Abstract
Gene-environment interactions involving exogenous environmental factors are known to shape behavior and personality development. Although gene-environment interactions involving endogenous environmental factors are hypothesized to play an equally important role, this conceptual approach has not been empirically applied in the study of early-developing temperament in humans. Here we report evidence for a gene-endoenvironment (i.e., resting frontal brain electroencephalogram, EEG, asymmetry) interaction in predicting child temperament. The DRD4 gene (long allele vs. short allele) moderated the relation between resting frontal EEG asymmetry (left vs. right) at 9 months and temperament at 48 months. Children who exhibited left frontal EEG asymmetry at 9 months and who possessed the DRD4 long allele were significantly more soothable at 48 months than other children. Among children with right frontal EEG asymmetry at 9 months, those with the DRD4 long allele had significantly more difficulties focusing and sustaining attention at 48 months than those with the DRD4 short allele. Resting frontal EEG asymmetry did not influence temperament in the absence of the DRD4 long allele. We discuss how the interaction of genetic and endoenvironment factors may confer risk and protection for different behavioral styles in children.
Although recent studies have reported gene-environment interactions that shape behavior and personality development (for reviews, see Caspi et al., 2002, 2003; Fox et al., 2005; Rutter, Moffitt, & Caspi, 2006), these studies have exclusively conceptualized the environment as outside of the individual and accordingly have measured exogenous environmental factors (e.g., parenting, exposure to maltreatment, stress, poverty). The endogenous environment (i.e., the environment inside the organism) is hypothesized to play an equally important role in gene expression and behavior in human and nonhuman animals (Gottlieb, 1998; see also Jasny, Kelner, & Pennisi, 2008, for a recent review) and has long been described in other biological disciplines (e.g., plant physiology) as critical to gene expression and resulting phenotypes (Meyer et al., 1992). However, the gene-environmental model in which the environment is conceptualized as comprising endogenous factors has not been empirically investigated in studies of early-developing temperament in humans. In the study reported here, we examined a gene-endoenvironment interaction (i.e., an interaction between the dopamine D4 receptor, or DRD4, gene and resting frontal electroencephalogram, EEG, asymmetry) in predicting child temperament.
DRD4 LONG ALLELE: MULTIPLE ROLES
The long allele of the DRD4 gene appears to play multiple roles in behavior. This allele has been linked to approach-related behaviors (e.g., novelty seeking), positive affect (e.g., feelings of euphoria and reward dependence), and stimulus responsivity in human and nonhuman animals. Two initial studies noted that human adults with longer versions (6–8 repeats) of the coding-sequence polymorphism self-reported higher novelty seeking than adults with shorter versions (2–5 repeats; Benjamin et al., 1996; Ebstein et al., 1996). The association between the DRD4 long allele and novelty seeking has been independently replicated in some studies (see Schinka, Letsch, & Crawford, 2002, for results of a meta-analysis), but associations in the opposite direction also have been noted (Malhotra et al., 1996). Most recently, the DRD4 long allele has been connected to novelty-seeking behaviors in nonhuman primates (Bailey, Breidenthal, Jorgensen, McCracken, & Fairbanks, 2007).
The DRD4 long allele has also been reliably implicated in attention-related problems in children. For example, researchers have reported associations of this allele with attention-related problems in typically developing human infants (Auerbach, Benjamin, Faroy, Geller, & Ebstein, 2001) and preschool and early-school-age children (Schmidt, Fox, Perez-Edgar, Hu, & Hamer, 2001), as well as in clinical samples of children with attention-deficit/hyperactivity disorder (see Faraone et al., 2005). The DRD4 long allele has also been linked to preschoolers’ aggression (Schmidt, Fox, Rubin, Hu, & Hamer, 2002), to externalizing behaviors in children with low IQ (DeYoung et al., 2006), and to disorganized attachment in healthy 12-month-old infants (Lakatos et al., 2000). Other researchers have found no association of the DRD4 gene with attachment status (Bakermans-Kranenburg & van IJzendoorn, 2004) or the opposite pattern of association between the DRD4 alleles and cognitive and behavioral problems in children (Birkas et al., 2005).
