Abstract
The Clade B lineage of the New World arenaviruses contains four viruses capable of causing severe hemorrhagic fevers in humans. Within this group, the B1 sub-lineage contains the pathogenic viruses Junin (JUNV) and Machupo (MACV), as well as the non-pathogenic Tacaribe virus (TCRV). In order to elucidate differences that may determine pathogenicity, we studied the entry pathways directed by the glycoproteins (GPs) from these related B1 viruses, using pseudotyped retroviral vectors and GP1 immunoadhesin constructs. Our data revealed variations in the efficiency with which different cell types could be transduced by B1 vectors, and this correlated with the ability of the immunoadhesins to bind to those cells. Interestingly, the tropism directed by the TCRV GP proved to be distinct from that of JUNV and MACV, in particular on lymphocyte cell lines. In addition, the GPs showed variations in their sensitivity to an inhibitor of endosome acidification, with the TCRV GP again being the outlier. Together these data suggest that more than one entry pathway can be used by these closely related viruses and that the ability to cause human disease may be highly dependent on receptor usage.
Keywords: arenavirus, Junin, Machupo, Tacaribe, cell receptor, tropism
Introduction
The arenaviruses are rodent-borne, single-stranded, enveloped RNA viruses. Five out of the twenty-two known members of this family cause severe hemorrhagic fevers in humans, with fatality rates up to 20% (Geisbert and Jahrling 2004), leading to their classification by the Centers for Disease Control and Prevention as Category A pathogens. They express four genes from a genome that consists of two ambisense strands of RNA. The viral glycoprotein (GP) alone is sufficient to direct the entry of these viruses into cells and retroviral vectors pseudotyped with GPs can recapitulate the entry pathway of the viruses (Reignier et al., 2006).
The arenaviruses are classified into two major groups, the Old World and the New World/Tacaribe complex viruses. The New World viruses are further subdivided into three Clades, with the Clade B lineage of particular interest since it contains four of the five significant human pathogens: Junin (JUNV), Machupo (MACV), Guanarito (GTOV) and Sabia viruses. These pathogens cause hemorrhagic fevers in Argentina, Bolivia, Venezuela and Brazil respectively, with the different geographical distributions of the viruses reflecting the habitats of their rodent hosts.
Phylogenetic analysis of the Clade B viruses revealed that the human pathogens do not group together but are distributed in 3 different sub-lineages, together with non-pathogenic members (Bowden et al., 1996; Charrel et al., 2002). For example, although JUNV and MACV are closely related, they also share a common ancestor with Tacaribe virus (TCRV) in the B1 sub-lineage (Fig. 1). JUNV and MACV both cause sever ehemorrhagic fevers in humans and are transmitted by Colomys spp. rodents, while TCRV was isolated from Artibeus spp. bats in Trinidad in the 1950s (Downs et al., 1963) and is unusual among the arenaviruses in having a non-rodent reservoir. Within the amino acid sequences of their respective GPs, JUNV and MACV are 69.4% identical, JUNV and TCRV are 65.3% identical, and MACV and TCRV are 61.3% identical. Despite the close relationship of TCRV with JUNV and MACV, it is not associated with human infections (Price et al., 1978), apart from occasional laboratory-acquired infections (Peters et al., 1996).
Fig. 1. Phylogeny of Clade B arenaviruses.
The strains that are pathogenic for humans (boxed) are distributed among the three sub-lineages. Figure adapted from phylogenetic analysis of amino acid sequences in GP based on pairwise distance algorithm and the neighbor-joining method, presented in Charrel et al., 2002.
It is becoming apparent that members of the arenavirus family have evolved to use more than one cellular receptor, and that differences in receptor usage and tropism can have a profound influence on the ability of the viruses to cause human disease (Smelt et al., 2001). While the Old World arenaviruses Lassa fever virus (LASV) and lymphocytic choriomeningitis virus (LCMV) can use α-dystroglycan (α-DG) as a cellular receptor, previous studies from our group and others have shown that the New World Clade B viruses do not require α-DG to enter cells (Reignier et al., 2006; Rojeck et al., 2006; Spiropoulou et al., 2002). Specifically, in mouse embryonic stem cells, the ability of retroviral vectors pseudotyped with Clade B GPs to transduce those cells was unaffected by a homozygous gene disruption of dystroglycan (Reignier et al., 2006; Rojeck et al., 2006). Further characterization of the nature of the alternative receptor(s) used by pathogenic Clade B viruses has determined that it is a protein, and that infection is not dependent on modification by N-glycans, O-glycans or glycosaminoglycans (Rojeck et al., 2006). It has also been suggested that the non-pathogenic B2 virus, Amapari (AMAV), uses a different receptor than JUNV, MACV or GTOV, since it shows distinct tropism patterns on a variety of cell lines and inactivated AMAV is unable to block transduction by JUNV, MACV or GTOV pseudotyped vectors (Rojeck et al., 2006).
We have previously reported that retroviral vectors pseudotyped with the GPs from JUNV and MACV display broad tropism, being able to transduce cell lines from a range of different species and tissue types (Reignier et al., 2006). However, we also observed variation in the efficiencies with which the different GP pseudotypes were able to transduce certain cell lines, with rodent cells in particular highlighting those differences. We hypothesized that this could be caused by the use of different receptors or co-receptors within the Clade B group or, alternatively, because of differential sensitivity to the form of a common receptor as it is presented on diverse cell lines. Such differences could include absolute levels of the receptor, differences at the primary amino acid sequence level, or be due to variations in post-translational modifications. There is a precedent for the latter, since differences in the glycosylation state of dystroglycan are thought to underlie the inability of LASV and LCMV to infect human lymphocytes (Imperiali et al., 2005; Kunz et al., 2005; Reignier et al., 2006).
