Abstract
Hepato-subcellular effect of Ang II and ethanol on the p42/44 MAP Kinase and MEK1/2 were investigated in the nucleus of rat hepatocytes. Hepatocytes were treated with ethanol (100 mM) for 24 hr and stimulated with angiotensin II (Ang II, 100 nM, 5 min). The levels of p42/44 MAPK and MEK1/2 were monitored in the nuclear fraction using antibodies. Ang II itself caused significant accumulation of phospho-p42/44 MAPK in the nucleus without any significant translocation of p42/44 MAPK protein there by suggesting activation of p42/44 MAPK in the nucleus. Ang II caused marked accumulation of phospho-MEK 1/2 in the nucleus without any significant accumulation of MEK1/2 protein. Ratio of phospho-MEK 1/2 to MEK 1/2 protein in the nucleus after Ang II treatment was 2.4 times greater than control suggesting phosphorylation of MEK 1/2 inside the nucleus. Ethanol had no effect on the protein level or the activation of p42/44 MAPK in the nucleus. Ethanol treatment potentiated nuclear activation of p42/44 MPAK by Ang II but not translocation of p42/44 MAPK protein. This was accompanied by potentiation of Ang II stimulated accumulation of phospho-MEK 1/2 in the nucleus by ethanol. MEK 1/2 inhibitor, U-0126 inhibited Ang II response or its potentiation by ethanol. These results suggest that Ang II mediated accumulation of phospho-p42/44 MAPK in the hepatocyte nucleus involves MEK 1/2 dependent activation and this effect is potentiated by ethanol.
Keywords: Angiotensin II, Ethanol, Mitogen activated protein kinase, MAP kinase kinase, Nuclear translocation, Hepatocytes
Introduction
Acute and chronic alcohol consumption results in a spectrum of altered hepatic functions including changes in redox state, alterations in lipid and carbohydrate metabolism, as well as protein and DNA synthesis (Lieber, 1988; Dey and Cederbaum, 2006). Although the biological effects of acute and chronic ethanol ingestion are well documented, the underlying mechanisms remain largely unknown. Among the key signaling pathways regulating mammalian cellular events is the p42/44 MAP kinase (p42/44 MAPK, ERK 1/2) cascade (Katz et al., 2007; Zebisch et al., 2007). The activation of p42/44 MAPK signaling has been reported to mediate stimulation of DNA synthesis, suppression of DNA synthesis, induction of c-myc, and suppression of apoptosis in hepatocytes depending on the tone, duration and stimulus (Adachi et al, 1996; Tombes et al., 1998; Qiao et al, 2002). There is overwhelming evidence that different MAP kinases are modulated by ethanol in hepatocytes (Aroor and Shukla, 2004; Zima and Kalousová, 2005; Venugopal et al., 2007). Ethanol potentiates serum stimulated p42/44 MAPK activation in embryonic liver cells (Reddy and Shukla, 1996) and enhances Gi-protein stimulated p42/44 MAPK activity in hepatic tumorigenic cells (McKillop, et al., 1999). Ethanol prolongs p42/44 MAPK activation induced by EGF, NGF, insulin and IGF in primary cultured hepatocytes (Chen et al., 1998; Tombes et al., 1998). Moreover, up regulation of p42/44 MAPK signaling has been reported in liver biopsy specimens from patients with alcoholic liver disease (Nguyen and Gao, 2002).
A key step in the p42/44 MAPK signaling cascade is its translocation into the nucleus where it phosphorylates transcription factors including c-myc, c-fos, Stat1/3 and Elk-1 involved in cell growth, differentiation and apoptosis (Chen et al., 1992; Brunet et al, 1999; Turjanski, et al., 2007; Whitmarsh et al., 2007). However, the nuclear translocation of p42/44 MAPK is stimulus specific (Traverse et al., 1992) and cell type specific (Menice et al., 1997). In addition, cytosolic and nuclear p42/44MAPKs can be differentially regulated (Wang et al., 1996; Whitehurst et al., 2004). Although nuclear activation of p42/44 MAPK was considered to be the consequence of nuclear translocation of p42/44 MAPK, recent studies indicate translocation of MEK1/2 and MEK1/2-dependent nuclear activation of p42/44 MAPK (Kim and Kahn, 1997; Tolwinski et al., 1999; Mizukami et al., 2000). Ethanol modulates serum activated p42/44 MAPK in the nucleus of BNLCL2 cells (Reddy and Shukla, 2000). Ethanol induces nuclear translocation of p42/44 MAPK and selective activation of p38 MAPK in hepatocyte nucleus (Lee and Shukla, 2008).
Angiotensin II (Ang II), the active component of renin-angiotensin system plays a major role in the regulation of blood pressure (Schulman et al., 2007). However, recent studies have shown role for Ang II in growth, inflammatory, hemodynamic and metabolic responses in various tissues including liver (Brasier et al., 2000; Bataller et al., 2005; McAllister-Lucas et al., 2007). Ang II increases glycogenolysis (Blackmore and Exton, 1985) and acts as a comitogen for hepatocyte DNA synthesis (Dajani et al., 1996). Angiotensin II causes induction of early genes (González-Espinosa and Garcia-Sainz, 1992) and activates NF-kB in hepatocytes (Brasier et al., 2000; McAllister-Lucas et al., 2007). Increases in plasma Ang II levels are seen during both acute and chronic alcohol consumption in humans (Collins et al., 1992).