Given the equivocal findings from research examining main effects of the DRD4 gene, some researchers have investigated whether child behavior and temperament can be predicted by interactions of this gene with exogenous environmental factors. Sheese, Voelker, Rothbart, and Posner (2007) recently reported that among children who possessed the DRD4 long allele, lower quality of parenting was associated with higher levels of sensation seeking. Parenting quality did not affect temperament in children without the long allele. Similarly, Bakermans-Kranenburg and van IJzendoorn (2006) reported that children with the long DRD4 genotype and insensitive caregivers exhibited more externalizing behaviors than children with other combinations of DRD4 genotype and caregiving. More recently, Bakermans-Kranenburg, van IJzendoorn, Pijlman, Mesman, and Femmie (2008) noted that the DRD4 gene moderated effects of intervention programs on toddlers’ externalizing behaviors. The effects of parenting were largest in toddlers who possessed the long allele of the DRD4 gene and whose parents used positive discipline.
FRONTAL BRAIN ASYMMETRY: AN ENDOENVIRONMENTAL FACTOR
One endogenous factor that can be measured relatively noninvasively in pediatric populations and that has not been examined in relation to molecular genetics and temperament in children is brain electrical activity. A number of studies have reported that the pattern of the resting frontal EEG reflects predisposition to experience positive or negative affect and is associated with individual differences in affective style in healthy infants, children, and adults, as well as in some clinical populations when their symptoms are in remission (for substantive reviews, see Coan & Allen, 2004; Davidson, 2000). Individual differences in the pattern of resting frontal EEG asymmetry appear during the 1st year of postnatal life, remain modestly stable across development, and in association with other endogenous factors (e.g., temperament) predict developmental outcome (Henderson, Fox, & Rubin, 2001).
Left frontal EEG asymmetry at rest is presumed to reflect individual dispositions in the experience of positive affect and the ability to regulate affect and behavior (Davidson, 2000). Infants who display greater left frontal EEG asymmetry at rest are characterized as having “easy” temperaments (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). These infants are easily soothed and calmed. In contrast, right frontal EEG asymmetry at rest is presumed to be a disposition to experience negative affect and the inability to regulate affect and behavior (Davidson & Fox, 1989). Infants who exhibit right frontal EEG asymmetry at rest are characterized as having “negative reactive” temperaments (Fox et al., 2001). These infants are easily distressed, are difficult to soothe, and have problems switching and focusing attention.
THE PRESENT STUDY
In the present study, we examined the interaction of the DRD4 gene and resting frontal EEG asymmetry in predicting temperament in typically developing children. When infants were 9 months old, continuous measures of frontal EEG activity were collected during a baseline condition and groups with relative left and right frontal asymmetry were formed. When the children were 48 months old, mothers completed ratings of their children’s temperament, and the children donated buccal cells. DNA was extracted from these cells and genotyped for the short (2–5 repeats) and long (6–8 repeats) allele of the DRD4 gene.
We predicted that the DRD4 gene would moderate the relation between resting frontal EEG asymmetry at 9 months and measures of regulation and attentional components of temperament at 48 months. We hypothesized that the DRD4 long allele in the presence of resting left frontal asymmetry (a marker of behavioral regulation) may confer a temperament characterized by the ability to regulate affect and behavior (i.e., soothability), given the predisposition to experience positive affect associated with left frontal EEG asymmetry and the stimulus responsivity associated with the DRD4 long allele. Specifically, we predicted that children who exhibited greater relative left frontal EEG activity at 9 months and who possessed the long allele of the DRD4 gene would score higher on soothability than children who possessed other combinations of the DRD4 gene and frontal EEG asymmetry. In contrast, we hypothesized that the DRD4 long allele in the presence of resting right frontal asymmetry (a marker of behavioral dysregulation) may confer a temperament characterized by difficulties focusing and switching attention and by problems regulating behavior. Specifically, we predicted that children who exhibited greater relative right frontal EEG activity at 9 months and who possessed the long allele of the DRD4 gene would score higher on attention-span difficulties and lower on soothability than children who possessed other combinations of the DRD4 gene and frontal EEG asymmetry.
METHOD
The participants in this study were 88 typically developing children for whom we had complete gene, frontal-EEG-asymmetry, and temperament data. These children had been followed since infancy as part of a larger longitudinal study examining psychophysiology and socioemotional development (see Fox et al., 2001, 2005). The children were primarily Caucasian and middle-class. All of the parents had completed high school, and the majority were college graduates. The children were living with their families in or near College Park, Maryland. Original exclusion criteria were that the child had no peri- or postnatal complications and no known neurological problems. All procedures were approved by the University of Maryland Research Ethics Board, and written consent was obtained for all procedures. The analyses we report focused on the EEG data collected at 9 months and gene and temperament measures collected at 48 months. The participants in this study did not differ on any behavioral or demographic measures from the originally recruited infants who did not return to the laboratory at 9 and 48 months of age.
