Abstract
Background and Purpose
The current gold standard for imaging intracranial AVMs involves catheter-based techniques, namely cerebral digital subtraction angiography (DSA). However, DSA presents some procedural risks to the patient. Unfortunately, AVM patients usually undergo multiple DSA exams throughout their diagnostic and therapeutic course, significantly increasing their procedural risk exposure. As such, high quality non-invasive imaging is desired. We hypothesize that 4D radial acquisition contrast-enhanced MRA approximates the vascular architecture and hemodynamics of AVMs compared to conventional angiography.
Methods
Thirteen consecutive AVM patients were assessed by 4D radial acquisition contrast-enhanced MRA and DSA. The 4D rCE-MRA images were independently assessed regarding the location, nidal size, Spetzler-Martin grade, and identification of arterial feeders, drainage pattern, and any other vascular anomalies.
Results
4D rCE-MRA correctly depicted the size, venous drainage pattern and prominent arterial feeders in all cases. Spetzler-Martin grade was correctly determined between reviewers and between the different imaging modalities in all cases except one. The nidus size was in good correlation between the reviewers, where r=0.99, p<0.000001. There was very good agreement between reviewers regarding the individual scans (k=0.63–1) while the agreement between the DSA and 4D rCE-MRA images was also good (k=0.61–0.85).
Conclusions
We have developed a 4D radial acquisition contrast-enhanced MRA sequence capable of imaging intracranial AVMs approximating that of DSA. Image analysis demonstrates equivalency in terms of grading AVMs using the Spetzler-Martin grading scale. This 4D rCE-MRA sequence has the potential to avoid some applications of DSA, thus saving patients from potential procedural risks.
Keywords: Arteriovenous Malformation, Vascular, Imaging, MRA, DSA, Angiography
Introduction
Intracranial arteriovenous malformations (iAVMs) are responsible for the majority of spontaneous intracranial hemorrhages and confer significant morbidity and mortality in young adults1, 2. Expectant management, eventual therapy, and post-procedural follow up of iAVMs require detailed vascular imaging studies. The current gold standard for imaging iAVMs involves catheter-based techniques, namely cerebral digital subtraction angiography (DSA), due mainly to its high spatial (0.2mm) and temporal (up to 24 frames/sec) resolution capabilities. However, acquiring DSA images presents some procedural risks to the patient (0.5–12.2%)3–5, including the risk of thromboembolic complications, vascular injury, and exposure to radiation and iodinated-contrast dyes. Unfortunately, iAVM patients usually undergo multiple DSA exams throughout their diagnostic and therapeutic course, whether it is for pre-procedural endovascular embolizations, surgical and/or radiosurgical planning, and/or follow-up imaging, which may be negative in many cases. Thus, multiple DSA examinations increase a patient’s procedural risk exposure. As such, non-invasive imaging techniques have been desired.
Non-invasive imaging of iAVMs using CT and MR angiography is not a novel concept6–10. Historically, the major disadvantages to both techniques have been inadequate spatial resolution and their inability to acquire dynamic information, i.e., the adequate separation of arterial, capillary, and venous phases. While CT angiography has the ability to demonstrate the iAVM nidus, inadequate slice thickness and spatial resolution, absence of sufficient dynamic information, as well as exposure to ionizing radiation and iodinated contrast agents makes CT less than desirable.
Time-of-flight magnetic resonance angiography (TOF-MRA), which uses the physiologic properties of blood flow, was developed to image the intracranial vasculature without the use of contrast agents or radiation. However, this technique lacks sufficient spatial and temporal resolution, requires a long acquisition period, and provides only a static, non-dynamic image of iAVMs. Furthermore, as a physiologic technique, image quality suffers from spin dephasing that occurs in complex or turbulent flow patterns, very common in iAVMs, as well as signal saturation in areas of slow flow11. To overcome these limitations, with the addition of contrast agents, dynamic contrast-enhanced MRA (dCE-MRA) relies on the T1 shortening of gadolinium, thus requiring shorter acquisition times per scan as well as boasting a higher signal-to-noise ratio (SNR), thereby improving image quality; but still falls short of the standard set by conventional DSA12–15. Recently, however, dCE-MRA sequences have been developed with higher temporal resolution through increased frame rates and higher spatial resolution using novel signal acquisition sequences, e.g., 4D CE-MRA with radial sliding window reconstruction and sliding mask subtraction (4D radial acquisition contrast-enhanced MRA (4D rCE-MRA))16, 17. This sequence allows the acquisition of diagnostic quality images at a high enough temporal resolution such that the phases of intracranial circulation are adequately separated. In this report, we describe the imaging of intracranial AVMs using 4D rCE-MRA at 3T and verified the grading of these AVMs between this sequence and DSA from the same patient. We hypothesize that 4D rCE-MRA can accurately image the vascular architecture and hemodynamics of iAVMs.
