Abstract
This study examined relationships between lobar volumes and performance on the D-KEFS Sorting Test, a standardized measure of concept formation. Participants included 89 subjects, 19 patients with probable Alzheimer's disease, 25 patients with frontotemporal dementia, 13 patients with semantic dementia, 12 patients with progressive nonfluent aphasia, 9 patients with probable progressive supranuclear palsy, 2 patients with possible progressive supranuclear palsy, and 9 healthy participants. We used BRAINS2 software to generate volumes of the right and left frontal, temporal, and parietal lobes. Multiple regression analysis indicated that, after controlling for Mini-Mental State Examination scores, intracranial volume, and demographic variables, only the left frontal lobe significantly predicted performance on the D-KEFS Sorting test.
Keywords: D-KEFS, Frontal lobes, Executive functioning, Card Sorting, Concept Formation
Introduction
The identification and formation of concepts refers to the ability to identify relationships among stimuli or “things in the world” (Hartman & Stratton-Salib, 2007). Common to most measures of concept formation is the requirement to identify the abstract or conceptual relationships shared by stimuli. Such tasks often require the ability to identify multiple conceptual relationships from a single set of stimuli, usually encompassing primarily perceptual (e.g., color of the stimuli) or conceptual (e.g., living things) relationships. As is the case with most higher-order cognitive operations falling under the rubric of “executive functions,” concept formation involves a diverse set of component cognitive operations, including the selection of the relevant semantic knowledge representing the abstract principles inherent in the stimuli, and the ability to generate hypotheses (Delis et al., 2001; Reverberi et al., 2005a; Reverberi et al., 2005b). Other cognitive abilities implicated in concept formation, include selective attention necessary for attending to multiple stimulus features and shifting between different concepts (Dias et al., 1996; Hartmann et al., 2004), and working memory, which is integral for the “on-line” maintenance of relevant stimulus attributes during task performance (Babcok, 1994; Babcock & Laguna, 1996).
In clinical neuropsychology, few standardized measures are available that assess concept identification and formation. The Wisconsin Card Sorting task (WCST) remains the gold standard and has been found to be sensitive to frontal dysfunction (for review see Demekis, 2003). The D-KEFS Sorting Test (DST; Delis et al., 2001), formally called the California Card Sorting Test (CCST; Delis et al., 1992) was designed to provide a standardized measure of specific component processes underlying concept formation that compliments information derived from the WCST. Specifically, the DST requires subjects to sort cards according to eight possible target rules, including five primarily perceptual or nonverbal rules (e.g., straight versus curved outer edges) and three primarily verbal rules (e.g., clothing versus body parts). Additionally, performance is evaluated both in terms of the total number of correct target concepts reflected in the examinee's sorts, as well as the accuracy and level of abstraction of the examinee's sort descriptions, which is useful in discerning the specific sorting strategies that were employed (Delis et al., 2001).
Several studies have revealed deficits on this task in a variety of clinical populations with either focal frontal lesions or compromise to, more broadly, frontal brain systems. For example, Delis et al. (1992) found that patients with either focal frontal lesions or Korsakoff's syndrome, a disorder that in part affects frontal brain systems, performed deficiently on a number of critical CCST indices, including total correct card sorts and the total free description score. Importantly, non-Korsakoff's amnestic patients who had lesions in regions other than the frontal lobes performed comparably to healthy control participants. Dimitrov et al. (1999) replicated and extended the Delis et al. (1992) findings by demonstrating that patients with focal frontal lesions and Parkinson's disease performed significantly worse than normal controls on the CCST. Beatty and Monson (1990) also found deficits on the CCST in Parkinson's patients. Performance difficulties on this task have also been associated with subcortical lacunar infarcts, which are thought to disrupt frontal-subcortical circuits, in non-demented elderly individuals (Kramer et al., 2002). Lastly, a recent study by Parmenter et al. (2007) found that patients with multiple sclerosis (MS) performed significantly worse than a normal control group on the DST, a finding that is consistent with a previous study of CCST performance in MS (Beatty et al., 1995).