We here report differences in the tropism directed by the GPs from the B1 lineage on cell lines from different species. In particular, we found striking differences between the GPs from the pathogenic B1 viruses, JUNV and MACV, compared to the related non-pathogenic virus TCRV, in their abilities to direct entry into human and mouse lymphocytes. This suggests that either these closely related viruses can utilize different primary receptors or co-receptors, or that if using a single common receptor for entry, that the viruses have evolved different requirements of that receptor. This, in turn, could underlie the differences in their ability to jump the species barrier and cause disease in humans.
Results
Retroviral vectors pseudotyped with Clade B1 glycoproteins display distinct tropism patterns
The GPs of the B1 arenaviruses JUNV, MACV and TCRV are closely related at the sequence level and constitute the B1 sub-lineage within the Clade B arenaviruses (Fig. 1). We have previously observed that retroviral vectors pseudotyped with the GPs from JUNV and MACV are able to transduce cell lines from a range of different species and tissue types, although, we also found some differences in their relative efficiencies (Reignier et al., 2006). To explore this further, we examined the titers of green fluorescent protein (GFP)-expressing retroviral vectors pseudotyped with the three B1 GPs on an expanded panel of cell lines. As a control we included vesicular stomatitis virus G protein (VSV G) pseudotyped vectors because this VSV G is known to have an extremely broad tropism and therefore provides a control for the level of transduction directed by the retroviral vector machinery in each cell line.
The efficiency of entry directed by each vector type was measured by FACS analysis of target cells 48 hours post-transduction, with up to 100,000 cells analyzed to ensure that even rare events could be detected. All of the vector stocks were initially titered on 293A cells, which are very permissive to all of the arenavirus GP pseudotypes that we have examined. The panel of test cell lines included various human and rodent cell lines, as well as one bat cell line (Fig. 2).
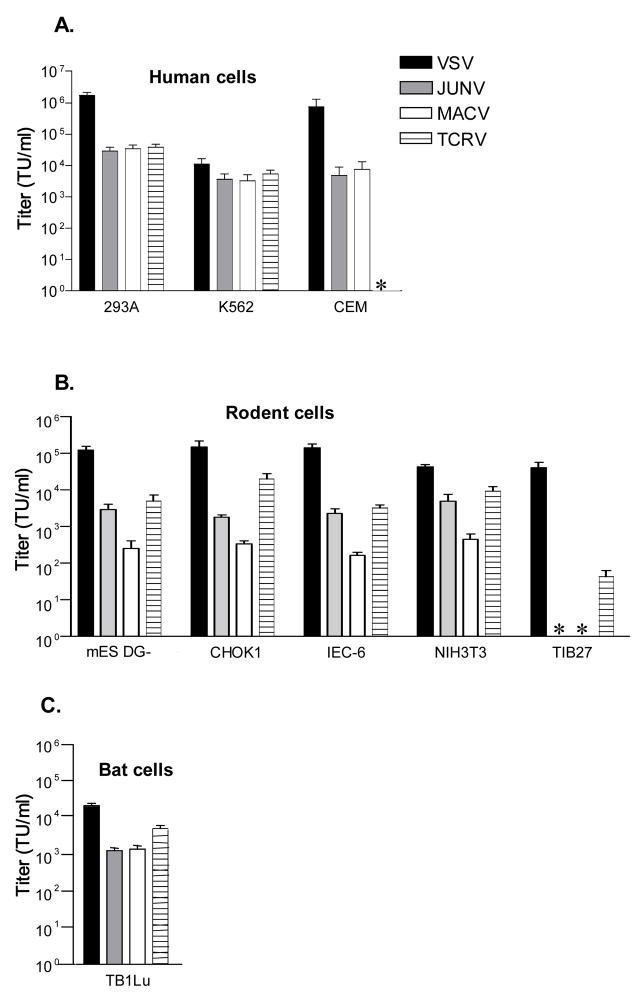
Fig. 2. Titers of pseudotyped retroviral vectors on (A) human, (B) rodent and (C) bat cells.
Titer was calculated by FACS analysis of cells for GFP expression 48 hours after transduction, and is expressed as transducing units (TU) per ml. The values shown are the mean +/− SEM for 3–6 independent experiments. *, no titer detected.
Examination of the data revealed several patterns. First, on the human cell lines, the B1 GPs directed similar titers (within the same order of magnitude) on human epithelial (293A) and erythroleukemia (K562) cells. However, on the human T lymphocytic cell line, CEM, we found that while the JUNV and MACV vectors gave titers of approximately 1 × 104 transducing units per ml, the TCRV vectors gave no titer.
In contrast, on the rodent cells lines, we observed a different but consistent pattern for the B1 GP vectors,. Here, the MACV GP was tyically one order of magnitude less efficient at directing entry than either the JUNV or TCRV GPs on mouse (mES DG-, NIH3T3), Chinese hamster (CHOK1) and rat (IEC-6) cell lines examined. An outlier to this general pattern was the mouse T lymphocyte cell line, TIB27, where neither JUNV or MACV vectors gave any titers, while the TCRV vectors resulted in a low but consistently positive titer. The single bat cell line that we examined (lung epithelial cells) was equally permissive for JUNV and MACV vectors, and slightly more so for the TCRV vectors. Together, these data highlight differences in the efficiency with which the different B1 GPs can direct entry into cell lines from different species and tissue types, and also suggest that human and rodent lymphocyte cell lines in particular can reveal differences in their requirements for entry.
