technologies to regenerate living tissues, has roots in many branches of science and engineering. Following its introduction to the broad scientific community over 15 years ago,1 the field has rapidly expanded and advanced, as evidenced by publication trends.2 Founded at the intersection of chemistry, materials science, systems level and molecular biology, and chemical and mechanical engineering,3, 4 the viability of technologies and products emerging from tissue engineering is apparent,5–11 and with continued expansion, tissue engineering based therapeutics will play a large role in advancing medicine in the 21st century.
Most iterations of the traditional tissue engineering paradigm describe the combination of cells, scaffold materials, and bioactive factors towards the de novo growth or induced regeneration of living tissues following damage or in conditions under which regeneration would not normally occur. For the last 16 years, our laboratory has investigated mainly orthopaedic and dental tissue engineering, focusing primarily on the regeneration of bone and cartilage. In doing so we have formulated new tissue engineering techniques, investigated key parameters for tissue growth within synthetic matrices, and developed novel biomaterials for use as tissue engineering scaffolds and bioactive factor delivery vehicles.
In 2005, over 2,300,000 procedures were performed in U.S. hospitals involving the partial excision of bone, treatment of fractures, or joint replacement.12 Many of these procedures were likely necessitated by or will result in a bony defect that will not regenerate. Most commonly due to trauma or neoplasm, these nonhealing or nonunion bone defects are costly and can adversely affect patient quality of life. Bone tissue engineering is a potential source of treatments for these defects. If successfully implemented, bone tissue engineering strategies will allow for the complete functional and morphological regeneration of healthy bone tissue without the need for residual or permanently indwelling synthetic materials or large amounts of donor tissue, the procurement of which typically involves either a risk of transmitted disease from allo- or xenogeneic tissues13, 14 or the necessity for additional surgeries15 and potential morbidity at the donor site for autologous tissues.16, 17
Regenerating bone tissue in vivo requires the consideration of a number of critical elements. First, bone regenerates or heals preferentially when under mechanical stimulation,18–20 possibly due to the differentiation of stem cells in response to their mechanical microenvironment.21 Thus, in addition to providing a three-dimensional template for tissue growth, a material used as a scaffold must be able to withstand the mechanical loading necessary to facilitate bone growth. Second, diffusional limitations on the delivery of oxygen and nutrients from the blood stream and the removal of waste products affect the size of defects that can be addressed by tissue engineering.22, 23 Appropriate material porosity and the allowance or induction of vascular ingrowth can mitigate these limitations.24–27 Finally, for the regenerated bone to be identical to natural bone, the scaffold material must degrade in vivo but must do so at a rate so as not to compromise the mechanical stability of the scaffold prior to sufficient bony ingrowth. Along with these key elements, cyto- and biocompatibility must obviously be addressed.
The requirements for engineering other tissue types are similarly specific, and thus as the field of tissue engineering progresses, it is unlikely that a single material will be capable of meeting the criteria necessary for successful application towards engineering many tissues. There is a distinct need for biomaterials and combinations of biomaterials, processing techniques, bioactive factors, and cells tailored for tissue specific applications.28 Early work in tissue engineering and within our laboratory focused predominantly on applications using the now FDA-regulated material poly(D,L-lactic-co-glycolic acid) (PLGA); however, as our laboratory and the field in general have evolved, more experimental work is being performed on novel biomaterials with parameters rendering them appropriate for specific applications.
Biomaterial and Scaffold Technology Development
Early work
Building upon prior work investigating preparation of polymer scaffolds,29–32 at its inception our laboratory began studying the interaction between osteoblasts and polymer matrices in collaboration with Dr. Rena Bizios, now of The University of Texas at San Antonio, first investigating the effect of varying the comonomer ratio in PLGA films on the expression of alkaline phosphatase (ALP) and collagen synthesis.33 Similar work investigating polymer composition and cell-polymer interactions was performed using poly(L-lactic acid) (PLLA) and PLGA films to culture human retinal pigment epithelial (RPE) cells.34, 35 The release of lactic acid from degrading PLLA membranes was modulated by varying the polydispersity of PLLA blends made from monodisperse PLLA of high and low molecular weights, allowing for controlled release of acidic degradation products to minimize fluctuations in pH.36 Biodegradable polymer particles fabricated from both PLLA and PLGA on the order of 1 μm in diameter were found to inhibit cell proliferation and matrix mineralization at high particle concentrations, possibly due to diffusional limitations imposed by the particles and cell culture; however, established cell cultures were less effected than cultures exposed to particles on the first culture day.37
Investigating the effect of pore size on fibrovascular tissue ingrowth, we found that varying the pore size in a polymer scaffold made of PLLA could drastically affect the rate at which surrounding tissues invaded the matrix25 and later the rate at which materials degrade both in vitro and in vivo.38, 39 While rapid tissue ingrowth and vascularization can modulate diffusional limitations within large scaffolds, the presence of this granulation tissue within the pores of the scaffold limits space available for guided tissue regeneration and was thus recognized as being potentially detrimental to success in some applications.
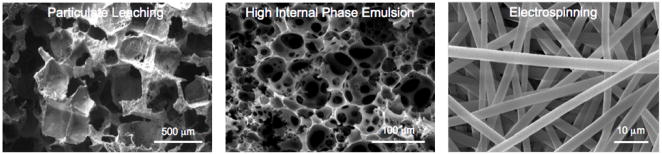
Noting the role pore size, morphology, and interconnectivity would no doubt have in abrogating diffusional limitations and facilitating tissue regeneration within defects of clinically relevant size, we developed a novel method of particulate leaching to create PLGA foams around degradable gelatin microspheres.40 Similar to other work involving fiber bonding29 and phase separation,41 this technique allowed control over pore sizes and overall porosity but did so through a process involving no additional organic solvents, making it particularly attractive for biomedical applications. This particulate leaching method was later employed using salt crystals as a porogen, and simple leaching of the crystals resulted in PLGA/poly(ethylene glycol) (PEG) blended scaffolds with varied shear moduli, porosities, and pore diameters.42 Solvent casting, salt leaching, and extrusion were used to create porous PLLA and PLGA conduits to investigate peripheral nerve regeneration.43–45 Continued work with our collaborators has applied the particulate leaching technique for bone tissue engineering with calcium phosphate (CaP) biomaterials.46–48 In addition to expansion into other areas of scaffold fabrication and tissue engineering, our laboratory and collaborators have continued to investigate processing techniques for creating scaffold porosity (Figure 1).49–51
Figure 1.
Examples of porous scaffolds. Techinques employing particulate leaching (left), high internal phase emulsion (middle), and electrospinning (right) have all been used to fabricate porous biodegradable polymer matrices for use as tissue engineering scaffolds.
Poly(propylene fumarate)
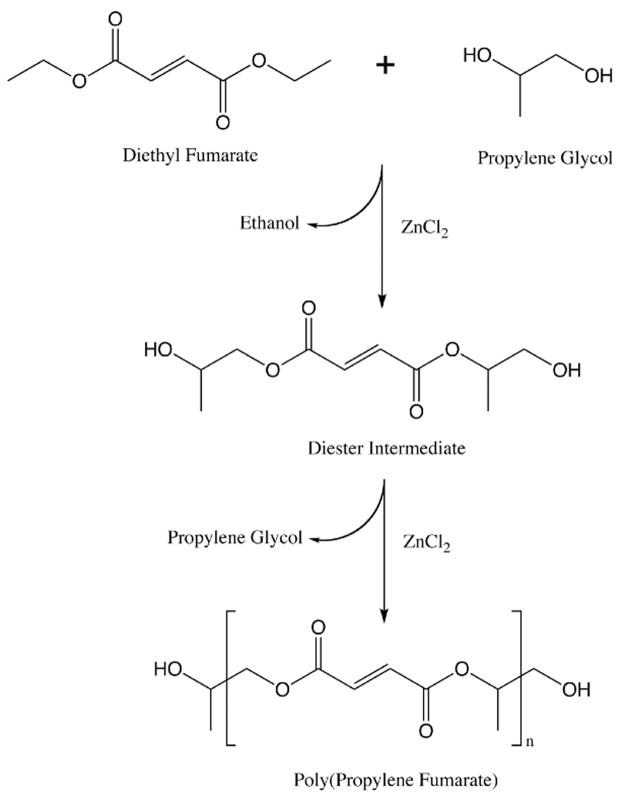
In 1995 we began developing novel biodegradable scaffolds based on poly(propylene fumarate) (Figure 2), an unsaturated linear polyester based on fumaric acid, a non-toxic intermediate in the citric acid cycle.52 Early work focused on characterizing PPF both alone53 and as a composite with the ceramic β-tricalcium phosphate (βTCP)54 and later with PLGA.55–57 Subsequent refinements in the synthesis of PPF were made,58 resulting in a step polymerization of diethyl fumarate and propylene glycol in the presence of zinc chloride (Figure 3).59 This one pot method yielded PPF with molecular weights up to 4600 after 12h.
Figure 2.
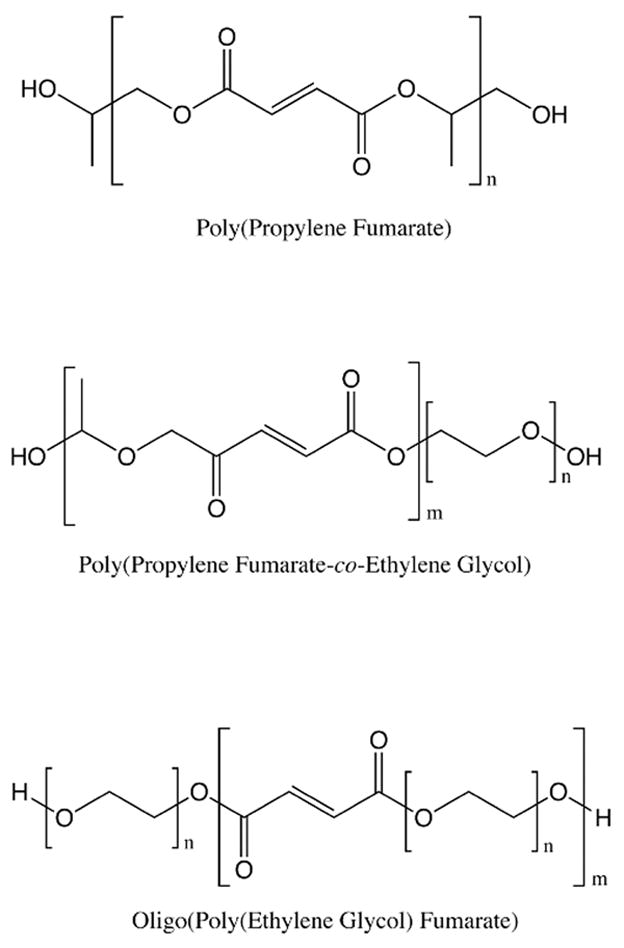
Structure of fumarate-based polymers, poly(propylene fumarate) (PPF), oligo(poly(ethylene glycol) fumarate) (OPF), and poly(propylene fumarate-co-ethylene glycol) (P(PF-co-EG)).
Figure 3.
Reaction schema showing the synthesis of poly(propylene fumarate) from diethyl fumarate and propylene glycol.
In addition to optimizing the synthesis of PPF, a series of studies investigating the development and characterization of cross-linked PPF scaffolds were performed. Using benzoyl peroxide (BP) as an initiator, cross-linked PPF networks were synthesized using PEG-dimethacrylate (PEG-DMA)60 or propylene fumarate-diacrylate (PF-DA).61 Using PPF/PF-DA networks as a model system, later work characterized the participation of acrylate and fumarate groups in network formation62 and found continual reactivity of unreacted fumarate groups at physiological temperatures, resulting in an increased compressive modulus after six weeks incubation.63 This unique process can lead to mechanical reinforcement of scaffolds with concurrent degradation, providing a possible mechanism for shape retention and maintained mechanical support with time.
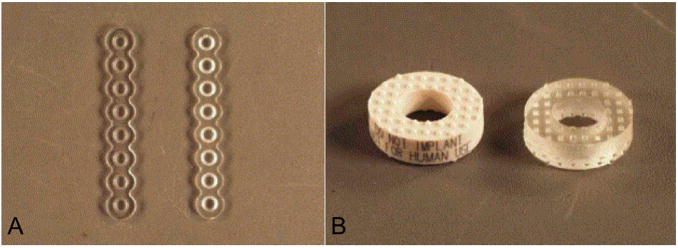
We also investigated photocross-linking using bis(2,4,6-trimethlybenzoyl) phenylphosphine oxide (BAPO) to cross-link PPF networks both with64 and without65, 66 the addition of porogen. Porous and non-porous formulations of photocross-linked PPF maintained their structure, strength, and porosity as the material degraded, even after 32 weeks and as mass losses approached 30%.67 A comparison of thermal- and photo-cross-linking of PPF networks found photocross-linking using BAPO yields a higher cross-linking density and higher double bond conversion than thermal cross-linking in the presence of BP;68 however, both methods offer utility in different applications. Networks that undergo thermal cross-linking close to physiological temperature hold potential as in situ cross-linkable materials for injectable applications,69, 70 while photocross-linking PPF/PF-DA networks within silicon molds71 or PPF/diethyl fumarate composites during stereolithography72 was successfully used to fabricate biodegradable orthopaedic implants (Figure 4). Using a rabbit model, photocross-linked PPF implants were also found to elicit only a mild inflammatory response 2 weeks after implantation in both soft and hard tissues, and this inflammatory response was largely resolved with surface degradation evident by 8 weeks post-implantation.73
Figure 4.
(A) 1.5 mm 8 hole adaption plates manufactured with 70:30 P(L/DL-LA) (left) and PPF/PF-DA with a double bond ratio of 0.5 (right). The PPF/PF-DA plate was fabricated with a transparent silicone mold formed with a P(L/DL-LA) master. (B) Plastic model (left) and PPF/PF-DA with double bond ratio 0.5 replicate (right) of a 5 mm lordotic anterior cervical fusion spacer. The plastic model has identical geometry as the bone allograft implant and was used to produce the silicone molds for the PPF/PF-DA device. Reprinted with permission from (71).
