Summary
3-Hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors, commonly known as statins, are widely used clinically for their lipid lowering properties. Recent evidence shows that statins are also effective in ameliorating cerebral vasospasm, which occurs as sequelae of subarachnoid haemorrhage. This review focuses on the pleiotropic effects of statins, and the putative mechanisms involved in statin mediated attenuation of cerebral vasospasm.
Keywords: Cerebral vasospasm, subarachnoid haemorrhage, statin, pleiotropic effects, Akt, Rho, eNOS
Introduction
Pleiotropic effects of statins
In addition to their cholesterol lowering properties, statins are well known to exhibit many pleiotropic actions. Statins improve the integrity of endothelial cells and preserve the endothelial function [18]. They enhance the stability of atherosclerotic plaques, decrease oxidative stress and inflammation, and inhibit the thrombogenic response. Statins are also believed to have extrahepatic effects on the immune system, CNS, and bone [20]. Furthermore, statins induce apoptosis of vascular smooth muscle [9] and inhibit vascular smooth muscle proliferation [6, 11].
Evidence suggests that many of these effects may be mediated by the inhibition of isoprenoids which serve as lipid attachments for intracellular signaling molecules [9, 19]. Statins are likely to protect against cerebral vasospasm by improving endothelial function [17], inhibiting Rho-kinase signaling pathway in endothelial cells and vascular smooth muscle, and decreasing oxidative stress and inflammation [40].
Clinical studies
There is limited clinical information on the effects of statins in cerebral vasospasm. Two randomized clinical trials investigating statins as treatment for vasospasm after aneurysmal subarachnoid haemorrhage [21, 37] tested the administration of 80 mg simvastatin within 48 h and 40 mg of pravastatin within 72 h of the clinical presentation of SAH, respectively. Each treatment regimen continued for 14 days and showed that acute treatment with statins after SAH is safe and ameliorates vasospasm. A prospective cohort study showed that prior statin users demonstrated lower transcranial doppler highest mean velocity values, and had a significantly lower incidence of delayed cerebral ischemia or stroke from vasospasm [31]. A retrospective study with SAH patients who received statin therapy for at least 1 month prior to SAH demonstrated an eleven fold decreased risk of developing symptomatic vasospasm after SAH [23]. On the other hand, however, another study reported that patients on statins before the onset of SAH had a higher risk for subarachnoid haemorrhage-related vasospasm [36]. The abrupt withdrawal of statins after SAH may have been responsible for the higher risk in this particular study.
Experimental studies
Preserving endothelial integrity
Restoration of eNOS activity which releases endothelial-derived nitric oxide
McGirt et al. recently showed that simvastatin (20 mg/kg) when administered as pretreatment for 14 days followed by 3 days treatment post SAH, ameliorated cerebral vasospasm with increased eNOS expression [24]. Another group of animals that received only the post treatment regimen also showed decreased vasospasm, however, without any associated increase in eNOS expression. The study, however, did not measure eNOS activity or elaborate upon the cellular mechanisms involved in statin induced eNOS upregulation in cerebral vasculature.
Our present understanding of signaling pathways involved in eNOS upregulation by statins is mostly provided by cardiovascular studies. Laufs et al. determined that statins upregulate eNOS expression by prolonging eNOS mRNA half-life but not eNOS gene transcription in human saphenous vein and aortic endothelial cell cultures [19].
Inhibition of Rho activation (geranylgeranylation)
Statins lead to the direct inhibition of geranylgeranyl-transferase or RhoA which leads to increased endothelial Akt phosphorylation [15]. Studies in cardiomyocytes have shown that statin inhibition of RhoA leads to increased eNOS expression through the activation of Akt [4, 5, 41]. It can be hypothesized that the same pathway may play a significant role in the effect of statins on cerebral vasculature during cerebral vasospasm.
Inhibition of caveolin
Caveolin-1 is a cholesterol binding protein that has been shown to bind to eNOS and inhibit its activity in the caveolae [3]. Pelat et al. revealed that rosuvastatin decreased cavelin-1 expression and promoted eNOS function in cardiac and aortic cells [32]. The inhibition of caveolin by statins in the cerebral endothelial cells may be a likely mechanism of prevention of cerebral vasospasm and must be further investigated.