Differences in tropism are most pronounced on human and rodent lymphocytes
To explore the differences that we had observed in more detail, we broadened our analysis to include a wider panel of human and mouse lymphocyte cell lines. We also generated concentrated stocks of vectors to increase the sensitivity of the assay, and allow us to be confident of the significance of relatively low titers. Controls were run to ensure that none of the titers were due to carryover of GFP or other types of pseudotransduction, as have been reported in the literature (Haas et al., 2000) (data not shown).
As shown in Table 1, the panel of human T and B lymphocyte cell lines examined showed the same pattern as the CEM cells, being susceptibility to the JUNV and MACV vectors, while being resistant to the TCRV vectors. The analysis of mouse lymphocytes comprised 3 cell lines derived from BALB/C strain mice: TIB27 and TIB53 are T lymphocyte lines derived from a lymphoma and thymoma respectively, while CRL1608 cells are B lymphoblasts. This expanded panel revealed further differences in the tropism of the B1 GPs; in all 3 cases, TCRV pseudotyped vectors were able to transduce the cells while MACV vectors did not. The pattern observed with the JUNV vectors was intermediate, being unable to transduce TIB27 cells, but giving titer on the other 2 cell types, albeit at very low levels.
Table 1. Titers of vectors on lymphocyte cell lines.
| Vector stock | 293A human epithelial | CEM human T | Jurkat human T | Nalm 6 human B | TIB27 mouse T | TIB53 mouse T | CRL1608 mouse B |
|---|---|---|---|---|---|---|---|
| JUNV | 2.4 × 104 | 4.9 × 103 | 5.0 × 102 | 1.2 × 102 | 0 | 9.8 × 101 | 1.6 × 101 |
| JUNV (conc.) | 2.5 × 106 | NT | NT | NT | 0 | 6.4 × 101 | 3.2 × 101 |
| MACV | 6.0 × 104 | 7.7 × 103 | 1.5 × 103 | 1.8 × 103 | 0 | 0 | 0 |
| MACV (conc.) | 1.1 × 106 | NT | NT | NT | 0 | 0 | 0 |
| TCRV | 4.1 × 104 | 0 | 0 | 0 | 4.3 × 101 | 3.9 × 102 | 2.6 × 102 |
| TCRV (conc.) | 1.2 × 106 | 0 | 1.3 × 101 | 0 | 1.9 × 103 | NT | NT |
Conc., concentrated vector stocks. NT, not tested.
Immunoadhesin binding patterns reflect the observed titer differences
To investigate whether the differences in tropism we observed were manifest at a binding or post-binding stage of the entry process, we next asked if the differences in titer were also observed at the level of binding/interaction with receptor. To do this, we constructed a panel of immunoadhesins for the Clade B1 GPs. These molecules comprise the GP1 subunit of the arenavirus GP which, by comparison to other class I viral fusion proteins, has been implicated in receptor binding (Parekh and Buchmeier, 1986), fused to the Fc portion of rabbit immunoglobulin (IgG). Similar constructs have been made using other viral fusion proteins and show good correlation between the range of cells that can be infected or transduced and the binding of the immunoadhesin (Adkins et al., 1997; Jones et al., 2002). As a control, we also included an immunoadhesin containing the LASV GP1. Since the GP1 component of each immunoadhesin is predicted to be extensively glycosylated, the actual molecular weight of the final immunoadhesins could not be predicted, but Western blotting revealed Fc-containing proteins in the expected range of molecular weights for the MACV, TCRV and LASV immunoadhesins, although not for JUNV (Fig. 3A). (The yield of immunoadhesins in the culture supernatant was too low to reliably observe the secreted proteins by Western blotting.)
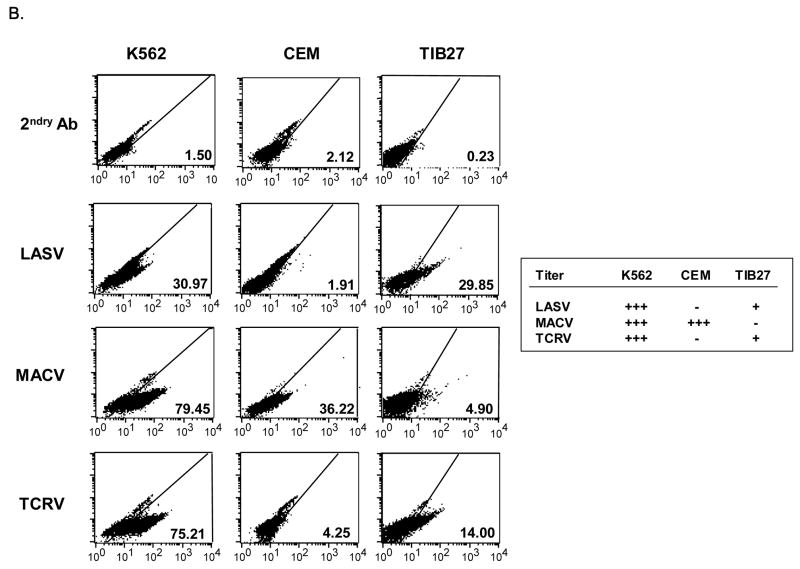
Fig. 3. GP1 immunoadhesin binding to cells.