The goal of the current study was to explore in greater precision the neuroanatomical basis of performance on the DST. Specifically, we employed quantitative MRI to characterize the relationships between the lobar volumes of multiple cortical brain regions and performance on a short-form of the DST in a mixed sample of patients with neurodegenerative diseases and in healthy participants. Based on the existing evidence implicating a relationship between frontal lobe functioning and the DST, we predicted that volumetric measures (i.e., the size) of the left and right frontal lobes would significantly predict DST performance.
Methods
Subjects
Participants included 89 subjects, 19 patients with probable Alzheimer's disease (AD), 25 patients with frontemporal dementia (FTD), 13 patients with Semantic Dementia (SD), 12 patients with progressive nonfluent aphasia (PNFA), 9 patients with probable progressive supranuclear palsy (PSP), 2 patients with possible PSP, and 9 healthy participants (HP). All participants underwent a standardized cognitive and neuroimaing evaluation. Inclusion criteria included a Mini-Mental State Examination (MMSE) score of 15 or higher and a magnetic resonance imaging (MRI) scan obtained within 90 days of the cognitive testing. The research diagnosis for each subject was made by a team consensus at the University of California, San Francisco, Memory and Aging Center based on medical, social, and psychiatric history; neurological evaluation; extensive family interview; visual inspection of a brain image (CT or MRI); and mental status testing. The Neary criteria were used for the diagnosis of FTD, SD, and PNFA (Neary et al., 1998; Neary et al., 2000). The primary clinical features in FTD are an early decline in social and interpersonal behavior, early impairment in regulation of personal conduct, early emotional blunting, and early loss of insight. A diagnosis of FTD is further supported by a presenile onset (late 50's or early 60's), the early presence of hyperorality and dietary changes, decline in personal hygiene, and predominant frontal and insular atrophy on neuroimaging. The primary clinical features of SD are a selective impairment of semantic memory, progressive, fluent, empty spontaneous speech, loss of word meaning, semantic paraphasias; anomia, and a relative sparing of syntax and phonology. The primary clinical features of PNFA are deficits in expressive language that includes effortful speech production, phonologic and grammatical errors, and word retrieval difficulties (Neary et al., 1998; Neary et al., 2000). The National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer's Disease and Related Disorders Associative criteria (McKhann et al., 1984) were used for the diagnosis of probable AD. The NINDS-SPSP (Society for Progressive Supranuclear Palsy) was used for the diagnosis of possible and probable PSP (Litvan et al., 1996). Specifically, the criteria for probable PSP are vertical (upward or downward gaze) supranuclear palsy and prominent postural instability with falls in the first year of the disease (Litvan et al., 1996). The criteria for possible PSP are either vertical (upward or downward gaze) supranuclear palsy or both slowing of vertical saccades and prominent postural instability with falls in the first year of disease onset (Litvan et al., 1996). Table 1 below summarizes the demographic, cognitive, and MRI data for the study participants.
Table 1.
Means and standard deviations for demographic, DST, and MRI volumetric data (in cubic centimeters)
| HP | AD | FTD | SD | PNFA | PSP | |
|---|---|---|---|---|---|---|
| n | 9 | 19 | 25 | 13 | 12 | 11 |
| Age | 57.1 (15.1) | 57.4 (5.8) | 58.6 (6.9) | 63.1 (7.8) | 63.7 (10.9) | 65.7 (7.6) |
| Education | 16.4 (1.9) | 15.9 (2.7) | 16.5 (2.2) | 16.4 (3.2) | 15.0 (2.8) | 16.6 (3.1) |
| MMSE | 29.3 (1.0) | 22.0 (4.0 | 25.8 (3.5) | 23.0 (5.5) | 23.17 (4.1) | 26.2 (3.0) |
| DST: Free Description score | 21.4 (6.5) | 7.8 (5.7) | 11.7 (7.2) | 11.5 (6.9) | 9.33 (8.8) | 10.8 (6.7) |
| DST: Total Confirmed Correct Sorts | 5.6 (1.0) | 2.3 (1.4) | 3.2 (2.0) | 3.5 (1.7) | 3.6 (2.2) | 3.2 (1.6) |
| Left frontal lobe | 197.3 (28.1) | 166.6 (26.5) | 165.8 (25.6) | 166.6 (20.1) | 147.7 (21.5) | 169.9 (17.4) |
| Right frontal lobe | 204.9 (29.3) | 176.4 (26.8) | 171.4 (29.5) | 180.3 (21.9) | 167.9 (21.2) | 181.5 (22.0) |
