Abstract
In this work, we detail a method whereby a polymeric hydrogel layer is grafted to the negative tone photoresist SU-8 in order to improve its wettability. A photoinitiator is first immobilized on freshly prepared SU-8 samples, acting as the starting point for various surface modifications strategies. Grafting of a 2-hydroxyethylmethacrylate-based hydrogel from the SU-8 surface resulted in the reduction of the static contact angle of a water droplet from 79 ± 1° to 36 ± 1°, while addition of a poly(ethylene glycol)-rich hydrogel layer resulted in further improvement (8 ± 1°). Wettability is greatly enhanced after 30 minutes of polymerization, with a continued but more gradual decrease in contact angle up to approximately 50 minutes. Hydrogel formation is triggered by exposure to UV irradiation, allowing for the formation of photopatterned structures using existing photolithographic techniques.
1. Introduction
Most often, BioMEMS devices or biofluidic chips are fabricated from either glass- or silicon-based materials such as those used in traditional semiconductor or ceramic manufacturing. Various processes have been developed to create on-chip structures enabling these materials to be used as microdevices [1], but their broad use in bioanalytical microdevices has been limited by the difficult processing steps associated with fabrication. Strong chemical etchants, for example, are needed to produce such basic fluidic features as channels. Furthermore, the wafer bonding processes to seal fluidic channels require harsh conditions such as high temperature and/or high voltage, which make integration with biomolecules (enzymes, antibodies, etc.) challenging. As a result, there has been growing interest in using organic-based, negative tone photoresists, such as the epoxy derived material, SU-8, in biofluidics applications [2]. Such photoresists allow for easy formation of high aspect ratio features with a simple expose-bake-develop procedure, even resulting in sealed channel structures formed under moderately low temperature [3]. However, for use in such applications it is of interest to improve the wettability of SU-8.
To date, researchers have focused on various methods to improve the aqueous wettability of SU-8. Measurements in our laboratory show that a freshly prepared SU-8 surface is not highly hydrophilic, with a static water contact angle of 79 ± 1°. In order to improve the hydrophilicity of SU-8, a number of strategies have been employed by various researchers, including modification of the SU-8 bulk chemistry [4], the catalyzed addition of ethanolamine to the material surface [5], the use of ceric ammonium nitrate to graft poly(ethylene glycol) [6], and reaction with oxygen plasma [7,8]. In this work we demonstrate a novel method for the modification of SU-8 that seeks to provide the following features a.) improved wettability, b.) absence of harsh processing conditions such as high temperatures or strong chemical agents, c.) ability to integrate bioanalytical functional layers (e.g. enzyme immobilized hydrogels) within a completed fluidic channel, and d.) spatial control of such layers using traditional photolithographic techniques. Another disadvantage of traditional structure materials for microfluidic channels is nonspecific adsorption of reagent/sample molecules from the surrounding fluid-human blood (so called “biofouling”). While biocompatibility of SU-8 has been previously evaluated [9], our work aims to further decrease biofouling through addition of grafted polymer layers which are known to be biocompatible. Poly(ethylene glycol) (PEG), one of most widely used biocompatible polymer, was chosen to modify the SU-8 surface. It has been shown that PEG modified surfaces, including those in nanochannels, gain the ability to minimize nonspecific protein adsorption and cell attachment [10–12].
In this work, a “graft from” technique – photoinitiator is first immobilized on the material surface in a method derived from work done by Ma et al. [13] —is used to produce thin polymer layers on the substrate (SU-8) layer. Once modified, the now photoreactive surface may be brought in contact with various monomers. Irradiation with UV light begins a free radical polymerization that originates from the surface and penetrates into the monomer liquid, resulting in covalent bound polymer chains. When this polymerization is done using hydrophilic monomers and crosslinker, the resulting grafted layer is a hydrogel – a material known for its wettability as well as its biocompatibility [14]. In this work we created grafted hydrogel layers composed of either 2-hydroxyethylmethacrylate (HEMA) monomer or of poly(ethylene glycol) (PEG) macromonomers. Polymeric HEMA (pHEMA) is known for its biocompatibility [14] and as such is found as the base material in soft contact lenses. Because of this, the transport of water and oxygen through pHEMA hydrogels has been studied [15] and it has subsequently attracted attention as a matrix for use in enzyme immobilization strategies [16–17]. PEG is a well studied polymer frequently used to improve its biocompatibility and wetting properties of various materials [18].