Although Ang II, vasopressin, insulin and epinephrine significantly increased p42/44 MAPK activity in hepatocytes, ethanol exposure potentiated only Ang II and epinephrine stimulated p42/44 MAPK, indicating an agonist selective effect of ethanol on p42/44 MAPK signaling in hepatocytes (Weng and Shukla, 2000). We report here the effects of ethanol and/or Ang II on upstream MEK1/2 signaling in the nucleus of primary cultures of hepatocytes.
Materials and methods
Materials
Ang II, benzamidine, β-glycerophosphate, aprotinin, leupeptin, and pepstatin A were obtained from Sigma-Aldrich (St. Louis, MO). The antibodies for phospho-p42/44 MAPK, p42/44 MAPK, phospho-MEK 1/2, MEK 1/2, phospho- p38 MAPK, phospho- JNK, phospho- Elk-1 were purchased from Cell Signaling Technology, Inc.(Beverly, MA). Anti-acetyl histone H3 K9 antibody was from Upstate (Charlottesville, VA) The goat anti-rabbit IgG antibody conjugated with horseradish peroxidase was purchased from Bio Rad (Herculeus, CA). U-0126 (MEK1/2 inhibitor) was purchased from EMD biosciences (Madison, WI).
Isolation and culture of hepatocytes
Hepatocytes were isolated from male Sprague-Dawley rats (150–200g) by collagenase-perfusion method as described previously (Seglen, 1976; Weng and Shukla, 2000). The use of rats for these experiments was approved by the University of Missouri Animal Care & Use Committee. Hepatocytes were plated on to collagen coated culture dishes (7.5 × 106 cells/100 mm dishes) in Dulbecco’s modified Eagles Medium (DMEM) containing 10% fetal bovine serum. Following an initial 2 h to allow the cells to attach, cells were washed with PBS and the media replaced with DMEM containing 0.1% FBS with or without ethanol. In order to avoid evaporation of ethanol, dishes were sealed with parafilm. After 24 hr, cells were subjected to desired treatments. We have shown earlier that after 24 hr treatment of cells with ethanol (100 mM) about 80 % of ethanol was still present in the medium (Weng and Shukla, 2000). Cell viability assessed by the exclusion of trypan blue was about 90 % before or after ethanol treatments (Weng and Shukla, 2000, 2002). Furthermore, hepatocytes isolated by this procedure showed Ang II activation of phosphorylase a/glycogenolysis, (Weng and Shukla, 2003) and elicited various receptor responses including Ca2+ mobilization, and MAP kinase, tyrosine kinase activations (Weng and Shukla, 2002). Hepatocytes used were therefore functionally viable.
Preparation of nuclear extracts
Following treatments, cells were washed with ice-cold PBS and then lysed using hypotonic lysis buffer (20 mM HEPES, pH 7.4, 10 mM β-glycerophosphate, 1 mM EDTA, 1 mM Na-orthovanadate, 2 mM MgCl2, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 1 mM benzamidine, and 10 μg/ml each of aprotinin, leupeptin and pepstatin A) containing 10% glycerol. Cells were allowed to swell for 15 min followed by homogenization by passing through a 26-gauge needle 10 times. The homogenate was centrifuged at 500 g for 10 min at 4 °C and the resulting nuclear pellet was resuspended in hypotonic lysis buffer containing 0.2% NP-40 but without glycerol. The nuclear suspension was passed through a 26-gauge needle 6 times, followed by centrifugation at 500 X g for 10 min. This step successfully removed contaminated endoplasmic reticulum. The nuclear pellet was resuspended in 0.5 ml hypotonic lysis buffer and layered over 45% sucrose cushion. After centrifugation at 1600 X g for 30 min, the pellet containing nuclei was washed once with hypotonic buffer and examined under light microscope for purity of nuclei that are devoid of membrane contamination and other subcellular organelles. The isolated nuclei preparations were solubilized using hypotonic lysis buffer containing 1% SDS and boiling for 5 min. The nuclear preparations were sonicated for 5 sec to reduce viscosity. After centrifugation at 14000 X g for 10 min, the supernatant was used as nuclear fraction. Protein concentration was measured using a Bio-Rad DC protein assay kit.
SDS-PAGE and immunoblotting
The nuclear fractions (40μg protein) were combined with equal volume of 2X Laemelli buffer and fractionated on 10% polyacrylamide gels. The proteins were electrophoretically transferred onto nitrocellulose membrane (Bio-Rad). The membrane was washed with 20 mM Tris, pH 7.5 containing 0.1 % Tween-20 and 150 mM NaCl (TBST) and incubated with TBST containing 5 % nonfat dry milk for 1 hour at room temperature. After washing, blots were incubated with specific primary antibodies in TBST buffer containing 3% BSA overnight at 4 °C. The blots were washed with TBST and incubated with 1:3000 diluted horse radish peroxidase conjugated sheep anti-rabbit secondary antibody for one hour at room temperature. The protein bands were detected by an enhanced chemiluminescence (ECL) reaction (Pierce) and exposed to X-ray film. Quantitative results were determined by laser densitometry. For repeat immunoblotting, membrane was stripped in 62.5 mM Tris-HCl, pH 6.7, 2 % SDS, and 0.1 M β-mercaptoethanol for 30–45 min at 50 °C.
Statistical Analysis
Data are expressed as mean ± S.E.M. Differences between control and experimental groups were checked for statistical significance (P < 0.05) by the Student’s t test (two-tailed, paired).