EEG Procedures
EEG Recording
At 9 months of age, each infant was seated on his or her mother’s lap, and a Lycra stretch cap (Electro-Cap, Inc., Eaton, OH) was positioned on the infant’s head. The cap electrodes were positioned according to the international 10–20 system of electrode placement. The experimenter used the blunt end of a cotton swab in combination with an abrasive gel and gently abraded each electrode surface. Each electrode site was then filled with a small amount of electrolyte gel, which served as a conductor. Electrode impedances below 10 kΩ at a given site and variation within 500 Ω between homologous sites were considered acceptable.
Resting EEG was recorded for 3 min while the infant attended to a spinning bingo wheel containing brightly colored balls; the wheel was positioned on a table directly in front of the infant. EEG was recorded from eight scalp locations: left and right midfrontal (F3, F4), central (C3, C4), parietal (P3, P4), and occipital (O1, O2) regions. These sites represent the left and right hemispheres and anterior and posterior regions of the brain. EEG data were collected in posterior regions to ensure that the interactions between asymmetry pattern and genotype were specific to the frontal region. All electrodes were referenced to the central vertex (Cz). The EEG channels were amplified by separate Grass AC bioamplifiers (7p511; West Warwick, RI), with filter settings for each channel set at 1 Hz (high pass) and 100 Hz (low pass). The data from all channels were digitized on-line at a sampling rate of 512 Hz.
EEG Data Reduction and Analysis
We used software to rereference the EEG data to an average reference configuration. The data were then visually scored for artifact due to eyeblinks, eye movements, and other motor movements, using software developed by James Long Company (EEG Analysis Program, Caroga Lake, NY). All artifact-free EEG data were analyzed using a discrete Fourier transform (DFT), with a Hanning window of 1-s width and 50% overlap. Power (microvolts squared) was then derived from the DFT output in single-hertz bins from 1 Hz to 12 Hz and was natural-log-transformed in order to reduce skewness. EEG power in the 4- to 6-Hz band was used in the analyses for two reasons: First, a majority of the EEG power was represented within this frequency band. Second, previous studies have related this frequency band to individual differences in temperament at the age of 9 months (e.g., Fox et al., 2001). A separate EEG asymmetry score was computed for each region (i.e., right EEG power minus left EEG power). Because EEG power is inversely related to activity, negative scores on this metric reflect greater relative right activity (Davidson, 2000). The children were divided into two groups on the basis of their frontal-EEG-asymmetry score: left frontal (positive scores; n = 51) and right frontal (negative scores; n = 37). Similar asymmetry scores were computed for all regions, but analyses involving interactions between the DRD4 gene and regional EEG asymmetry at other regions were not significant and are not discussed further.
Maternal Report of Temperament
When the children were 48 months old, their mothers completed the Colorado Childhood Temperament Inventory (CCTI; Rowe & Plomin, 1977). The CCTI is a 30-item measure that is widely used to index childhood temperament. It comprises six subscales that index different aspects of temperament: activity level (e.g., “Child goes from toy to toy quickly”), attention-span difficulties (e.g., “Child gives up easily when difficulties are encountered”), emotionality (e.g., “Child gets easily upset”), shyness (e.g., “Child tends to be shy”), sociability (e.g., “Child makes friends easily”), and soothability (e.g., “When upset by an unexpected situation, child quickly calms down”). The items are rated on a 5-point Likert scale from 1 (not at all, strongly disagree) to 5 (a lot, strongly agree). Scores for the 5 items from each subscale were summed and averaged. The Cronbach alphas for this sample were .77 for activity level, .75 for attention-span difficulties, .76 for emotionality, .82 for shyness, .63 for sociability, and .73 for soothability. These values are similar to those originally reported by Rowe and Plomin (1977).