| Left temporal lobe | 116.2 (13.8) | 98.8 (12.9) | 103.8 (16.1) | 84.0 (10.3) | 92.7 (9.2) | 103.7 (11.2) |
| Right temporal lobe | 115.8 (15.3) | 98.6 (13.0) | 99.8 (17.9) | 91.7 (18.0) | 98.3 (8.1) | 101.4 (14.5) |
| Left parietal lobe | 118.7 (13.9) | 99.4 (14.9) | 112.4 (13.6) | 103.5 (12.6) | 98.1 (14.8) | 106.7 (14.6) |
| Right parietal lobe | 122.5 (14.3) | 101.3 (13.6) | 112.1 (15.2) | 109.0 (13.8) | 104.6 (14.2) | 107.6 (12.8) |
Abbreviations: HP = Healthy participants; AD = Alzheimer's disease; FTD = Frontotemporal dementia; PNFA = Progressive Nonfluent Aphasia; PSP = Progressive Supranuclear Palsy; SD = Semantic dementia.
D-KEFS Sorting Test Procedure
The D-KEFS Sorting test (DST; Delis et al., 2001) requires participants to sort six cards into two groups of three cards. The cards vary along several dimensions (e.g., size, shape, color, meanings of words printed on cards), with a total of 8 different sorting concepts or rules for each card set. A short-form of the DST involving the administration of only Card Set 1 was used in the present study. In the free-sort condition, the examinee sorts the cards based on rules or concepts that he or she identifies on the cards. The highest possible total confirmed correct sorts for the free sorting condition for the first card deck is 8 correct sorts; the total confirmed correct sorts represent the number of correct sorts for which the verbal description is awarded one or more points. Following each sort, the examinee is queried regarding the sorting strategy that was employed. The examinees verbal description is scored in terms of its accuracy and the level of abstraction, with a maximum of 4 points per description of a particular sorting rule (Free Description score); see Delis et al. (2001) for detailed scoring procedures. For the current study, the DST variables of interest were the total free description score and the total confirmed correct sorts.
Neuroimaging
MRI scans were obtained on a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ) equipped with a standard quadrature head coil. Structural MRI sequences included: (a) 2D FLASH MRI along three orthogonal directions, 3 mm thick slices, ∼ 15 slices in each direction to obtain scout views of the brain for positioning subsequent MRI slices. (b) A double spin echo sequence [repetition time/echo time 1/echo time 2 (TR/TE1/TE2) = 5000/20/80 ms] to obtain proton density and T2-weighted MRIs, 51 contiguous axial slices (3 mm) covering the entire brain and angulated -10° from the AC-PC line; 1.0 × 1.25 mm2 in-plane resolution. (c) Volumetric magnetization prepared rapid gradient echo MRI [MPRAGE, repetition time/echo time/inversion time (TR/TE/TI) = 10/4/300 ms] to obtain T1-weighted images of the entire brain, 15° flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness.
Magnetic resonance images were processed on Linux workstations using the BRAINS2 software package, which is developed and made freely available by the Mental Health – Clinical Research Center at the University of Iowa (Magnotta et al., 2002). The T1 weighted images were spatially normalized and resampled to 1.0 mm3 voxels so that the anterior-posterior axis of the brain was realigned parallel to the anterior commissure-posterior commissure line and the inter-hemispheric fissure aligned on the other two axes. Next, the outermost boundaries of the cortex, as well as the anterior commissure and posterior commissure, are identified in order to warp the Talairach grid (Talairach & Tournoux, 1988) onto the current brain. The T2 and PD weighted images were then realigned to the spatially normalized T1 weighted image using an automated image registration program (Woods et al., 1992). The resampled images were then segmented into grey matter, white matter, and CSF using the coregistered images and a discriminant analysis method based on automated training class selection (Harris et al., 1999). This tissue classification algorithm uses a Bayesian classifier based on discriminant analysis in order to reduce the variability in signal intensity across individual image sets and to correct for partial voluming. This step requires the manual tracing of venous blood, but is able to perform “plug” selection for grey matter, white matter, and cerebrospinal fluid automatically.