2. Materials and Methods
2.1 Materials
The photoinitiator 1-hydroxycyclohexyl phenyl ketone (HCPK), HEMA monomer, and tetraethyleneglycol dimethacrylate (TEGDMA) crosslinker were purchased from Sigma Aldrich (St. Louis, MO). PEG-rich hydrogels were formed using macromonomers of poly(ethylene glycol). Poly(ethylene glycol) monomethyl ether methacrylate (PEGMA) and poly(ethylene glycol) dimethacrylate (PEGDMA) were purchased from Sigma Aldrich. The macromonomers featured short PEG chains of Mn = 475 and Mn = 875 respectively. HEMA monomer was vacuum distilled prior to use in order to remove the free radical inhibitor hydroquinone monomethyl ether. Silicon wafers were purchased from University Wafer (South Boston, MA) and the negative tone photoresist SU-8 (formulation 2050) and propylene glycol methyl ether acetate were purchased from MicroChem (Newton, MA).
2.2 Preparation of SU-8 Samples
An SU-8 layer was added to the silicon wafers by spin coating at 2000 rpm for 15 seconds, followed by 3000 rpm for 38 seconds. A prebaking step was carried out at 65 °C for 4 min followed 95 °C for 8 min on a hotplate. The SU-8 initiator was activated by flood exposure to UV light in a mask aligner for 38 seconds for a total exposure of 209 mJ/cm2. Post irradiation, the samples were then baked at 65 °C for one minute and then ramped to 95°C for nine minutes. Finally, the samples were developed with propylene glycol methyl ether acetate for 8 minutes and then rinsed with isopropanol followed by deionized water. This silicon wafer was cut into 1.5×1.5 cm small pieces, each of them with 1×1 cm SU-8 patterned layer. Those small pieces were soaked in ethanol for 12 hours and dried to constant weight.
2.3 Characterization of Modified Surfaces
The static contact angle was measured with a research goniometer (Ramé-Hart, Netcong, NJ). A 2μl droplet of deionized water was placed on the sample surface and the resulting angle read. All measurements were done in triplicate. Analysis of the surface chemistry was done using attenuated total reflectance (ATR) FTIR (Nicolet 6700, Thermo Electron, Madison, WI), with the sample placed on a single bounce 45° Ge crystal (Smart Performer, Thermo Electron).
2.4. Photografting
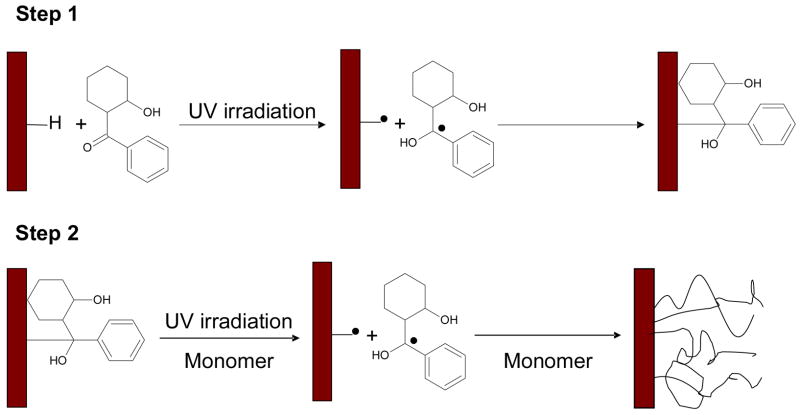
A “graft-from” technique was used to grow various layers on the SU-8 surface (Figure 1), starting with the formation of a surface bound initiator. The addition of a surface bound initiator molecule is done via a hydrogen abstraction technique [8]. Newly prepared SU-8 was soaked in a 5% (w/w) HCPK in ethanol solution and later place in a nitrogen filled glovebox. UV irradiation (UV LED, 27mW/cm2 at 375nm emission, Opto Technologies, Wheeling, IL) of the SU-8 surface was performed for 30 minutes (total exposure of 48,600 mJ/cm2), resulting in the formation of surface bound photoinitiator. Once reacted, the modified samples were washed with ethanol to remove any excess HCPK, and allowed to dry. Grafted hydrogel layers were then grown on the substrate by covering the modified SU-8 samples with a_mixture of monomer (HEMA, PEGMA) and/or crosslinker (TEGDMA, PEGDMA). Polymerization followed when the material and monomer mixture were exposed to UV irradiation again in a nitrogen atmosphere. These resulting hydrogel modified materials were then washed with DI water, dried, and then analyzed.