Other fumarate based materials
While developing PPF, we also investigated other fumarate-based materials. Poly(propylene fumarate-co-ethylene glycol) (P(PF-co-EG), Figure 2), a block copolymer hydrogel of PPF and PEG, was synthesized and found to have tunable mechanical properties controlled by varying the PPF molecular weight and PEG content.74 In vitro and in vivo degradation studies of P(PF-co-EG) hydrogels, performed in collaboration with Dr. James M. Anderson of Case Western Reserve University, determined that increasing the weight percent of PEG decreased material degradation rates, while changes in PEG molecular weight had only minimal effects on degradation.75 Similarly, the biocompatibility of the hydrogels increased with increasing weight percent of PEG,76 and both PEG weight percent and molecular weight influenced platelet attachment at the material surface.77 The ability to tune the mechanical, degradative, and biointeractive characteristics of this injectable material make it an attractive substrate for engineering a variety of tissues. Later work using this copolymer included the introduction of a novel method for creating in situ crosslinked macroporous hydrogels using generated carbon dioxide as a porogen.78, 79 Substitution of methoxy poly(ethylene glycol) for PEG yielded biodegradable copolymers that undergo both physical and chemical gelation,80 a concept that has continued to be investigated in our laboratory.81
In addition to P(PF-co-EG), hydrogels based on oligo(poly(ethylene glycol) fumarate) (OPF, Figure 2), a novel oligomer developed by our laboratory,82 were investigated as tissue engineering and drug delivery substrates. A PEG-based macromer with unsaturated double bonds along its macromolecular chain, OPF synthesized with PEG of different molecular weights allows for modulation of tensile mechanical properties, swelling characteristics, mesh size, and cell attachment.83, 84 Cross-linking of OPF hydrogels using redox radical initiatiors such as ammonium persulfate (APS) with a reducing agent was found to be a feasible method for fabricating cross-linked hydrogel networks in the presence of mesenchymal stem cells (MSCs),85 a subpopulation of bone marrow stromal cells or adherent cells found within the bone marrow space, these adult progenitor cells hold great promise for tissue engineering and other medical applications. OPF would later be used for a number of tissue engineering and drug delivery applications, most notably in the area of cartilage regeneration.
3D Cell-Scaffold Constructs
Beyond material characterization such as cytotoxicity evaluation, the development of materials for tissue engineering applications requires application specific investigation of cellular function on or within the material being developed. As we sought to develop materials for bone tissue engineering, the function and interaction of osteoblasts and osteoblast progenitors with the biomaterial scaffold was critical to the success of the material. Our early work found that osteoblasts proliferated and deposited a mineralized matrix on PLGA foams both in vitro86, 87 and in vivo,88 even when implanted into an ectopic anatomical site (one in which bone growth does not naturally occur). While this early work found no correlation between scaffold pore size and mineralized matrix deposition, subsequent work relating matrix deposition and cell culture period to pore morphology found that highly porous scaffolds seeded with osteoblasts tended to collapse after only one week in culture.89 This work was important in shaping future studies, as a balance is needed between highly porous scaffolds that allow rapid tissue ingrowth and minimize diffusional limitations and less porous materials that retain both construct shape and the ability to bear mechanical loads in a complex biochemical and mechanical environment.
In continuing to investigate interactions between cells and scaffolds, a study of the attachment and proliferation of bone marrow derived osteoblasts on end-capped and non-end-capped poly(D,L-lactic acid) (PLA) and a diblock copolymer of PEG-monomethyl ether and PLA found that cells on PLA adhered and proliferated equally well, while cells on the copolymer did not proliferate but were more highly differentiated and more metabolically active than cells on PLA alone.90 This was attributed to decreased protein adsorption on the copolymer and provided an early example of the influence of material properties on cell differentiation and activity. Comparing OPF and PPF within a rabbit tooth socket defect, we found that implantation of the hydrophilic OPF constructs inhibited bone healing as compared to PPF and control groups.91 Immunohistochemistry during the wound healing process found that the presence of OPF relative to PPF blunted the response of fibroblast growth factor-2 (FGF-2), an important factor in bone and wound healing, indicating that the interaction of biomaterials and growth factor cascades may be critical to wound healing and tissue regeneration.
In addition to OPF and PPF, P(PF-co-EG) was studied as a carrier for endothelial cells. In situ cross-linking of P(PF-co-EG) did not blunt the wound healing response in vivo and in vitro cross-linking with encapsulated endothelial cells confirmed the viability of the copolymer as an injectable cell carrier.92
3D Composite Scaffolds
Building upon the knowledge that bulk material properties and surface characteristics are critical in biological applications, our laboratory has continually explored various composite materials as tissue engineering scaffolds. Initial work primarily focused on polymer composites and polymer-ceramic composites, but as new advances have been made in chemistry and materials science, we have incorporated new materials into tissue engineering constructs.
Polymer-ceramic composites
As previously described, PPF/βTCP composites were among the earliest materials investigated in our laboratory. PPF/βTCP composites were found to have increased compressive moduli and strength compared to PPF alone, and the composites degraded in vivo with a mild inflammatory response following implantation.93 In collaboration with Dr. Michael J. Yaszemski of the Mayo Clinic in Rochester, Minnesota, we investigated moldable PPF/βTCP paste as an alternative to the currently used poly(methyl methacrylate) (PMMA) bone cements. PPF/βTCP pastes had comparable mechanical properties to PMMA and, in contrast to PMMA, were biodegradable and cross-linked below 50 °C, well below 94 °C, the potentially toxic curing temperature of PMMA.94 Layered composites were also fabricated,95 and adherent marrow stromal cells attached and proliferated well on the composites.96
Our more recent studies of polymer-ceramic composites have been performed with our collaborator, Dr. John A. Jansen of Radboud University Nijmegen Medical Center in the Netherlands. Most of this work has focused on CaP cements augmented with PLGA microspheres. These materials exhibit excellent biocompatibility, have improved biodegradability over CaP cements alone, and can be used as injectable materials.48, 97, 98 In vivo bone regeneration using these materials has been promising,99 and more recently our laboratories have been investigating both release of the osteogenic growth factor bone morphogenetic protein 2 (BMP-2) from PLGA microspheres100 as well as the inclusion of gelatin in CaP cements as porogens or drug delivery vehicles.46, 101 With the importance of biological interactions at the nanoscale becoming more apparent, we have also developed methods for the dispersion of calcium phosphate nanocrystals within OPF hydrogels.102
Nanocomposite scaffolds
Commensurate with the need for scaffolds with enhanced compressive mechanical properties, our laboratory has investigated methods to reinforce polymer scaffolds. Earlier attempts in this direction used short hydroxyapatite fibers to reinforce PLGA foams and succeeded in enhancing the compressive yield strength;103 more recent attempts at composite scaffold reinforcement have focused on nanoscale scaffold reinforcement. A variety of surface modified carboxylate alumoxane nanoparticles were dispersed within PPF/PF-DA scaffolds and found to cause a 3-fold increase in flexural modulus over polymer resin reinforced scaffolds.104 Following an accelerated degradation protocol, alumoxane reinforced PPF scaffolds degraded faster than nonreinforced scaffolds, and the inclusion of alumoxane nanoparticles did not affect scaffold cytotoxicity or biocompatibility.105 After 12 weeks of in vitro degradation, porous PPF/alumoxane composites maintained their compressive mechanical properties, pore morphology, and overall scaffold size despite mass losses of over 5% due to degradation.106 Following 12 weeks implantation within a goat hindlimb condylar defect, PPF/alumoxane composites were found to degrade and regenerate bone similar to control PPF/PF-DA scaffolds, indicating that the improved mechanical properties of PPF/alumoxane nanocomposites are gained without a concomitant decrease in degradation or bone healing in vivo.107 Despite these positive results, other efforts in our laboratory involving nanoreinforced scaffolds aimed to achieve superior scaffold mechanical properties with corresponding improvements in in vivo bone regeneration.
Carbon nanotube composite scaffolds
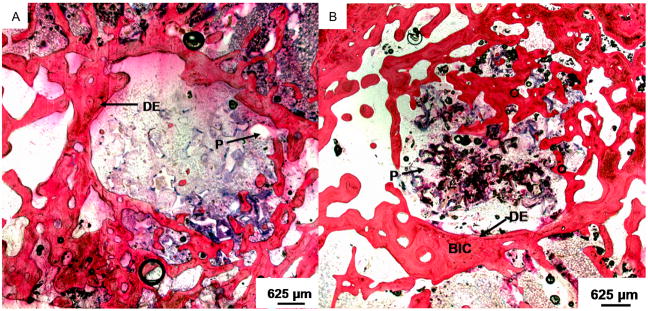
We have also investigated the incorporation of single-walled carbon nanotubes (SWNTs) dispersed within PPF scaffolds. With respect to enhancement of mechanical properties, an ideal concentration of 0.05 wt% resulted in a 74% increase in compressive modulus and a 69% increase in flexural modulus.108 At higher than 0.05 wt%, nanotube aggregates were observed. Functionalized SWNTs were synthesized and interacted with PPF chains, increasing cross-linking densities of PPF networks and facilitating load transfer between the polymer and the nanotubes thereby resulting in 3-fold increases in compressive and flexural moduli and 2-fold increases in compressive and flexural strengths over non-reinforced PPF.109 Incorporation of nanotubes did not increase the cytotoxicity of the material,110 and marrow stromal cells attached and proliferated on porous scaffolds augmented with ultrashort nanotubes.111 Additionally, these materials have the potential to be used as injectable biomaterials.112 Twelve weeks following implantation in a rabbit condylar defect, PPF/ultrashort nanotube composites displayed a 3-fold greater bone tissue ingrowth than control PPF/PF-DA scaffolds, and additionally, markedly fewer infiltrating inflammatory cells and more highly organized connective tissue were observed around the nanocomposites (Figure 5).113 Because of their greatly increased mechanical properties combined with improved in vivo capacity for bone regeneration compared to PPF alone, PPF/SWNT composites are a promising material for bone and other hard tissue engineering applications. Additionally, the development of methods for functionalization and dispersion of nanotubes within biomaterial matrices could be used to augment other biomaterials or to make materials with limited mechanical strength appropriate for new applications within tissue engineering and biology.
Figure 5.
Representative histological sections of scaffolds implanted in femoral condyle defects: (A) a PPF/PF-DA scaffold after 12 weeks, and (B) an ultrashort carbon nanotube/PPF scaffold at 12 weeks post-implantation. The images are presented at 1.6× magnification. The PPF scaffold (P) appears as white areas in all images. The original defect edge (DE) is visible in the low magnification images. Bone-like tissue appears red; direct bone-implant contact (BIC) and bony ingrowth occurred with the nanocomposite scaffold 12 weeks after implantation. Reprinted with permission from (113).
Injectable Carriers for Cellular Delivery
An area of research that holds great promise in biomaterials science and tissue engineering is the development of injectable materials and systems for clinical applications. Injectable systems can be delivered to a patient using minimally invasive methods and can fill complex tissue defects without the need for extensive imaging and prefabrication. Perhaps most importantly, injectable systems that use water as a solvent can potentially be used to deliver cells and water soluble growth factors or drugs for therapeutic purposes.114 In biology, cell based technology is rapidly expanding and cellular therapeutics are moving closer to becoming clinical reality, making the need for delivery vehicles and injectable matrices increasingly apparent.
We investigated PPF/gelatin microparticle composites as an injectable system for tissue engineering. Marrow stromal cells, a subpopulation of which are osteoblastic precursors or MSCs, maintain their viability and differentiation capacity when encapsulated within gelatin microparticles.115 This encapsulation process protects MSCs during PPF crosslinking,116, 117 and amelioration of the potentially toxic effects of being exposed to uncross-linked macromers and initiators renders the system viable for injectable cell delivery.
Cell adhesion to P(PF-co-EG) hydrogels has also been investigated along with cell viability during in situ cross-linking using the water soluble APS initiator system described previously.118, 119 Cells adhered to the cross-linked gels and the viability of cells present during cross-linking was above 30% for some formulations, making cell delivery for in vivo applications possible.
OPF has been extensively studied as a carrier for cell delivery. OPF incorporating low molecular weight PEG chains was originally found to elicit a minimal inflammatory response in vivo120 and had cytotoxicity below 25% after 24 hours exposure in vitro.121 These promising results led to a series of studies investigating cell encapsulation within OPF hydrogels.
Because of their high water content, hydrogels are often thought of as ideal carriers for cell encapsulation and delivery; however, a number of parameters must be considered and optimized to ensure appropriate material properties in combination with maintained cell viability and differentiation. Our early encapsulation studies focused on identifying radical initiators and reducing agents as well as proper concentrations of chemicals from both classes that would achieve ideal gelation kinetics without large fluctuations in pH or decreased encapsulated cell viability. The effects of two persulfate oxidizing agents and three reducing agents derived from ascorbic acid were measured with respect to encapsulated rat marrow stromal cell survival, and initiator concentration and final pH were recognized as key parameters affecting cell survival.85 Encapsulation of rat marrow stromal cells in the presence of cell culture media containing osteogenic supplements demonstrated that encapsulated cells retained the ability to differentiate into osteoblast-like cells,122 and OPF hydrogels with greater swelling due to incorporation of higher molecular weight PEG support greater osteoblastic differentiation of encapsulated cells than OPF hydrogels that undergo less swelling.123
Native cartilage consists of a largely acellular matrix composed primarily of water with interspersed chondrocytes, a structure that is well approximated by encapsulating chondrocytes within a hydrogel matrix. OPF hydrogels have been used to deliver encapsulated chondrocytes and MSCs for cartilage regeneration, although this delivery has typically been in conjunction with the delivery of growth factors for inducing differentiation of encapsulated cells and surrounding host cells into chondrocytes.124, 125 This and other examples of biomaterial facilitated delivery of bioactive molecules has been an expanding focus of our work and has led to a number of diverse and novel techniques for bioactive factor generation and delivery.
Growth Factor Carriers and Scaffolds
The continued focus and progressive discoveries in areas such as stem cell biology, cell signaling, and tissue specific microenvironments or niches has led to the identification of a number of key growth factors necessary for tissue repair or regeneration. In addition to studying the release of common pharmaceuticals from biomaterial matrices and particles to treat localized infection or disease,126–130 we along with many other researchers in tissue engineering have been attempting to mimic natural healing or development by delivering exogenous growth factors, often simultaneously with the cells upon which they are intended to act, to speed or enable tissue regeneration.
Transforming growth factor-β1 (TGF-β1) is a 25 kilodalton cytokine that is nearly ubiquitously expressed by immune cells and likely plays an important role in cell differentiation and wound healing.131 Consequently, it is frequently investigated in tissue engineering applications. We have investigated TGF-β1 coating of scaffolds and release from PLGA/PEG microparticles and found increased osteoblast proliferation and enhanced deposition of osteogenic markers.132–135 Gelatin microparticles, a popular drug delivery vehicle,136 have also been utilized for TGF-β1 release from OPF hydrogels137, 138 and injectable calcium phosphate cements.101
We have extensively studied the release of TGF-β1 from OPF hydrogels for cartilage tissue engineering. Bilayered OPF hydrogels with TGF-β1 loaded into the superficial layer of the gel were used to repair osteochondral defects in a rabbit model,139 and in vitro studies were undertaken to investigate the simultaneous delivery of chondrocytes and TGF-β1.124 Following promising early results in these studies, including a noted maintenance of chondrocytic phenotype for encapsulated cells, we, in collaboration with Dr. Arnold I. Caplan of Case Western Reserve University, demonstrated that rabbit mesenchymal stem cells encapsulated in OPF with TGF-β1 loaded gelatin microparticles differentiate into chondrocyte-like cells with upregulated collagen II and aggrecan expression.125 Expanding on successful TGF-β1 release systems for cartilage engineering, we explored the tandem release of TGF-β1 and insulin-like growth factor 1 (IGF-1). By varying the cross-linking extent and isoelectric point of gelatin microparticles, TGF-β1 and IGF-1 release from OPF hydrogels was tailored to mimic the release profiles found naturally.140 In vitro studies showed that marrow stromal cells exposed to both TGF-β1 and IGF-1 had significantly upregulated expression of chondrogenesis-related genes compared to controls (Figure 6).141 In vivo assessment of this dual release technique showed improved cartilage healing in defects treated with IGF-1 releasing hydrogels; however, this improvement was not observed with scaffolds releasing both growth factors (Figure 7).142 This surprising result confirmed that a complex interplay between growth factors and the native healing site is at work, and careful evaluation of delivery strategies, even those that seemingly approximate a natural healing response, is necessary.