We generated a brain mask using a previously trained artificial neural network, one of the features of the BRAINS2 software package. We generated a brain mask using a previously trained artificial neural network, one of the features of the BRAINS2 software package. Then, lobar volumes were calculated using an automated Talairach-based method of regional classification (Andreasen et al., 1996; Magnotta et al., 2002). To avoid tissue misclassification that can result from vascular degeneration, an important consideration when studying geriatric populations, whole-tissue volumes (grey matter + white matter) were used in this study, rather than tissue-classified volumes that measure grey matter and white matter separately.
Statistics
For the analysis of the relationships between specific MRI lobar volumes and DST measures, we conducted two separate hierarchical repression analyses, one with the free description score as the dependent measure and the second with total number of confirmed correct sorts serving as the dependent measure. For both regression models, potential moderator variables (age, MMSE score, gender, and education) were entered into the first step. Total intracranial volume was entered into the second step to control for differences in head size. In the third and final step, lobar volumes (right frontal, left frontal, right temporal, left temporal, right parietal, and left parietal) were entered simultaneously as predictor variables.
Results of regression analyses
Model 1: Total Free Description Score
In the first step of the model examining predictors of the free description score, age, education, gender, and MMSE total score explained 49.5% of the variance in the free description score. The addition of total brain volume did not result in a significant increase in explained variance (Fchange = .129; p = 721). In the final step, the inclusion of the lobar volumes accounted for an additional 8.6% of the variance (Fchange = 2.586; p = .029). Of the six lobar volumes, only the left frontal volume significantly predicted the total free description score (β = .533; t = 2.216; p = .030; a scatterplot illustrating the relationship between the total free description score and left frontal lobar volumes is shown in fig. 1; complete results for regression analysis examining the relationship between lobar volumes and the total free description score are summarized in Table 2). When entered alone into the regression model (after entering age, education, gender, total MMSE score, and total brain volume) left frontal volume accounted for an additional 5% of the variance in the total free description score.
Figure 1.
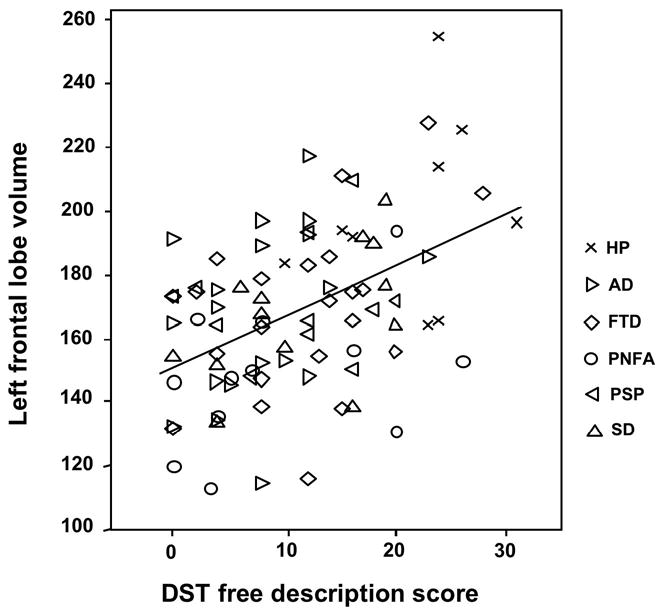
Scatterplot showing the relationship between left frontal lobe volumes and D-KEFS Sorting Test (DST) free description scores; Abbreviations: HP = Healthy participants; AD = Alzheimer's disease; FTD = Frontotemporal dementia; PNFA = Progressive Nonfluent Aphasia; PSP = Progressive Supranuclear Palsy; SD = Semantic dementia.
Table 2.