Materials and Methods
Consecutive iAVM patients, that were scheduled to undergo stereotactic radiosurgery as well as patients who presented to the neurovascular clinic with prior DSA imaging, were enrolled in a HIPPA-compliant, IRB-approved study to undergo a 4D rCE-MRA scan during a 12 month period. The inclusion criteria for patients were presence of a previously untreated iAVM, aged between 12–75 years-old, and normal (GFR>60) renal function. Patients were only excluded if they did not meet the above criteria or chose not to participate in the study. No in-hospital patients were examined. Stereotactic DSA imaging was performed on a Neurostar biplane angiography unit (Siemens AG Healthcare Sector, Erlangen, Germany) by selective contrast injection of all territories feeding the iAVM at 6 frames/sec in standard orthogonal anteroposterior, lateral and oblique projections.
4D rCE-MRA was performed on a 3T Whole-body MR-scanner (MAGNETOM Trio, Siemens AG Healthcare Sector, Erlangen, Germany) within 2–4 weeks of the DSA examination. A single injection of intravenous gadolinium (0.1 mmol/kg, Magnevist, Berlex, Wayne, NJ) for each anatomic (sagittal and coronal) plane was used and injected at a rate of 4ml/sec. Our 4D rCE-MRA acquisition technique included radial k-space undersampling and pseudorandom view ordering, sliding scale windowing and a sliding mask subtraction technique16, 17. We achieved a Field of View (FOV) of 220×220×75mm with pixel resolution of ~ 1mm with the temporal resolution at the equivalent of 6 frames per second by imaging the sagittal and coronal planes separately. A dynamic series of maximum-intensity-projection (MIP) images were generated in the sagittal and coronal planes and stored on a workstation.
The 4D rCE-MRA images were independently assessed by a neuroradiologist, a neurosurgeon, and an interventional radiologist blinded to the patient and clinical information. No image sequences were viewed together, i.e., DSA and MRA images from the same patient were viewed 2–3 weeks apart. An iAVM imaging questionnaire was provided to each physician regarding the location, nidal size, Spetzler-Martin (SM) grade, identification of arterial feeders, drainage pattern, and any vascular anomalies, e.g., flow-related aneurysms, venous stenosis, and varices. Each response was assigned a yes (1) or no (0) value except for the nidal size, which was a numerical value in centimeters. Each binary categorical response (e.g. presence of deep drainage) was compared between DSA and MRA images as well between the 3 reviewers using a 2 reviewer Kappa analysis. The intra- and inter-reviewer Kappa analysis was performed using the MiniTab statistics program (Minitab Inc, State College, PA).
Results
Thirteen consecutive patients were assessed by 4D rCE-MRA and DSA. The patient demographics are listed in Table 1 (Mean age was 44.2 +/− 14.7 with a male to female ratio of 6:7). DSA demonstrated 10 supratentorial and 3 infratentorial (cerebellar hemisphere) iAVMs.
Table 1.