Figure 6.
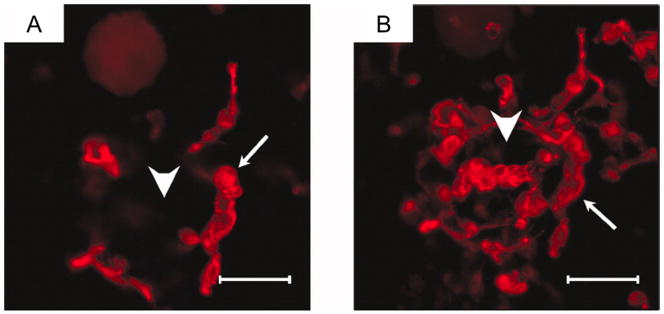
2D image (A) and z-axial projection stack image (B) of rabbit marrow MSCs in OPF hydrogel composites with both IGF-1-loaded MPs and TGF-β1-loaded microparticles at day 14. Samples were incubated with rhodamine phalloidin solution for 1 h and imaged with confocal fluorescence microscopy. Scale bar represents 100 μm. Small arrows indicate rabbit marrow MSCs and large arrows indicate microparticles in hydrogel composites. Reprinted with permission from (141).
Figure 7.
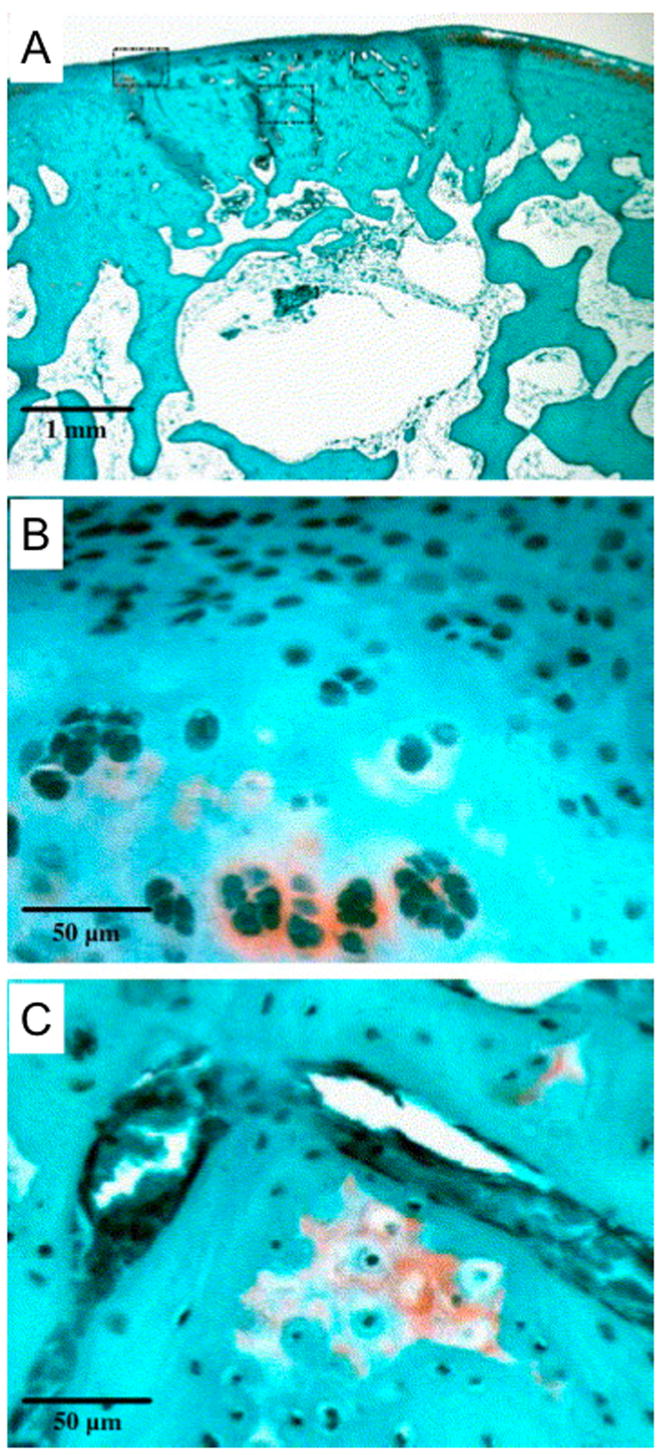
Histological section displaying fibrocartilage in-growth near the chondral defect margins and significant subchondral restoration. The boxed regions in (A) (2× magnification) are shown at 20× magnification to illustrate the spherical shape of cartilage cells in the neo-surface (B) and small regions of remodeling tissue in subchondral region (C). This defect was treated by IGF-1 delivery. Reprinted with permission from (142).
We have also studied dual growth factor delivery for bone tissue engineering. Using the well characterized gelatin microparticle system for release of BMP-2 and vascular endothelial growth factor (VEGF),143, 144 we, in collaboration with Dr. Yasuhiko Tabata of Kyoto University in Japan, found after 4 weeks implantation within a rat cranial defect, dual release of VEGF and BMP-2 regenerated significantly more bone tissue than release of either growth factor alone (Figure 8).27 After 12 weeks scaffolds releasing BMP-2 alone produced similar tissue regeneration to dual release scaffolds, indicating that neovascularization can promote improved bone regeneration early in the healing process. Subsequent studies found that vascularization as induced by VEGF is insufficient to rescue bone growth in scaffolds releasing lower doses of BMP-2, illustrating the need for a balanced interplay between angiogenesis and osteogenesis to yield a therapeutic effect.145 Previous studies have demonstrated the efficacy of BMP-2 release from different scaffold based systems for bone tissue engineering applications.146–148
Figure 8.
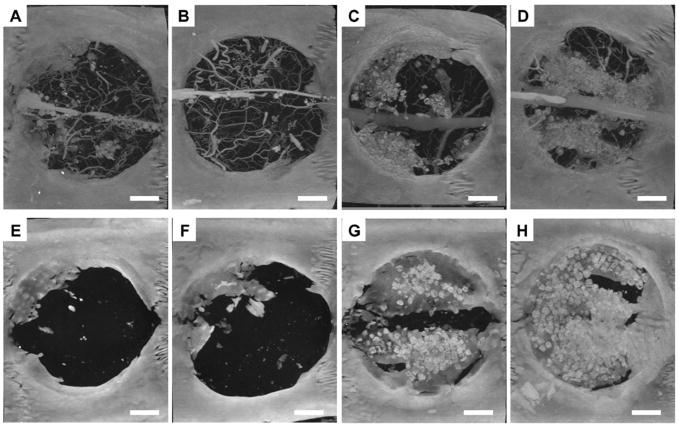
Microcomputed tomography images (maximum intensity projections) of cranial defects taken at 4 and 12 weeks after implantation. Panels A-D represent scaffolds releasing no growth factor (control), VEGF, BMP-2, and both VEGF and BMP-2 respectively at 4 weeks prior to decalcification. Blood vessels were filled with a silicon based radiopaque material so that both blood vessels and mineralized tissue are visible. Panels E–H represent scaffolds releasing no growth factor (control), VEGF, BMP-2, and both VEGF and BMP-2 respectively at 12 weeks; no blood vessels were visible because perfusion with the radiopaque material was not done at this time point. Bar represents 200 μm for all panels. Scaffolds releasing both growth factors exhibited significantly greater amounts of bone-like tissue regeneration after 4 weeks but were not significantly different from scaffolds releasing BMP-2 only after 12 weeks. Reprinted with permission from (27).
Work in our laboratory elucidating the release of angiogenic and osteogenic growth factors during bone healing did not begin with the study of VEGF and BMP-2 release. Prior work studied the release of TP508, an angiogenic and osteogenic 23 amino acid peptide sequence derived from thrombin.149 TP508 released from PPF-based composite scaffolds enabled healing of segmental long bone defects in rabbits, and we found that the release kinetics of the peptide were of critical importance to bone healing, with a burst release of the peptide facilitating bony bridging in over 80% of the defects after 12 weeks.150 More recent work in this area in collaboration with Dr. Achim Goepferich of the University of Regensburg in Germany has studied whether amino acid or bisphosphonate modification can target peptides selectively to bony sites, possibly decreasing necessary dosing by improving the efficiency of delivery.151
Biomimetic Hydrogels
Peptide sequences have been utilized in our laboratory for purposes other than controlled release to stimulate tissue regeneration. Many of our aforementioned studies of material-cell interactions noted varying levels of cell attachment based on material properties, most notably decreases in cell attachment as the hydrophilicity of OPF and P(PF-co-EG) hydrogels increased. In addition to modulating material-cell interactions by varying material compositions, surface patterning of materials was previously used in our laboratory to regulate RPE attachment and morphology.152, 153 Cyclic Arg-Gly-Asp (RGD) peptide sequences, long known to bind surface integrins necessary for cell attachment and migration in vivo, were first used in our laboratory to block integrin domains to study growth factor stimulated migration.154 A later series of work built upon our previous work in surface patterning and also the relationship of RGD to cell attachment by modifying hydrogels with RGD sequences to promote cell attachment and allow the hydrophilic materials to be more suitable for cell-based therapies and tissue regeneration.
Using 4-nitrophenyl chloroformate as an activator, OPF surfaces were modified by covalently attaching Gly-Arg-Gly-Asp (GRGD) peptides.155 Later incorporating a PEG spacer to avoid steric obscuration of the peptide, we found that marrow stromal cells adhered significantly better to GRGD-modified OPF hydrogels, and the sequence specific role of GRGD was confirmed by competitive inhibition of cell attachment following incubation with free GRGD.156 RGDS modification of P(PF-co-EG) hydrogels found concentration dependent attachment of marrow derived osteoblasts.157 While other work investigating surface modification of hydrogels to improve cell attachment using agmatine118, 158, 159 and osteopontin derived peptides,160 an important discovery that followed our ongoing work in the area of bioreactors was the role surface peptide sequences played towards influencing cell differentiation. RGD-modified hydrogels were capable of inducing progenitor cells to differentiate down an osteoblastic lineage, even in the absence of culture supplements normally required for differentiation.161, 162
Generated Matrix with Bioreactors
Flow perfusion bioreactor systems
The seemingly anomalous decrease in bone mineral density found in astronauts returning from prolonged missions in space163 coupled with the prevention of this bone loss by repeated mechanical loading164 are indicative of the importance of mechanical stimulation on the maintenance of healthy bone tissue. Comparing a spinner flask, rotary vessel, and flow perfusion bioreactor, we began investigating the effects of convection on bone growth within biomaterial scaffolds, surmising that convection of media could both overcome diffusional limitations encountered during static culture of large scaffolds and provide a mechanical stimulus possible of augmenting bone formation.165, 166 Flow perfusion systems were determined to be the most appropriate method for achieving these effects165 and designed specifically for bone tissue engineering applications within our laboratory.167
When seeded on titanium fiber mesh scaffolds within a flow perfusion bioreactor, marrow stromal cells differentiate into osteoblast-like cells and deposit far greater mineralized matrix than cells in constructs cultured under static conditions (Figure 9).20, 168 Similar results were observed using PLLA fiber mesh scaffolds,169 starch based scaffolds,170 and CaP scaffolds.171 Furthermore, by varying the fluid viscosity such that fluid shear stress could be varied but transport of nutrients and removal of waste remained constant, we found that mineral deposition increased and extracellular matrix distribution was more even, revealing a dose dependent relationship between shear stresses and mineralized tissue generation.172 In vivo implantation of cell-scaffold constructs following culture in a flow perfusion bioreactor showed the constructs to be osteoinductive and that the in vitro culture period can affect in vivo outcomes following implantation.173
Figure 9.
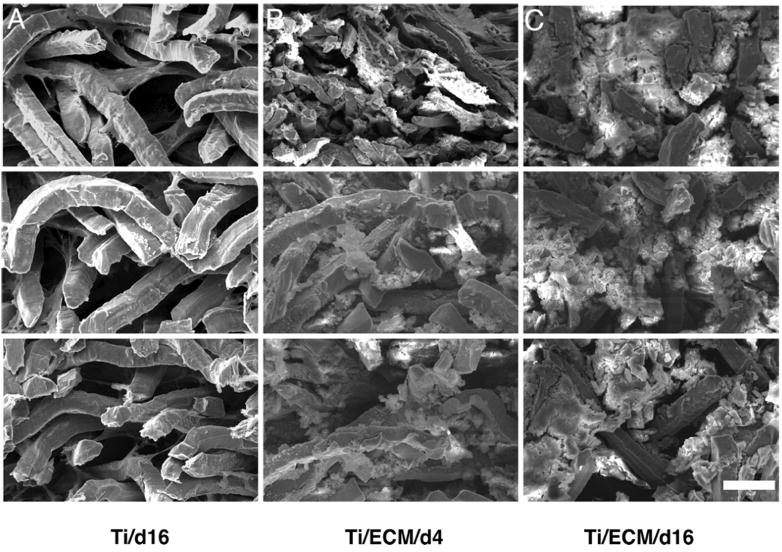
SEM images obtained from the cross-sections of 0.8 mm thick constructs cultured in vitro in a flow-perfusion bioreactor. Each image consists of three panels. (Top) One-hundred micrometers from the top of the construct. (Middle) The middle of the construct. (Bottom) One-hundred micrometers from the bottom surface. These images show the plain Ti construct after 16 days of culture (A), the Ti/ECM construct after 4 days of culture (B), and the Ti/ECM construct after 16 days of culture (C). (Scale bar, 50 μm for all SEM images.) Reprinted with permission from (178).
Continuing work using the flow perfusion bioreactor system determined a time dependent affect of titanium fiber network mesh size on cell differentiation and matrix deposition,174 and increasing the overall porosity of degradable starch based scaffolds yielded significantly greater matrix deposition and cell proliferation when cultured under flow conditions.175 In the absence of culture in the flow perfusion system, implantation of undifferentiated stromal cells on titanium fiber meshes led to poor bone regeneration when compared to cells precultured in dexamethasone, a factor known to induce osteogenic differentiation of MSCs.176 These results showed that without flow perfusion, culture on titanium fiber meshes, even in the presence of RGD peptide to facilitate cell attachment, was not sufficient to differentiate cells to an extent at which bone regeneration in vivo was similar to that of constructs seeded with differentiated cells.