Figure 1.
Schematic diagram for the photoinduced graft polymerization of monomer onto a SU-8 surface.
2.5 Micropatterning
The photoinitiator modified SU-8 materials were covered with a thin film of monomers by spin-coating. All monomer solutions used in the experiment were bubbled with nitrogen for 20 minutes to remove oxygen. A chromium-plated glass mask was then placed on the top of the sample tightly. The sample was then irradiated with UV light through a collimating lens for 30 min at an energy dose of 9,900 mJ/cm2 (5.5 mW/cm2 at 365 nm). Finally, the exposed sample was developed and washed by ethanol to get rid of the unreacted monomers.
The resulting patterns were examined several ways. The height and profile of the pattern was determined by a profilometer (Alpha-step 200). SEM images were taken (Hitachi S-570 SEM) to examine the pattern formation on SU-8 surface. Before imaging, a thin layer of gold and palladium was sputtered on the sample for 30 seconds to prevent electron oxidation corrosion for polymer pattern.
3. Discussion
3.1 Effect of Hydrogel Composition on Wettability
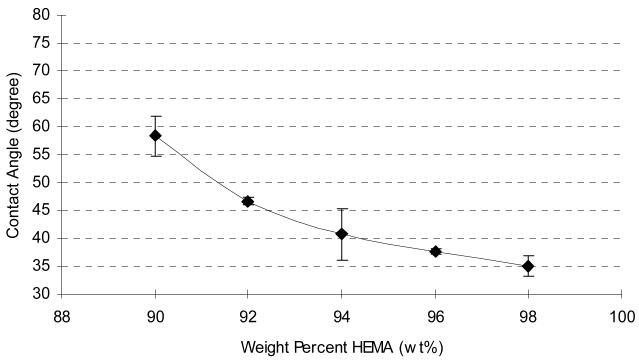
Various amounts of crosslinker were studied to determine any possible effect on wettability. Hydrogel modified SU-8 samples were prepared using various ratios of HEMA: TEGDMA, ranging from 2 to 10% TEGDMA. Greater amounts of the hydrophilic HEMA monomer resulted in surfaces with enhanced wettability (Figure 2). In general, lower degrees of crosslinking allow for greater water uptake in hydrogels [10]. This result was confirmed in these experiments, as it was ultimately determined that a 98% HEMA: 2% TEGDMA (w/w) mixture provided the best wettability, as reflected by the static contact angle measurement of 36 ± 1°.
Figure 2.
Static water contact angle decreases with increasing percentage of HEMA in prepolymerization mixture.
3.2 Effect of polymerization time
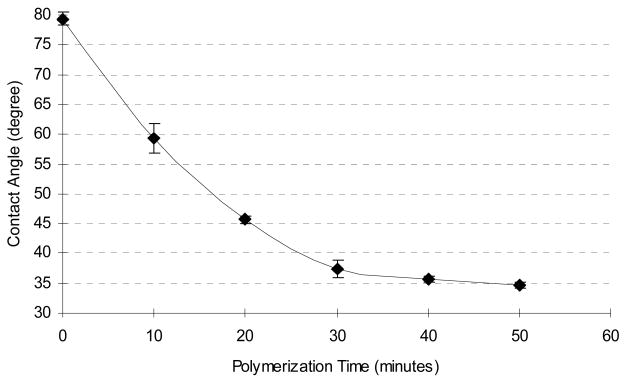
It should be noted that surface initiation techniques rely on monolayers of reactive species, which means inherently low initiator concentrations and therefore slow reaction kinetics. This disadvantage is outweighed, however, by the great deal of spatial control gained when coupling a “graft from” technique with photolithographic processing. In order to determine optimal reaction times, we studied the dynamic growth of the hydrogel layer. Before modification, the static contact angle of a freshly prepared SU-8 sample was measured as 79 ± 1°. One specific hydrogel formulation (95% HEMA, 5% TEGDMA) was used to study the dynamic growth of the grafted hydrogel layer. Samples were created with varying reaction times, ranging from 10 to 50 minutes, and the resulting contact angles measured (Figure 3). The data is best described as a monotonic decrease in contact angle as a function of time. The wettability increases rapidly in the first few minutes but then slows as surface initiation sites are consumed (monomer is present in great excess and is not likely to limit the reaction).