Results from regression model for lobar volume predictors of DST free description score
| Predictor | β | T |
|---|---|---|
| Left frontal | .533 | 2.21* |
| Right frontal | -.118 | -.478 |
| Left temporal | -.352 | -1.62 |
| Right temporal | .186 | .789 |
| Left parietal | -.239 | -.774 |
| Right parietal | .219 | .725 |
p <.05
Model 2: Total Confirmed Correct Sorts
In the first step of the model examining predictors of the total confirmed correct sorts, age, education, gender, and MMSE score explained 35% of the variance in total correct sorts. The addition of total brain volume did not result in a significant increase in explained variance (Fchange = .012; p = .913). In the final step, the inclusion of the lobar volumes accounted for an additional 10% of the variance (Fchange = 2.412; p = .035). However, unlike the findings with total free description score, none of the individual lobar volumes significantly predicted the total confirmed correct sorts; this indicates less specificity in the relationship between lobar volumes and total correct sorts compared to the free description score. In addition, consistent with performance data on the DST from the D-KEFS normative sample (Delis et al., 2001), the partial correlation between the free description score and the total confirmed correct sorts (after controlling for age, education, gender, and MMSE score) was highly significant (r = .7725; p <.0001).
Exploratory Analyses of Group Differences on the DST
In addition to the repression analyses examining lobar volume predictors of DST performance, as an exploratory analysis of the potential groups differences on the primary DST performance measures we submitted both the DST total free description score and the total confirmed correct sorts to one-way analysis of variance (ANOVA). It should be emphasized, however, that the present study was not designed specifically to evaluate differences on the DST in the different patient groups, as the sample sizes for the various groups were relatively small and variable, and the patients groups were not matched in terms of their overall level of cognitive functioning.
The one-way ANOVA with total free description score as the dependent measure was significant, F(5, 83) = 4.946; p = .001 (see table 1 above for means and standard deviations). Follow-up pairwise tests after applying a Tukey's correction for multiple comparisons revealed that the healthy participant's (HP) total free description score was significantly higher than all the patients groups (HP vs. AD, p = <.0001; HP vs. FTD, p = .009; HP vs. SD, p =.018; HP vs. PPA, p = .002; HP vs. PSP, p = .014)), and there were no significant pairwise differences between any patient groups. Similarly, the ANOVA with total confirmed correct sorts was significant, F(5,83) = 4.022; p = .003 (see table 1 above for means and standard deviations). Follow-up pairwise tests after applying a Tukey's correction for multiple comparisons indicate that the healthy participants produced significantly higher score for total confirmed correct sorts on the DST than the AD group (p = <.0001), the FTD group (p =.013), and the PSP group (p = .045). There were no significant pairwise differences for any comparisons between patient groups for total confirmed correct sorts.
Discussion
The goal of the present study was to examine the neuroanatomical basis of performance on the short-form of the D-KEFS Sorting Test (Delis et al., 2001), a standardized measure of concept formation. The primary finding of this study was that left frontal lobe volumes significantly predicted total free description score, a DST measure that reflects the accuracy and level of abstraction of an examinee's description of their sorting strategies. However, there were no significant relationships between any of the lobar volumes and the total number of confirmed correct sorts produced, despite the fact that total correct sorts and the free description score are highly correlated. The significant correlation between the total confirmed correct sorts and the free description score suggests that these DST performance indices tap similar underlying abilities, which would be expected given that the total free description score reflects the ability both to sort the cards accurately (reflected in the total confirmed correct sorts) and to accurately describe ones sorting strategy. Therefore, the failure to observe any significant unique relationships between lobar volumes and the total confirmed correct sorts might reflect the more restricted range of scores for the total confirmed correct sorts (observed range = 0-8) than for the total free description score (observed range = 0-31).
