To the Editor
Shin and colleagues detail an elegant study of collateral perfusion in the ischemic penumbra of mice treated with induced hypertension after distal middle cerebral artery occlusion (MCAO).1 Using optical imaging techniques, they demonstrate that early augmentation of systemic blood pressure with phenylephrine after MCAO improves collateral flow from ipsilateral leptomeningeal branches of the anterior and posterior cerebral arteries. Such collateral perfusion is noted to markedly improve cerebral blood flow (CBF) in penumbra and core, with associated enhancement of oxygenation and cerebral metabolic rate of oxygen in these ischemic regions. After prompt reversal of distal MCAO, infarct size is reduced by induced hypertension in concentric fashion around a focus emanating from the site of distal MCAO. There are subtle yet critical details embedded in this sophisticated cerebrovascular pathophysiology experiment that must be considered regarding the potential of induced hypertension and other therapeutic approaches using collateral perfusion in acute ischemic stroke patients.2
Changes in collateral perfusion due to induced hypertension included an increase in CBF without corresponding alteration of cerebral blood volume (CBV). Most prior animal studies analyzed CBF without multiparametric evaluation of perfusion. The authors note that this finding of unaltered CBV has been replicated in the few published cases of induced hypertension in human stroke. CBV is often markedly elevated in peripheral regions of the ischemic territory, especially at early time points. Under such conditions, further elevation of CBV is unlikely and, in fact, the effects of induced hypertension may be inherently linked to the pressure-passive nature of collateral perfusion in these peripheral borderzones. In deeper regions and with increasing time from onset, CBV instability or collapse and the resultant increase in downstream resistance may preclude any potential beneficial effects of induced hypertension. Realization of induced hypertension as an effective treatment option for stroke patients will likely depend on discerning these other dimensions of cerebral perfusion beyond CBF.
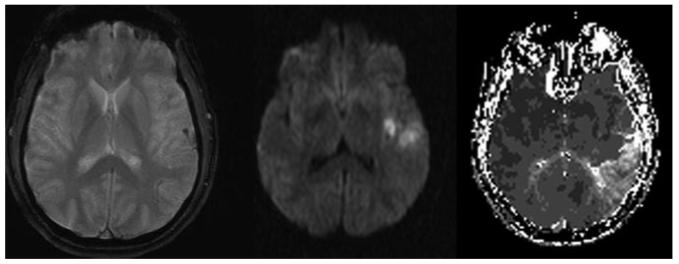
The spatiotemporal characterization of collateral perfusion in mice also illustrated marked discrepancy with collateral circulation in humans.3 Shin et al reveal that the ischemic core widely distributed across the cortical surface of the ischemic field beyond distal MCAO is situated only microns from penumbra at the borderzones fed by arterial anastomoses. The location or topography of ischemic core and penumbra may be quite different in humans compared to mice. Distal MCAO in humans may demonstrate extensive regions of penumbra with core restricted to the immediate vicinity of the arterial occlusion (Figure). The site of arterial occlusion and corresponding collateral flow patterns, Willisian or leptomeningeal, will also influence strategies such as induced hypertension. The pattern of infarct reduction due to hypertensive therapy in mice overtly demonstrated a direct correlation with collateral topography. Variability of collateral flow across species, strains, and individuals, however, will ultimately determine the effect of collateral therapeutics. Prior animal studies have accounted for arterial collateral anastomoses, yet downstream determinants of effective perfusion such as venous anatomy have not been considered.
Figure.
Distal left MCAO in the setting of acute stroke. GRE sequence (left) reveals blooming artifact associated with thrombus in a cortical arterial branch. DWI (middle) shows ischemic changes approximating the ischemic core in a limited region of tissue immediately beyond the MCAO. Time-to-peak perfusion MRI sequence (right) illustrates the delayed flow of collateral perfusion to a far more extensive region of penumbra.
Other aspects of this experimental study underscore potential obstacles for translation of induced hypertension as a practical and effective treatment in acute ischemic stroke. Shin et al initiated hypertensive therapy only 10 or 60 minutes after arterial occlusion, an incredibly early time point in acute stroke cases. In this hyperacute epoch, revascularization would likely be top priority. Furthermore, successful reperfusion after 60 minutes as used in this study also remains an elusive goal in the clinical realm. Distal MCAO is also not the most common scenario in humans where collateral failure, penumbral hemodynamics, and evolving infarction predominate. In acute MCAO, systemic blood pressure is often highest in those individuals with poor collaterals demonstrated at angiography.4 Induced hypertension would likely not be an option in such cases.
Despite these translational obstacles, Shin et al unequivocally demonstrated the most effective neuroprotective approach possible—delivery of oxygenated blood to the penumbra. They also inadvertently established evidence for successful vasoprotection in the ischemic penumbra. CBF and oxygen delivery to the penumbra progressively increased throughout the duration of induced hypertension. This effect may be due to amelioration of endothelial ischemia in distal arterial segments, enhancing retrograde collateral flow. Cyclic iterations of this vasoprotective mechanism may underlie this unusual and novel finding. Consideration of these neurovascular concepts and further study in humans may advance the role of various collateral therapeutics, from hemodynamic approaches to hyperoxia and hypothermia.
Footnotes
Disclosures
None.
References
- 1.Shin HK, Nishimura M, Jones PB, Ay H, Boas DA, Moskowitz MA, Ayata C. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39:1548–1555. doi: 10.1161/STROKEAHA.107.499483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liebeskind DS. Collateral therapeutics for cerebral ischemia. Expert Rev Neurother. 2004;4:255–265. doi: 10.1586/14737175.4.2.255. [DOI] [PubMed] [Google Scholar]
- 3.Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Liebeskind DS, Starkman S, Jo KD, Ohanian AG, Sayre JW, Yun S, Kim D, Ali LK, Ovbiagele B, Towfighi A, Shah SH, Jahan R, Duckwiler GR, Vinuela F, Saver JL. Blood pressure in acute stroke is inversely related to the extent of collaterals. Stroke. 2008;39:538. [Google Scholar]