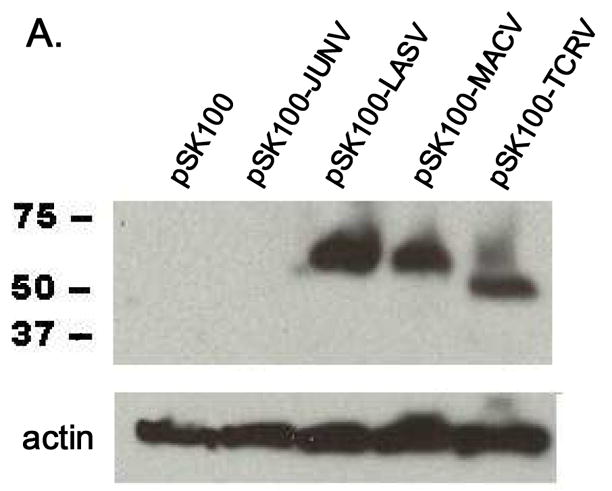
(B) Western blot of 293T cell lysates expressing immunoadhesins, probed with anti-rabbit IgG antibody. pSK100 is the Fc cloning vector alone. The JUNV construct was not detected and is presumably not expressed or unstable. (B) Binding of LASV, MACV and TCRV immunoadhesins to indicated cell lines. The Fc portion of the immunoadhesin was detected with a goat anti-rabbit secondary antibody labeled with FITC, followed by FACS analysis. The percentage of cells staining positive in each sample is shown. For comparison, the table indicates whether the various GP vector/cell combinations resulted in titer: +++ is > 103 TU/ml, + is 101 TU/ml.
Examination of the patterns of immunoadhesin binding revealed that, in general, the binding activity of the immunoadhesins mirrored the titer results we observed (Fig. 3B). For example, on K562 cells, which are susceptible to transduction by LASV, MACV and TCRV vectors, all three immunoadhesins showed good binding. In contrast, human lymphocytes such as CEM cells showed no binding over the background control sample for either the LASV or TCRV immunoadhesins, in agreement with the lack of titer we observed on these cells, while the MACV immunoadhesin was able to bind to these cells efficiently. Finally, on the rodent lymphocyte cell line TIB27, we found that the TCRV immunoadhesin was able to bind, while the MACV reagent did not, again recapitulating the vector transduction observations. The only exception to this general pattern was found with the LASV immunoadhesin and TIB27 cells. Here we observed reasonably good binding by the immunoadhesin although the titers of LASV GP vectors on this cell are extremely low (data not shown). Taken together our data suggest that the different tropism we observe for the Clade B1 GPs is primarily determined at the level of GP1 interaction with the cell surface receptor.
The pH dependence of B1 entry pathways is cell-type specific
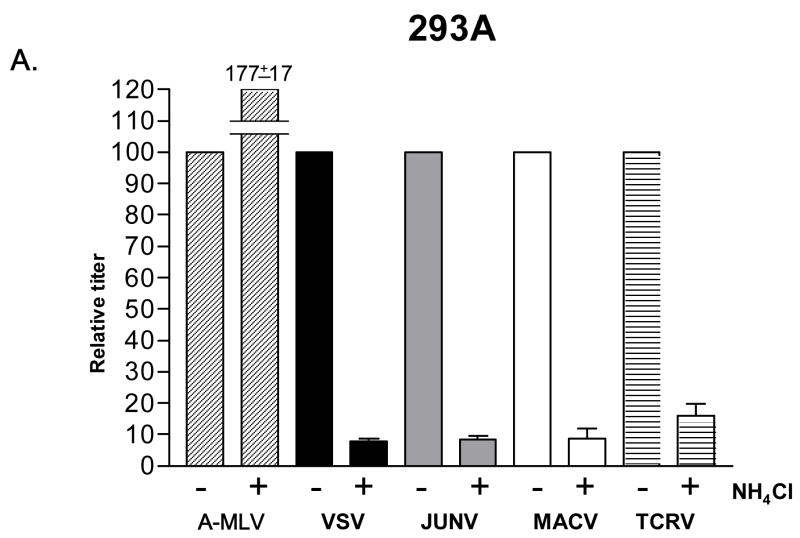
Viral entry pathways can be classified as pH-dependent or pH-independent, depending on whether the acidic environment of an endosome is a necessary condition to trigger virus-cell fusion (Eckert and Kim, 2001). We examined the requirements for low pH in the entry pathways directed by the Clade B1 GPs by measuring the titers achieved in the presence and absence of ammonium chloride (NH4Cl), a lysosomotropic agent that blocks endosome acidification. As controls we included VSV G and amphotropic murine leukemia virus Envelope (A-MLV Env) pseudotyped vectors, since their entry is known to be pH-dependent and independent respectively (Carneiro et al., 2001; Katen et al., 2001; Nussbaum et al., 1993; McClure et al., 1990). Cell viability, as analyzed by flow cytometry, revealed no significant differences between control and treated cells.