Results
We have shown earlier that primary cultures of hepatocytes can be exposed to ethanol concentrations up to 200 mM for 24 h without affecting their viability (Weng and Shukla, 2000). It was also shown that at least 12 hr of treatment with ethanol is required to observe the potentiating effects of ethanol on Ang II stimulated phosphorylation of p42/44 MAPK. The maximum potentiation is noted at 24 hr. The potentiating effects are seen at 50–200 mM ethanol (Weng and Shukla, 2000). The concentration of Ang II (100 nM) and the time of stimulation (5 min) were thus selected based on observations from this laboratory (Weng and Shukla, 2000; 2002; 2003, Park et al; 2006) to investigate ethanol effects on nuclear translocation of the kinases. Accordingly, hepatocytes were treated with 100 mM ethanol for 24 h and were subsequently challenged with 100 nM Ang II for 5 min and the samples were then processed as needed. We have selected 100 mM ethanol to increase the sensitivity of the detection of nuclear translocation of p42/44 MAPK and MEK 1/2. In vivo concentrations of ethanol in chronic alcoholics have been observed as high as 300 mM (Deitrich and Harris 1996; Shukla et al., 2007).
Nuclear p42/44 MAPK after ethanol and/or Ang II treatment of hepatocytes
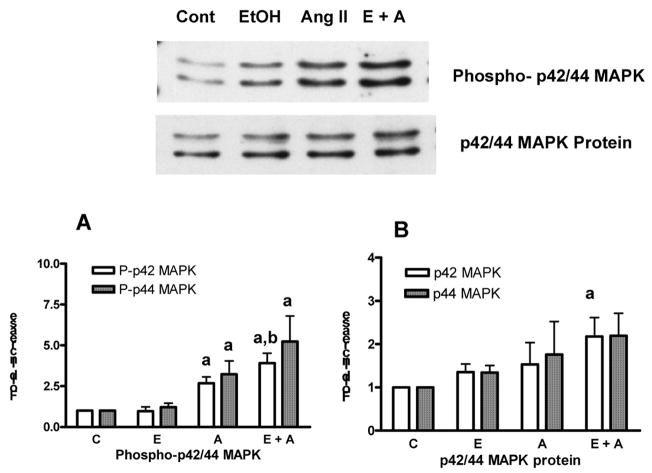
In this study, we first determined the levels of activated p42/44 MAPK in the nucleus of hepatocytes using polyclonal antibody specific for phospho-p42/44 MAPK that recognizes the activated form. Treatment of hepatocyte with ethanol (100 mM) for 24 h did not significantly affect phosphorylation of p42/p44 MAPK (Fig.1A). Next we determined p42/44 MAPK protein levels in the nuclear extracts. The p42/44 MAPK protein level was moderately increased (1.4 and 1.3 fold respectively) in ethanol treated cells compared to controls (Fig. 1B). However, the magnitude of increase in p42/44 MAPK protein accumulation observed by ethanol was not statistically significant. We next investigated whether ethanol modulated Ang II responses in the nucleus. Ang II (100 nM) treatment for 5 min increased phosphorylation of p42 and p44 MAPK by 2.7 fold and 3.2 fold, respectively in hepatocytes. Ang II increased p42 and p44 MAPK phosphorylation by 3.9 and 5.2 fold, respectively, in hepatocytes pretreated with ethanol for 24 h (Fig 1A) over the control. Thus, ethanol potentiated Ang II stimulated p42 and p44 MAPK activity by 1.4 and 1.5 fold respectively, over Ang II alone (no ethanol) stimulated phsopho-p42 and p 44 MAPK. Nuclear accumulation of phospho-p42/44 MAPK after ethanol pretreatment followed by Ang II stimulation was statistically significant for phospho-p42 MAPK and the values reached near significance for phospho-p44 MAPK (0.051) compared to nuclear accumulation of phospho-p42 MAPK and phospho-p44 MAPK stimulated by Ang II alone (no ethanol). Ang II stimulation (100 nM, 5 min) also caused a moderate increase in p42/44 MAPK protein (1.5 and 1.8 fold) in the nuclear fraction (Fig. 1B). After treatments with ethanol (100 mM, 24 h), further increase in the magnitude of p42/44 MAPK protein accumulation in the nucleus (2.2 and 2.2 fold, respectively) was seen when hepatocytes were stimulated with Ang II (Fig. 1B). Although the magnitude of increase in p42 MAPK protein observed with ethanol pretreatment followed by Ang II stimulation was statistically significant compared to controls, the nuclear accumulation of p44 MAPK protein after ethanol pretreatment followed by Ang II stimulation was not statistically significant (p=0.07) compared to controls. The nuclear accumulation of p42/44 MAPK protein after ethanol pretreatment followed by Ang II stimulation was not significant compared to stimulation by Ang II alone (no ethanol).
Fig. 1. Nuclear accumulation of phospho-p42/44 MAPK and p42/44 MAPK protein in response to ethanol and/or Ang II.
Hepatocytes were exposed to 100 mM ethanol for 24 hr followed by stimulation with Ang II for 5 min. Nuclear extracts were prepared and aliquots were examined by Western blotting using phospho-p42/44 MAPK and p42/44 MAPK antibodies as described under “Methods”. Upper panel shows a representative western blot image. The lower panel is data compiled using densitometric analysis. A: Phospho-p42/44 MAPK; B: p42/44 MAPK protein. The Values represented are mean ± S.E.M. (bars), n = 6. a, p < 0.05 compared with corresponding unstimulated samples and b, p < 0.05 compared with Ang II stimulated samples (C; control, E; ethanol, and A; Ang II)
We have previously reported persistent accumulation of phospho-p38 MAPK at 24 hr after ethanol treatment and transient accumulation of phospho-JNK at 15 min to 2 hr in hepatocyte nucleus (Lee and Shukla, 2008). However, Ang II alone did not cause significant activation of either p38 MAPK or JNK under the conditions employed for the activation of phospho p42/44 MAPK (100 nM Ang II, 5 min). Moreover, ethanol did not modulate Ang II induced activation of p38 MAPK and JNK in rat hepatocytes (data not shown).