Based on previous studies demonstrating that patients with frontal lobe-lesions or disorders primarily affecting frontal brain systems perform significantly worse than healthy individuals on the DST or on an earlier version of this task (California Card Sorting Test [CCST]; Beatty & Monson (1990); Beatty et al., 1995; Bondi et al., 1993; Cato et al., 2004; Delis et al., 1992; Dimitrov et al., 1998; Kramer et al., 2002; Parmenter et al., 2007), we hypothesized that volumetric measures (i.e., the size) of the left and right frontal lobes would significantly predict DST performance. The results from the current study, namely that only the left frontal lobe volumes significantly predicted aspects of performance on the DST (i.e., the free description score), is nevertheless consistent with evidence from functional neuroimaging studies demonstrating that regions within the left frontal lobe are involved in specific higher-order cognitive operations germane to performance on the DST. For example, several functional neuroimaging studies have demonstrated that regions within the left prefrontal cortex (i.e., left inferior prefrontal cortex and left ventrolateral prefrontal cortex) are involved in the controlled retrieval and selection of relevant semantic information (Badre et al., 2005; Demb et al., 1995; Fletcher et al., 2000; Martin, 2007; Wagner et al., 2001) that is stored in other cortical regions (for review, see Martin & Chao, 2001; Martin, 2007). These findings have been further interpreted as reflecting a specific role for the left prefrontal cortex in what has been described as “semantic working memory,” which refers specifically to the ability to maintain in working memory semantic information germane for semantic analysis (for review, see Gabrieli et al., 1998). These left frontal-mediated processes are highly pertinent to DST performance. Concepts, such as those reflected by the sorting principles included in the DST, are constructed from semantic representations, representations that must be appropriately retrieved from long-term semantic stores and maintained in working memory during DST performance. Other functional neuroimaging and neuropsychological studies also implicate the left dorsolateral prefrontal cortex in generating hypotheses based on abstract rules (Goel et al., 1997; Goel & Dolan, 2004; Osherson et al., 2004; Reverberi et al., 2005), which is another higher-order cognitive operation integral for successful DST performance. It is likely these cognitive processes -- the retrieval and selection of semantic information, the maintenance of semantic information in working memory, and hypothesis generation -- are important to successful DST performance.
As noted above, the regression analyses failed to reveal a significant relationship between the total confirmed correct sorts measure on the DST and any of the cortical brain regions, which we suggested might reflect the restricted range of scores for this DST measure. It is important to emphasize that the pattern of findings from the regression analyses (i.e., only left frontal lobar volumes predicted total free description scores on the DST) is unlikely related to regional differences in the distribution of lobar volume measurements for the following reasons. First, only lobar volume measurements of the left frontal lobe significantly predicted DST total free description score, despite the fact the means and standard deviations for the left and right frontal lobar volumes were nearly identical (lt. frontal: Mean = 167.36; STD = 26.2 vs. rt frontal: Mean = 177.9; STD = 27.2). Second, although the range of scores for measures of right and left frontal lobar volumes were larger than for the other cortical regions, which would be expected given that the frontal lobe is the largest cortical structure, the standard deviations of the lobar volumes expressed as a percentage of their means were quite comparable (lt. frontal = 15.7%; rt. frontal = 15.3%; lt. temporal = 15.6%; rt. temporal = 16%; lt. parietal = 14.8%; rt. parietal = 14.6%). These two findings argue against the possibility that the failure to observe significant relationships between the DST total free description and any of the other cortical brain regions were false negatives, given that the observed differences in the relative variability in the distribution of lobar volume measurements were minimal.
The limitations of the present study include the fact that measures of lobar volumes do not provide sufficient information regarding the contributions of specific frontal regions to DST performance. Thus, a more concise understanding of the brain-behavior relationships reflected in tasks like the DST requires comparisons of patients with circumscribed focal frontal and non-frontal lesions. Although our results implicate the left frontal lobe in DST performance, given that concept formation is a multi-component process, it is likely subserved, as is the case with other “executive” functions, by a distributed neural network that includes both anterior and posterior brain regions (for example, see Carpenter et al., 2000; Andrés & Van der Linden, 2002). Therefore, to characterize the dynamics and spatial extent of the neural system underlying concept formation, complimentary neuroimaging techniques, such as functional magnetic resonance imaging (FMRI), are needed. Another limitation of the study design is that it did not allow for a rigorous examination of possible group differences on the DST, as the sample sizes for the various groups were relatively small and variable, and the groups were not matched in terms of their overall level of cognitive functioning. Future studies examining DST performance in clinical groups matched in terms of overall dementia severity would likely provide insights into differences in the profiles of executive functioning deficits in individuals with various neurodegenerative diseases. Finally, to enhance our understanding of the component processes underlying concept formation, experimental measures should be employed along with clinical measures such as the DST. Thus, the present study does not establish the specific cognitive contributions of the left frontal lobe to DST performance.