Activation of phosphatidylinositol 3-kinase/protein kinase Akt (PI3K/Akt) pathway
Statins rapidly activate the protein kinase Akt in endothelial cells [15]. Wang et al. showed that treatment of human umbilical endothelial cells with pitavastatin induced eNOS phosphorylation at Ser-1177, activated Akt phosphorylation at Ser-473 in a time-and dose-dependent manner, and increased NO production [39]. Simvastatin reduced myocardial injury after acute ischemia and reperfusion in an NO- dependent manner by activating the PI3K/Akt pathway [41]. Thus statins may attenuate vasospasm by activating the PI3K/Akt pathway directly or through the inhibition of RhoA in cerebral endothelial cells. Further studies are required to examine this pathway in cerebral vasculature.
Antioxidant effects
Reactive oxygen species (ROS) contribute to vascular dysfunction in various ways, such as reducing the bio-availability of NO, impairing endothelium-dependent vasodilatation [13, 14, 27], endothelial cell growth, causing apoptosis or anoikis, stimulating endothelial cell migration, and activating adhesion molecules and inflammatory reaction [43]. Fluvastatin has been shown to have a strong free radical scavenging activity in vitro; it recovered endothelium-dependent relaxation responses to acetylcholine in vivo [35]. Statins are known as an inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity [29, 42]. Erdos et al. showed that rosuvastatin improved cerebrovascular function in rats by inhibiting NADPH oxidase-dependent superoxide production [7]. The implications of the antioxidant effects of statins need to be studied with respect to cerebral vasospasm.
Inhibition of platelet aggregation
There is increased platelet consumption in patients presenting with cerebral vasospasm [12]. Platelet aggregation also plays an important role in subarachnoid clot formation. The nitric oxide mediated inhibition of platelet aggregation [34] after statin administration needs to be further explored.
Inhibit vascular inflammation
Statins have been suggested to have anti-inflammatory effects [38]. The anti-inflammatory mechanisms may involve the inhibition of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) [28].
An in vivo report in rabbits has shown that simvastatin (40 mg/kg) administrated after SAH ameliorated basilar artery vasospasm and attenuated perivascular granulocyte (CD18 cell) migration [25].
Inhibit the expression of endothelin-1
Endothelin-1 is a well-known vasoconstrictor [8, 22, 33]. Simvastatin and atorvastatin inhibited pre-pro endothelin-1 mRNA expression in a concentration- and time-dependent fashion and reduced immunoreactive endothelin-1 levels in bovine aortic endothelial cells [10]. Fluvastatin also reduced the production of endothelin-1 and pre-pro endothelin-1 mRNA expression in human umbilical vein endothelial cells [30]. Endothelin-1 has been suggested as a putative spasmogen in cerebral vasospasm [16] and statins may be effective in decreasing endothelin-1.
Effects on vascular smooth muscle cell
Inhibition of the Rho/Rho kinase pathway
Treatment with simvastatin abolished Rho activation mediated by endothelin-1 in the endothelium-denuded rat aorta preparations [26]. Thus, statins may affect this pathway both in endothelial and vascular smooth muscle cells.
Inhibition of vascular smooth muscle proliferation
Borel et al. concluded that cellular proliferation and subsequent vessel wall thickening after SAH may contribute to the syndrome of delayed cerebral vasospasm [2]. Simvastatin inhibited the proliferation of rat aorta myocytes [6]. It also inhibited the migration of cultured porcine smooth muscle cells [11]. Thus, the inhibition of vascular smooth muscle proliferation by statins may be an important pathway in cerebral vasospasm.
Vascular smooth muscle apoptosis
Bochaton-Piallat et al. showed that apoptosis is an important mechanism in the regulation of intimal thickening [1]. Atorvastatin was reported to induce apoptosis of rat thoracic aorta smooth muscle cells [9]. The anti-apoptotic effects and mechanisms of statins need to be further investigated in cerebral vasospasm.
Future research direction
There are many studies about statins and their effects in the cardiovascular field. Much of this knowledge may be applicable to the understanding of cerebral vasospasm; however, there are limited studies showing this evidence. More in vivo evidence is needed to elucidate the role of statins in cerebral vasospasm and the underlying cellular mechanisms.