Nuclear translocation of MEK ½ after ethanol and Ang II
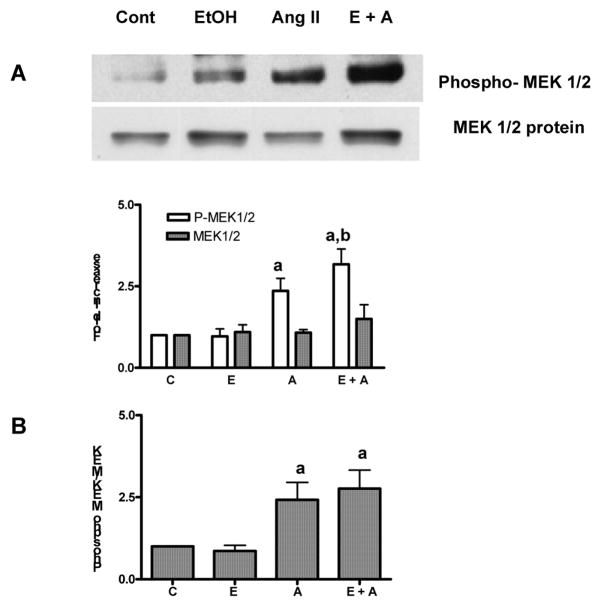
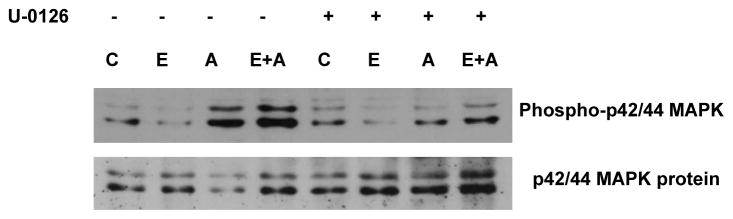
MEK 1/2 is upstream kinase, which phosphorylates p42/44 MAPK. MEK1/2 itself is phosphorylated to be active. Phosphorylated MEK 1/2 gets translocated into the nucleus and can activate p42/44 MAPK (Tolwinski et al., 1999). Although MEK 1/2 is localized mainly in the cytosol because of the nuclear export signal in MEK (Fukuda et al., 1997), there is also a transient or constitutive increase in the phospho MEK 1/2 in the nuclear compartment (Mizukami et al., 2000). Therefore, we monitored the nuclear activation and localization of phospho-MEK 1/2, using antiphospho-MEK 1/2 antibody that recognizes the phosphorylation site (ser-217/ser-222) necessary for the activation. Accumulation of phospho-MEK1/2 was not significant after exposure of hepatocytes to ethanol for 24 h (Fig. 2A). Ang II (100 nM) treatment caused significant increase (2.4 fold) in phospho-MEK1/2 in control hepatocytes. The accumulation of Ang II activated phospho-MEK1/2 was increased by 3.2 fold in hepatocytes pre-treated with ethanol. Thus, ethanol significantly (p < 0.02) potentiated Ang II stimulated nuclear accumulation of phospho-MEK 1/2 activity by 1.3 fold over its accumulation by Ang II (no ethanol) alone. The level of MEK 1/2 protein was not significantly different in the nuclear fraction after ethanol treatment. In contrast to significant accumulation of phospho- MEK1/2 by Ang II, the level of MEK1/2 protein was not increased in the hepatocyte nucleus after Ang II stimulation (Fig. 2A). Although nuclear accumulation of MEK1/2 protein was moderately increased (1.5 fold) by combined treatment of ethanol and Ang II. (Fig. 2A), the magnitude of increase was not statistically significant. It is striking that Ang II caused a large increase in phospho-MEK 1/2 in the nuclear fraction but a corresponding increase in MEK1/2 protein was not observed (Fig. 2A). Therefore, we compared the ratio of the increases in phosphorylated MEK 1/2 to MEK 1/2 protein. It became apparent that Ang II caused a substantial increase in the activation of MEK1/2 inside the nucleus (2.4 fold, Fig. 2B) These results suggest that increased levels of nuclear phospho-p42/44 MAPK, after Ang II stimulation of hepatocytes, may be due to nuclear p42/44 MAPK activation by phospho-MEK 1/2. The ratio of increase in phosphorylated MEK 1/2 to MEK protein is further increased after ethanol exposure (2.8 fold, Fig. 2B), but the magnitude of increase by ethanol plus Ang II was not statistically significant compared to Ang II alone. Although potentiation of nuclear accumulation of phospho-p42/44 MAPK by ethanol is through potentaition of Ang II mediated MEK 1/2 phosphorylation, nuclear translocation of p42/44 MAPK to nuclear compartment may be secondary to MEK1/2 activation. This is likely because moderate increase of p42/44 MAPK protein in the nucleus was observed after combined Ang II and ethanol treatment of hepatocytes. In order to examine the role of MEK1/2 activation in translocation of p42/44 MAPK protein, hepatocytes were treated with MEK 1/2 inhibitor U-0126 before the addition of ethanol and Ang II. Nuclear accumulation of phospho–p42/44 MAPK was significantly decreased after Ang II or ethanol treatment followed by Ang II stimulation but nuclear translocation of p42/44 MAPK protein was not affected after treatment with MEK 1/2 inhibitor (Fig.3).