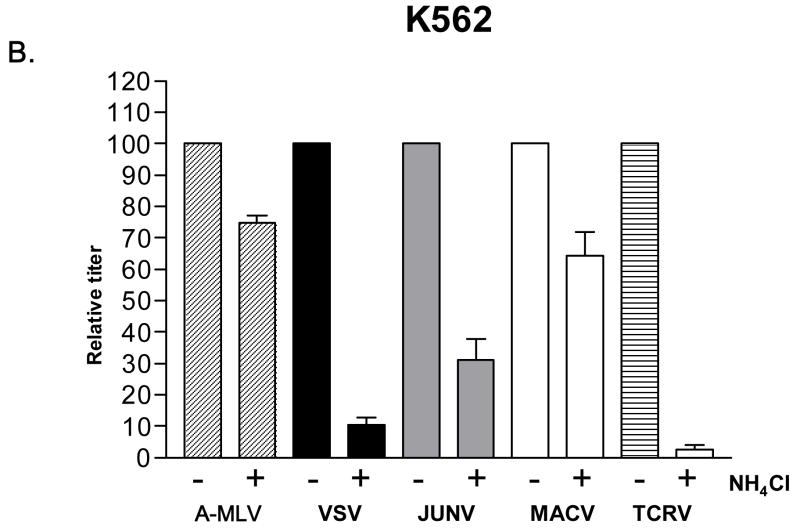
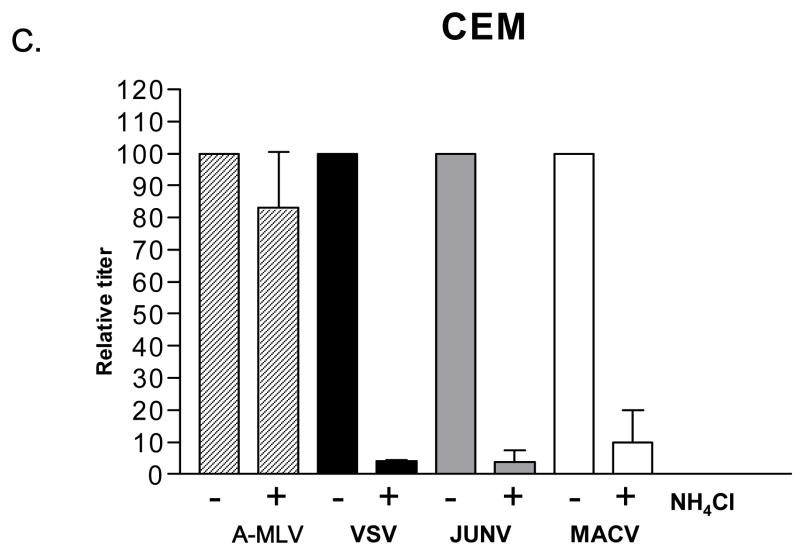
When this analysis was performed on human 293A cells (Fig. 4A), all of the Clade B1 GPs exhibited pH-dependence, with titers dropping one order of magnitude in the presence of NH4Cl. This is a typical response for a pH-dependent virus (Katen et al., 2001; Kahn et al., 2990) and a similar drop was seen for the VSV G control, but not for the A-MLV Env control. However, when we repeated this analysis using K562 cells, we found that only TCRV vectors remained strongly pH-dependent, with the JUNV and MACV pseudotyped vectors being only partially inhibited (40–65%). Finally, analysis on CEM cells revealed that the B1 viruses that can infect these cells (JUNV and MACV) are using a pH-sensitive entry pathway. Together, these findings suggest that the entry pathways directed by the B1 GPs may differ between the different viruses, and on different cell types.
Fig. 4.
NH4Cl sensitivity. Effect of 50 mM NH4Cl treatment on the titer of indicated vectors on (A) 293A cells, (B) K562 cells and (C) CEM cells. Stocks of VSV G, JUNV, MACV and TCRV vectors were used that gave titers on untreated 293A cells that were equivalent to those shown in Figure 2A; the A-MLV Env vector stock used had an average titer of 1.6 × 106 TU/ml on untreated 293A cells. In each case, the titers obtained in the presence of NH4Cl were made relative to the values obtained in the absence of the drug (100%). The values shown are the mean +/− SEM for n>4 independent experiments.
Discussion
The tropism or host range of a virus is a key determinant of its pathogenicity. In the arenaviruses, the Old World viruses LASV and LCMV, as well as the New World Clade C viruses, are able to use the broadly expressed cellular protein α-dystroglycan as a cellular receptor (Cao et al., 1998; Spiropoulou et al., 2002). LASV in particular seems to be highly dependent on this molecule (Kunz et al., 2005; Reignier et al., 2006), although LCMV can clearly use another receptor(s) (Smelt et al., 2001; Kunz et al., 2004). The other human pathogens in the arenavirus family reside in the Clade B lineage. The GPs from these viruses also exhibit broad tropism, suggesting a widely expressed receptor, but do not require α-DG for entry (Reignier et al., 2006. Rojeck et al., 2006; Spiropoulou et al., 2002).
In this study, we analyzed the tropism directed by the GPs from the Clade B1 viruses JUNV, MACV and TCRV. We used pseudotyped retroviral vectors that act as surrogates for the entry pathway used by the native viruses, and GP1-Fc immunoadhesin constructs to study the binding of the GP1 subunit to both permissive and resistant cell lines. These studies revealed interesting differences between these three related GPs, both in their host ranges and the pH sensitivity of the entry pathways that they use, most notably that the TCRV GP displayed properties that were distinct from the MACV and JUNV GPs.
The main difference in tropism that we observed amongst the B1 viruses was manifest in lymphocytes. Specifically, TCRV GP pseudotyped vectors could not transduce human B or T cell lines, while MACV and JUNV vectors could. Conversely, TCRV vectors could transduce mouse T and B cell lines while MACV, and to a lesser extent JUNV vectors, were restricted on these lines. The titer pattern that we observed was mirrored by the behavior of immunoadhesins containing only the GP1 subunit, with binding efficiency correlating well with titer. Therefore, GP1 alone is able to bind to cells in a pattern that recapitulates the tropism of the complete GP when carried by retroviral vectors. This suggests that the defects in entry we observed are at the level of binding of GP1 to a receptor.