Characteristics of patients imaged with intracranial AVMs.
| Patient | Age | Sex | Location | Presentation | Treatment |
|---|---|---|---|---|---|
| 1 | 33 | F | R Medial Parietal | Hemorrhage | GK |
| 2 | 20 | M | R Cerebellar | Incidental | Surgery |
| 3 | 51 | M | L Inferior Frontal | Vertigo | GK |
| 4 | 63 | F | L Cerebellar | L Facial Numbness | GK |
| 5 | 33 | F | R TPO | Headache | GK/Surgery |
| 6 | 56 | F | R Posterior Frontal | Incidental | GK |
| 7 | 52 | F | L Posterior Frontal | Hemorrhage | GK |
| 8 | 42 | M | R Medial Occipital | Seizure | GK/Surgery |
| 9 | 31 | F | L Parietal | Hemorrhage | GK |
| 10 | 24 | M | R Posterior Frontal | L Parasthesias | GK |
| 11 | 65 | M | R Posterior Frontal | Seizure | Embo/Surgery |
| 12 | 50 | M | R Cerebellar | Ataxia | Embo/Surgery |
| 13 | 54 | F | L Mesial Temporal | Hemorrhage | GK |
TPO – Tempo-parieto-occipital, GK – Gamma Knife, Embo – embolization.
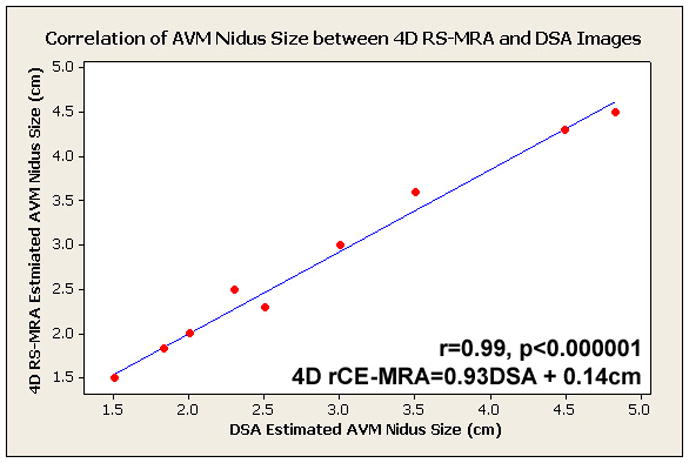
4D rCE-MRA correctly depicted the size, venous drainage pattern and prominent arterial feeders in all cases. Spetzler-Martin grade was correctly determined between reviewers and between the different imaging modalities in all cases except one, where the size of the nidus was underestimated resulting in an SM grade that was greater than the grade assigned using DSA images. The nidus size was in good correlation between the reviewers (Figure 1), where r=0.99, p<0.000001, and 4D RACE MRA=0.93 DSA + 0.14cm. There was very good agreement between raters regarding the individual scans (k=0.63–1) (Table 2) while the agreement between the DSA and 4D rCE-MRA images was also good (k=0.61–0.85) (Table 3). Figures 2 and 3 represent case examples. Figure 4 demonstrates the discovery of an incidental aneurysm on both DSA and 4D rCE-MRA images.
Figure 1.
Scatter plot of nidal size as measured in 4D rCE-MRA versus DSA.
Table 2.
MRA/DSA Comparison Inter-reviewer Kappa values
| Reviewer 1 | Reviewer 2 | Reviewer 3 | |
|---|---|---|---|
| Reviewer 1 | - | 0.63–1 | 0.5–1 |
| Reviewer 2 | 0.63–1 | - | 0.63–1 |
| Reviewer 3 | 0.5–1 | 0.63–1 | - |
Table 3.
MRA/DSA Comparison Intra-reviewer Kappa values
| Reviewer 1 | 0.61 |
| Reviewer 2 | 0.68 |
| Reviewer 3 | 0.85 |
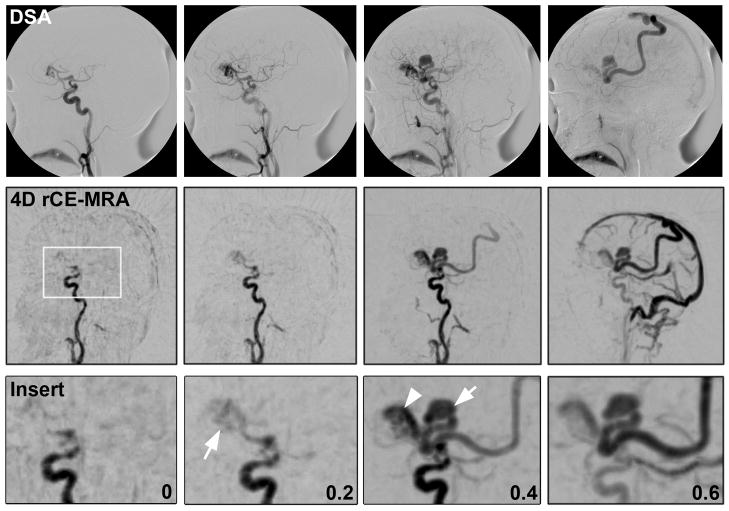
Figure 2.