In vitro generated ECM
The presence of fluid shear stresses within the flow perfusion bioreactor, however, was found to be sufficient to induce osteoblast-like differentiation of marrow stromal cells in the absence of dexamethasone.177 While dexamethasone and shear stresses in tandem have a synergistic effect on cell differentiation, a series of pivotal studies determined the utility of culture within the flow perfusion bioreactor towards differentiating cells in the absence of media supplements.
Titanium fiber meshes seeded with marrow stromal cells were cultured for 12 days in the flow perfusion system to generate bone-like extracellular matrix (ECM), after which the scaffolds were decellularized using repeated freeze-thaw cycles. Subsequent culture of MSCs in the absence of dexamethasone on scaffolds with previously deposited ECM showed a 40-fold increase in mineralization compared with control scaffolds. Similar increases were found compared to scaffolds with denatured ECM. The presence of dexamethasone further enhanced matrix deposition; however, this work demonstrated the powerful capacity of flow and in vitro generated ECM to guide progenitor cells down an osteogenic lineage (Figure 10).178 Previous work had shown that ECM/titanium composites enhanced differentiation of MSCs cultured statically,179 but the drastic increases in matrix deposition in the absence of dexamethasone were most apparent when ECM was combined with mechanical forces imparted by the flow perfusion system. Localization of growth factors following flow perfusion on starch-based scaffolds found TGF-β1, fibroblast growth factor, VEGF, and BMP-2 deposited on scaffolds cultured under flow, with increases in deposition area found following culture at higher flow rates.180 In vivo implantation of ECM/titanium composite scaffolds revealed an increase in scaffold vascularity corresponding to increases in the amount of deposited matrix present.181 Marrow stromal cells cultured on matrices with previously deposited ECM showed significant increases in osteoblast-specific genes, likely due to the prior deposition of growth factors and matrix molecules by cells during the original perfusion induced mineralization.182 Using electrospinning, we have also fabricated and characterized poly(ε-caprolactone) fiber mesh scaffolds with mixed micro- and nanofiber layers to simulate the scale of the ECM, and this physical mimicry of the ECM facilitated increased cell spreading compared to scaffolds without the nanofiber domains (Figure 11).51
Figure 10.
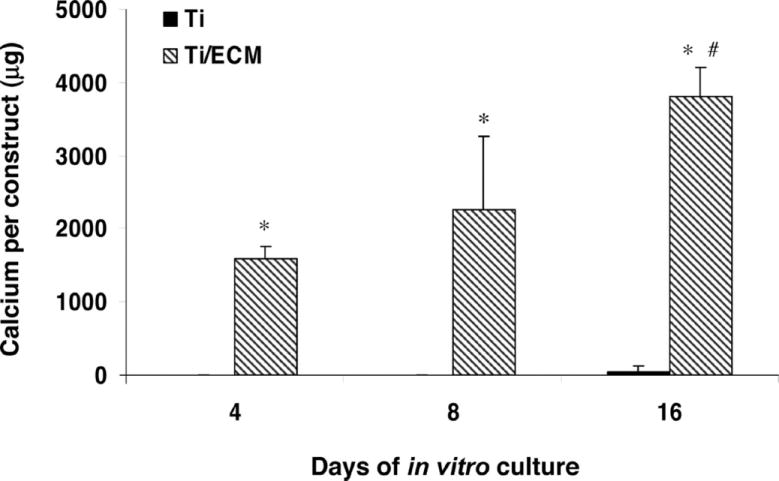
The calcium content of Ti and Ti/ECM constructs cultured in the flow-perfusion bioreactor after 4, 8, and 16 days of culture. The data represent means of four samples, with the error bars representing the standard deviations. Statistical differences (P < 0.05) between Ti and Ti/ECM constructs are indicated with an asterisk; # designates a statistical difference (P < 0.05) between all other data points. Reprinted with permission from (178).
Figure 11.
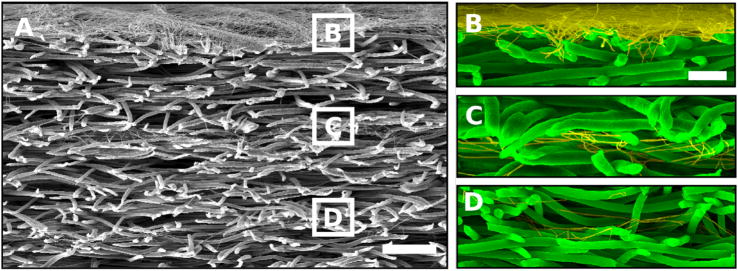
Scanning electron micrographs of cross-sections of layered scaffolds generated by sequential electrospinning. (A) Cross-section illustrating (from top to bottom) a nano-micro-nano-micro-nano-microfiber layered scaffold. The white boxes correspond to the nanofiber layers and their respective magnified images shown to the right. (B) Magnification of the nanolayer electrospun for 5 min. (C) Magnification of the nanolayer electrospun for 90 s. (D) Magnification of the nanolayer electrospun for 30 s. The scale bar shown for (A) is 100 μm, and for (B–D) it is 25 μm. Microfibers are false colored green while nanofibers are false colored yellow to enhance contrast. Reprinted with permission from (51). Copyright (2006) American Chemical Society.
Injectable Plasmid DNA Carriers
Tuning of material properties, growth factor delivery, culture in supplemented media, and induction via exposure to generated extracellular matrix have all been demonstrated as viable methods for influencing cell behavior and specifically for promoting the differentiation of progenitor cells down tissue specific lineages. All of these approaches have indirect effects that likely influence or regulate gene transcription and/or expression. More directly towards this end, we have also investigated material mediated methods for the delivery of plasmid DNA. Our earliest work related to this area used PLGA microparticles to deliver antisense oligodeoxy-nucleotides to inhibit smooth muscle cell proliferation and migration, phenomena which are among the leading causes of restenosis after vascular intervention.183
Poly(ethylenimine)/DNA complexes
Subsequent studies focused on the delivery of plasmid DNA using the polycation poly(ethylenimine) (PEI) as a delivery vehicle. Modification of standard PEI/DNA complex delivery ameliorated PEI toxicity and improved packing of PEI around plasmid DNA. The end result was improvement in PEI transfection efficiency from 37% to 53%.184 PEI size matters as well; transfection efficiency using green fluorescent protein transfection of human endothelial cells increased with increasing PEI molecular weight. PEI with molecular weights of 70,000 Da successfully transfected 25.6% of cells, while PEI with molecular weights under 1,800 Da did not transfect any cells.185 A mechanistic study of PEI transfection revealed the pathway of transfection from cellular uptake via endocytosis through nuclear localization and transfection (Figure 12).186 This study also found that PEI, independently of complexation with DNA, localizes to the nucleus through the same pathway, and a later study determined that contrary to the commonly held hypothesis that endocytosed PEI merges with lysosomes, endocytosed PEI enters the nucleus without binding to lysosomes.187 When used to transfect endothelial cells with genes encoding three naturally expressed gene products, we found a bimodally distributed pattern of cell death, attributable to death from free PEI and PEI/DNA complexes, although transfection was successful and translation increased.188 More recently we have used branched PEI conujugated with hyaluronic acid to improve cell viability and transfection efficiency over branched PEI alone.189
Figure 12.
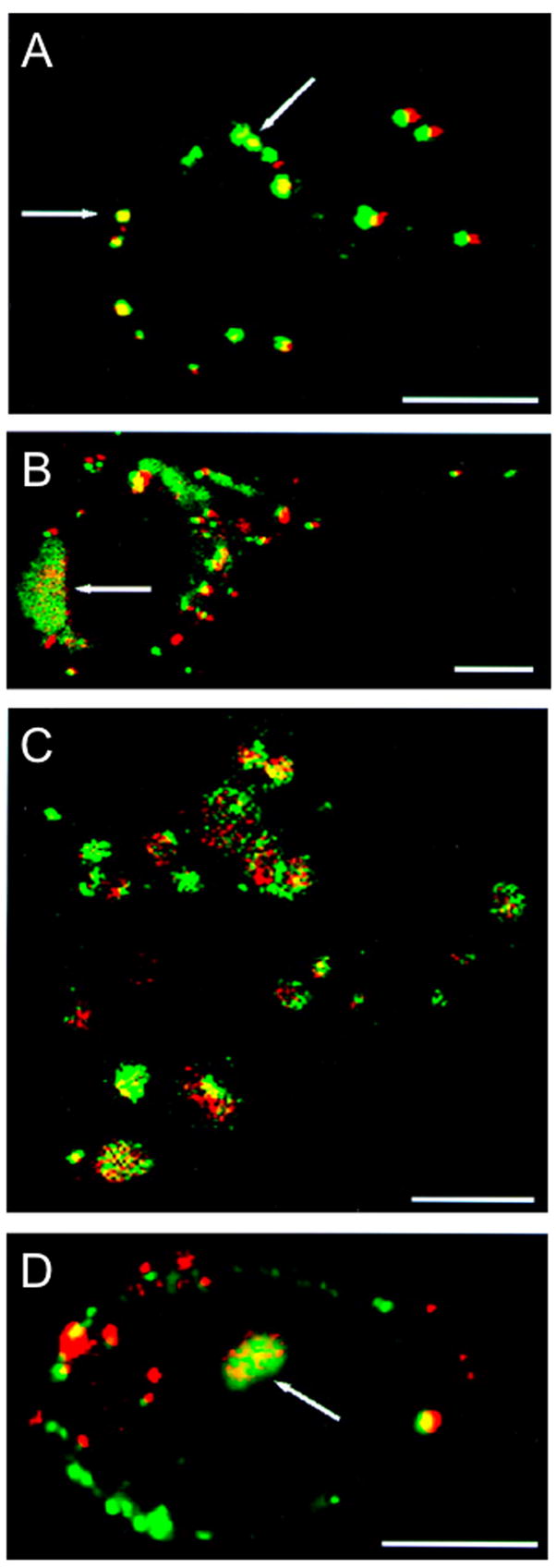
Tracking of double-labeled PEI/DNA complexes. The fluorescence patterns for single-labeled complexes are also seen for double-labeled complexes. (a) At 2 hours post-transfection, visible complexes appear as clumps on the exterior of cells, as indicated by arrows. (b) At 3 hours post-transfection, both surface aggregation of complexes and endosomal formation (indicated by an arrow) are visible. The arrow indicates endosomal formation. (c) At 4 hours post-transfection, endosomes containing both PEI and DNA are visible throughout the cell cytoplasm. (d) At 4.5 hours post-transfection, fluorescent structures containing both PEI and DNA inside the cell nucleus are present, as indicated by the arrow. (Bar = 10 μm.) Reprinted with permission from (186).
Gene transfection for tissue engineering applications
In addition to polycationic gene delivery using PEI as a model delivery vehicle, we have also studied more conventional methods of gene transfection for tissue engineering. In the first study comparing transfection vectors for the delivery of osteogenic genes, adenoviral, retroviral, and cationic lipid vectors were used to transfect rat marrow stromal cells with the gene encoding human BMP-2. Both in vitro and in vivo, cells transfected with adenoviral vectors displayed enhanced osteogenic and bone healing potential versus those transfected with other vectors and controls.190 Culture in dexamethasone led to a 3-fold increase in transgene expression over culture in nonsupplemented media when using adenoviral vectors but did not increase transgene expression from other vector types.191
Sustained plasmid DNA release from gelatin microspheres embedded within OPF hydrogels was studied for use in bone tissue engineering applications. Entrapment within cationized gelatin microspheres and subsequent embedding within OPF extended DNA bioavailability, and release was primarily mediated by OPF degradation.192–194 Attempts to regenerate critical-sized bone defects in vivo using plasmid DNA encoding BMP-2 released from gelatin microparticles was unsuccessful compared to controls, perhaps due to poor release or most likely the lack of a vector.195 Future improvements to the scaffold based delivery system, such as incorporation of adenoviral or PEI based vectors, may improve tissue regeneration; however, the vector technologies developed hold great potential in future applications.
Enabling Technologies and Translational Approaches
As the fields of tissue engineering and regenerative medicine evolve, need arises for reliable tools to evaluate emerging technologies. Although reporting of specific combinations of cells, bioactive factors, and biomaterials as a whole help to advance the field, the emergence of new techniques in material and scaffold fabrication, drug and cell delivery, and the interaction of these factors continue to be the driving forces pushing strategies closer to the clinic. While difficult to specifically categorize, our work in enabling technologies and new approaches to addressing problems that potentially can be solved by tissue engineering have continued relevance today. Work in the areas of cell culture techniques196, 197, nanomaterials for high resolution MRI,198 and evaluation of scaffold and cellular technologies199, 200 have proven useful for the advancement of the field. Strategies similar to those based on cell lines created through our collaborations201 have been used to evaluate new strategies in tissue engineering.202, 203 The development of animal models204–206 and modalities to evaluate tissue regeneration within those models207 will continue to be necessary as expansion into engineering a variety of tissues and multiple tissues occurs. Through collaboration with Dr. Mark Wong of the University of Texas Health Science Center Dental Branch in Houston, we have recently developed a model to investigate alveolar bone regeneration within a non-healing defect in the rabbit mandible,204 and through this collaboration we continue to explore further clinically relevant animal models and tissue engineering strategies. An animal model developed early in the course of studies in our laboratory represents a different in vivo approach; rather than the evaluation of tissue engineering strategies, a sheep model for bone flap creation was developed to provide transplantable vascularized bone flaps.208
The sheep model developed in collaboration with Drs. Michael J. Miller and Alan W. Yasko, now at The Ohio State University and Northwestern University’s Feinberg School of Medicine respectively, utilized a seldom used but clinically relevant approach to tissue engineering.208 Rather than the more common approach of regenerating tissues at the site where they are needed and a defect exists, PMMA chambers filled with morcellized bone graft were implanted adjacent to the ribs of a sheep, with the chamber open to the rib periosteum. New, well formed bone penetrated the implants after 6 weeks, and removal of the newly formed bone with an attached vascular pedicle yielded a vascularized implant that could be used for autologous tissue augmentation at a distant defect site (Figure 13). PLGA foam filled chambers were also investigated for use in this technique.209 Recently, Cheng et al. applied a similar technique to reconstruct the mandible of a patient with a locally invasive squamous cell carcinoma, and after one year the implanted bone flap remained viable such that dental implants could be placed within it, allowing a near complete reconstruction of the patient’s mandible.210 Strategies such as this may prove useful in treating traumatic injuries where inflammation or localized infection may require treatment before attempts at tissue reconstruction or regeneration can be made.
Figure 13.
(A) Molded block of bone harvested after 6 weeks conforming to a 10 × 40 × 10-mm PMMA chamber and attached to the vascularized periosteal bed. Note the polytetrafluoroethylene (PTFE) cuff bonded to the perimeter of the chamber, used to sew the implant to the periosteum. Reprinted with permission from (208). (B) Molded bone flap attached to vascularized pedicle (artery and vein) removed from a 10 × 40 × 10-mm chamber implanted for 6 weeks. Reprinted from (211) with permission of John Wiley & Sons, Inc., Copyright © (1998).