Differences in the ability of native viruses or pseudotyped retroviral vectors to enter cells can arise for various reasons. In the simplest case, it is caused by the absence of the required cellular receptor or co-receptor on the surface of the cells. Other possibilities relate to the location of the receptor on a cell, for example whether it has an apical or basolateral distribution on polarized cells (Fuller et al., 1984; Fuller et al., 1985), and the presence or absence of any post-translational modifications. We and others have previously shown that the LASV GP cannot direct entry into human lymphocytes (Kunz et al., 2005; Reignier et al., 2006) and it has been suggested that this is due to the glycosylation state of α-DG in these cells (Imperiali et al., 2005; Kunz et al., 2005). Lymphocytes exhibit varied glycosylation states that are related to their differentiation state (Daniels et al., 2002). It is possible therefore that a similar situation exists with regard to the ability of B1 viruses to use receptors on lymphocytes. Alternatively, different levels of receptor expression in these cells may underlie the tropism differences that we observed.
Viral entry can also be blocked at a post-binding stage, as the virus-receptor interaction can sometimes lead to sequestration into non-productive entry pathways (Nilson et al., 1996). In particular, the fusion proteins from many enveloped viruses require an additional step in their entry process beyond simple receptor binding, for example requiring the low pH environment of an endosome to trigger the post-binding conformational changes needed for virus-cell fusion (Eckert and Kim, 2001). Accordingly we examined the pH-dependence of B1 entry using a classic inhibitor of endosome acidification, NH4Cl. Previously it has been reported that JUNV is sensitive to such inhibitors (Castilla et al., 1994). In human 293A cells, all of the B1 pseudotyped vectors exhibited the typical one order of magnitude decrease in titer in the presence of NH4Cl that indicates a pH-sensitive entry pathway. However, human K562 cells revealed a different pattern, with only the TCRV vectors remaining strongly pH-dependent among the three Clade B1 GPs. Finally, we also examined the pH-sensitivity of entry for JUNV and MACV vectors on CEM cells (which cannot be transduced by TCRV vectors). In both cases the vectors were sensitive to NH4Cl treatment.
It is possible that the different sensitivities to the presence of NH4Cl that we observed within the B1 GPs arose because of variations in the stability of these GPs. The ability to complete entry when NH4Cl is removed will be dependent on the overall stability of a viral fusion protein, and this can lead to the appearance of different pH sensitivities. For example, this has been shown to underlie the differences between the ecotropic and amphotropic subtypes of MLV Env (Katen et al., 2001), which had previously been considered pH-dependent and independent respectively (McClure et al., 1990). However we believe that the situation with the different Clade B1 GPs is more complicated than this explanation would allow, since the different responses to NH4Cl treatment were cell line specific as well as GP specific, and are therefore more likely to result from the use of distinct entry pathways by the GPs, at least in the K562 cell line. Instead, our findings suggest that JUNV and MACV may be able to access an alternate, non pH-dependent pathway in K562 cells that is not available to the TCRV GP in these same cells, and which is also not available to any of the GPs in either 293A or CEM cells.
Overall, we repeatedly observed that the TCRV GP behaved differently than either the JUNV or MACV GP, despite the relatedness of these three viruses. It is tempting to suggest that the acquisition of the ability to use the JUNV/MACV entry pathway was critical in allowing these viruses to cross the species barrier and infect humans. Similarly intriguing are the observations of Kunz et al., (2006), who reported evidence that the non-pathogenic B2 virus, Amapari (AMAV), uses a different receptor than either JUNV, MACV, or the other known B2 virus, GTOV. Together these findings suggest that the New World Clade B viruses have evolved to use more than one receptor. Whether TACV and AMAV can use the same alternate receptor, and whether there is a correlation between receptor use and ability to cause human disease remains to be seen. But if so, it suggests that receptor use will be a good predictor of pathogenicity for any new viruses that may emerge within this family, and that the GP-receptor interaction will be an important therapeutic target.
Materials and methods
Cell lines
293A, 293T, NIH3T3, CHOK1, TIB27 and TIB53 cells were maintained in D10 medium: Dulbeccos modified Eagle’s medium (DMEM) (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and 2 mM glutamine (Gemini Bio-Products, West Sacramento, CA). IEC-6 cells were maintained in D10 medium supplemented with 0.1 U/ml of bovine insulin (Sigma, St. Louis, MO). K562, Jurkat, CEM, TB1Lu and CRL1608 cells were maintained in R10 medium: RPMI (Mediatech) supplemented with 10% FBS and 2 mM glutamine. Nalm-6 cells were maintained in D10 supplemented with 0.02% β-mercaptoethanol (Sigma) and FBS tested for B cell culture. Dystroglycan knockout (−/−) R1 murine embryonic stem cells (mES DG-) (Williamson et al., 1997) were generously provided by Dr. Kevin Campbell (University of Iowa) and cultured in DMEM supplemented with 20% FBS, 2 mM glutamine, 1 mM non-essential amino acids (Chemicon, Temecula, CA), 0.001% β-mercaptoethanol (Sigma) and 103 units/ml of murine leukemia inhibiting factor (ESGRO, Chemicon). All cells were maintained in 5% CO2 except TIB27, TIB53, CRL1608, IEC-6 and TB1Lu cells, which were maintained in 10% CO2.
Retroviral vector production and titer determination
Pseudotyped retroviral vectors displaying arenavirus GPs were produced as described (Christodoulopoulos and Cannon, 2001; Reignier et al., 2006). The retroviral vector genome used was pMND-eGFP, which expresses enhanced green fluorescent protein (eGFP) as a reporter gene. The arenavirus GPs used were from LASV (Josiah strain, accession no. AY628203), JUNV (Parodi strain, accession no. U70799), MACV (Carvallo strain, accession no. AY129248) and TCRV (TRVL 11598 strain (accession no. P31840, with substitutions at two positions, R233H and E446R) (Reignier et al., 2006). All GPs were cloned into the expression vector pCAGGS (Niwa et al., 1991).