Fig. 2. Nuclear accumulation of phospho-MEK1/2 and MEK 1/2 in response to ethanol and/or Ang II.
Hepatocytes were exposed to 100 mM ethanol for 24 hr followed by stimulation with Ang II for 5 min. Nuclear extracts were prepared and aliquots were examined by Western blotting using phospho-MEK1/2 or MEK 1/2 antibody as described under “ Methods ”. A. Upper panel shows a representative western blot image. The lower panel is data compiled using densitometric analysis. B. Ratio of the fold increases in phospho-MEK 1/2 to MEK 1/2 protein after different treatments are presented. Values represented are mean ± S.E.M. (bars), n = 6. a, p < 0.05 compared with corresponding unstimulated samples and b, p < 0.05 compared with Ang II stimulated samples (C; control, E; ethanol, and A; Ang II)
Fig. 3. Effect of MEK 1/2 inhibitor on nuclear accumulation of phospho-p42/44 MAPK and p42/44 MAPK protein in response to ethanol and/or Ang II.
Hepatocytes were pretreated with 10 μM MEK 1/2 inhibitor U-0126 for 1 hr and exposed to 100 mM ethanol for 24 hr. After 24 treatment with ethanol, hepatocytes were stimulated with 100 nM Ang II for 5 min. Nuclear extracts were prepared and aliquots were examined by Western blotting using phospho-p42/44 MAPK and p42/44 MAPK antibodies as described under “Methods”. Upper panel shows a representative phospho-p42/44 MAPK western blot image and lower panel shows representative of p42/44 MAPK protein. Results are representative of three independent experiments from three different hepatocyte preparations. C; control, E; ethanol, and A; Ang II
We have reported inhibition of ethanol induced histone H3 K9 acetylation by MEK 1/2 inhibitor in hepatocytes (Park et al., 2005). Although Ang II caused modest increase in histone H3 K9 acetylation, ethanol did not potentiate Ang II induced histone H3 acetylation (data not shown). We have also examined phosphorylation of nuclear p42/44 MAPK substrate Elk-1. Ang II caused increase in phosphorylation of Elk-1 and this was inhibited by U-0126. However, phosphorylation of Elk-1 was lower in hepatocytes treated with ethanol followed by Ang II stimulation compared to Ang II alone treated cells (data not shown).
Discussion
This is the first report on activation of phospho MEK 1/2 and phospho-p42/44 MAPK induced by Ang II in hepatocyte nucleus and their potentiation by ethanol. The presence of phospho-p42/44 MAPK and p42/44 MAPK protein in the nucleus of unstimulated cell has been reported in several cells including hepatocytes (Kim and Kahn, 1997; Goetze, et al, 1999., Tolwinski et al., 1999; Mizukami et al., 2000., Rosseland et al., 2005). This may be due to a basal level of shuttling of p42/44 MAPK and MEK 1/2 between cytoplasm and nucleus (Fukuda et al., 1997; Adachi et al., 2002). We have previously reported activation of p42/44 MAPK by ethanol and accumulation of phospho-p42/44 MAPK in hepatocyte nucleus between 1 and 3 h after exposure of hepatocytes to ethanol (Lee and Shukla, 2005; Lee and Shukla, 2008). However, accumulation of phosphorylated p42/44 MAPK was not significant at 24 hr after ethanol treatment. In this regard, phosphatases that were thought to inactivate p42/44 MAPK in the nuclear compartment were shown to be newly synthesized in response to p42/44 MAPK activation. It remains to be known whether the nuclear inactivation of p42/44 MAPK were mediated by the inducible nuclear MKPs encoded by dual specificity phosphatases such as MKP-1, MKP-2 and MKP-5 (Brunet et al., 1999., Whitehurst et al., 2004., Owens et al., 2007). Ethanol has been shown to increase the expression of MKP-1 in gastric mucosal cells (Kawanaka et al; 2001) and human hepatoma cell line (Venugopal et al., 2007).
Notwithstanding ethanol potentiation of nuclear activation of p42/44 MAPK by Ang II by ethanol, nuclear accumulation of phospho-p42/44 MAPK by Ang II alone is intriguing. Nuclear accumulation of p42/44 MAPK can occur by nuclear translocation of phosphorylated p42/44 MAPK after its activation by MEK1/2 in the cytosol; or nuclear activation of through translocation of MEK 1/2; or nuclear activation of MEK 1/2 in the nuclear compartment by upstream kinase eg. PKC. Although, previous studies failed to show nuclear translocation of MEK1/2 in response to activation, these results were later modified demonstrating translocation of MEK1/2 to the nucleus independently of activation and these were rapidly removed from the nucleus by its nuclear export signal (Fukuda et al., 1997). Reactivation of inactive form of p42/44 MAPK retained in the nucleus by nuclear targeting of MEK1/2 has also been reported (Mandl et al., 2005) Protein kinase C (PKC) has been reported to modulate both nuclear translocation of p42/44 MAPK and nuclear accumulation of MEK1/2 (Mizukami et al., 2000). Ang II has been shown to cause PKC ζ dependent activation and nuclear accumulation of p42/44 MAPK (Goetze et al., 1999). Moreover, src mediated p42/44 MAPK activation by Ang II was mainly localized in the cytosolic compartment where as G-protein and PKCζ mediated p42/44 MAPK by Ang II was localized to the nuclear compartment (Godney and Sayeski, 2006). In contrast to nuclear activation of p42/44 MAPK by Ang II alone, the nuclear accumulation of p42/44 MAPK protein by Ang II was not significant suggesting mechanisms independent of nuclear translocation of p42/44 MAPK are contributing to increased accumulation of phospho-p42/44 MAPK. On the other hand, accumulation of phospo-p42/44 MAPK mainly occurs by nuclear activation of MEK 1/2 without any significant nuclear translocation of MEK 1/2. It may be noted that MEK1/2 can be activated within the nucleus by protein kinase Cζ (Mizukami et al., 2000). We have previously reported role of PKC in Ang II stimulation of p42/44 MAPK phosphorylation in hepatocytes (Weng and Shukla, 2002). Therefore a role of PKC in the activation of MEK1/2 in hepatocyte nucleus cannot be excluded at present.