Future Directions in Biomaterials Science and Tissue Engineering
Within the fields of biomaterials and tissue engineering, a number of emerging and recent trends will likely shape the future and lead to even greater successes in both the laboratory and also the clinic. Tissue engineering has always been tied to discoveries in a number of diverse fields, and this trend will continue.
From biology, greater knowledge about the role stem cells play in tissue regeneration and healing will play a pivotal role in translating tissue engineering technologies into the clinic. As we better understand the capabilities and limitations of these progenitor cells, as well as gain better understanding of the extra- and intracellular pathways that determine their differentiation in vivo, researchers in biomaterials science and tissue engineering will be able to fabricate better materials upon which stem cells can proliferate and differentiate, devise more tailored cell delivery systems, and understand the applications for which these cells are best suited. Similarly, an increased knowledge of the role more diffuse biological processes, such as inflammation and development, play in tissue regeneration will allow future work to focus on crafting materials and identifying bioactive factor delivery regimes to modulate, recreate, or exploit these natural phenomena.
Materials science and the specific field of biomaterials science will continue to develop under a reciprocal exchange of ideas and new technologies. As nanotechnology continues to develop and we better appreciate and understand the importance of nanoscale stimuli and interactions in biology, biomaterials with tailored nanoscale properties will be fabricated and used to mimic, stimulate, and augment biological processes. Given the clinical success of many growth factors and growth factor delivery systems, spatiotemporal control of bioactive molecule release and the release of multiple factors simultaneously and in series to mimic useful biological cascades must be explored.
Finally, in the near future we predict advances in two broad areas. The first, as described above, will be advances in our understanding of natural biological processes critical to tissue engineering, the integration of this understanding into specific tissue engineering technologies, and a continued pursuit of new biomaterial technologies integrating advances in chemistry, biology, and materials science. The second area for major advancement will be in harnessing existing technologies for clinical use. Motivated by the clinical success of products related to tissue engineering, clinicians and researchers are now gaining a better understanding of the toolbox available to them in the form of FDA regulated technologies. Even as better, more application-specific approaches are being developed and in their early stages, significant translational advances will come from novel integration of existing technologies. These advances will be the nidus for bringing new and better technologies in tissue engineering through the regulatory process and will guarantee a continued commitment to and recognition of biomaterials and tissue engineering as viable and promising fields with a truly impactful scope.
Summary
From beginnings involved in the somewhat disparate studies of synthesizing and characterizing new biomaterials and developing new technologies and techniques for tissue engineering based on existing materials, work in our laboratory has evolved over the last 16 years to encompass scaffold fabrication and tissue regeneration using novel biomaterials, drug, DNA, and growth factor delivery, bioreactor technology and bioactive ECM generation, and the development of animal models for the evaluation and development of tissue engineering constructs. During this time, biomaterials science has similarly expanded to include many sophisticated technologies and methods from the pure biological sciences as well as the emerging nanosciences, and advances in tissue engineering have followed progress in these fields.
Our laboratory specifically has developed tissue engineering approaches using fumarate-based materials. Working with PPF, we have developed a variety of methods towards ex vivo fabrication of porous hydrophobic scaffolds with properties suitable for bone tissue engineering. PPF, as well as OPF and P(PF-co-EG), has been used as an injectable, in situ cross-linkable material, and we continue to investigate injectable materials for tissue engineering and cell delivery. The model hydrogel OPF has been examined to elucidate the interplay between hydrophobic and hydrophilic domains and their interaction with cells. Modification of OPF to mimic naturally occurring substrates encouraged cell attachment, and cartilage tissue engineering through the incorporation of cells and growth factors in OPF has been extensively investigated. We continue studying the delivery of growth factors for bone tissue engineering, building upon the use of flow perfusion generated ECM and gene delivery strategies for the overexpression of osteogenic factors, and the impact of our early work in scaffold fabrication and bone flap generation is widely implemented in research throughout the field and in preliminary clinical applications. Finally, areas for future growth and promising advances within the field have been described, and we expect continued success for and realization of the promise of biomaterials and tissue engineering.
Acknowledgments
We acknowledge financial support by the National Institutes of Health, National Science Foundation, NASA, Whitaker Foundation, and other agencies and companies for research towards the development of new biomaterials (AGM). We thank all the current and past undergraduate students, graduate students, and postdoctoral fellows for their hard work during the past 16 years. We also thank our colleagues at Rice University and our collaborators around the world for their significant contributions over the years. JDK acknowledges support by the National Institutes of Health and Rice University Institute of Biosciences and Bioengineering through the Biotechnology Training Grant (T32 GM008362). JDK is a student in the Baylor College of Medicine Medical Scientist Training Program and was also supported by the National Institute of General Medical Sciences (T32 GM007330).
Abbreviations
- ALP
alkaline phosphatase
- APS
ammonium persulfate
- BAPO
bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide
- BMP-2
bone morphogenetic protein-2
- BP
benzoyl peroxide
- βTCP
β-tricalcium phosphate
- CaP
calcium phosphate
- ECM
extracellular matrix
- FDA
Food and Drug Administration
- FGF-2
fibroblast growth factor-2
- GRGD
Gly-Arg-Gly-Asp
- IGF-1
insulin-like growth factor-1
- MSC
mesenchymal stem cell
- OPF
oligo(poly(ethylene glycol) fumarate)
- PEG
poly(ethylene glycol)
- PEG-DMA
poly(ethylene glycol)-dimethacrylate
- PEI
poly(ethylenimine)
- PF-DA
propylene fumarate-diacrylate
- PLA
poly(D,L-lactic acid)
- PLGA
poly(D,L-lactic-co-glycolic acid)
- PLLA
poly(L-lactic acid)
- PMMA
poly(methyl methacrylate)
- P(PF-co-EG)
poly(propylene fumarate-co-ethylene glycol)
- PPF
poly(propylene fumarate)
- PTFE
polytetrafluoroethylene
- RGD
Arg-Gly-Asp
- RPE
retinal pigment epithelium
- SWNT
single-walled carbon nanotube
- TGF-β1
transforming growth factor-β1
- TP508
thrombin peptide 508
- VEGF
vascular endothelial growth factor
Footnotes
This invited contribution presents a 16-year perspective of the research conducted in the laboratory of Professor Antonios G. Mikos of Rice University in the biomaterials and tissue engineering fields. Professor Mikos was the recipient of the 2007 Alpha Chi Sigma Award for Chemical Engineering Research of the American Institute of Chemical Engineers.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Hacker MC, Mikos AG. Trends in tissue engineering research. Tissue Eng. 2006;12(8):2049–2057. doi: 10.1089/ten.2006.12.2049. [DOI] [PubMed] [Google Scholar]
- 3.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263(5154):1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 4.Peppas NA, Langer R. Origins and development of biomedical engineering within chemical engineering. AIChE Journal. 2004;50(3):536–546. [Google Scholar]
- 5.Eaglstein WH, Falanga V. Tissue engineering and the development of Apligraf, a human skin equivalent. Clin Ther. 1997;19(5):894–905. doi: 10.1016/s0149-2918(97)80043-4. [DOI] [PubMed] [Google Scholar]
- 6.Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27(21):2396–2408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Purdue GF, Hunt JL, Still JM, Jr, Law EJ, Herndon DN, Goldfarb IW, Schiller WR, Hansbrough JF, Hickerson WL, Himel HN, Kealey GP, Twomey J, Missavage AE, Solem LD, Davis M, Totoritis M, Gentzkow GD. A multicenter clinical trial of a biosynthetic skin replacement, Dermagraft-TC, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil. 1997;18(1 Pt 1):52–57. doi: 10.1097/00004630-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Suppl 1Pt 2):S151–158. [PMC free article] [PubMed] [Google Scholar]
- 9.Vaccaro AR, Anderson DG, Patel T, Fischgrund J, Truumees E, Herkowitz HN, Phillips F, Hilibrand A, Albert TJ, Wetzel T, McCulloch JA. Comparison of OP-1 Putty (rhBMP-7) to iliac crest autograft for posterolateral lumbar arthrodesis: a minimum 2-year follow-up pilot study. Spine. 2005;30(24):2709–2716. doi: 10.1097/01.brs.0000190812.08447.ba. [DOI] [PubMed] [Google Scholar]
- 10.Vaccaro AR, Patel T, Fischgrund J, Anderson DG, Truumees E, Herkowitz HN, Phillips F, Hilibrand A, Albert TJ, Wetzel T, McCulloch JA. A pilot study evaluating the safety and efficacy of OP-1 Putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885–1892. doi: 10.1097/01.brs.0000137062.79201.98. [DOI] [PubMed] [Google Scholar]
- 11.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. The Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 12.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) www.hcup-us.ahrq.gov/nisoverview.jsp.
- 13.Buck BE, Malinin TI, Brown MD. Bone Transplantation and Human Immunodeficiency Virus: An Estimate of Risk of Acquired Immunodeficiency Syndrome (AIDS) Clin Orthop Relat Res. 1989;240:129–136. [PubMed] [Google Scholar]
- 14.Last chance to stop and think on risks of xenotransplants. Nature. 1998;391(6665):320–324. doi: 10.1038/34749. [DOI] [PubMed] [Google Scholar]
- 15.Kessler P, Thorwarth M, Bloch-Birkholz A, Nkenke E, Neukam FW. Harvesting of bone from the iliac crest--comparison of the anterior and posterior sites. Br J Oral Maxillofac Surg. 2005;43(1):51–56. doi: 10.1016/j.bjoms.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, Vaccaro AR, Albert TJ. Donor Site Morbidity After Anterior Iliac Crest Bone Harvest for Single-Level Anterior Cervical Discectomy and Fusion. Spine. 2003;28(2):134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann C, Borner B, Hasse A, Sieg P. Donor site morbidity after microvascular fibula transfer. Clin Oral Investig. 2001;5(4):214–219. doi: 10.1007/s00784-001-0140-5. [DOI] [PubMed] [Google Scholar]
- 18.Mofid MM, Inoue N, Atabey A, Marti G, Chao EY, Manson PN, Vander Kolk CA. Callus stimulation in distraction osteogenesis. Plast Reconstr Surg. 2002;109(5):1621–1629. doi: 10.1097/00006534-200204150-00020. [DOI] [PubMed] [Google Scholar]
- 19.Mauney JR, Sjostorm S, Blumberg J, Horan R, O’Leary JP, Vunjak-Novakovic G, Volloch V, Kaplan DL. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74(5):458–468. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 20.Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99(20):12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Botchwey EA, Dupree MA, Pollack SR, Levine EM, Laurencin CT. Tissue engineered bone: measurement of nutrient transport in three-dimensional matrices. J Biomed Mater Res A. 2003;67(1):357–367. doi: 10.1002/jbm.a.10111. [DOI] [PubMed] [Google Scholar]
- 23.Malda J, Klein TJ, Upton Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007;13(9):2153–2162. doi: 10.1089/ten.2006.0417. [DOI] [PubMed] [Google Scholar]
- 24.Mikos AG, Sarakinos G, Lyman MD, Ingber DE, Vacanti JP, Langer R. Prevascularization of porous biodegradable polymers. Biotechnol Bioeng. 1993;42(6):716–723. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 25.Wake MC, Patrick CW, Jr, Mikos AG. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994;3(4):339–343. doi: 10.1177/096368979400300411. [DOI] [PubMed] [Google Scholar]
- 26.Wake MC, Mikos AG, Sarakinos G, Vacanti JP, Langer R. Dynamics of fibrovascular tissue ingrowth in hydrogel foams. Cell Transplant. 1995;4(3):275–279. doi: 10.1177/096368979500400305. [DOI] [PubMed] [Google Scholar]
- 27.Patel ZS, Young S, Tabata Y, Jansen JA, Wong M, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008 doi: 10.1016/j.bone.2008.06.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klouda L, Kretlow JD, Mikos A. Tailored Biomaterials for Tissue Engineering Needs and Their Clinical Translation. In: Mao JJ, Vunjak-Novakovic G, Mikos AG, Atala A, editors. Translational Approaches in Tissue Engineering and Regenerative Medicine. Boston, MA: Artech House; 2008. pp. 325–337. [Google Scholar]
- 29.Mikos AG, Bao Y, Cima LG, Ingber DE, Vacanti JP, Langer R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J Biomed Mater Res. 1993;27(2):183–189. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- 30.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14(5):323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 31.Mikos AG, Lyman MD, Freed LE, Langer R. Wetting of poly(L-lactic acid) and poly(DL-lactic-co-glycolic acid) foams for tissue culture. Biomaterials. 1994;15(1):55–58. doi: 10.1016/0142-9612(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 32.Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, Vacanti JP. Preparation and characterization of poly(L-lactic acid) foams. Polymer. 1994;35(5):1068–1077. [Google Scholar]
- 33.Ishaug SL, Yaszemski MJ, Bizios R, Mikos AG. Osteoblast function on synthetic biodegradable polymers. J Biomed Mater Res. 1994;28(12):1445–1453. doi: 10.1002/jbm.820281210. [DOI] [PubMed] [Google Scholar]
- 34.Thomson RC, Giordano GG, Collier JH, Ishaug SL, Mikos AG, Lahiri-Munir D, Garcia CA. Manufacture and characterization of poly(alpha-hydroxy ester) thin films as temporary substrates for retinal pigment epithelium cells. Biomaterials. 1996;17(3):321–327. doi: 10.1016/0142-9612(96)85570-0. [DOI] [PubMed] [Google Scholar]
- 35.Lu L, Garcia CA, Mikos AG. Retinal pigment epithelium cell culture on thin biodegradable poly(DL-lactic-co-glycolic acid) films. J Biomater Sci Polym Ed. 1998;9(11):1187–1205. doi: 10.1163/156856298x00721. [DOI] [PubMed] [Google Scholar]