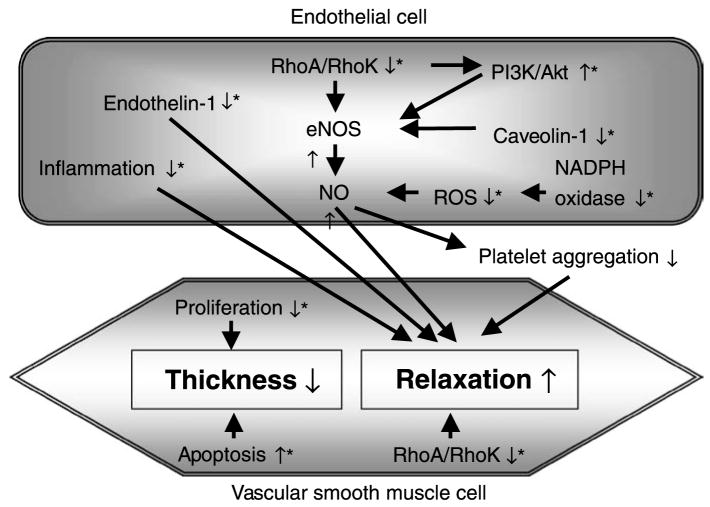
Fig. 1.
Proposed pathways of protection against cerebral vasospasm by statins. eNOS Endothelial nitric oxide synthase; NADPH oxidase nicotinamide adenine dinucleotide phosphate oxidase; NO nitric oxide; PI3K phosphatidyl-inositol-3 kinase; RhoK Rho kinase; ROS reactive oxygen species; ↑ upregulation; ↓ inhibition or down-regulation; *proposed targets of statins
Acknowledgments
This study is partially supported by grants from NIH NS53407, NS45694, NS43338, and HD43120 to J. H. Zhang.
References
- 1.Bochaton-Piallat ML, Gabbiani F, Redard M, Desmouliere A, Gabbiani G. Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am J Pathol. 1995;146:1059–1064. [PMC free article] [PubMed] [Google Scholar]
- 2.Borel CO, McKee A, Parra A, Haglund MM, Solan A, Prabhakar V, Sheng H, Warner DS, Niklason L. Possible role for vascular cell proliferation in cerebral vasospasm after subarachnoid hemorrhage. Stroke. 2003;34:427–433. [PubMed] [Google Scholar]
- 3.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 4.Budzyn K, Marley PD, Sobey CG. Opposing roles of endothelial and smooth muscle phosphatidylinositol 3-kinase in vasoconstriction: effects of rho-kinase and hypertension. J Pharmacol Exp Ther. 2005;313:1248–1253. doi: 10.1124/jpet.104.082784. [DOI] [PubMed] [Google Scholar]
- 5.Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006;27:97–104. doi: 10.1016/j.tips.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Corsini A, Raiteri M, Soma M, Fumagalli R, Paoletti R. Simvastatin but not pravastatin inhibits the proliferation of rat aorta myocytes. Pharmacol Res. 1991;23:173–180. doi: 10.1016/s1043-6618(05)80119-7. [DOI] [PubMed] [Google Scholar]
- 7.Erdos B, Snipes JA, Tulbert CD, Katakam P, Miller AW, Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol. 2006;290:H1264–H1270. doi: 10.1152/ajpheart.00804.2005. [DOI] [PubMed] [Google Scholar]
- 8.Gray GA, Webb DJ. The endothelin system and its potential as a therapeutic target in cardiovascular disease. Pharmacol Ther. 1996;72:109–148. doi: 10.1016/s0163-7258(96)00101-5. [DOI] [PubMed] [Google Scholar]
- 9.Guijarro C, Blanco-Colio LM, Ortego M, Alonso C, Ortiz A, Plaza JJ, Diaz C, Hernandez G, Egido J. 3-Hydroxy-3-methylglutaryl coenzyme a reductase and isoprenylation inhibitors induce apoptosis of vascular smooth muscle cells in culture. Circ Res. 1998;83:490–500. doi: 10.1161/01.res.83.5.490. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, Diaz C, Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hidaka Y, Eda T, Yonemoto M, Kamei T. Inhibition of cultured vascular smooth muscle cell migration by simvastatin (MK-733) Atherosclerosis. 1992;95:87–94. doi: 10.1016/0021-9150(92)90179-k. [DOI] [PubMed] [Google Scholar]
- 12.Hirashima Y, Hamada H, Kurimoto M, Origasa H, Endo S. Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2005;102:882–887. doi: 10.3171/jns.2005.102.5.0882. [DOI] [PubMed] [Google Scholar]
- 13.Katusic ZS. Superoxide anion and endothelial regulation of arterial tone. Free Radic Biol Med. 1996;20:443–448. doi: 10.1016/0891-5849(96)02116-8. [DOI] [PubMed] [Google Scholar]
- 14.Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- 15.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwan AL, Lin CL, Yen CP, Winardi W, Su YF, Winardi D, Dai ZK, Jeng AY, Kassell NF, Howng SL. Prevention and reversal of vasospasm and ultrastructural changes in basilar artery by continuous infusion of CGS 35066 following subarachnoid hemorrhage. Exp Biol Med (Maywood) 2006;231:1069–1074. [PubMed] [Google Scholar]