Comparison of DSA and 4D rCE-MRA images in a patient with a deep iAVM associated with a verix. White box in 4D rCE-MRA image shows magnified area represented by insert row. Numbers represent approximate elapsed time between frames in all rows. Arrow in 0.2s frame demonstrates initial nidal filling. Arrow in 0.4s frame points to venous verix, arrowhead points to main draining vein.
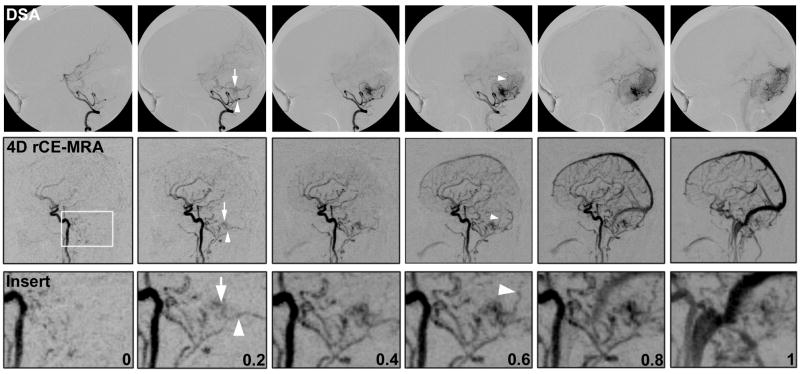
Figure 3.
Comparison of DSA and 4D rCE-MRA images in a patient with a small, infratentorial iAVM. White box in 4D rCE-MRA image shows magnified area represented by insert row. Numbers represent approximate elapsed time between frames in all rows. Arrow in 0.2s frame demonstrates initial nidal filling. Arrowhead in 0.2s frame represents en passage vessel identified. Arrowhead in 0.6s frame points to small draining vein.
Figure 4.
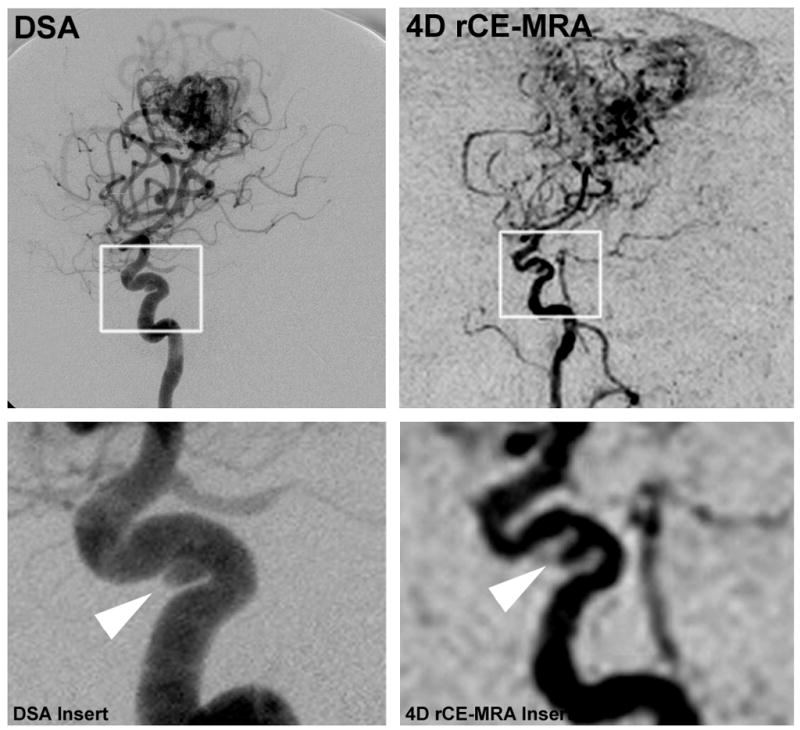
Demonstration of incidental aneurysm on DSA and 4D rCE-MRA images. White box shows area magnified in insert row. Arrowhead points to cavernous aneurysm.