- 36.von Recum HA, Cleek RL, Eskin SG, Mikos AG. Degradation of polydispersed poly(L-lactic acid) to modulate lactic acid release. Biomaterials. 1995;16(6):441–447. doi: 10.1016/0142-9612(95)98816-w. [DOI] [PubMed] [Google Scholar]
- 37.Wake MC, Gerecht PD, Lu L, Mikos AG. Effects of biodegradable polymer particles on rat marrow-derived stromal osteoblasts in vitro. Biomaterials. 1998;19(14):1255–1268. doi: 10.1016/s0142-9612(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 38.Lu L, Peter SJ, Lyman MD, Lai HL, Leite SM, Tamada JA, Vacanti JP, Langer R, Mikos AG. In vitro degradation of porous poly(L-lactic acid) foams. Biomaterials. 2000;21(15):1595–1605. doi: 10.1016/s0142-9612(00)00048-x. [DOI] [PubMed] [Google Scholar]
- 39.Lu L, Peter SJ, Lyman MD, Lai HL, Leite SM, Tamada JA, Uyama S, Vacanti JP, Langer R, Mikos AG. In vitro and in vivo degradation of porous poly(DL-lactic- co-glycolic acid) foams. Biomaterials. 2000;21(18):1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 40.Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J Biomater Sci Polym Ed. 1995;7(1):23–38. doi: 10.1163/156856295x00805. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q, Hedberg EL, Liu Z, Bahulekar R, Meszlenyi RK, Mikos AG. Preparation of macroporous poly(2-hydroxyethyl methacrylate) hydrogels by enhanced phase separation. Biomaterials. 2000;21(21):2163–2169. doi: 10.1016/s0142-9612(00)00137-x. [DOI] [PubMed] [Google Scholar]
- 42.Wake MC, Gupta PK, Mikos AG. Fabrication of pliable biodegradable polymer foams to engineer soft tissues. Cell Transplant. 1996;5(4):465–473. doi: 10.1177/096368979600500405. [DOI] [PubMed] [Google Scholar]
- 43.Widmer MS, Gupta PK, Lu L, Meszlenyi RK, Evans GR, Brandt K, Savel T, Gurlek A, Patrick CW, Jr, Mikos AG. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 1998;19(21):1945–1955. doi: 10.1016/s0142-9612(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 44.Evans GR, Brandt K, Widmer MS, Lu L, Meszlenyi RK, Gupta PK, Mikos AG, Hodges J, Williams J, Gurlek A, Nabawi A, Lohman R, Patrick CW., Jr In vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials. 1999;20(12):1109–1115. doi: 10.1016/s0142-9612(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 45.Evans GR, Brandt K, Katz S, Chauvin P, Otto L, Bogle M, Wang B, Meszlenyi RK, Lu L, Mikos AG, Patrick CW., Jr Bioactive poly(L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002;23(3):841–848. doi: 10.1016/s0142-9612(01)00190-9. [DOI] [PubMed] [Google Scholar]
- 46.Habraken WJ, de Jonge LT, Wolke JG, Yubao L, Mikos AG, Jansen JA. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31703. In press. [DOI] [PubMed] [Google Scholar]
- 47.Habraken WJ, Zhang Z, Wolke JG, Grijpma DW, Mikos AG, Feijen J, Jansen JA. Introduction of enzymatically degradable poly(trimethylene carbonate) microspheres into an injectable calcium phosphate cement. Biomaterials. 2008;29(16):2464–2476. doi: 10.1016/j.biomaterials.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Habraken WJ, Wolke JG, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2006;17(9):1057–1074. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 49.Christenson EM, Soofi W, Holm JL, Cameron NR, Mikos AG. Biodegradable fumarate-based polyHIPEs as tissue engineering scaffolds. Biomacromolecules. 2007;8(12):3806–3814. doi: 10.1021/bm7007235. [DOI] [PubMed] [Google Scholar]
- 50.Hacker M, Ringhofer M, Appel B, Neubauer M, Vogel T, Young S, Mikos AG, Blunk T, Gopferich A, Schulz MB. Solid lipid templating of macroporous tissue engineering scaffolds. Biomaterials. 2007;28(24):3497–3507. doi: 10.1016/j.biomaterials.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 52.Yaszemski MJ, Payne RG, Hayes WC, Langer RS, Aufdemorte TB, Mikos AG. The Ingrowth of New Bone Tissue and Initial Mechanical Properties of a Degrading Polymeric Composite Scaffold. Tissue Eng. 1995;1(1):41–52. doi: 10.1089/ten.1995.1.41. [DOI] [PubMed] [Google Scholar]
- 53.Peter SJ, Yaszemski MJ, Suggs LJ, Payne RG, Langer R, Hayes WC, Unroe MR, Alemany LB, Engel PS, Mikos AG. Characterization of partially saturated poly(propylene fumarate) for orthopaedic application. J Biomater Sci Polym Ed. 1997;8(11):893–904. doi: 10.1163/156856297x00074. [DOI] [PubMed] [Google Scholar]
- 54.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. In vitro degradation of a poly(propylene fumarate)-based composite material. Biomaterials. 1996;17(22):2127–2130. doi: 10.1016/0142-9612(96)00008-7. [DOI] [PubMed] [Google Scholar]
- 55.Dean D, Topham NS, Meneghetti SC, Wolfe MS, Jepsen K, He S, Chen JE, Fisher JP, Cooke M, Rimnac C, Mikos AG. Poly(propylene fumarate) and poly(DL-lactic-co-glycolic acid) as scaffold materials for solid and foam-coated composite tissue-engineered constructs for cranial reconstruction. Tissue Eng. 2003;9(3):495–504. doi: 10.1089/107632703322066679. [DOI] [PubMed] [Google Scholar]
- 56.Hedberg EL, Kroese-Deutman HC, Shih CK, Crowther RS, Carney DH, Mikos AG, Jansen JA. In vivo degradation of porous poly(propylene fumarate)/poly(DL-lactic-co-glycolic acid) composite scaffolds. Biomaterials. 2005;26(22):4616–4623. doi: 10.1016/j.biomaterials.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 57.Hedberg EL, Shih CK, Lemoine JJ, Timmer MD, Liebschner MA, Jansen JA, Mikos AG. In vitro degradation of porous poly(propylene fumarate)/poly(DL-lactic-co-glycolic acid) composite scaffolds. Biomaterials. 2005;26(16):3215–3225. doi: 10.1016/j.biomaterials.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Peter SJ, Suggs LJ, Yaszemski MJ, Engel PS, Mikos AG. Synthesis of poly(propylene fumarate) by acylation of propylene glycol in the presence of a proton scavenger. J Biomater Sci Polym Ed. 1999;10(3):363–373. doi: 10.1163/156856299x00423. [DOI] [PubMed] [Google Scholar]
- 59.Shung AK, Timmer MD, Jo S, Engel PS, Mikos AG. Kinetics of poly(propylene fumarate) synthesis by step polymerization of diethyl fumarate and propylene glycol using zinc chloride as a catalyst. J Biomater Sci Polym Ed. 2002;13(1):95–108. doi: 10.1163/156856202753525963. [DOI] [PubMed] [Google Scholar]
- 60.He S, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Injectable biodegradable polymer composites based on poly(propylene fumarate) crosslinked with poly(ethylene glycol)-dimethacrylate. Biomaterials. 2000;21(23):2389–2394. doi: 10.1016/s0142-9612(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 61.He S, Timmer MD, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Synthesis of biodegradable poly(propylene fumarate) networks with poly(propylene fumarate)-diacrylate macromers as crosslinking agents and characterization of their degradation products. Polymer. 2001;42(3):1251–1260. [Google Scholar]
- 62.Timmer MD, Jo S, Wang C, Ambrose CG, Mikos AG. Characterization of the cross-linked structure of fumarate-based degradable polymer networks. Macromolecules. 2002;35(11):4373–4379. [Google Scholar]
- 63.Timmer MD, Horch RA, Ambrose CG, Mikos AG. Effect of physiological temperature on the mechanical properties and network structure of biodegradable poly(propylene fumarate)-based networks. J Biomater Sci Polym Ed. 2003;14(4):369–382. doi: 10.1163/156856203321478874. [DOI] [PubMed] [Google Scholar]
- 64.Fisher JP, Holland TA, Dean D, Engel PS, Mikos AG. Synthesis and properties of photocross-linked poly(propylene fumarate) scaffolds. J Biomater Sci Polym Ed. 2001;12(6):673–687. doi: 10.1163/156856201316883476. [DOI] [PubMed] [Google Scholar]
- 65.Fisher JP, Dean D, Mikos AG. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials. 2002;23(22):4333–4343. doi: 10.1016/s0142-9612(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 66.Fisher JP, Timmer MD, Holland TA, Dean D, Engel PS, Mikos AG. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part I. Determination of network structure. Biomacromolecules. 2003;4(5):1327–1334. doi: 10.1021/bm030028d. [DOI] [PubMed] [Google Scholar]
- 67.Fisher JP, Holland TA, Dean D, Mikos AG. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part II. In vitro degradation. Biomacromolecules. 2003;4(5):1335–1342. doi: 10.1021/bm0300296. [DOI] [PubMed] [Google Scholar]
- 68.Timmer MD, Ambrose CG, Mikos AG. Evaluation of thermal- and photo-crosslinked biodegradable poly(propylene fumarate)-based networks. J Biomed Mater Res A. 2003;66(4):811–818. doi: 10.1002/jbm.a.10011. [DOI] [PubMed] [Google Scholar]
- 69.Timmer MD, Shin H, Horch RA, Ambrose CG, Mikos AG. In vitro cytotoxicity of injectable and biodegradable poly(propylene fumarate)-based networks: unreacted macromers, cross-linked networks, and degradation products. Biomacromolecules. 2003;4(4):1026–1033. doi: 10.1021/bm0300150. [DOI] [PubMed] [Google Scholar]
- 70.Timmer MD, Ambrose CG, Mikos AG. In vitro degradation of polymeric networks of poly(propylene fumarate) and the crosslinking macromer poly(propylene fumarate)-diacrylate. Biomaterials. 2003;24(4):571–577. doi: 10.1016/s0142-9612(02)00368-x. [DOI] [PubMed] [Google Scholar]
- 71.Timmer MD, Carter C, Ambrose CG, Mikos AG. Fabrication of poly(propylene fumarate)-based orthopaedic implants by photo-crosslinking through transparent silicone molds. Biomaterials. 2003;24(25):4707–4714. doi: 10.1016/s0142-9612(03)00364-8. [DOI] [PubMed] [Google Scholar]
- 72.Cooke MN, Fisher JP, Dean D, Rimnac C, Mikos AG. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J Biomed Mater Res B Appl Biomater. 2003;64(2):65–69. doi: 10.1002/jbm.b.10485. [DOI] [PubMed] [Google Scholar]
- 73.Fisher JP, Vehof JW, Dean D, van der Waerden JP, Holland TA, Mikos AG, Jansen JA. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J Biomed Mater Res. 2002;59(3):547–556. doi: 10.1002/jbm.1268. [DOI] [PubMed] [Google Scholar]
- 74.Suggs LJ, Kao EY, Palombo LL, Krishnan RS, Widmer MS, Mikos AG. Preparation and characterization of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomater Sci Polym Ed. 1998;9(7):653–666. doi: 10.1163/156856298x00073. [DOI] [PubMed] [Google Scholar]
- 75.Suggs LJ, Krishnan RS, Garcia CA, Peter SJ, Anderson JM, Mikos AG. In vitro and in vivo degradation of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res. 1998;42(2):312–320. doi: 10.1002/(sici)1097-4636(199811)42:2<312::aid-jbm17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 76.Suggs LJ, Shive MS, Garcia CA, Anderson JM, Mikos AG. In vitro cytotoxicity and in vivo biocompatibility of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res. 1999;46(1):22–32. doi: 10.1002/(sici)1097-4636(199907)46:1<22::aid-jbm3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 77.Suggs LJ, West JL, Mikos AG. Platelet adhesion on a bioresorbable poly(propylene fumarate-co-ethylene glycol) copolymer. Biomaterials. 1999;20(7):683–690. doi: 10.1016/s0142-9612(98)00226-9. [DOI] [PubMed] [Google Scholar]
- 78.Behravesh E, Jo S, Zygourakis K, Mikos AG. Synthesis of in situ cross-linkable macroporous biodegradable poly(propylene fumarate-co-ethylene glycol) hydrogels. Biomacromolecules. 2002;3(2):374–381. doi: 10.1021/bm010158r. [DOI] [PubMed] [Google Scholar]
- 79.Behravesh E, Timmer MD, Lemoine JJ, Liebschner MA, Mikos AG. Evaluation of the in vitro degradation of macroporous hydrogels using gravimetry, confined compression testing, and microcomputed tomography. Biomacromolecules. 2002;3(6):1263–1270. doi: 10.1021/bm020067+. [DOI] [PubMed] [Google Scholar]
- 80.Behravesh E, Shung AK, Jo S, Mikos AG. Synthesis and characterization of triblock copolymers of methoxy poly(ethylene glycol) and poly(propylene fumarate) Biomacromolecules. 2002;3(1):153–158. doi: 10.1021/bm010137x. [DOI] [PubMed] [Google Scholar]
- 81.Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Synthesis and Characterization of Injectable, Thermally and Chemically Gelable, Amphiphilic Poly(N-isopropylacrylamide)-Based Macromers. Biomacromolecules. 2008;9(6):1558–1570. doi: 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- 82.Jo S, Shin H, Shung AK, Fisher JP, Mikos AG. Synthesis and characterization of oligo(poly(ethylene glycol) fumarate) macromer. Macromolecules. 2001;34(9):2839–2844. [Google Scholar]
- 83.Temenoff JS, Athanasiou KA, LeBaron RG, Mikos AG. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res. 2002;59(3):429–437. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 84.Temenoff JS, Steinbis ES, Mikos AG. Effect of drying history on swelling properties and cell attachment to oligo(poly(ethylene glycol) fumarate) hydrogels for guided tissue regeneration applications. J Biomater Sci Polym Ed. 2003;14(9):989–1004. doi: 10.1163/156856203322381465. [DOI] [PubMed] [Google Scholar]
- 85.Temenoff JS, Shin H, Conway DE, Engel PS, Mikos AG. In vitro cytotoxicity of redox radical initiators for cross-linking of oligo(poly(ethylene glycol) fumarate) macromers. Biomacromolecules. 2003;4(6):1605–1613. doi: 10.1021/bm030056w. [DOI] [PubMed] [Google Scholar]
- 86.Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36(1):17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 87.Ishaug-Riley SL, Crane-Kruger GM, Yaszemski MJ, Mikos AG. Three- dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998;19(15):1405–1412. doi: 10.1016/s0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 88.Ishaug-Riley SL, Crane GM, Gurlek A, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Ectopic bone formation by marrow stromal osteoblast transplantation using poly(DL-lactic-co-glycolic acid) foams implanted into the rat mesentery. J Biomed Mater Res. 1997;36(1):1–8. doi: 10.1002/(sici)1097-4636(199707)36:1<1::aid-jbm1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 89.Goldstein AS, Zhu G, Morris GE, Meszlenyi RK, Mikos AG. Effect of osteoblastic culture conditions on the structure of poly(DL-lactic-co-glycolic acid) foam scaffolds. Tissue Eng. 1999;5(5):421–434. doi: 10.1089/ten.1999.5.421. [DOI] [PubMed] [Google Scholar]
- 90.Gopferich A, Peter SJ, Lucke A, Lu L, Mikos AG. Modulation of marrow stromal cell function using poly(D,L-lactic acid)-block-poly(ethylene glycol)-monomethyl ether surfaces. J Biomed Mater Res. 1999;46(3):390–398. doi: 10.1002/(sici)1097-4636(19990905)46:3<390::aid-jbm12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 91.Fisher JP, Lalani Z, Bossano CM, Brey EM, Demian N, Johnston CM, Dean D, Jansen JA, Wong ME, Mikos AG. Effect of biomaterial properties on bone healing in a rabbit tooth extraction socket model. J Biomed Mater Res A. 2004;68(3):428–438. doi: 10.1002/jbm.a.20073. [DOI] [PubMed] [Google Scholar]
- 92.Suggs LJ, Mikos AG. Development of poly(propylene fumarate-co-ethylene glycol) as an injectable carrier for endothelial cells. Cell Transplant. 1999;8(4):345–350. doi: 10.1177/096368979900800402. [DOI] [PubMed] [Google Scholar]