Vector supernatants were harvested 48 hrs post-transfection, filtered through a 0.45 μm filter (Millipore, Bedford, MA) and stored in aliquots at −80°C. When necessary, supernatants were also concentrated by layering over 6 ml of 20% w/v sucrose, followed by ultracentrifugation at 25,000 rpm for 2 hrs. in a Beckman SW28 rotor. Titers were determined by incubation of serially diluted vector stocks with target cells for 48 hrs., followed by FACS analysis for eGFP expression and calculation of percent positive cells. The titer of vector stocks was expressed as transducing units per ml (TU/ml).
Immunoadhesin production, Western blot and binding assay
Immunoadhesin constructs were generated by fusing GP1 sequences, including the native signal peptide, upstream of a rabbit IgG heavy chain Fc sequence (hinge-CH2-CH3), contained in the expression plasmid pSK100 (generously provided by Dr. J. Young, Salk Institute). 293T cells seeded in poly-L-lysine-coated 10-cm plates were transiently transfected with 20 μg of plasmid using the calcium phosphate transfection technique (Christodoulopoulos and Cannon 2001). Forty-eight hrs. post-transfection, supernatants were collected (15ml per plate), filtered through 0.45 μm syringe filters, and concentrated using Centricon Plus-20 30 kD MWCO centrifugal filter units (Millipore). Briefly, 19 ml of supernatant was added to each filter unit and centrifuged at 3000 rpm for 45 min. The units were reloaded 1 or 2 times with up to 19 ml of additional supernatant. To elute the immunoadhesins, the units were inverted and centrifuged for 1 min. at 1000 rpm. Eluates were frozen in 30 μl aliquots at −80°C until use.
Immunoadhesin production was analyzed by Western blot. Cell lysates were harvested and 25 μg total protein resolved on 4–20% gradient SDS-polyacrylamide gels (Bio-Rad). Following transfer to a PVDF membrane, immunoadhesins were detected using HRP-conjugated anti-rabbit IgG (Sigma) diluted 1:5000 in phosphate buffered saline (PBS) plus 0.1% Tween-20 (Sigma), and specific bands were visualized using ECL detection reagent (GE/Amersham Biosciences, Piscataway, NJ). To ensure equal protein loading, membranes were also probed with mouse anti-actin IgG diluted 1:5000, followed by HRP-conjugated anti-mouse IgG (Sigma) diluted 1:10,000.
The binding activity of each batch of immunoadhesin was determined by staining 5 × 106 K562 cells with up to 30 μl of a thawed aliquot, as described below. This allowed samples to be normalized between batches. Binding of the immunoadhesins to various human, rodent and bat cell lines was assayed as follows: between 5 × 105 and 1 × 106 cells were resuspended in blocking buffer (0.5% bovine serum albumin in PBS) and incubated 20 min. on ice. After washing in 1 ml of PBS, cells were resuspended in an aliquot of thawed immunoadhesin, incubated 30 min. on ice, washed again, resuspended in 50 μl of a 1:50 dilution of FITC-conjugated anti-rabbit IgG (BD Pharmingen, San Jose, CA) in PBS, and incubated 20 min. on ice. Cells were washed twice with 1 ml of PBS, resuspended in 400 μl of 4% paraformaldehyde and analyzed on a FACSCAN flow cytometer (BD, Franklin Lakes, NJ). Unstained cells were initially gated by forward and side scatter (FCS and SSC) to identify the viable cell population. Cells stained with only the secondary antibody were analyzed and used to set the gate for the test samples (R2 population) and the percentage of positive cells in the R2 population in each test sample was measured.
pH-dependence assay
Five × 105 K562 or 293A cells were pre-treated for 1 hr. with 50 mM NH4Cl (Sigma). One ml of vector supernatant, containing 50 mM NH4Cl, was added to the cells and incubated for 2 hrs. The vector supernatants were removed and the cells were incubated for 3 hrs. in 2 ml D10 medium containing 50 mM NH4Cl. The media was then removed, the cells were washed in PBS, and replated in 2 ml fresh D10 medium (no drug). Following incubation for a further 48 hrs., the vector titers were determined by FACS analysis as described above. Negative (no drug) controls were run in parallel, and the percentage titer for the drug treatment arm compared to control cells was calculated for each vector and cell line combination.