Discussion
Imaging
We have found that the vascular architecture and hemodynamics of iAVMs can be determined using 4D rCE-MRA exams. Intracranial AVMs present a unique challenge for MRA because of the high-flow hemodynamics, i.e., AV shunt transit times on the order of <0.5s. As such, conventional dynamic MR signal acquisition techniques, e.g., contrast-enhanced timing-robust angiography [CENTRA], keyhole and parallel imaging, and sensitivity encoding [SENSE], have improved the temporal resolution of MRA; however, these techniques have been less successful at achieving the necessary temporal resolution required to adequately separate the hemodynamic phases of iAVMs, i.e., separate arterial and venous phases6–8, 11, 14, 18–21. Several groups have reported temporal resolutions equivalent to image acquisition speeds as low as ~600ms/frame22–25. However, acquiring adequate temporal resolution comes at the expense of spatial resolution, which for iAVMs, requires high spatial resolution. Only recently however has sufficiently high spatial resolution been achieved using novel acquisition sequences such as rapid radial undersampling techniques16, 17, 26.
MR undersampling techniques, while aiding in the improvement of the temporal resolution in recent reports of dCE-MRA of iAVMs, have still been ultimately limited by the necessity of enough contrast signal to capture all of the relevant physiological information within the time frame desired. In order to maintain both an acceptable signal-to-noise ratio along with adequate temporal resolution, we employed a combination of several MR techniques, namely radial undersampling, sliding window reconstruction, and sliding mask subtraction. Radial undersampling involves sampling a higher density of the center of k-space, where the concentration of image energy resides, and less of the outer parts. Each radially acquired line of the image contains the center of k-space, in contrast to Cartesian sampling, which does not, and thus allows fewer acquisitions without significant signal loss. Further, this technique has been shown to increase the temporal resolution with minimal degradation of spatial resolution26, 27. The sliding window reconstruction technique allows multiple frames between two consecutive independent acquisitions to be reconstructed by combining data from the two acquisitions. As a result, each reconstructed frame has an equal amount of data, but involves various combinations of consecutive acquisitions26, 27. The sliding mask subtraction technique allows the continuous subtraction of stagnate signal similar to digital subtraction angiography which allows the dynamic phases of iAVM filling to be separately imaged15. When these images are put together in a series, the dynamic filling of iAVMs can be clearly delineated with both high spatial and temporal resolution.
Limitations
The limitations of this study are several-fold. The current spatial resolution of 4D rCE-MRA is just on the order of ~1mm, less than that of DSA. While many intracranial AVMs involve arterial feeders (<1mm) that may not be completely resolved using this 4D rCE-MRA technique, this limitation did not affect the grading of the iAVMs examined in this study, as arterial feeder size or location are not considered in the classification system. Further, current improvements in contrast agents and signal acquisition techniques continue to increase the visualization of these small arterial feeders. In the cases of iAVMs which involve the deeper parts of the brain, namely the thalamus, basal ganglia, and brain stem that are notorious for having arterial feeders that are even beyond the spatial resolution of DSA, 4D rCE-MRA may not be as useful a technique. However, the shape and volume of the nidus, which is what is used for stereotactic radiosurgery planning, could possibly be delineated. Lastly, iAVMs imaged with 4D rCE-MRA are shown to fill with contrast simultaneously from all arterial feeders, which could possibly obscure some arterial feeders in the same imaged plane, given that only the MIP images are used. However, this limitation can be overcome by either multi-plane or 3D-rotational imaging, which is currently being examined at our institution.