- 93.Peter SJ, Miller ST, Zhu G, Yasko AW, Mikos AG. In vivo degradation of a poly(propylene fumarate)/beta-tricalcium phosphate injectable composite scaffold. J Biomed Mater Res. 1998;41(1):1–7. doi: 10.1002/(sici)1097-4636(199807)41:1<1::aid-jbm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 94.Peter SJ, Kim P, Yasko AW, Yaszemski MJ, Mikos AG. Crosslinking characteristics of an injectable poly(propylene fumarate)/beta-tricalcium phosphate paste and mechanical properties of the crosslinked composite for use as a biodegradable bone cement. J Biomed Mater Res. 1999;44(3):314–321. doi: 10.1002/(sici)1097-4636(19990305)44:3<314::aid-jbm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 95.Wolfe MS, Dean D, Chen JE, Fisher JP, Han S, Rimnac CM, Mikos AG. In vitro degradation and fracture toughness of multilayered porous poly(propylene fumarate)/beta-tricalcium phosphate scaffolds. J Biomed Mater Res. 2002;61(1):159–164. doi: 10.1002/jbm.10058. [DOI] [PubMed] [Google Scholar]
- 96.Peter SJ, Lu L, Kim DJ, Mikos AG. Marrow stromal osteoblast function on a poly(propylene fumarate)/beta-tricalcium phosphate biodegradable orthopaedic composite. Biomaterials. 2000;21(12):1207–1213. doi: 10.1016/s0142-9612(99)00254-9. [DOI] [PubMed] [Google Scholar]
- 97.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Biocompatibility and degradation of poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composites. J Biomed Mater Res A. 2005;74(4):533–544. doi: 10.1002/jbm.a.30341. [DOI] [PubMed] [Google Scholar]
- 98.Link DP, van den Dolder J, van den Beucken JJ, Cuijpers VM, Wolke JG, Mikos AG, Jansen JA. Evaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle composites. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31831. [DOI] [PubMed] [Google Scholar]
- 99.Ruhe PQ, Hedberg-Dirk EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006;12(4):789–800. doi: 10.1089/ten.2006.12.789. [DOI] [PubMed] [Google Scholar]
- 100.Bodde EW, Boerman OC, Russel FG, Mikos AG, Spauwen PH, Jansen JA. The kinetic and biological activity of different loaded rhBMP-2 calcium phosphate cement implants in rats. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31830. [DOI] [PubMed] [Google Scholar]
- 101.Link DP, van den Dolder J, van den Beucken JJ, Wolke JG, Mikos AG, Jansen JA. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-beta 1 loaded gelatin microparticles. Biomaterials. 2008;29(6):675–682. doi: 10.1016/j.biomaterials.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 102.Leeuwenburgh SC, Jansen JA, Mikos AG. Functionalization of oligo(poly(ethylene glycol)fumarate) hydrogels with finely dispersed calcium phosphate nanocrystals for bone-substituting purposes. J Biomater Sci Polym Ed. 2007;18(12):1547–1564. [PubMed] [Google Scholar]
- 103.Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Hydroxyapatite fiber reinforced poly(alpha-hydroxy ester) foams for bone regeneration. Biomaterials. 1998;19(21):1935–1943. doi: 10.1016/s0142-9612(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 104.Horch RA, Shahid N, Mistry AS, Timmer MD, Mikos AG, Barron AR. Nanoreinforcement of poly(propylene fumarate)-based networks with surface modified alumoxane nanoparticles for bone tissue engineering. Biomacromolecules. 2004;5(5):1990–1998. doi: 10.1021/bm049768s. [DOI] [PubMed] [Google Scholar]
- 105.Mistry AS, Mikos AG, Jansen JA. Degradation and biocompatibility of a poly(propylene fumarate)-based/alumoxane nanocomposite for bone tissue engineering. J Biomed Mater Res A. 2007;83(4):940–953. doi: 10.1002/jbm.a.31280. [DOI] [PubMed] [Google Scholar]
- 106.Mistry AS, Cheng SH, Yeh T, Christenson EM, Jansen JA, Mikos AG. Fabrication and in vitro degradation of porous fumarate-based polymer/alumoxane nanocomposite scaffolds for bone tissue engineering. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32010. In press. [DOI] [PubMed] [Google Scholar]
- 107.Mistry AS, Pham QP, Schouten C, Yeh T, Christenson EM, Mikos AG, Jansen JA. In vivo bone biocompatibility and degradation of porous fumarate-based polymer/alumoxane nanocomposites for bone tissue engineering. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32371. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Rheological behaviour and mechanical characterization of injectable poly(propylene fumarate)/single-walled carbon nanotube composites for bone tissue engineering. Nanotechnology. 2005;16(7):531–538. doi: 10.1088/0957-4484/16/7/030. [DOI] [PubMed] [Google Scholar]
- 109.Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Injectable nanocomposites of single-walled carbon nanotubes and biodegradable polymers for bone tissue engineering. Biomacromolecules. 2006;7(7):2237–2242. doi: 10.1021/bm060391v. [DOI] [PubMed] [Google Scholar]
- 110.Shi X, Sitharaman B, Pham QP, Spicer PP, Hudson JL, Wilson LJ, Tour JM, Raphael RM, Mikos AG. In vitro cytotoxicity of single-walled carbon nanotube/biodegradable polymer nanocomposites. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31671. [DOI] [PubMed] [Google Scholar]
- 111.Shi X, Sitharaman B, Pham QP, Liang F, Wu K, Edward Billups W, Wilson LJ, Mikos AG. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials. 2007;28(28):4078–4090. doi: 10.1016/j.biomaterials.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sitharaman B, Shi X, Tran LA, Spicer PP, Rusakova I, Wilson LJ, Mikos AG. Injectable in situ cross-linkable nanocomposites of biodegradable polymers and carbon nanostructures for bone tissue engineering. J Biomater Sci Polym Ed. 2007;18(6):655–671. doi: 10.1163/156856207781034133. [DOI] [PubMed] [Google Scholar]
- 113.Sitharaman B, Shi X, Walboomers XF, Liao H, Cuijpers VM, Wilson LJ, Mikos AG, Jansen JA. In vivo biocompatibility of ultra-short single walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering. Bone. 2008 doi: 10.1016/j.bone.2008.04.013. In press. [DOI] [PubMed] [Google Scholar]
- 114.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 115.Payne RG, Yaszemski MJ, Yasko AW, Mikos AG. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 1. Encapsulation of marrow stromal osteoblasts in surface crosslinked gelatin microparticles. Biomaterials. 2002;23(22):4359–4371. doi: 10.1016/s0142-9612(02)00184-9. [DOI] [PubMed] [Google Scholar]
- 116.Payne RG, McGonigle JS, Yaszemski MJ, Yasko AW, Mikos AG. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 2. Viability of encapsulated marrow stromal osteoblasts cultured on crosslinking poly(propylene fumarate) Biomaterials. 2002;23(22):4373–4380. doi: 10.1016/s0142-9612(02)00185-0. [DOI] [PubMed] [Google Scholar]
- 117.Payne RG, McGonigle JS, Yaszemski MJ, Yasko AW, Mikos AG. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 3. Proliferation and differentiation of encapsulated marrow stromal osteoblasts cultured on crosslinking poly(propylene fumarate) Biomaterials. 2002;23(22):4381–4387. doi: 10.1016/s0142-9612(02)00186-2. [DOI] [PubMed] [Google Scholar]
- 118.Tanahashi K, Mikos AG. Cell adhesion on poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res. 2002;62(4):558–566. doi: 10.1002/jbm.10284. [DOI] [PubMed] [Google Scholar]
- 119.Shung AK, Behravesh E, Jo S, Mikos AG. Crosslinking characteristics of and cell adhesion to an injectable poly(propylene fumarate-co-ethylene glycol) hydrogel using a water-soluble crosslinking system. Tissue Eng. 2003;9(2):243–254. doi: 10.1089/107632703764664710. [DOI] [PubMed] [Google Scholar]
- 120.Shin H, Ruhe QP, Mikos AG, Jansen JA. In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene glycol) fumarate) hydrogels. Biomaterials. 2003;24(19):3201–3211. doi: 10.1016/s0142-9612(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 121.Shin H, Temenoff JS, Mikos AG. In vitro cytotoxicity of unsaturated oligo[poly(ethylene glycol) fumarate] macromers and their cross-linked hydrogels. Biomacromolecules. 2003;4(3):552–560. doi: 10.1021/bm020121m. [DOI] [PubMed] [Google Scholar]
- 122.Temenoff JS, Park H, Jabbari E, Conway DE, Sheffield TL, Ambrose CG, Mikos AG. Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules. 2004;5(1):5–10. doi: 10.1021/bm030067p. [DOI] [PubMed] [Google Scholar]
- 123.Temenoff JS, Park H, Jabbari E, Sheffield TL, LeBaron RG, Ambrose CG, Mikos AG. In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A. 2004;70(2):235–244. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 124.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF-beta 1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26(34):7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 125.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28(21):3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ueda H, Hacker MC, Haesslein A, Jo S, Ammon DM, Borazjani RN, Kunzler JF, Salamone JC, Mikos AG. Injectable, in situ forming poly(propylene fumarate)- based ocular drug delivery systems. J Biomed Mater Res A. 2007;83(3):656–666. doi: 10.1002/jbm.a.31226. [DOI] [PubMed] [Google Scholar]
- 127.Haesslein A, Hacker MC, Mikos AG. Effect of macromer molecular weight on in vitro ophthalmic drug release from photo-crosslinked matrices. Acta Biomater. 2008;4(1):1–10. doi: 10.1016/j.actbio.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 128.Ambrose CG, Gogola GR, Clyburn TA, Raymond AK, Peng AS, Mikos AG. Antibiotic microspheres: preliminary testing for potential treatment of osteomyelitis. Clin Orthop Relat Res. 2003;(415):279–285. doi: 10.1097/01.blo.0000093920.26658.ae. [DOI] [PubMed] [Google Scholar]
- 129.Ambrose CG, Clyburn TA, Louden K, Joseph J, Wright J, Gulati P, Gogola GR, Mikos AG. Effective treatment of osteomyelitis with biodegradable microspheres in a rabbit model. Clin Orthop Relat Res. 2004;(421):293–299. doi: 10.1097/01.blo.0000126303.41711.a2. [DOI] [PubMed] [Google Scholar]
- 130.Hacker MC, Haesslein A, Ueda H, Foster WJ, Garcia CA, Ammon DM, Borazjani RN, Kunzler JF, Salamone JC, Mikos AG. Biodegradable fumarate-based drug-delivery systems for ophthalmic applications. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31942. In press. [DOI] [PubMed] [Google Scholar]
- 131.Letterio JJ, Roberts AB. Regulation of Immune Responses by TGF--β1. Annu Rev Immunol. 1998;16(1):137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 132.Vehof JW, Fisher JP, Dean D, van der Waerden JP, Spauwen PH, Mikos AG, Jansen JA. Bone formation in transforming growth factor beta-1-coated porous poly(propylene fumarate) scaffolds. J Biomed Mater Res. 2002;60(2):241–251. doi: 10.1002/jbm.10073. [DOI] [PubMed] [Google Scholar]
- 133.Lu L, Stamatas GN, Mikos AG. Controlled release of transforming growth factor beta1 from biodegradable polymer microparticles. J Biomed Mater Res. 2000;50(3):440–451. doi: 10.1002/(sici)1097-4636(20000605)50:3<440::aid-jbm19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 134.Peter SJ, Lu L, Kim DJ, Stamatas GN, Miller MJ, Yaszemski MJ, Mikos AG. Effects of transforming growth factor beta1 released from biodegradable polymer microparticles on marrow stromal osteoblasts cultured on poly(propylene fumarate) substrates. J Biomed Mater Res. 2000;50(3):452–462. doi: 10.1002/(sici)1097-4636(20000605)50:3<452::aid-jbm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 135.Lu L, Yaszemski MJ, Mikos AG. TGF-beta1 release from biodegradable polymer microparticles: its effects on marrow stromal osteoblast function. J Bone Joint Surg Am. 2001;83-A(Suppl 1Pt 2):S82–91. [PubMed] [Google Scholar]
- 136.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109(1–3):256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 137.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91(3):299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 138.Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta 1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release. 2004;94(1):101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 139.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75(1):156–167. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 140.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101(1–3):111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 141.Park H, Temenoff JS, Tabata Y, Caplan AI, Raphael RM, Jansen JA, Mikos AG. Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31948. In press. [DOI] [PubMed] [Google Scholar]
- 142.Holland TA, Bodde EW, Cuijpers VM, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage. 2007;15(2):187–197. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 143.Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008 doi: 10.1016/j.actbio.2008.04.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patel ZS, Ueda H, Yamamoto M, Tabata Y, Mikos AG. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res. 2008 doi: 10.1007/s11095-008-9685-1. In press. [DOI] [PubMed] [Google Scholar]
- 145.Young S. The Effect of Simultaneous, Controlled Release of Angiogenic and Osteogenic Growth Factors on the Enhancement of Osteogenesis within Craniofacial Defects. Houston, TX: Ph.D. Thesis, Department of Bioengineering, Rice University; 2008. [Google Scholar]
- 146.Oldham JB, Lu L, Zhu X, Porter BD, Hefferan TE, Larson DR, Currier BL, Mikos AG, Yaszemski MJ. Biological activity of rhBMP-2 released from PLGA microspheres. J Biomech Eng. 2000;122(3):289–292. doi: 10.1115/1.429662. [DOI] [PubMed] [Google Scholar]
- 147.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. rhBMP- 2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85-A(Suppl 3):75–81. doi: 10.2106/00004623-200300003-00013. [DOI] [PubMed] [Google Scholar]
- 148.Ruhe PQ, Boerman OC, Russel FG, Spauwen PH, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release. 2005;106(1–2):162–171. doi: 10.1016/j.jconrel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 149.Hedberg EL, Tang A, Crowther RS, Carney DH, Mikos AG. Controlled release of an osteogenic peptide from injectable biodegradable polymeric composites. J Control Release. 2002;84(3):137–150. doi: 10.1016/s0168-3659(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 150.Hedberg EL, Kroese-Deutman HC, Shih CK, Crowther RS, Carney DH, Mikos AG, Jansen JA. Effect of varied release kinetics of the osteogenic thrombin peptide TP508 from biodegradable, polymeric scaffolds on bone formation in vivo. J Biomed Mater Res A. 2005;72(4):343–353. doi: 10.1002/jbm.a.30265. [DOI] [PubMed] [Google Scholar]
- 151.Murphy MB, Hartgerink JD, Goepferich A, Mikos AG. Synthesis and in vitro hydroxyapatite binding of peptides conjugated to calcium-binding moieties. Biomacromolecules. 2007;8(7):2237–2243. doi: 10.1021/bm070121s. [DOI] [PubMed] [Google Scholar]