In summary, the current study revealed that left frontal lobe volumes significantly predicted performance on the short-form of the DST. This finding is consistent with the accumulating evidence suggesting that the DST is sensitive to dysfunction in frontal-subcortical brain systems (Beatty & Monson, 1990; Beatty et al., 1995; Bondi et al., 1993; Cato et al., 2004; Delis et al., 1992; Dimitrov et al., 1998; Kramer et al., 2002; Parmenter et al., 2007) and with results from cognitive neuroscience regarding the role of the left frontal lobe in certain higher-order cognitive operations. The current study also suggests that the short-form of the DST is useful in terms of research studies and in clinical practice for identifying frontal systems dysfunction.
Acknowledgments
This research was supported in part by NIA Grants AG019724, AG02351, AG12435, AG22983, The State of California Alzheimer's Disease Research Center of California (ARCC) Grant 01-154-20, and the Hillblom Foundation Grant 2002/2F. Disclosure: Dr. Delis and Dr. Kramer are co-authors of the D-KEFS Sorting Test and receive royalties from the test.
References
- Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW, Jr, Flashman LA. Automatic Atlas-Based Volume Estimation of Human Brain Regions from MR Images. Journal of Computer Assisted Tomography. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Andrés P, Van der Linden M. Are Central Executive Functions Working in Patients with Focal Frontal Lesions? Neuropsychologia. 2002;40:835–845. doi: 10.1016/s0028-3932(01)00182-8. [DOI] [PubMed] [Google Scholar]
- Babcock R. Analysis of Adult Age Differences on the Raven's Advanced Progressive Matrices Test. Psychology and Aging. 1994;2:303–314. doi: 10.1037//0882-7974.9.2.303. [DOI] [PubMed] [Google Scholar]
- Babcock R, Laguna KD. An Examination of the Adult Age-Related Differences on the Raven's Advanced Progressive Matrices: A Structural Equations Approach. Aging, Neuropsychology, and Cognition. 1996;3:187–200. [Google Scholar]
- Badre D, Wagner AD. Frontal Lobe Mechanisms that Resolve Proactive Interference. Cerebral Cortex. 2005;15(12):2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Hames KA, Blanco CR, Paul RH, Wilbanks SL. Verbal Abstraction Defecit in Multiple Sclerosis. Neuropsychology. 1995;9(2):198–205. [Google Scholar]
- Beatty WW, Monson N. Problem Solving in Parkinson's Disease: Comparison of Performance on the Wisconsin and California Card Sorting Tests. Journal of Geriatric Psychiatry and Neurology. 1990;3:163–171. doi: 10.1177/089198879000300308. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Kaszniak AW, Bayles KA, Vance KT. Contributions of frontal system dysfunction to memory and perceptual abilities in Parkinson's disease. Neuropsychology. 1993;7:89–102. [Google Scholar]
- Carpenter P, Just MA, Reichle ED. Working Memory and Executive Function: Evidence From Neuroimaging. Current Opinion in Neurobiology. 2000;10:195–199. doi: 10.1016/s0959-4388(00)00074-x. [DOI] [PubMed] [Google Scholar]
- Cato MA, Delis DC, Abildskov TJ. Assessing the elusive cognitive deficits associated with ventromedial prefrontal damage: a case of a modern-day Phineas Gage. 2004;10:453–465. doi: 10.1017/S1355617704103123. [DOI] [PubMed] [Google Scholar]
- Delis D, Squire LR, Bihrle A, Massman P. Componential Analysis of Problem-Solving Ability: Performance of Patients with Frontal Lobe Damage and Amnesic Patients on a New Sorting Test. Neuropsychologia. 1992;30(8):683–697. doi: 10.1016/0028-3932(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. D-KEFS Executive Function System: Examiners Manual. San Antonio: Psychological Corporation; 2001. [Google Scholar]
- Demakis GJ. A Meta-Analytic Review of the Sensitivity of the Wisconsin Card Sorting Test to Frontal and Laterized Frontal Brain Damage. Neuropsychology. 2003;17(2):255–264. doi: 10.1037/0894-4105.17.2.255. [DOI] [PubMed] [Google Scholar]