Figure 3.
Influence of polymerization reaction time on surface wettability for 95% HEMA hydrogel on SU-8.
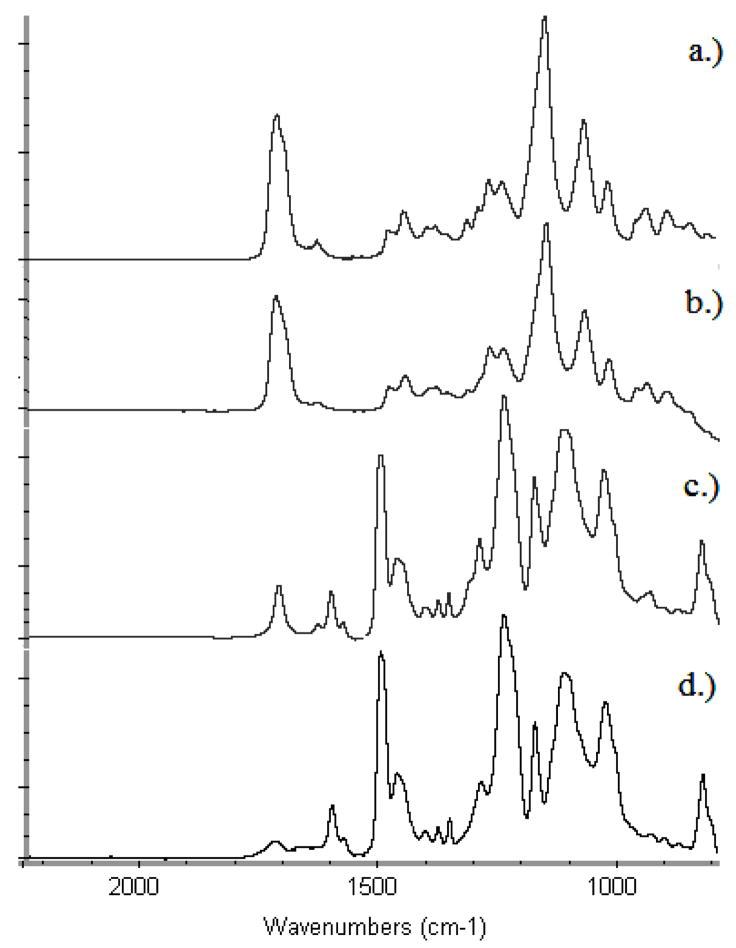
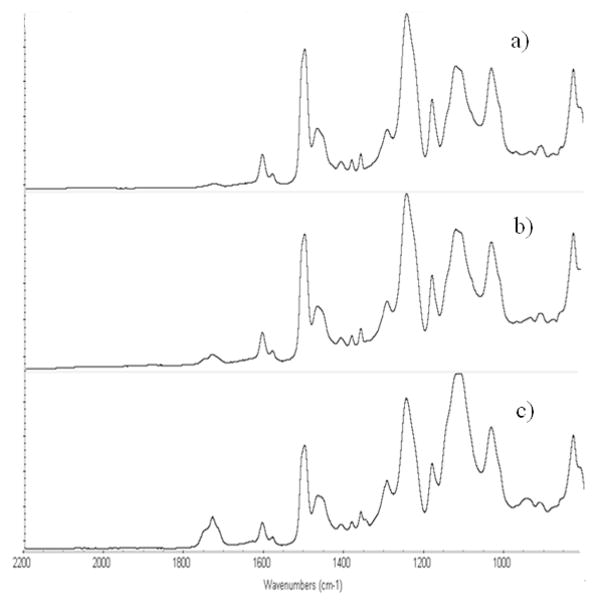
Verification of this result was accomplished through a surface chemical detection technique, ATR-FTIR (Figure 4). Shown are unmodified SU-8 substrate 4(d), surface after 10 minutes of polymerization 4(c), surface after 50 minutes of polymerization 4(b), and a reference spectrum of a bulk prepared hydrogel (also 95% HEMA: 5% TEGDMA). After 50 minutes of polymerization, the SU-8-grafted-hydrogel spectrum appears nearly identical to that of the bulk hydrogel material, indicating the presence of a distinct pHEMA layer.
