Abstract
The protein kinase C (PKC) family of serine/threonine kinases regulates diverse cellular function, including cell death, proliferation and survival. In particular, PKCδ governs the cellular homeostatic response against hypoxic stress. Autophagy, a lysosome-dependent degradative pathway, and apoptosis are two fundamental cellular pathways that respond to stress conditions, such as hypoxia, oxidative stress, and nutrient starvation. Recently, we uncovered a novel role for PKCδ in the early stage of hypoxic response where PKCδ activates autophagy by promoting JNK1-mediated Bcl-2 phosphorylation and dissociation of the Bcl-2/Beclin 1 complex. Whereas acute hypoxic stress promotes autophagy, we have previously reported that prolonged hypoxic stress caused the cleavage of PKCδ by caspase-3, resulting in the nuclear translocation of a constitutively active catalytic fragment of PKCδ, PKCδ-CF. Moreover, PKCδ-CF also serves a feed-forward function for the reciprocal PKCδ and caspase-3 proteolytic activation. Here, we discussed the requirement for PKCδ and JNK1 for hypoxia-induced autophagy, and the kinetic relationship among Bcl-2/Beclin 1 interaction, caspase-3 activation and the steady-state level of Beclin 1 during hypoxic exposure. Based on these results, we propose a model for understanding the PKCδ-dependent crosstalk mechanisms between autophagy and apoptosis, both induced by hypoxic stress. These findings collectively support a pivotal role for PKCδ in regulating hypoxic stress with hitherto unappreciated significance.
Keywords: PKCδ, JNK1, Bcl-2, Beclin 1, caspase-3, hypoxia, autophagy, apoptosis
It has been established that mammalian cells activate the autophagy pathway as an adaptive response to extrinsic signals, such as hypoxia and starvation, or they proceed towards basal self-eating process for homeostatic maintenance.1-3 Autophagy processes parallel with apoptosis mechanisms, but autophagy and apoptosis are also connected through Bcl-2.4 Bcl-2 is an anti-apoptotic factor governing the responses to cell death stimuli by fine-tuning mitochondria membrane dynamics and triggering the release of cytochrome c and other apoptotic effectors from mitochondria.5 Beclin 1, a BH3 domain-containing protein, was originally identified as a Bcl-2-interacting protein and demonstrated to be essential for autophagy induction.6 The interaction of Bcl-2 with Beclin 1 was subsequently demonstrated to inhibit autophagy,7, 8 and the relative amounts of Beclin 1 and Bcl-2 appear to modulate crosstalk between autophagy and apoptosis.1 A non-mitochondrial pool of Bcl-2 and Bcl-XL inhibits the function of Beclin 1 in autophagosome formation. Moreover, we and other laboratories further demonstrated that Bcl-2/Beclin 1 complexes are dissociated during starvation and hypoxia by Bcl-2 phosphorylation that is mediated by JNK1.9, 10
Role of PKCδ and JNK1 in Acute Hypoxic Stress-induced Autophagy
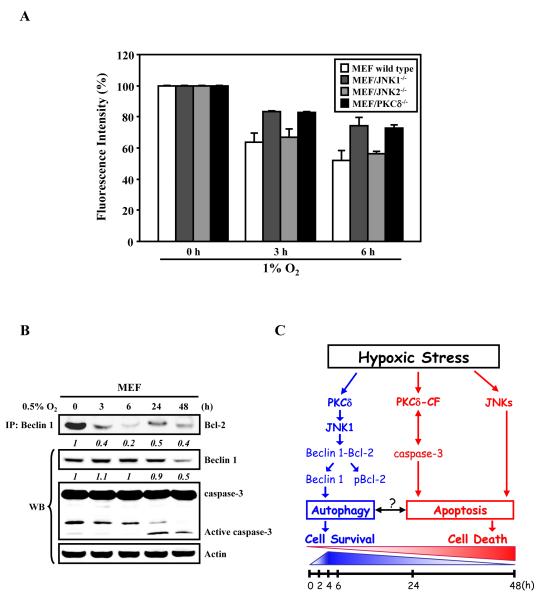
Our results suggested that the cell’s decision on death or survival in the context of hypoxic stress is determined, at least in part, by the level of autophagy that is measurable with the dissociation of Beclin 1 from phosphorylated Bcl-2, which in turn is mediated by PKCδ/JNK1.9 As a “proof of concept”, we confirmed the role of PKCδ and JNK1 in the acute hypoxia-induced autophagic process herein, by using a new quantitative assay to assess autophagic activity in living MEF(WT), MEF/PKCδ-/-, MEF/JNK1-/-, and MEF/JNK2-/- cells that have been transduced with lentivirus carrying GFP-LC3, a well-established autophagosomal marker. The assay determines the time-dependent degradation of GFP-LC3 in the autolysosomes using Fluorescence Activated Cell Sorter (FACS) analysis as described by Shvets et al.11 The reduction in GFP-LC3 fluorescence reflects its delivery into the lysosomes.11 Consistent with results of GFP-LC3 puncta formation and Western analyses of the steady-state levels of endogenous LC3-I/II,9 it was clearly demonstrated that the fluorescent signal is reduced during acute hypoxia-induced autophagic processes in a time-dependent manner (Fig. 1A). As predicted, the reduction in GFP-LC3 levels at both 3 h and 6 h post-hypoxic stress was only clearly observed in MEF wild-type and MEF/JNK2-/-, but was less evident in MEF/PKCδ-/- and MEF/JNK1-/- cells, confirming the indispensable involvement of PKCδ/JNK1 activation in the acute hypoxia-induced autophagic response.