Ethanol potentiation of MEK 1/2 phosphorylation induced by Ang II suggests role of activation of MEK 1/2 activation for enhanced phosphorylation of p42/44 MAPK in the hepatocyte nucleus in cells pretreated with ethanol followed by Ang II stimulation. Although mean increase of p42/44 MAPK protein by ethanol plus Ang II was significant compared to control, the magnitude of increase by ethanol pretreatment followed by Ang II stimulation was not significant compared to treatment with Ang II alone (no ethanol). These results suggest nuclear translocation of p42/44 MAPK does not play significant role in ethanol potentiation of Ang II stimulated nuclear accumulation of phospho-p42/44 MAPK.
MAPK pathways provide an important link between agonist induced cell stimulation and the nucleus through phosphorylation of nuclear substrates including transcription factors. A perplexing aspect of p42/44 MAPK signaling in general is the magnitude and duration of p42/44 MAPK signaling in the nuclear compartment in regulating different responses in the same cell. For example, proliferative response is characterized by robust but transient activation of p42/44 MAPK in the nucleus (Tolwinski et al., 1999). In contrast, cell cycle arrest and differentiation were shown to be associated with low but persistent activation of nuclear p42/44MAPK (Adachi et al., 2002). We have reported the antiapoptotic role of ethanol induced p42/44MAPK activation (Lee and Shukla, 2005). MAP kinases also modulate ethanol induced epigenetic histone modifications. We have recently shown dependence of ethanol induced histone H3 acetylation on p42/44 MAPK (Park et al., 2005) and of histone H3 phosphorylation on p38 MAPK (Lee and Shukla, 2008). However, acetylation of histone H3 induced by Ang II alone was not marked and acetylation of histone H3 in cells pretreated with ethanol followed by Ang II was lower than sum of Ang II and ethanol induced acetylation. We have also examined the effects of ethanol and Ang II on phosphorylation of Elk-1 (a downstream target of p42/44 MAPK). Phosphorylation of Elk-1 was lower in cells pretreated with ethanol followed by Ang II stimulation compared to cells treated with Ang II alone. Dissociation of Elk-1 phosphorylation from p42/44 MAPK activation has recently been reported. In PC12 cells, Ndrg4 enhanced NGF-induced p42/44 MAPK activation but this was not coupled with Elk-1 activation (Hongo et al., 2006). Recently, we have reported dissociation of ethanol potentiation of Ang II stimulated p42/44 MAPK phosphorylation from p42/44 MAPK dependent phosphorylation of serine 727 of STAT3 in hepatocytes (Weng et al., 2008). It will be of interest to study the significance of ethanol induced persistent nuclear accumulation of p42/44 MAPK and ethanol potentiation of Ang II stimulated nuclear p42/44 MAPK activation through nuclear activation of MEK1/2 in the context of downstream targets, gene expression, metabolic function and survival of hepatocytes.
Acknowledgments
This work was supported by grant RO1-AA11962 from NIAAA of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Nakashima S, Saji S, Nakamura T, Nozawa Y. Mitogen-activated protein kinase activation in hepatocyte growth factor-stimulated rat hepatocytes: Involvement of protein tyrosine kinase and protein kinase C. Hepatology. 1996;23:1244–1253. doi: 10.1053/jhep.1996.v23.pm0008621160. [DOI] [PubMed] [Google Scholar]
- Adachi T, Kar S, Wang M, Carr BI. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. J Cell Physiol. 2002;192:151–159. doi: 10.1002/jcp.10124. [DOI] [PubMed] [Google Scholar]
- Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Bataller R, Gabel E, Parsons CJ, Morris T, Yang L, Schoonhoven R, Brenner DA, Rippe RA. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046–1055. doi: 10.1002/hep.20665. [DOI] [PubMed] [Google Scholar]
- Blackmore PF, Exton JH. Assessment of effects of vasopressin, angiotensin II, and glucagon on Ca2+ fluxes and phosphorylase activity in liver. Methods Enzymol. 1985;109:550–558. doi: 10.1016/0076-6879(85)09113-3. [DOI] [PubMed] [Google Scholar]
- Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem. 2000;212:155–69. [PubMed] [Google Scholar]
- Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor- induced gene expression and cell cycle entry. EMBO Journal. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk- encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ishac EJN, Dent P, Kunos G, Gao B. Effects of ethanol on mitogen- activated protein kinase and stress-activated protein kinase cascades in normal and regenerating liver. Biochem J. 1998;334:669–676. doi: 10.1042/bj3340669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GB, Brosnihan KB, Zuti RA, Messina M, Gupta MK. Neuroendocrine, fluid balance, and thirst responses to alcohol in alcoholics. Alcohol Clin Exp Res. 1992;16:228–233. doi: 10.1111/j.1530-0277.1992.