Figure 4.
ATR-FTIR spectra: pHEMA photo-grafting on SU-8 surface:
a.) Reference spectrum of hydrogel (95% HEMA, 5% TEGDMA).
b.) Surface after 50 minutes of polymerization.
c.) Surface after 10 minutes of UV initiated polymerization.
d.) Unmodified SU-8 surface.
3.3 PEG surface layer
PEG is a molecule of substantial interest and is frequently used to improve the biocompatibility of materials. Modification of the SU-8 surface with PEG macromonomers resulted in a substantial decrease in the static water contact angle. As shown in Table 1, the average static contact angle for PEGDMA modified SU-8 is 8 ± 1°, which indicates high surface wettability. The PEGMA modified surface also shows a significant contact angle decrease from 79 ±1° to 23 ± 1°. The significant decrease in contact angle indicates that PEG chains have been successfully attached to SU-8 surface and make it distinctly wettable.
Table 1.
Contact angle measurement of PEG modified SU-8 surface
| Materials | Measured Contact Angle |
|---|---|
| Fresh SU-8 substrate | 79 ±1° |
| PEGDMA hydrogel on SU-8 | 8 ± 1° |
| PEGMA hydrogel on SU-8 | 23 ± 1° |
| 95% PEGMA+5% PEGDMA hydrogel on SU-8 | 22 ±2° |
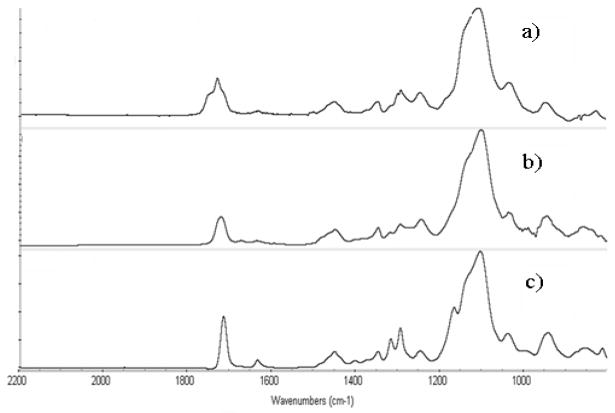
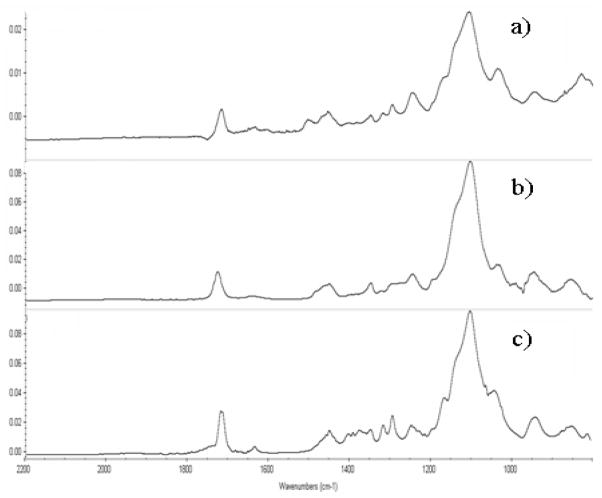
The results were confirmed using ATR-FTIR. The ATR-FTIR spectra of fresh and PEGDMA modified SU-8 is shown in Figure 5 and 6. In Figure 5(c), the characteristic adsorption band for O-C=O stretch (1727 cm −1), attributable to the methacrylated groups in the grafted PEGDMA polymer appear stronger than that in Figure 5(a). Another characteristic adsorption band for C-O stretch (1100 cm−1) was also strengthened in Figure 5(c) compared with the spectra of blank SU-8. These two differences strongly indicate that PEGDMA chains have been successfully covalently bound to SU-8 surface. To further confirm this result, a subtraction spectrum between PEGDMA modified and HCPK initiated SU-8 was determined. As shown in Figure 6, the PEGDMA subtraction spectrum is nearly identical to that of the reference spectrum, indicating the formation of PEGDMA layer. The difference between the spectrum in Figure 6(a), (b) and Figure 6(c) shows that majority of PEG monomers were polymerized on the SU-8 surface. The similar conclusion can be reached by analysis of Figure 7. Shown are subtraction spectrum between PEGDMA modified and fresh SU-8 7(a), Polymerized PEGMA hydrogel (99% PEGMA+1% HCPK) 7(b), and PEGMA monomer (Mn=475) 7(c). ATR-FTIR confirm the presence of poly(PEGMA) on the SU-8 surface.
Figure 5.
ATR - FTIR spectra: PEGDMA hydrogel on SU-8 surface:
a) Unmodified SU-8 surface after development and hard bake
b) Scan of initiator HCPK bound SU-8 surface
c) Surface after 30 minutes of polymerization of PEGDMA on SU-8 surface
Figure 6.
ATR - FTIR subtraction spectra: PEGDMA hydrogel on SU-8 surface:
a) Subtraction spectrum of PEGDMA (reference spectrum of initiator HCPK bound SU-8 surface).
b) Polymerized PEGDMA hydrogel (99%PEGMA+1% HCPK)
c) PEGDMA monomer (Mn=875)
Figure 7.
ATR - FTIR subtraction spectra: PEGMA hydrogel on SU-8 surface:
a) Subtraction spectrum of PEGMA (reference spectrum of initiator HCPK bound SU-8 surface).
b) Polymerized PEGMA hydrogel (99%PEGMA+1% HCPK)
c) PEGMA monomer (Mn=475)
3.4 Verification of Proposed Reaction
A series of comparative experiments were conducted to verify the proposed mechanism of Figure 1, and the results are shown in Table 2. Static water contact angle measurements were made on samples to illustrate the need for the various steps. In each case, polymerization was done to produce a grafted pHEMA film, with any unreacted monomer removed via washing. First, the need for an oxygen free environment is shown through loss of wettability when the steps were conducted in air. Next, the role of the surface bound initiator was established through a set of experiments. The contact angle of irradiated pHEMA on SU-8 in the absence of HCPK was not significantly different than that of freshly prepared SU-8, indicating the need for photoinitiator. Next, a set of experiments were conducted where the material was incubated in photoinitiator solution without activation with UV irradiation. This set also produced materials with no significant wettability enhancement over the control (freshly prepared SU-8) material. These results verified that the reaction proceeded as desired and that addition of activated photoinitiator is necessary for film formation.
Table 2.
Static water contact angle measurements of HEMA modified SU-8 for triple repeats in air and in oxygen free environments to verify surface initiator role
| without HCPK treatment a | without UV irradiation b | with HCPK treatment and UV irradiation c | |
|---|---|---|---|
| air | 79±1° | 78±1° | 75±3° |
| Oxygen free environment | 78±3° | 77±2° | 35±1° |
Contact angle of fresh SU-8: 79±1°
All samples received the same UV dosage and HEMA amount during the polymerization step. Only the addition of surface bound photoinitiator was altered:
Monomer and fresh SU-8 were exposed to UV light without the initial step of adding surface bound photoinitiator
SU-8 material was soaked in photoinitiator solution but no UV irradiation was used for activation.