Several limitations regarding the future implementation of such an MR technique for imaging iAVMs exist. Many patients, including those with iAVMs, cannot undergo routine MR imaging due to the presence of ferromagnetic aneurysm clips, devices, or implants. Furthermore, medical co-morbidities, such as severe renal failure, put patients at risk for nephrogenic systemic fibrosis, a serious syndrome involving extensive fibrosis of skin, joints, and internal organs associated with exposure to gadolinium28. Therefore, caution must be undertaken with renal patients by either decreasing the amount of contrast used or abandoning the use of contrasted imaging altogether. Fortunately, this syndrome is rare and even rarer in the young patients that often present with iAVMs. Moreover, contrast agents continue to improve in terms of increased signal (higher relaxivity) at lower doses, thus reducing the patients’ risks for this rare syndrome29.
Future Considerations
While DSA imaging of iAVMs will unlikely ever be replaced as long as endovascular options in their treatment exist, several clinical applications of this 4D rCE-MRA sequence are possible. Some centers continue to utilize stereotactic DSA in conjunction with MRI to plan radiosurgical treatment of iAVMs and other vascular lesions, including dAVFs18, 30. Given the high correlation of Spetzler-Martin grade between the two imaging modalities, this would allow radiosurgical planning utilizing these MRA sequences and avoid any DSA procedural risks. Furthermore, DSA is an invasive procedure which requires increased costs compared to the acquisition of these image sequences. These studies are currently underway at our institution. Another important potential clinical application is using the 4D rCE-MRA sequences for preoperative planning. Localization of specific details of the angio-architecture can be difficult and potentially problematic when working near/around eloquent areas of the brain. Having the ability to visualize the high-definition angio-architecture overlaid onto either cortical brain images or diffusion-tensor maps could allow the neurosurgeon to avoid unnecessary surgical complications. Furthermore, these sequences can be used in conjunction with stereotactic-guidance such that important aspects of the iAVM nidus or corresponding vasculature can be noted throughout the resection. Finally, iAVM patients often require multiple follow-up images, where some institutions still perform routine DSA. While MR has been shown to be a viable option with regard to post-radiosurgical treatment of iAVMs, this imaging modality could potentially improve the detection of residual nidus or early draining veins and minimize the number of DSA procedures required.
Summary
We have developed a 4D rCE-MRA sequence capable of imaging intracranial AVMs at sufficiently high spatial resolution and a temporal resolution at the equivalent of a frame rate acquisition of at least 6 frames/s, approximating that of DSA. Image analysis demonstrates equivalency in terms of grading iAVMs using the Spetzler-Martin grading scale. This 4D rCE-MRA sequence has the potential to avoid some applications of DSA, thus saving patients from potential procedural risks. Further use of this MRA sequence in different clinical applications is currently underway.
Footnotes
No portions of this manuscript have been previously presented.
No financial or material support for this manuscript and the data within were used.