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Dajani OF, Rottingen JA, Sandnes D, Horn RS, Refsnes M, Thoresen GH, Iversen JG, Christoffersen T. Growth-promoting effects of Ca2+-mobilizing agents in hepatocytes: lack of correlation between the acute activation of phosphoinositide-specific phospholipase C and the stimulation of DNA synthesis by angiotensin II, vasopressin, norepinephrine, and prostaglandin F2α. J Cell Physiol. 1996;168:608–617. doi: 10.1002/(SICI)1097-4652(199609)168:3<608::AID-JCP13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcoholism Clin Exp Res. 1996;20:1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO Journal. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeny MD, Sayeski PP. Ang II-induced cell proliferation is dually mediated by c-Src/Yes/Fyn-regulated ERK1/2 activation in the cytoplasm and PKCzeta-controlled ERK1/2 activity within the nucleus. Amer J Physiol Cell Physiol. 2006;291:C1297–307. doi: 10.1152/ajpcell.00617.2005. [DOI] [PubMed] [Google Scholar]
- Goetze S, Xi X, Graf K, Fleck E, Hsueh WA, Law RE. Troglitazone inhibits angiotensin II-induced extracellular signal-regulated kinase 1/2 nuclear translocation and activation in vascular smooth muscle cells. FEBS Lett. 1999;452:277–282. doi: 10.1016/s0014-5793(99)00624-9. [DOI] [PubMed] [Google Scholar]
- González-Espinosa C, García-Sáinz JA. Angiotensin II and active phorbol esters induce proto-oncogene expression in isolated rat hepatocytes. Biochim Biophys Acta. 1992;1136:309–314. doi: 10.1016/0167-4889(92)90122-r. [DOI] [PubMed] [Google Scholar]
- Hongo S, Watanabe T, Takahashi K, Miyazaki A. Ndrg4 enhances NGF-induced ERK activation uncoupled with Elk-1 activation. J Cell Biochem. 2006;98:185–193. doi: 10.1002/jcb.20763. [DOI] [PubMed] [Google Scholar]
- Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta. 2007;1773:1161–1176. doi: 10.1016/j.bbamcr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanaka H, Tomikawa M, Jones MK, Szabo IL, Pai R, Baatar D, Tsugawa K, Sugimachi K, Sarfeh IJ, Tarnawski AS. Defective mitogen-activated protein kinase (ERK2) signaling in gastric mucosa of portal hypertensive rats: potential therapeutic implications. Hepatology. 2001;34:990–999. doi: 10.1053/jhep.2001.28507. [DOI] [PubMed] [Google Scholar]
- Kim S, Kahn CR. Insulin regulation of mitogen-activated protein kinase (MEK), mitogen-activated protein kinase and casein kinase in the cell nucleus: a possible role in the regulation of gene expression. Biochem J. 1997;323:621–627. doi: 10.1042/bj3230621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Shukla SD. Pro- and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes. Eur J Pharmacol. 2005;508:31–45. doi: 10.1016/j.ejphar.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Shukla SD. Histone H3 phosphorylation at serine 10 and serine 28 is mediated by p38 MAPK in rat hepatocytes exposed to ethanol and acetaldehyde. Eur J Pharmacol. 2008;573:29–38. doi: 10.1016/j.ejphar.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. Biochemical and molecular basis of alcohol-induced injury to liver and other tissues. N Engl J Med. 1988;319:1639–1650. doi: 10.1056/NEJM198812223192505. [DOI] [PubMed] [Google Scholar]
- Mandl M, Slack DN, Keyse SM. Specific inactivation and nuclear anchoring of extracellular signal-regulated kinase 2 by the inducible dual-specificity protein phosphatase DUSP5. Mol Cell Biol. 2005;25:1830–1845. doi: 10.1128/MCB.25.5.1830-1845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop IH, Vyas N, Schmidt CM, Cahill PA, Sitzmann JV. Enhanced Gi- protein-mediated mitogenesis following chronic ethanol exposure in a rat model of experimental hepatocellular carcinoma. Hepatology. 1999;29:412–420. doi: 10.1002/hep.510290218. [DOI] [PubMed] [Google Scholar]
- McAllister-Lucas LM, Ruland J, Siu K, Jin X, Gu S, Kim DS, Kuffa P, Kohrt D, Mak TW, Nunez G, Lucas PC. CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Med Sci USA. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menice CB, Hulvershorn J, Adam LP, Wang CLA, Morgan KG. Calponin and mitogen-activated protein kinase signaling in differentiated vascular smooth muscle. J Biol Chem. 1997;272:25157–25161. doi: 10.1074/jbc.272.40.25157. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Kobayashi S, Überall F, Hellbert K, Kobayashi N, Yoshida K. Nuclear mitogen-activated protein kinase activation by protein kinase Cζ during reoxygenation after ischemic hypoxia. J Biol Chem. 2000;275:19921–19927. doi: 10.1074/jbc.M907901199. [DOI] [PubMed] [Google Scholar]
- Nguyen VA, Gao B. Expression of interferon alfa signaling components in human alcoholic liver disease. Hepatology. 2002;35:425–432. doi: 10.1053/jhep.2002.31169. [DOI] [PubMed] [Google Scholar]
- Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual- specificity protein phosphatases. Oncogene. 2007;26:3203–13. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- Park PH, Lim RW, Shukla SD. Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: potential mechanism for gene expression. Amer J Physiol Gastrointestinal Liver Physiol. 2005;289:1124–1136. doi: 10.1152/ajpgi.00091.2005. [DOI] [PubMed] [Google Scholar]