Figure 1. Hypoxic stress induces autophagy and apoptosis through PKCδ/JNK1-mediated signaling pathways.
(A) PKCδ and JNK1 are involved in hypoxia-induced degradation of GFP-LC3 fluorescence intensity via autophagy. MEF wild type, PKCδ-/-, JNK1-/-, and JNK2-/- cells were transduced by lentiviral GFP-LC3 for 24 h, followed by exposure to 1% O2 for the indicated time periods, and subsequently subjected to FACS analysis. The relative levels of GFP-LC3 intensity measured by FACS analyses were normalized to that of control cells as described in Chen et al.9 Results from at least three independent experiments are shown as relative mean ± S.D. (i.e., reported as percentage of fluorescence intensity where control level was designed as 100%). (B) Acute hypoxic stress induces autophagy through disrupting the Bcl-2 complex, whereas prolonged hypoxia induces caspase-3 activation to promote apoptotic cell death. MEF cells were exposed to 0.5% O2 for the indicated time periods. Equal amounts of whole cell lysates were subjected to immunoprecipitation with an anti-Beclin 1 antibody, followed by Western blot analyses with an anti-Bcl-2 antibody. A fraction (i.e., 5%) of total cell lysates was subjected to Western blot analyses with specific antibodies as indicated. The relative levels (numbers in italic) are calculated by normalizing observed data against their corresponding internal loading controls of actin, where the non-treatment value is set as 1. (C) A proposed model of a PKCδ/JNK1-mediated signaling pathway network that mediates autophagy and apoptosis crosstalk/switch in response to hypoxic stress. In response to acute hypoxia, PKCδ/JNK1-mediated phosphorylation of Bcl-2 results in the dissociation of the Beclin 1/Bcl-2 complex and the activation of autophagy (shown in blue). Upon prolonged hypoxic exposure, PKCδ and caspase-3 are proteolytically activated, leading to apoptotic cell death (shown in red). The schemes shown at the bottom of the figure illustrate the hypoxic exposure time frames.