SU-8 was modified as described previously – photoinitiator was present and activated with UV light
3.5 Photopatterning of PEGDMA
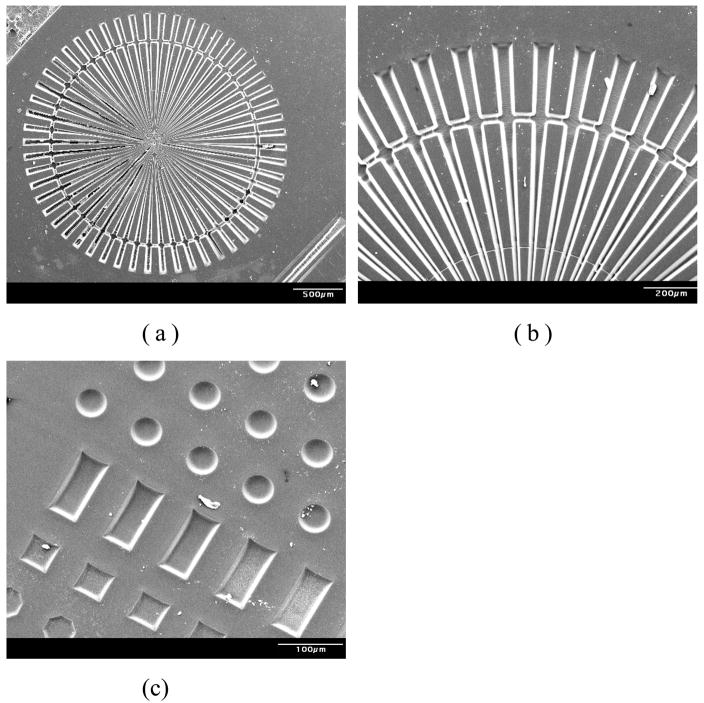
In this experiment the monomer PEGDMA was spin-coated onto an activated SU-8 surface at 600 rpm for 15 seconds. The sample was then covered with a patterning mask and exposed to UV light through a collimating lens for 30 min. The mask used here contained many various features. After exposure, PEGDMA patterns were developed with ethanol and dried to constant weight. Then samples were then stored in D.I. water for two days to observe if any delamination would occur (none seen). As these films are significantly hydrophilic and swellable, lack of delamination indicated a strong bond between the substrate and grafted film. SEM images of the PEGDMA patterns on are shown in Figures 8(a) and (b).
Figure 8.
Scanning electron micrographs of PEGDMA(Mn=875) gel microstructures on SU-8 surface.
(a) Wheel-like PEG microstructure consists of 50 μm wide rod (at outer side)
(b) SEM image of wheel-like PEG microstructure (tilt angle=60°)
(c) SEM image of photopatterned grafted layer on SU-8 surface (tilt angle=30°)
The same procedure was also used with a test mask containing circles with diameter of 50 μm, rectangles (50 μm wide and 100 μm long), and 50 μm×50 μm squares. Uniform hydrogel patterns are shown in Figure 8(c) and clearly demonstrate the precision that was attained with this technology. Profilometry results reveal that the height of patterns was approximately 7 μm.
4. Conclusions
We have presented a new technique to improve the wettability of negative-tone photoresist, SU-8 used to build the microfluidic channels on silicon wafer. This technique is amenable to photolithography and therefore affords spatial control. This technique also attempts to avoid any harsh conditions (strong oxidizers, plasma, high temperatures, etc.) that are incompatible with various molecules of interest; especially proteins (enzymes, antibodies, etc.) used in manufacture of bioMEMS devices. The ability to grow covalently bound polymer layers opens a broad range of modification techniques to integrate functional components for bioanalytical device applications. These components include photografted hydrophilic polymer brushes for reduction of protein adsorption or incorporation of various biorecognition layers such as enzyme immobilized hydrogel.
Acknowledgments
This work was funded by grants from the NSF (ECS0427360) and NIH (EB006611-01) as well as by the Intelligent System Center, Missouri University of Science and Technology (formerly University of Missouri-Rolla).
Footnotes
Publisher's Disclaimer: This is an author-created, un-copyedited version of an article accepted for publication in Journal of Micromechanics and Microengineering. IOP Publishing Ltd is not responsible for any errors or omissions in this version of the manuscript or any version derived from it. The definitive publisher authenticated version is available online at DOI#10.1088/0960-1317/18/4/045013.