Although the CCTI indexes six individual temperamental characteristics, we were particularly interested in the subscales indexing attention-span difficulties and soothability for several reasons. First, these two subscales are conceptually related to the hypothesized executive-control processes of attention and regulation, respectively, thought to underlie individual differences in temperament (Rothbart & Posner, 2005). Second, the most reliable empirical evidence to date has related the DRD4 gene to attention-related processes (see Faraone et al., 2005). Third, there has been relatively little theoretical and empirical evidence linking the DRD4 gene or dopamine to constructs assessed by the other CCTI subscales, which reflect the socio-affective components of temperament. Much of the work on these other subscales has involved the serotonin transporter gene, and in particular has related it to emotionality and shyness in children (Fox et al., 2005) and adults (Lesch et al., 1996). Fourth, it is plausible that attention and regulation are endogenous aspects of temperament, which are likely more influenced by endogenous factors than by exogenous factors (although see Kopp, 1982, for discussion of exogenous factors influencing the development of self-regulation). In contrast, emotionality, shyness, and sociability are more complex social aspects of temperament, and are later developing and possibly more susceptible to exogenous factors (e.g., socialization, parenting, and early peer relationships; Fox et al., 2005) than attention and regulation.
DNA Preparation and Genotyping
Buccal-Cell Collection
Genomic DNA was prepared from buccal swabs (Epicentre Technologies, Madison, WI). Children were instructed by an investigator or parent to roll a buccal brush firmly on the inside of the cheek, approximately 20 times on each side. The brushes were air-dried for 10 to 20 min and then sent within 3 days to the laboratory at the National Institutes of Health for extraction within 3 days. DNA was prepared by absorption to a bead matrix and heat elution according to the manufacturer’s instructions.
DRD4 Genotype
The DRD4 gene exon III polymorphism was assayed by polymerase chain reaction (PCR), using the primers and reaction conditions, combined with a “hot start,” described by Lichter et al. (1993). The number of repeats was determined by electrophoresis through a 3.5% agarose gel and ethidium bromide staining. Allele frequencies were 6.2% for allele 2, 1.9% for allele 3, 68.3% for allele 4, 1.6% for allele 5, 0.3% for allele 6, 21.4% for allele 7, and 0.3% for allele 8. On the basis of previous results (Benjamin et al., 1996), we classified genotypes into two groups (s = 2–5 repeats; l = 6–8 repeats): Infants in the short group had the s/s genotype (n = 52), and those in the long group had the s/l or l/l genotype (n = 36). Indistinguishable results were obtained when genotypes were coded according to the presence or absence of the seven-repeat alleles (Ebstein et al., 1996) or as the sum of allele lengths.
RESULTS
Correlations Among Measures
The Pearson correlation between the attention-span-difficulties and soothability subscales of the CCTI was .03, confirming that the two subscales were largely independent, as in prior research (Rowe & Plomin, 1977). We also computed Pearson correlations between resting frontal EEG asymmetry at 9 months and CCTI soothability and attention-span difficulties. These relations were not significant.
Between-Group Analyses
Table 1 presents the mean scores (and standard errors) on the attention-span-difficulties and soothability CCTI subscales, for each combination of DRD4 genotype (short, long) and frontal EEG asymmetry (left, right). An analysis of variance with DRD4 group and frontal-EEG-asymmetry group as the between-subjects factors was performed separately on each of the two CCTI subscales.
TABLE 1.
Mean Soothability and Attention-Span Difficulties at Age 48 Months in the Four Participant Groups
| Long DRD4 allele | Short DRD4 allele | |||||||
|---|---|---|---|---|---|---|---|---|
| Left frontal asymmetry (n = 14) | Right frontal asymmetry (n = 22) | Left frontal asymmetry (n = 37) | Right frontal asymmetry (n = 15) | |||||
| CCTI measure | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
| Soothability | 3.74a | 0.10 | 3.12b | 0.13 | 3.30b | 0.11 | 3.36b | 0.15 |
| Attention-span difficulties | 3.31 | 0.20 | 3.69a | 0.13 | 3.48 | 0.09 | 3.17b | 0.20 |
Note. CCTI = Colorado Childhood Temperament Inventory (Rowe & Plomin, 1977); DRD4 = dopamine D4 receptor. Frontal asymmetry was assessed at age 9 months. Within a row, means with different subscripts are significantly different from one another, p <.05
As illustrated in Figure 1a, the DRD4 × Frontal EEG Asymmetry interaction was significant for the CCTI soothability subscale, F(1, 87) = 6.19, p = .015. As predicted, children who exhibited greater relative left frontal EEG activity at 9 months and who possessed the DRD4 long allele were reported by their mothers to be significantly more soothable at 48 months than children who exhibited greater relative left frontal EEG activity at 9 months and who possessed the DRD4 short allele, t(49) = 2.30, p = .026, d = 0.66; they were also reported to be significantly more soothable than children who exhibited right frontal EEG activity at 9 months and who possessed the DRD4 long allele, t(34) = 3.51, p = .001, d = 1.20, or the DRD4 short allele, t(27) = 2.13, p = .043, d = 0.82. As expected, the children with the DRD4 long allele and right frontal asymmetry had the lowest soothability score.