Prolonged Hypoxia Attenuated Beclin 1-dependent Autophagic Response
We have previously reported that prolonged hypoxic stress leads to the proteolytic and feed-forward activation of PKCδ and caspase-3, resulting in the irreversible commitment to apoptosis.12 These observations led us to speculate as to the reasons underlying the observation that hypoxia-stressed cells eventually failed to survive during prolonged hypoxic stress even if they underwent an earlier autophagic process. One possibility is that hypoxia-induced autophagy is a transient event. Recent reports that autophagy occurs when caspase-3 is inhibited by zVAD-fmk,13 and that Beclin 1 is cleaved by caspase-3 to inactivate autophagy,14 suggest that the proteolytic activation of caspase-3 during prolonged hypoxia may lead to inhibition of autophagy by cleaving Beclin 1. To examine these possibilities, MEF cells were subjected to treatment with 0.5% O2 for different time periods, followed by analyses of the levels of Beclin 1, caspase-3 activation and the degree of interaction between Beclin 1 and Bcl-2 (Fig. 1B). We found that the amount of Bcl-2 that can be co-immunoprecipitated with Beclin 1 gradually decreases to almost the undetectable level after 6 h of hypoxic exposure. Of particular note, we also observed that Beclin 1 is re-complexed with Bcl-2 at 24 h post-exposure, suggesting that Beclin 1-dependent autophagy is gradually attenuated. In addition, the steady-state level of Beclin 1 was found to be decreased to 50% of the control at 48 h post-hypoxic exposure, concomitantly with proteolytic activation of caspase-3. Accordingly, the lack of autophagic response to acute hypoxic stress increases cell death.9
PKCδ-dependent and -independent JNK Activation
JNKs are the other factor that plays a critical role in hypoxia, leading to activation of PKCδ that governs autophagy-apoptosis crosstalk. Our findings demonstrated that JNK1-mediated Bcl-2 phosphorylation upon acute hypoxic stress is PKCδ-dependent. Recently, JNK1 has also been demonstrated to mediate starvation- and ceramide-induced autophagy by phosphorylating Bcl-2, causing the subsequent dissociation of Beclin 1 from Bcl-2.10,15 Whether or not starvation also activates JNK1 via PKCδ is still unclear. However, JNK1 and JNK2 were also activated in a PKCδ-independent manner in response to sustained hypoxic stress.9 Thus, consistent with the pro-survival function of autophagy and pro-cell death function of prolonged JNK1 activation,16 transient activation of JNK1 by hypoxic stress via PKCδ is a cell survival mechanism, whereas the delayed PKCδ-independent activation of JNK promotes apoptosis in response to sustained hypoxic stress.
Conclusion
Taken together, our data suggest that PKCδ/JNK1 signaling mechanisms mediate Bcl-2 phosphorylation in response to hypoxic stress, which leads to the dissociation of the complex comprised of Bcl-2 and Beclin 1, resulting in the activation of autophagy. Furthermore, prolonged hypoxic stress causes the proteolytic activation of PKCδ and caspase-3, which is associated with the onset of apoptosis. Based on these findings, we propose a model for understanding the interrelationship between hypoxic stress-induced autophagy and apoptosis regulated by PKCδ (Fig. 1C). To our knowledge, this model represents the first demonstration of a time-dependent switch from autophagy to apoptosis in response to hypoxic stress. According to this model, during early time points under hypoxic stress, PKCδ-dependent JNK1 activation serves to promote autophagy in an attempt to protect cells from cell death. With sustained hypoxic stress, by contrast, the increased proteolytic activation of PKCδ and caspase-3 stops the protection from autophagy and causes cells to irreversibly commit to apoptosis. Thus, PKCδ-dependent signaling not only represents a mechanism for inducing autophagy for protecting cells from stress conditions and a mechanism for promoting apoptosis to eliminate irreversibly damaged cells, but also provides a mechanism for switching or regulating cells between survival and death.
Acknowledgements
This work was supported in part by the Hastings Foundation and National Institutes of Health Research Grants DE 10742, DE 14183 (to DK Ann), HL 38658 and HL 64365 (to KJ Kim).
Abbreviations
- PKCδ
protein kinase Cδ
- JNK
c-Jun N-terminal protein kinase
- FACS
Fluorescence Activated Cell Sorter
Footnotes
Addendum to: Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK. Novel roles for protein kinase Cδ-dependent signaling pathways in acute hypoxic stress-induced autophagy. J Biol Chem 2008; 283:34432-44.
References
- 1.Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell. 2008;15:344–57. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–6. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 6.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 7.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 8.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK. Novel roles for protein kinase Cδ-dependent signaling pathways in acute hypoxic stress-induced autophagy. J Biol Chem. 2008;283:34432–44. doi: 10.1074/jbc.M804239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shvets E, Fass E, Elazar Z. Utilizing flow cytometry to monitor autophagy in living mammalian cells. Autophagy. 2008;4:621–8. doi: 10.4161/auto.5939. [DOI] [PubMed] [Google Scholar]
- 12.Clavijo C, Chen JL, Kim KJ, Reyland ME, Ann DK. Protein kinase Cδ-dependent and -independent signaling in genotoxic response to treatment of desferroxamine, a hypoxia-mimetic agent. Am J Physiol Cell Physiol. 2007;292:C2150–60. doi: 10.1152/ajpcell.00425.2006. [DOI] [PubMed] [Google Scholar]
- 13.Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–97. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.09.004. e-publication. [DOI] [PubMed] [Google Scholar]
- 15.Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Condogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautphagy. J Biol Chem. 2008 doi: 10.1074/jbc.M805920200. e-publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventura JJ, Hubner A, Zhang C, Flavell RA, Shokat KM, Davis RJ. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21:701–10. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]