References
- 1.Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A. Natural history of brain arteriovenous malformations: A long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63:823–829. doi: 10.1227/01.NEU.0000330401.82582.5E. discussion 829–831. [DOI] [PubMed] [Google Scholar]
- 2.Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: A 24-year follow-up assessment. J Neurosurg. 1990;73:387–391. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 3.Burger IM, Murphy KJ, Jordan LC, Tamargo RJ, Gailloud P. Safety of cerebral digital subtraction angiography in children: Complication rate analysis in 241 consecutive diagnostic angiograms. Stroke. 2006;37:2535–2539. doi: 10.1161/01.STR.0000239697.56147.77. [DOI] [PubMed] [Google Scholar]
- 4.Dawkins AA, Evans AL, Wattam J, Romanowski CA, Connolly DJ, Hodgson TJ, Coley SC. Complications of cerebral angiography: A prospective analysis of 2,924 consecutive procedures. Neuroradiology. 2007;49:753–759. doi: 10.1007/s00234-007-0252-y. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann TJ, Huston J, 3rd, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: Evaluation of 19,826 consecutive patients. Radiology. 2007;243:812–819. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- 6.Duran M, Schoenberg SO, Yuh WT, Knopp MV, van Kaick G, Essig M. Cerebral arteriovenous malformations: Morphologic evaluation by ultrashort 3d gadolinium-enhanced mr angiography. Eur Radiol. 2002;12:2957–2964. doi: 10.1007/s00330-002-1418-y. [DOI] [PubMed] [Google Scholar]
- 7.Farb RI, McGregor C, Kim JK, Laliberte M, Derbyshire JA, Willinsky RA, Cooper PW, Westman DG, Cheung G, Schwartz ML, Stainsby JA, Wright GA. Intracranial arteriovenous malformations: Real-time auto-triggered elliptic centric-ordered 3d gadolinium-enhanced mr angiography--initial assessment. Radiology. 2001;220:244–251. doi: 10.1148/radiology.220.1.r01jn15244. [DOI] [PubMed] [Google Scholar]
- 8.Gauvrit JY, Leclerc X, Oppenheim C, Munier T, Trystram D, Rachdi H, Nataf F, Pruvo JP, Meder JF. Three-dimensional dynamic mr digital subtraction angiography using sensitivity encoding for the evaluation of intracranial arteriovenous malformations: A preliminary study. AJNR Am J Neuroradiol. 2005;26:1525–1531. [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta V, Chugh M, Walia BS, Vaishya S, Jha AN. Use of ct angiography for anatomic localization of arteriovenous malformation nidal components. AJNR Am J Neuroradiol. 2008;29:1837–1840. doi: 10.3174/ajnr.A1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamm KD, Klisch J, Surber G, Kleinert G, Eger C, Aschenbach R. Special aspects of diagnostic imaging for radiosurgery of arteriovenous malformations. Neurosurgery. 2008;62:A44–52. doi: 10.1227/01.neu.0000325936.00982.0a. discussion A52. [DOI] [PubMed] [Google Scholar]
- 11.Ozsarlak O, Van Goethem JW, Maes M, Parizel PM. Mr angiography of the intracranial vessels: Technical aspects and clinical applications. Neuroradiology. 2004;46:955–972. doi: 10.1007/s00234-004-1297-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Maki JH, Prince MR. 3d contrast-enhanced mr angiography. J Magn Reson Imaging. 2007;25:13–25. doi: 10.1002/jmri.20767. [DOI] [PubMed] [Google Scholar]
- 13.Unlu E, Temizoz O, Albayram S, Genchellac H, Hamamcioglu MK, Kurt I, Demir MK. Contrast-enhanced mr 3d angiography in the assessment of brain avms. Eur J Radiol. 2006;60:367–378. doi: 10.1016/j.ejrad.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya K, Katase S, Yoshino A, Hachiya J. Mr digital subtraction angiography of cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 2000;21:707–711. [PMC free article] [PubMed] [Google Scholar]
- 15.Cashen TA, Carr JC, Shin W, Walker MT, Futterer SF, Shaibani A, McCarthy RM, Carroll TJ. Intracranial time-resolved contrast-enhanced mr angiography at 3t. AJNR Am J Neuroradiol. 2006;27:822–829. [PMC free article] [PubMed] [Google Scholar]