- Demb JB, Wagner JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic Encoding and Retrieval in the Left Interior Prefrontal Cortex: A Functional MRI Study of Task Difficulty and Process Specificity. Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov M, Grafman J, Soares AHR, Clark K. Concept Formation and Concept Shifting in Frontal Lesion and Parkinson's Disease Patients Assessed with the California Card Sorting Test. Neuropsychology. 1999;13(1):135–143. doi: 10.1037//0894-4105.13.1.135. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. “Sculpting the Response Space” -An Account of Left Prefrontal Activation at Encoding. NeuroImage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The Role of Left Prefrontal Cortex in Language and Memory. Proc National Academy of Science. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. The Seats of Reason? An Imaging Study of Dedecutive and Inductive Reasoning. NeuroReport. 1997;8(5):1305–1310. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Differential Involvement of Left Prefrontal Cortex in Inductive and Deductive Reasoning. Cognition. 2004;93:109–121. doi: 10.1016/j.cognition.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: A three-dimensional multi-spectral discriminant analysis method with automated training class selection. Journal of Computer Assisted Tomography. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Hartman M, Nielsen C, Stratton B. The Contributions of Attention and Working Memory to Age Differences in Concept Identification. Journal of Clinical and Experimental Neuropsychology. 2004;26(2):227–245. doi: 10.1076/jcen.26.2.227.28083. [DOI] [PubMed] [Google Scholar]
- Hartman M, Stratton-Salib BC. Age Differences in Concept Formation. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):198–214. doi: 10.1080/13803390600630294. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Reed BR, Mungas D, Weiner MW, Chui HC. Executve Dysfunction in Subcortical Ischaemic Vascular Disease. Journal of Neurology, Neurosurgery, Psychiatry. 2002;72:217–220. doi: 10.1136/jnnp.72.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, DuBois B, Duvoisin RC, Goetz CG, Golbe LL, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical Research Criteria for the Diagnosis of Progressive Supranuclear Palsy (Steele-Richardson-Olszewski Syndrome): Report of the NINDS-SPSP International Workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR Image Processing Using the Brains2 Toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and brain: structure and processes. Current Opinion in Neurobiology. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Martin A. The Representation of Object Concepts in the Brain. The Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer's Disease: Report of the NINCDS-ADRDA Work Group Under the Auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;43:289–292. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Mann DM. Classification and description of frontotemporal dementias. Annals of the New York Academy of Science. 2000;920:46–51. doi: 10.1111/j.1749-6632.2000.tb06904.x. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Osherson D, Tentori K, Bonini N. The Conjunction Fallacy: A Misunderstanding About Conjunction? Cognitive Science. 2004;28:467–477. [Google Scholar]
- Parmenter B, Zivadinov R, Kerenyi L, Gavett R, Weinstock-Guttman B, Dwyer M, Garg N, Munschauer F, Benedict RHB. Validity of the Wisconsin Card Sorting Test and Delis-Kaplan Exective Function System (DKEFS) Sorting Test in Multiple Sclerosis. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):215–223. doi: 10.1080/13803390600672163. [DOI] [PubMed] [Google Scholar]
- Reverberi C, D'Agostini S, Skrap M, Shallice T. Generation and Recognition of Abstract Rules in Different Frontal Lobe Subgroups. Neuropsychologia. 2005a;43(3):460–472. doi: 10.1016/j.neuropsychologia.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reverberi C, Lavaroni A, Gigli GL, Skrap M, Shallice T. Specific Impairments of Rule Induction in Different Frontal Lobe Subgroups. Neuropsychologia. 2005b;43(3):460–472. doi: 10.1016/j.neuropsychologia.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A Co-Planar Stereotactic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering Meaning: Left Prefrontal Cortex Guides Controlled Semantic Retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid Automated Algorithm for Aligning and Reslicing PET Images. Journal of Computer Assisted Tomography. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]