- Park PH, Aroor AR, Shukla SD. Role of Ras in ethanol modulation of angiotensin II activated p42/p44 MAP kinase in rat hepatocytes. Life Sci. 2006;79:2357–2363. doi: 10.1016/j.lfs.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Qiao L, Yacoub A, Studer E, Gupta S, Pei XY, Grant S, Hylemon PB, Dent P. Inhibition of the MAPK and PI3K pathways enhances UDCA-induced apoptosis in primary rodent hepatocytes. Hepatology. 2002;35:779–789. doi: 10.1053/jhep.2002.32533. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Shukla SD. Potentiation of mitogen-activated protein kinase by ethanol in embryonic liver cells. Biochem Pharmacol. 1996;51:661–668. doi: 10.1016/s0006-2952(95)02239-2. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Shukla SD. Nuclear activation and translocation of mitogen-activated protein kinases modulated by ethanol in embryonic liver cells. Biochim Biophys Acta. 2000;1497:271–278. doi: 10.1016/s0167-4889(00)00058-6. [DOI] [PubMed] [Google Scholar]
- Rosseland CM, Wierød L, Oksvold MP, Werner H, Ostvold AC, Thoresen GH, Paulsen RE, Huitfeldt HS, Skarpen E. Cytoplasmic retention of peroxide-activated ERK provides survival in primary cultures of rat hepatocytes. Hepatology. 2005;42:200–207. doi: 10.1002/hep.20762. [DOI] [PubMed] [Google Scholar]
- Schulman IH, Zhou MS, Raij L. Cross-talk between angiotensin II receptor types 1 and 2: potential role in vascular remodeling in humans. Hypertension. 2007;49:270–271. doi: 10.1161/01.HYP.0000253966.21795.d3. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Lee YJ, Park P, Aroor AR. Acetaldehyde alters MAP kinase signaling and epigenetic histone modifications in hepatocytes. In: Chadwick DJ, Goode J, editors. ‘Acetaldehyde related pathology: bridging the trans-disciplinary divide’ (Novartis Foundation Symposium) John Wiley & Sons; 2007. pp. 217–228. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Shapiro PS, Goueli S, Ahn NG. Nuclear localization of mitogen- activated protein kinase kinase 1 (MKK1) is promoted by serum stimulation and G2-M progression. Requirement for phosphorylation at the activation lip and signaling downstream of MKK. J Biol Chem. 1999;274:6168–6174. doi: 10.1074/jbc.274.10.6168. [DOI] [PubMed] [Google Scholar]
- Tombes RM, Auer KL, Mikkelsen R, Valerie K, Wymann MP, Marshall CJ, McMahon M, Dent P. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem J. 1998;330:1451–1460. doi: 10.1042/bj3301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Gomez S, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski AG, Vaque JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- Venugopal SK, Chen J, Zhang Y, Clemens D, Follenzi A, Zern MA. Role of MAPK phosphatase-1 in sustained activation of JNK during ethanol-induced apoptosis in hepatocyte-like VL-17A cells. J Biol Chem. 2007;282:31900–31908. doi: 10.1074/jbc.M703729200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Schramek H, Dunn MJ. Cytosolic and nuclear mitogen-activated protein kinases are regulated by distinct mechanisms. Exp Cell Res. 1996;225:382–388. doi: 10.1006/excr.1996.0189. [DOI] [PubMed] [Google Scholar]
- Weng YI, Shukla SD. Ethanol alters angiotensin II stimulated mitogen activated protein kinase in hepatocytes: agonist selectivity and ethanol metabolic independence. Eur J Pharmacol. 2000;398:323–333. doi: 10.1016/s0014-2999(00)00313-7. [DOI] [PubMed] [Google Scholar]
- Weng YI, Shukla SD. Angiotensin II activation of focal adhesion kinase and pp60c-Src in relation to mitogen-activated protein kinases in hepatocytes. Biochim Biophys Acta. 2002;1589:285–297. doi: 10.1016/s0167-4889(02)00189-1. [DOI] [PubMed] [Google Scholar]
- Weng YI, Shukla SD. Effects of chronic ethanol treatment on the angiotensin II-mediated p42/p44 mitogen-activated protein kinase and phosphorylase a activation in rat hepatocytes. Alcohol. 2003;29:83–90. doi: 10.1016/s0741-8329(02)00325-7. [DOI] [PubMed] [Google Scholar]
- Weng YI, Aroor AR, Shukla SD. Ethanol inhibition of angiotensin II stimulated Tyr705 and Ser 727 STAT 3 phosphorylation in cultured rat hepatocytes: Relevance to activation of p42/44 mitogen activated protein kinase. Alcohol. 2008;42:397–406. doi: 10.1016/j.alcohol.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Whitehurst A, Cobb MH, White MA. Stimulus-coupled spatial restriction of extracellular signal-regulated kinase 1/2 activity contributes to the specificity of signal-response pathways. Mol Cell Biol. 2004;24:10145–10150. doi: 10.1128/MCB.24.23.10145-10150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta. 2007;1773:1285–1298. doi: 10.1016/j.bbamcr.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Zebisch A, Czernilofsky AP, Keri G, Smigelskaite J, Sill H, Troppmair J. Signaling through RAS-RAF-MEK-ERK: from basics to bedside. Curr Med Chem. 2007;14:601–623. doi: 10.2174/092986707780059670. [DOI] [PubMed] [Google Scholar]
- Zima T, Kalousova M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29(Suppl):110S–115S. doi: 10.1097/01.alc.0000189288.30358.4b. [DOI] [PubMed] [Google Scholar]