Acknowledgments
This work was supported by PHS grant 1U54 AI065359 to the Pacific Southwest Regional Center of Excellence (PSWRCE) for Biodefense and Emerging Infectious Diseases. We thank our colleagues within the PSWRCE for helpful discussions and reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins HB, Brojatsch J, Naughton J, Rolls MM, Pesola JM, Young JA. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc Natl Acad Sci USA. 1997;94:11617–11622. doi: 10.1073/pnas.94.21.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MD, Peters CJ, Nichol ST. The phylogeny of New World (Tacaribe complex) arenaviruses. Virology. 1996;219:285–290. doi: 10.1006/viro.1996.0248. [DOI] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MBA. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–81. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Carneiro FA, Ferradosa AS, Da Poian AT. Low pH-induced conformational changes in vesicular stomatitis virus glycoprotein involve dramatic structure reorganization. J Biol Chem. 2001;276:62–67. doi: 10.1074/jbc.M008753200. [DOI] [PubMed] [Google Scholar]
- Castilla V, Mersich SE, Candurra NA, Damonte EB. The entry of Junin virus into Vero cells. Arch Virol. 1994;136:363–374. doi: 10.1007/BF01321064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel RN, Feldmann H, Fulhorst CF, Khelifa R, de Chesse R, de Lamballerie XK. Phylogeny of New World arenaviruses based on the complete coding sequences of the small genomic segment identified an evolutionary lineage produced by intrasegmental recombination. Biochem Biophys Res Commun. 2002;296:1118–1124. doi: 10.1016/s0006-291x(02)02053-3. [DOI] [PubMed] [Google Scholar]
- Christodoulopoulos I, Cannon PM. Sequences in the cytoplasmic tail of the gibbon ape leukemia virus envelope protein that prevent its incorporation into lentivirus vectors. J Virol. 2001;75:4129–4138. doi: 10.1128/JVI.75.9.4129-4138.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- Downs WG, Anderson CR, Spence L, Aitken THG, Greenhall AM. Tacaribe virus, a new agent isolated from Artibeus bats and mosquitoes in Trinidad, West Indies. Am J Trop Med Hyg. 1963;12:640–646. doi: 10.4269/ajtmh.1963.12.640. [DOI] [PubMed] [Google Scholar]
- Eckert DM, Kim PS. Mechanism of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- Fuller S, von Bonsdorff CH, Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984;38:65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Fuller SD, von Bonsdorffm CH, Simons K. Cell surface influenza haemagglutinin can mediate infection by other animal viruses. EMBO J. 1985;4:2475–2485. doi: 10.1002/j.1460-2075.1985.tb03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10(12 Suppl):S110–121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Haas DL, Case SS, Crooks GM, Kohn DB. Critical factors influencing stable transduction of human CD34(+) cells with HIV-1-derived lentiviral vectors. Mol Ther. 2000;2:71–80. doi: 10.1006/mthe.2000.0094. [DOI] [PubMed] [Google Scholar]
- Imperiali M, Thoma C, Pavoni E, Brancaccio A, Callewaert N, Oxenius A. O Mannosylation of alpha-dystroglycan is essential for lymphocytic choriomeningitis virus receptor function. J Virol. 2005;79:14297–14308. doi: 10.1128/JVI.79.22.14297-14308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KS, Nath M, Petrow-Sadowski C, Baines AC, Dambach M, Huang Y, Ruscetti FW. Similar regulation of cell surface human T-cell leukemia virus type 1 (HTLV-1) surface binding proteins in cells highly and poorly transduced by HTLV-1-pseudotyped virions. J Virol. 2002;76:12723–12734. doi: 10.1128/JVI.76.24.12723-12734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katen LJ, Januszeski MM, Anderson WF, Hasenkrug KJ, Evans LH. Infectious entry by amphotropic as well as ecotropic murine leukemia viruses occurs through an endocytic pathway. J Virol. 2001;75:5018–5026. doi: 10.1128/JVI.75.11.5018-5026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JS, Schnell MJ, Buonocore L, Rose JK. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology. 1999;254:81–91. doi: 10.1006/viro.1998.9535. [DOI] [PubMed] [Google Scholar]
- Kunz S, Sevilla N, Rojek JM, Oldstone MBA. Use of alternative receptors different than α–dystroglycan by selected isolates of lymphocytic choriomeningitis virus. Virology. 2004;325:432–445. doi: 10.1016/j.virol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Kunz S, Rojek JM, Kanagawa M, Spiropoulou CF, Barresi R, Campbell KP, Oldstone MBA. Posttranslational modification of alpha-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol. 2005;79:14282–14296. doi: 10.1128/JVI.79.22.14282-14296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson BHK, Morling FJ, Cosset FL, Russell SJ. Targeting of retroviral vectors by protease-substrate interactions. Gene Ther. 3:280–286. [PubMed] [Google Scholar]
- Nussbaum O, Roop A, Anderson WF. Sequences determining the pH dependence of viral entry are distinct from the host range-determining region of the murine ecotropic and amphotropic retrovirus envelope proteins. J Virol. 1993;67:7402–7405. doi: 10.1128/jvi.67.12.7402-7405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure MO, Sommerfelt MA, Marsh M, Weiss RA. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990;71:767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Parekh BS, Buchmeier MJ. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology. 1986;153:168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Price JL. Serological evidence of infection of Tacribe virus and arbovirus in Triinidadian bats. Am J Trop Med Hyg. 1978;27:162–167. doi: 10.4269/ajtmh.1978.27.162. [DOI] [PubMed] [Google Scholar]
- Peters CJ, Buchmeier M, Rollin PE, Ksiazek TG. Arenaviruses. In: Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman R, Straus SE, editors. Fields Virology. 3. Lippincott-Raven Publishers; Philadelphia, PA: 1996. pp. 1521–1551. [Google Scholar]
- Reignier T, Oldenburg J, Noble B, Lamb E, Romanowski V, Buchmeier MJ, Cannon PM. Receptor use by pathogenic arenaviruses. Virology. 2006;353:111–120. doi: 10.1016/j.virol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Rojek JM, Spiropoulou CF, Kunz S. Characterization of the cellular receptors for the South American hemorrhagic fever viruses Junin, Guanarito, and Machupo. Virology. 2006;349:476–491. doi: 10.1016/j.virol.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Smelt SC, Borrow P, Kunz S, Cao W, Tishon A, Lewicki H, Campbell KP, Oldstone MBA. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics. J Virol. 2001;75:448–57. doi: 10.1128/JVI.75.1.448-457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MBA. New World arenavirus clade C, but not clade A and B viruses, utilizes a-dystroglycan as its major receptor. J Virol. 2002;76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]