Fig. 1.
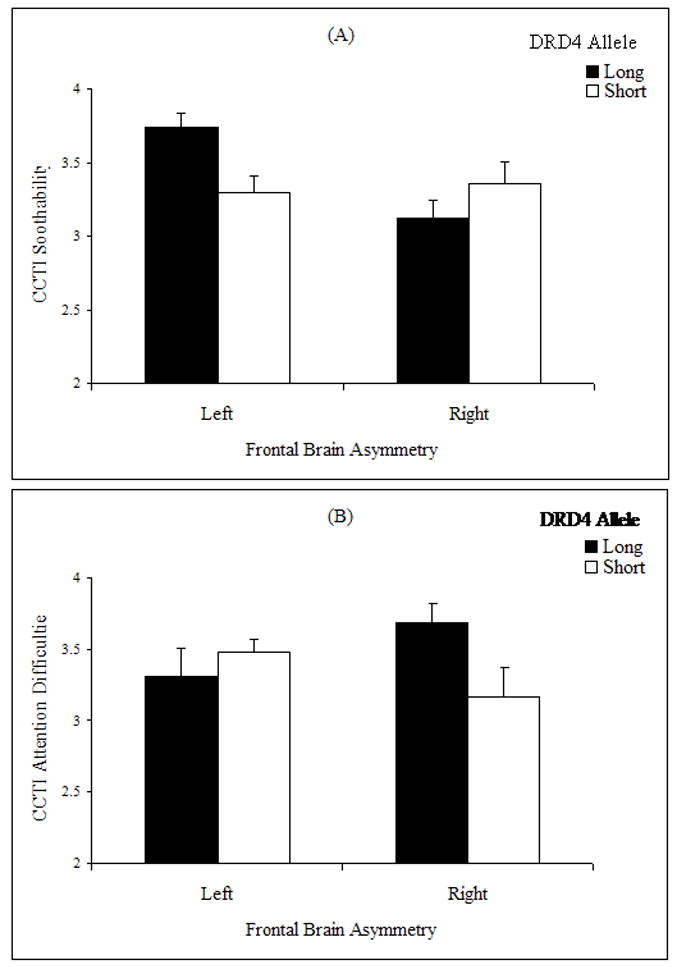
Mean (a) soothability and (b) attention difficulties among 48-month-old children as a function of dopamine D4 receptor (DRD4) gene (long vs. short allele) and resting frontal electroencephalogram asymmetry (left vs. right) at 9 months. Soothability and attention difficulties were assessed by maternal report on the Colorado Childhood Temperament Inventory (CCTI; Rowe & Plomin, 1977). Error bars represent standard errors.
As illustrated in Figure 1b, the DRD4 × Frontal EEG Asymmetry interaction was also significant for the CCTI attention-span-difficulties subscale, F(1, 87) = 5.12, p = .026. As predicted, infants who exhibited greater relative right frontal EEG activity at 9 months and who possessed the DRD4 long allele were reported by their mothers to have significantly greater difficulty focusing and sustaining attention at 48 months than children who exhibited greater relative right frontal EEG activity at 9 months and who possessed the DRD4 short allele, t(35) = 2.22, p = .033, d = 0.75.
DISCUSSION
We found evidence of a gene-endoenvironment interaction in predicting two components of temperament in a sample of typically developing children. The DRD4 long allele moderated the relation between the pattern of resting frontal EEG asymmetry at 9 months and regulation and attention at 48 months. Children who possessed the DRD4 long allele and who exhibited left frontal EEG asymmetry were significantly more soothable than children who possessed the DRD4 long allele and who exhibited right frontal EEG asymmetry or those who possessed the short allele and exhibited either left or right frontal EEG asymmetry. Children who had the DRD4 long allele and who exhibited right frontal EEG asymmetry had significantly more difficulty focusing and sustaining attention than children who had the DRD4 short allele and who exhibited right frontal EEG asymmetry group; they also had the lowest soothability scores. Resting frontal EEG asymmetry did not influence temperament in the absence of the DRD4 long allele.