- 16.Cashen TA, Jeong H, Shah MK, Bhatt HM, Shin W, Carr JC, Walker MT, Batjer HH, Carroll TJ. 4d radial contrast-enhanced mr angiography with sliding subtraction. Magn Reson Med. 2007;58:962–972. doi: 10.1002/mrm.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong HH, Cashen TA, Hurley MC, Eddleman CS, Getch CG, Batjer HH, Carroll TJ. Radial sliding window mra with hypr. Magn Reson Med. 2009;58:962–972. doi: 10.1002/mrm.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauvrit JY, Oppenheim C, Nataf F, Naggara O, Trystram D, Munier T, Fredy D, Pruvo JP, Roux FX, Leclerc X, Meder JF. Three-dimensional dynamic magnetic resonance angiography for the evaluation of radiosurgically treated cerebral arteriovenous malformations. Eur Radiol. 2006;16:583–591. doi: 10.1007/s00330-005-0011-6. [DOI] [PubMed] [Google Scholar]
- 19.Summers PE, Kollias SS, Valavanis A. Resolution improvement in thick-slab magnetic resonance digital subtraction angiography using sense at 3t. J Magn Reson Imaging. 2004;20:662–673. doi: 10.1002/jmri.20156. [DOI] [PubMed] [Google Scholar]
- 20.Taschner CA, Gieseke J, Le Thuc V, Rachdi H, Reyns N, Gauvrit JY, Leclerc X. Intracranial arteriovenous malformation: Time-resolved contrast-enhanced mr angiography with combination of parallel imaging, keyhole acquisition, and k-space sampling techniques at 1.5 t. Radiology. 2008;246:871–879. doi: 10.1148/radiol.2463070293. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya K, Aoki C, Fujikawa A, Hachiya J. Three-dimensional mr digital subtraction angiography using parallel imaging and keyhole data sampling in cerebrovascular diseases: Initial experience. Eur Radiol. 2004;14:1494–1497. doi: 10.1007/s00330-004-2281-9. [DOI] [PubMed] [Google Scholar]
- 22.Hadizadeh DR, von Falkenhausen M, Gieseke J, Meyer B, Urbach H, Hoogeveen R, Schild HH, Willinek WA. Cerebral arteriovenous malformation: Spetzler-martin classification at subsecond-temporal-resolution four-dimensional mr angiography compared with that at dsa. Radiology. 2008;246:205–213. doi: 10.1148/radiol.2453061684. [DOI] [PubMed] [Google Scholar]
- 23.Reinacher P, Reinges MH, Simon VA, Hans FJ, Krings T. Dynamic 3-d contrast-enhanced angiography of cerebral tumours and vascular malformations. Eur Radiol. 2007;17 (Suppl 6):F52–62. doi: 10.1007/s10406-007-0229-2. [DOI] [PubMed] [Google Scholar]
- 24.Reinacher PC, Stracke P, Reinges MH, Hans FJ, Krings T. Contrast-enhanced time-resolved 3-d mra: Applications in neurosurgery and interventional neuroradiology. Neuroradiology. 2007;49 (Suppl 1):S3–13. doi: 10.1007/s00234-007-1468-6. [DOI] [PubMed] [Google Scholar]
- 25.Saleh RS, Lohan DG, Villablanca JP, Duckwiler G, Kee ST, Finn JP. Assessment of craniospinal arteriovenous malformations at 3t with highly temporally and highly spatially resolved contrast-enhanced mr angiography. AJNR Am J Neuroradiol. 2008;29:1024–1031. doi: 10.3174/ajnr.A0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumashiro M, Murase K, Oda K, Fukushige M, Ito O, Nagayama M, Watanabe Y. Assessment of time-resolved, dynamic, contrast-enhanced mrdsa using radial sliding-window reconstruction. Magn Reson Med Sci. 2008;7:1–12. doi: 10.2463/mrms.7.1. [DOI] [PubMed] [Google Scholar]
- 27.d’Arcy JA, Collins DJ, Rowland IJ, Padhani AR, Leach MO. Applications of sliding window reconstruction with cartesian sampling for dynamic contrast enhanced mri. NMR Biomed. 2002;15:174–183. doi: 10.1002/nbm.755. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Rodriguez J, Lai S, Ehst BD, Fine DM, Bluemke DA. Nephrogenic systemic fibrosis: Incidence, associations, and effect of risk factor assessment--report of 33 cases. Radiology. 2009;250:371–377. doi: 10.1148/radiol.2502080498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juluru K, Vogel-Claussen J, Macura KJ, Kamel IR, Steever A, Bluemke DA. Mr imaging in patients at risk for developing nephrogenic systemic fibrosis: Protocols, practices, and imaging techniques to maximize patient safety. Radiographics. 2009;29:9–22. doi: 10.1148/rg.291085072. [DOI] [PubMed] [Google Scholar]
- 30.St George EJ, Butler P, Plowman PN. Can magnetic resonance imaging alone accurately define the arteriovenous nidus for gamma knife radiosurgery? J Neurosurg. 2002;97:464–470. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]