References
- 1.Madou M. Fundamentals of Microfabrication. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 2.Lorenz H, Despont M, Fahrni N, Brugger J, Vettiger P, Renaud P. High-aspect-ratio, ultrathick, negative-tone near-UV photoresist and its applications for MEMS. Sensors and Actuators A. 1998;63:33–9. [Google Scholar]
- 3.Bilenberg B, Hielson T, Clausen B, Kristensen A. PMMA to SU-8 bonding for polymer based lab-on-a-chip systems with integrated optics. J Micromech Microeng. 2004;14:814–818. [Google Scholar]
- 4.Wu CL, Chen MH, Tseng FG. SU-8 hydrophilic modification by forming copolymer with hydrophilic epoxy molecule. 7th International Conference on Miniaturized Chemical and Biochemical Analysis Systems; Squaw Valley, CA, USA. Oct, 2003. pp. 1117–20. [Google Scholar]
- 5.Nordstrom M, Marie R, Calleja M, Boisen A. Rendering SU-8 hydrophilic to facilitate use in micro channel fabrication. J Micromech Microeng. 2004;14:1614–7. [Google Scholar]
- 6.Wang Yuli, Pai Jeng-Hao, Lai Hsuan-Hong, Sims Christopher E, Bachman Mark, Li GP, Allbritton Nancy L. Surface graft polymerization of Su-8 for bio-MEMS applications. J Micromech Microeng. 2007;17:1371–80. [Google Scholar]
- 7.Tseng F-G, Lin K-H, Hsu H-T, Chieng C-C. A surface-tension driven fluidic network for precise enzyme batch-dispensing and glucose detection. Sensors and Actuators A. 2004;111:107–117. [Google Scholar]
- 8.Walther F, Davydovskaya P, Zurcher S, Kaiser M, Herberg H, Gigler AM, Stark RW. Stability of the hydrophilic behavior of oxygen plasma activated SU-8. J Micromech Microeng. 2007;17:524–31. [Google Scholar]
- 9.Sung C, Sobarzo MR, Merrill EW. Synthesis and characterization of polymer networks made from poly(ethylene oxide) and polysiloxane. Polymer. 1990;31:556–63. [Google Scholar]
- 10.Kim P, Jeong HE, Suh KY. Fabrication of non-biofouling polyethylene glycol micro- and nanochannels; Korea-US NanoForum; Seoul, Korea. April 3–4, 2006; [DOI] [PubMed] [Google Scholar]
- 11.Lan S, Mandana V, Zhang M. Surface modification of silicon and gold-patterned silicon surfaces for improved biocompatibility and cell patterning selectivity. Biosensors and Bioelectronics. 2005;20:1697–708. doi: 10.1016/j.bios.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Voskerician G, Shive MS, Shawgo RS, Recum HV, Anderson JM, Cima MJ, Langer R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials. 2003;24:1959–67. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 13.Ma H, Davis RH, Bowman CN. A novel sequential photoinduced living graft polymerization. Macromolecules. 2000;33:331–5. [Google Scholar]
- 14.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 15.Fornasiero F, Krull F, Prausnitz JM, Radke CJ. Steady-state diffusion of water through soft-contact-lens materials. Biomaterials. 2005;26:5704–16. doi: 10.1016/j.biomaterials.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Arica MY, Hasirci VN. Immobilization of glucose oxidase in poly(2-hydroxyethyl methacrylate) membranes. Biomaterials. 1987;8:489–95. doi: 10.1016/0142-9612(87)90087-1. [DOI] [PubMed] [Google Scholar]
- 17.Arica MY, Hasirci V. Immobilization of glucose oxidase: a comparison of entrapment and covalent bonding. J Chem Technol Biotechnol. 1993;58:287–92. doi: 10.1002/jctb.280580313. [DOI] [PubMed] [Google Scholar]
- 18.Harris JM, Zalipsky S. Poly(ethylene glycol) chemistry and biological application. Washington DC: American Chemical Society; 1997. [Google Scholar]