What role does the DRD4 long allele play in children’s temperament? Our results indicate that this allele acts as a moderator or susceptibility factor: Carriers of the long allele are more sensitive to both the positive influence of left frontal EEG asymmetry on soothability and the negative influence of right frontal EEG asymmetry on attention difficulties. These results are consistent with the recent findings of Bakermans-Kranenburg and her colleagues, pointing to the DRD4 long allele as a “susceptibility” allele (see Bakermans-Kranenburg & Van IJzendoorn, 2006, 2007; Bakermans-Kranenburg et al., 2008).
The current findings are also in line with, and extend, the idea of genetic susceptibility for certain behaviors that are expressed in different environments (e.g., Belsky, Bakermans-Kranenburg, & Van IJzendoorn, 2007). Belsky et al. argued that a gene may be expressed for better or for worse depending on environmental conditions. Our results suggest that it is possible that the DRD4 long allele plays different roles (for better and for worse) in child temperament as a function of endoenvironmental conditions: The DRD4 long allele may be expressed for better and serve as a protective factor if the endoenvironmental condition is left frontal asymmetry, but this genotype may be expressed for worse or be an additional risk factor if the endoenvironmental condition is right frontal asymmetry.
It is also important to point out that the DRD4 gene was not related to resting frontal EEG asymmetry. The main effect of genotype group (long vs. short DRD4 allele) on the continuous measure of frontal EEG asymmetry at 9 months was not significant. Rutter et al. (2006) argued that gene-environment interactions should not be discussed in the presence of a gene-environment correlation. Our results raise the possibility of decoupled biological processes (i.e., genetics and physiology) that may interact on some level to confer a particular behavioral phenotype.
Limitations
The present study has several limitations. First, although the sample size was relatively large compared with the samples in other recent studies examining associations between molecular genetics and complex traits in children, and although the effect sizes were medium to large, the results need to be replicated to ensure their reliability. Second, temperament was assessed using a single maternal report when the children were 48 months old. Maternal-report measures may be inherently biased. Third, causation cannot be inferred, and the findings should be interpreted with caution. For example, we do not know the direction of the influence between children’s temperament and the pattern of frontal EEG activity or between gene expression and frontal EEG activity. Future research using repeated objective measures of temperament and psychophysiology over an extended time period would be informative.
One last caveat also warrants discussion. It is important to point out that many EEG parameters appear to have a genetic basis (for a review, see van Beijsterveldt & Boomsma, 1994). Accordingly, our findings might reflect complex gene-gene interactions involving the DRD4 gene and a gene linked to midfrontal EEG asymmetry. In two recent twin studies, genetic factors have accounted for a small but significant amount of variance in midfrontal (F4–F3) EEG asymmetry in adults (Anokhin, Heath, & Myers, 2006) and children (Gao, Tuvblad, Raine, Lozano, & Baker, in press).
Conclusions and Implications
Individual differences in human temperament are undoubtedly multiply and complexly determined by processes involving multiple genes, biological systems, and experiences. To fully understand the brain mechanisms underlying individual differences in temperament, researchers need to consider the many variables that may moderate brain-behavior relations. One logical source of individual variability that moderates these relations is genes involved in modulating neurochemicals presumed to underlie cognitive and affective processes (see Rothbart & Posner, 2005). Studies of humans and nonhuman animals have shown that within the frontal cortex, dopamine plays key roles in cognitive and affective processes involved in individual differences in behavior and temperament (for reviews, see Berridge, 2003; Depue & Collins, 1999). We found evidence that a gene involved in the regulation of dopamine moderates the relation between frontal brain activity and two basic components of early temperament, one cognitive (i.e., attention) and one affective (i.e., regulation). We conceptualized resting frontal brain asymmetry as an endogenous environmental condition and used a relatively noninvasive psychophysiological measure to index brain activity. Our data serve as a starting point for considering the impact of endogenous factors and other exogenous environmental factors on gene expression to fully understand the complexities of temperament and other complex human traits.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (HD 17899) awarded to Nathan A. Fox. The authors wish to thank the parents and children for their participation; Stacy Barton, Susan Calkins, Genevieve Erb, Heather Henderson, Lisa Perry-Moss, Ariana Shahinfar, and Cindy Smith for their help with behavioral data collection at the University of Maryland; Stella Hu for her help with genotyping at the National Institutes of Health; and Alison Niccols and two anonymous reviewers for their helpful comments.