- 152.Lu L, Kam L, Hasenbein M, Nyalakonda K, Bizios R, Gopferich A, Young JF, Mikos AG. Retinal pigment epithelial cell function on substrates with chemically micropatterned surfaces. Biomaterials. 1999;20(23–24):2351–2361. doi: 10.1016/s0142-9612(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 153.Lu L, Nyalakonda K, Kam L, Bizios R, Gopferich A, Mikos AG. Retinal pigment epithelial cell adhesion on novel micropatterned surfaces fabricated from synthetic biodegradable polymers. Biomaterials. 2001;22(3):291–297. doi: 10.1016/s0142-9612(00)00179-4. [DOI] [PubMed] [Google Scholar]
- 154.Liu G, Eskin SG, Mikos AG. Integrin alpha(v)beta(3) is involved in stimulated migration of vascular adventitial fibroblasts by basic fibroblast growth factor but not platelet-derived growth factor. J Cell Biochem. 2001;83(1):129–135. doi: 10.1002/jcb.1208. [DOI] [PubMed] [Google Scholar]
- 155.Jo S, Shin H, Mikos AG. Modification of oligo(poly(ethylene glycol) fumarate) macromer with a GRGD peptide for the preparation of functionalized polymer networks. Biomacromolecules. 2001;2(1):255–261. doi: 10.1021/bm000107e. [DOI] [PubMed] [Google Scholar]
- 156.Shin H, Jo S, Mikos AG. Modulation of marrow stromal osteoblast adhesion on biomimetic oligo[poly(ethylene glycol) fumarate] hydrogels modified with Arg-Gly-Asp peptides and a poly(ethyleneglycol) spacer. J Biomed Mater Res. 2002;61(2):169–179. doi: 10.1002/jbm.10193. [DOI] [PubMed] [Google Scholar]
- 157.Behravesh E, Zygourakis K, Mikos AG. Adhesion and migration of marrow- derived osteoblasts on injectable in situ crosslinkable poly(propylene fumarate- co-ethylene glycol)-based hydrogels with a covalently linked RGDS peptide. J Biomed Mater Res A. 2003;65(2):260–270. doi: 10.1002/jbm.a.10461. [DOI] [PubMed] [Google Scholar]
- 158.Tanahashi K, Mikos AG. Effect of hydrophilicity and agmatine modification on degradation of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res A. 2003;67(4):1148–1154. doi: 10.1002/jbm.a.10147. [DOI] [PubMed] [Google Scholar]
- 159.Tanahashi K, Mikos AG. Protein adsorption and smooth muscle cell adhesion on biodegradable agmatine-modified poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res A. 2003;67(2):448–457. doi: 10.1002/jbm.a.10077. [DOI] [PubMed] [Google Scholar]
- 160.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials. 2004;25(5):895–906. doi: 10.1016/s0142-9612(03)00602-1. [DOI] [PubMed] [Google Scholar]
- 161.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Modulation of differentiation and mineralization of marrow stromal cells cultured on biomimetic hydrogels modified with Arg-Gly-Asp containing peptides. J Biomed Mater Res A. 2004;69(3):535–543. doi: 10.1002/jbm.a.30027. [DOI] [PubMed] [Google Scholar]
- 162.Shin H, Temenoff JS, Bowden GC, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg-Gly-Asp modified hydrogels without dexamethasone and beta-glycerol phosphate. Biomaterials. 2005;26(17):3645–3654. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 163.Leach CS, Dietlein LF, Pool SL, Nicogossian AE. Medical considerations for extending human presence in space. Acta Astronautica. 1990;21(9):659–666. doi: 10.1016/0094-5765(90)90077-x. [DOI] [PubMed] [Google Scholar]
- 164.Goodship AE, Cunningham JL, Oganov V, Darling J, Miles AW, Owen GW. Bone loss during long term space flight is prevented by the application of a short term impulsive mechanical stimulus. Acta Astronautica. 1998;43(3–6):65–75. doi: 10.1016/s0094-5765(98)00144-1. [DOI] [PubMed] [Google Scholar]
- 165.Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22(11):1279–1288. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 166.Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62(1):136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- 167.Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9(3):549–554. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 168.van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PH, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal osteoblasts in titanium fiber mesh. J Biomed Mater Res A. 2003;64(2):235–241. doi: 10.1002/jbm.a.10365. [DOI] [PubMed] [Google Scholar]
- 169.Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MA, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33(1):63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- 170.Gomes ME, Sikavitsas VI, Behravesh E, Reis RL, Mikos AG. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J Biomed Mater Res A. 2003;67(1):87–95. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- 171.Holtorf HL, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal cells seeded on porous biphasic calcium phosphate ceramics. Ann Biomed Eng. 2005;33(9):1238–1248. doi: 10.1007/s10439-005-5536-y. [DOI] [PubMed] [Google Scholar]
- 172.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100(25):14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sikavitsas VI, van den Dolder J, Bancroft GN, Jansen JA, Mikos AG. Influence of the in vitro culture period on the in vivo performance of cell/titanium bone tissue- engineered constructs using a rat cranial critical size defect model. J Biomed Mater Res A. 2003;67(3):944–951. doi: 10.1002/jbm.a.10126. [DOI] [PubMed] [Google Scholar]
- 174.Holtorf HL, Datta N, Jansen JA, Mikos AG. Scaffold mesh size affects the osteoblastic differentiation of seeded marrow stromal cells cultured in a flow perfusion bioreactor. J Biomed Mater Res A. 2005;74(2):171–180. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 175.Gomes ME, Holtorf HL, Reis RL, Mikos AG. Influence of the porosity of starch- based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12(4):801–809. doi: 10.1089/ten.2006.12.801. [DOI] [PubMed] [Google Scholar]
- 176.Holtorf HL, Jansen JA, Mikos AG. Ectopic bone formation in rat marrow stromal cell/titanium fiber mesh scaffold constructs: effect of initial cell phenotype. Biomaterials. 2005;26(31):6208–6216. doi: 10.1016/j.biomaterials.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 177.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72(3):326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 178.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103(8):2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26(9):971–977. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 180.Gomes ME, Bossano CM, Johnston CM, Reis RL, Mikos AG. In vitro localization of bone growth factors in constructs of biodegradable scaffolds seeded with marrow stromal cells and cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12(1):177–188. doi: 10.1089/ten.2006.12.177. [DOI] [PubMed] [Google Scholar]
- 181.Pham QP, Kasper FK, Mistry AS, Sharma U, Yasko AW, Jansen JA, Mikos AG. Analysis of the osteoinductive capacity and angiogenicity of an in vitro generated extracellular matrix. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31875. In press. [DOI] [PubMed] [Google Scholar]
- 182.Pham QP, Kurtis Kasper F, Baggett LS, Raphael RM, Jansen JA, Mikos AG. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29(18):2729–2739. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 183.Cleek RL, Rege AA, Denner LA, Eskin SG, Mikos AG. Inhibition of smooth muscle cell growth in vitro by an antisense oligodeoxynucleotide released from poly(DL-lactic-co-glycolic acid) microparticles. J Biomed Mater Res. 1997;35(4):525–530. doi: 10.1002/(sici)1097-4636(19970615)35:4<525::aid-jbm12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 184.Godbey WT, Wu KK, Hirasaki GJ, Mikos AG. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther. 1999;6(8):1380–1388. doi: 10.1038/sj.gt.3300976. [DOI] [PubMed] [Google Scholar]
- 185.Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999;45(3):268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 186.Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci U S A. 1999;96(9):5177–5181. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Godbey WT, Barry MA, Saggau P, Wu KK, Mikos AG. Poly(ethylenimine)-mediated transfection: A new paradigm for gene delivery. J Biomed Mater Res. 2000;51(3):321–328. doi: 10.1002/1097-4636(20000905)51:3<321::aid-jbm5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 188.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22(5):471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 189.Saraf A, Hacker MC, Sitharaman B, Grande-Allen KJ, Barry MA, Mikos AG. Synthesis and conformational evaluation of a novel gene delivery vector for human mesenchymal stem cells. Biomacromolecules. 2008;9(3):818–827. doi: 10.1021/bm701146f. [DOI] [PubMed] [Google Scholar]
- 190.Blum JS, Barry MA, Mikos AG, Jansen JA. In vivo evaluation of gene therapy vectors in ex vivo-derived marrow stromal cells for bone regeneration in a rat critical-size calvarial defect model. Hum Gene Ther. 2003;14(18):1689–1701. doi: 10.1089/104303403322611719. [DOI] [PubMed] [Google Scholar]
- 191.Blum JS, Parrott MB, Mikos AG, Barry MA. Early osteoblastic differentiation induced by dexamethasone enhances adenoviral gene delivery to marrow stromal cells. J Orthop Res. 2004;22(2):411–416. doi: 10.1016/j.orthres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 192.Kasper FK, Jerkins E, Tanahashi K, Barry MA, Tabata Y, Mikos AG. Characterization of DNA release from composites of oligo(poly(ethylene glycol) fumarate) and cationized gelatin microspheres in vitro. J Biomed Mater Res A. 2006;78(4):823–835. doi: 10.1002/jbm.a.30736. [DOI] [PubMed] [Google Scholar]
- 193.Kasper FK, Kushibiki T, Kimura Y, Mikos AG, Tabata Y. In vivo release of plasmid DNA from composites of oligo(poly(ethylene glycol)fumarate) and cationized gelatin microspheres. J Control Release. 2005;107(3):547–561. doi: 10.1016/j.jconrel.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 194.Kasper FK, Seidlits SK, Tang A, Crowther RS, Carney DH, Barry MA, Mikos AG. In vitro release of plasmid DNA from oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2005;104(3):521–539. doi: 10.1016/j.jconrel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 195.Kasper FK, Young S, Tanahashi K, Barry MA, Tabata Y, Jansen JA, Mikos AG. Evaluation of bone regeneration by DNA release from composites of oligo(poly(ethylene glycol) fumarate) and cationized gelatin microspheres in a critical-sized calvarial defect. J Biomed Mater Res A. 2006;78(2):335–342. doi: 10.1002/jbm.a.30698. [DOI] [PubMed] [Google Scholar]
- 196.Peter SJ, Liang CR, Kim DJ, Widmer MS, Mikos AG. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta- glycerolphosphate, and L-ascorbic acid. J Cell Biochem. 1998;71(1):55–62. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 197.van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PH, Mikos AG, Jansen JA. Effect of fibronectin- and collagen I-coated titanium fiber mesh on proliferation and differentiation of osteogenic cells. Tissue Eng. 2003;9(3):505–515. doi: 10.1089/107632703322066688. [DOI] [PubMed] [Google Scholar]
- 198.Sitharaman B, Tran LA, Pham QP, Bolskar RD, Muthupillai R, Flamm SD, Mikos AG, Wilson LJ. Gadofullerenes as nanoscale magnetic labels for cellular MRI. Contrast Media Mol Imaging. 2007;2(3):139–146. doi: 10.1002/cmmi.140. [DOI] [PubMed] [Google Scholar]
- 199.Behravesh E, Sikavitsas VI, Mikos AG. Quantification of ligand surface concentration of bulk-modified biomimetic hydrogels. Biomaterials. 2003;24(24):4365–4374. doi: 10.1016/s0142-9612(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 200.Blum JS, Li RH, Mikos AG, Barry MA. An optimized method for the chemiluminescent detection of alkaline phosphatase levels during osteodifferentiation by bone morphogenetic protein 2. J Cell Biochem. 2001;80(4):532–537. doi: 10.1002/1097-4644(20010315)80:4<532::aid-jcb1007>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 201.Blum JS, Temenoff JS, Park H, Jansen JA, Mikos AG, Barry MA. Development and characterization of enhanced green fluorescent protein and luciferase expressing cell line for non-destructive evaluation of tissue engineering constructs. Biomaterials. 2004;25(27):5809–5819. doi: 10.1016/j.biomaterials.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 202.Oshima Y, Watanabe N, Matsuda K, Takai S, Kawata M, Kubo T. Behavior of transplanted bone marrow-derived GFP mesenchymal cells in osteochondral defect as a simulation of autologous transplantation. J Histochem Cytochem. 2005;53(2):207–216. doi: 10.1369/jhc.4A6280.2005. [DOI] [PubMed] [Google Scholar]
- 203.Kajikawa Y, Morihara T, Watanabe N, Sakamoto H, Matsuda K, Kobayashi M, Oshima Y, Yoshida A, Kawata M, Kubo T. GFP chimeric models exhibited a biphasic pattern of mesenchymal cell invasion in tendon healing. J Cell Physiol. 2007;210(3):684–691. doi: 10.1002/jcp.20876. [DOI] [PubMed] [Google Scholar]
- 204.Young S, Bashoura AG, Borden T, Baggett LS, Jansen JA, Wong M, Mikos AG. Development and characterization of a rabbit alveolar bone nonhealing defect model. J Biomed Mater Res A. 2008;86A(1):182–194. doi: 10.1002/jbm.a.31639. [DOI] [PubMed] [Google Scholar]
- 205.Bodde EW, Spauwen PH, Mikos AG, Jansen JA. Closing capacity of segmental radius defects in rabbits. J Biomed Mater Res A. 2008;85(1):206–217. doi: 10.1002/jbm.a.31549. [DOI] [PubMed] [Google Scholar]
- 206.Young S, Kretlow JD, Nguyen C, Bashoura A, Baggett LS, Jansen JA, Wong M, Mikos AG. Microcomputed Tomography Characterization of Neovascularization in Bone Tissue Engineering Applications. Tissue Engineering Part B: Reviews. 2008 doi: 10.1089/ten.teb.2008.0153. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hedberg EL, Kroese-Deutman HC, Shih CK, Lemoine JJ, Liebschner MA, Miller MJ, Yasko AW, Crowther RS, Carney DH, Mikos AG, Jansen JA. Methods: a comparative analysis of radiography, microcomputed tomography, and histology for bone tissue engineering. Tissue Eng. 2005;11(9–10):1356–1367. doi: 10.1089/ten.2005.11.1356. [DOI] [PubMed] [Google Scholar]
- 208.Miller MJ, Goldberg DP, Yasko AW, Lemon JC, Satterfield WC, Wake MC, Mikos AG. Guided Bone Growth in Sheep: A Model for Tissue-Engineered Bone Flaps. Tissue Eng. 1996;2(1):51–59. doi: 10.1089/ten.1996.2.51. [DOI] [PubMed] [Google Scholar]
- 209.Thomson RC, Mikos AG, Beahm E, Lemon JC, Satterfield WC, Aufdemorte TB, Miller MJ. Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials. 1999;20(21):2007–2018. doi: 10.1016/s0142-9612(99)00103-9. [DOI] [PubMed] [Google Scholar]
- 210.Cheng MH, Brey EM, Ulusal BG, Wei FC. Mandible augmentation for osseointegrated implants using tissue engineering strategies. Plast Reconstr Surg. 2006;118(1):1e–4e. doi: 10.1097/01.prs.0000221120.11128.1a. [DOI] [PubMed] [Google Scholar]
- 211.Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Polymer concepts in tissue engineering. J Biomed Mater Res. 1998;43(4):422–427. doi: 10.1002/(sici)1097-4636(199824)43:4<422::aid-jbm9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]