References
- Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: A twin study. Biological Psychology. 2006;71:289–295. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach JG, Benjamin J, Faroy M, Geller V, Ebstein R. DRD4 related to infant attention and information processing: A developmental link to ADHD? Psychiatric Genetics. 2001;11:31–35. doi: 10.1097/00041444-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Bailey JN, Breidenthal SE, Jorgensen MJ, McCracken JT, Fairbanks LA. The association of DRD4 and novelty seeking is found in a nonhuman primate model. Psychiatric Genetics. 2007;17:23–27. doi: 10.1097/YPG.0b013e32801140f2. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. No association of the dopamine D4 receptor (DRD4) and –521 C/T promoter polymorphisms with infant attachment disorganization. Attachment & Human Development. 2004;6:211–218. doi: 10.1080/14616730412331281584. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Genetic vulnerability or differential susceptibility in child development: The case of attachment. Journal of Child Psychology and Psychiatry. 2007;48:1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Pijlman FTA, Mesman J, Femmie J. Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between D4 dopamine receptor gene and measures of novelty seeking. Nature Genetics. 1996;12:81–84. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain and Cognition. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Birkas E, Lakatos K, Nemoda Z, Ney K, Toth I, Novak A, et al. Effects of the D4 dopamine receptor gene variation on behavior problems at 6 years of age. Neuropsychopharmacologia Hungarica. 2005;7:125–131. [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ responses to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Seguin JR, Mejia JM, Pihl RO, Beitchman JH, et al. The dopamine D4 receptor gene and moderation of the association between externalizing behavior and IQ. Archives of General Psychiatry. 2006;63:1410–1416. doi: 10.1001/archpsyc.63.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, et al. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nature Genetics. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Faraone S, Perlis R, Doyle A, Smoller J, Goralnick J, Holmgren M, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin KH, Schmidt LA, Hamer D, et al. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Tuvblad C, Raine A, Lozano DI, Baker LA. Genetic and environmental influences on frontal EEG asymmetry and alpha power in 9–10-year-old twins. Psychophysiology. doi: 10.1111/j.1469-8986.2009.00815.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Normally occurring environmental and behavioral influences on gene activity: From central dogma to probabilistic epigenesis. Psychological Review. 1998;105:792–802. doi: 10.1037/0033-295x.105.4.792-802. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Jasny BR, Kelner KL, Pennisi E, editors. Science. Vol. 322. 2008. From genes to social behavior [Special section] pp. 891–914. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Lakatos K, Toth I, Nemoda Z, Ney K, Sasvari-Szekely M, Gervai J. Dopamine D4 receptor (DRD4) gene polymorphism is associated with attachment disorganization in infants. Molecular Psychiatry. 2000;5:633–637. doi: 10.1038/sj.mp.4000773. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lichter JB, Barr CL, Kennedy JL, Van Tol HH, Kidd KK, Livak KJ. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Human Molecular Genetics. 1993;2:767–773. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Virkkunen M, Rooney W, Eggert M, Linnoilia M, Goldman D. The association between the dopamine D4 receptor (D4DR) 16 amino acid repeat polymorphism and novelty seeking. Molecular Psychiatry. 1996;1:388–391. [PubMed] [Google Scholar]
- Meyer P, Linn F, Heidmann I, Meyer H, Niedenhof I, Saedler H. Endogenous and environmental factors influence 35S promoter methylation of a maize A1 gene construct in transgenic petunia and its colour phenotype. Molecular Genetics and Genomics. 1992;231:345–352. doi: 10.1007/BF00292701. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI. Genes and experience in the development of executive attention and effort control. New Directions for Child and Adolescent Development. 2005;109:101–108. doi: 10.1002/cd.142. [DOI] [PubMed] [Google Scholar]
- Rowe DC, Plomin R. Temperament in early childhood. Journal of Personality Assessment. 1977;41:150–156. doi: 10.1207/s15327752jpa4102_5. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Letsch EA, Crawford FC. DRD4 and novelty seeking: Results of meta-analyses. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2002;114:643–648. doi: 10.1002/ajmg.10649. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hu S, Hamer DH. Association of DRD4 with attention problems in normal childhood development. Psychiatric Genetics. 2001;11:25–29. doi: 10.1097/00041444-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Hu S, Hamer DH. Molecular genetics of shyness and aggression in preschoolers. Personality and Individual Differences. 2002;33:227–238. [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): A review. Human Genetics. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]