- 17.Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 18.Laufs U, La FV, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 19.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 20.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch JR, Wang H, McGirt MJ, Floyd J, Friedman AH, Coon AL, Blessing R, Alexander MJ, Graffagnino C, Warner DS. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36:2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 22.Masaki T. Possible role of endothelin in endothelial regulation of vascular tone. Annu Rev Pharmacol Toxicol. 1995;35:235–255. doi: 10.1146/annurev.pa.35.040195.001315. [DOI] [PubMed] [Google Scholar]
- 23.McGirt MJ, Blessing R, Alexander MJ, Nimjee SM, Woodworth GF, Friedman AH, Graffagnino C, Laskowitz DT, Lynch JR. Risk of cerebral vasopasm after subarachnoid hemorrhage reduced by statin therapy: A multivariate analysis of an institutional experience. J Neurosurg. 2006;105:671–674. doi: 10.3171/jns.2006.105.5.671. [DOI] [PubMed] [Google Scholar]
- 24.McGirt MJ, Lynch JR, Parra A, Sheng H, Pearlstein RD, Laskowitz DT, Pelligrino DA, Warner DS. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 25.McGirt MJ, Pradilla G, Legnani FG, Thai QA, Recinos PF, Tamargo RJ, Clatterbuck RE. Systemic administration of simvastatin after the onset of experimental subarachnoid hemorrhage attenuates cerebral vasospasm. Neurosurgery. 2006;58:945–951. doi: 10.1227/01.NEU.0000210262.67628.7E. [DOI] [PubMed] [Google Scholar]
- 26.Mraiche F, Cena J, Das D, Vollrath B. Effects of statins on vascular function of endothelin-1. Br J Pharmacol. 2005;144:715–726. doi: 10.1038/sj.bjp.0706114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa S, Totsuka T, Hayashi S. Inhibitory effect of fluvastatin, an HMG-CoA reductase inhibitor, on the expression of adhesion molecules on human monocyte cell line. Int J Immunopharmacol. 1996;18:669–675. doi: 10.1016/s0192-0561(96)00068-9. [DOI] [PubMed] [Google Scholar]
- 29.Otto A, Fontaine J, Tschirhart E, Fontaine D, Berkenboom G. Rosuvastatin treatment protects against nitrate-induced oxidative stress in eNOS knockout mice: implication of the NAD(P)H oxidase pathway. Br J Pharmacol. 2006;148:544–552. doi: 10.1038/sj.bjp.0706738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozaki K, Yamamoto T, Ishibashi T, Matsubara T, Nishio M, Aizawa Y. Regulation of endothelial nitric oxide synthase and endothelin-1 expression by fluvastatin in human vascular endothelial cells. Jpn J Pharmacol. 2001;85:147–154. doi: 10.1254/jjp.85.147. [DOI] [PubMed] [Google Scholar]
- 31.Parra A, Kreiter KT, Williams S, Sciacca R, Mack WJ, Naidech AM, Commichau CS, Fitzsimmons BF, Janjua N, Mayer SA. Effect of prior statin use on functional outcome and delayed vasospasm after acute aneurysmal subarachnoid hemorrhage: a matched controlled cohort study. Neurosurgery. 2005;56:476–484. doi: 10.1227/01.neu.0000153925.96889.8a. [DOI] [PubMed] [Google Scholar]
- 32.Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107:2480–2486. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 33.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J. 1995;9:1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- 34.Radomski MW, Rees DD, Dutra A, Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992;107:745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rikitake Y, Kawashima S, Takeshita S, Yamashita T, Azumi H, Yasuhara M, Nishi H, Inoue N, Yokoyama M. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 2001;154:87–96. doi: 10.1016/s0021-9150(00)00468-8. [DOI] [PubMed] [Google Scholar]
- 36.Singhal AB, Topcuoglu MA, Dorer DJ, Ogilvy CS, Carter BS, Koroshetz WJ. SSRI and statin use increases the risk for vasospasm after subarachnoid hemorrhage. Neurology. 2005;64:1008–1013. doi: 10.1212/01.WNL.0000154523.21633.0E. [DOI] [PubMed] [Google Scholar]
- 37.Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 38.Vaughan CJ, Gotto AM, Jr, Basson CT. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol. 2000;35:1–10. doi: 10.1016/s0735-1097(99)00525-2. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Tokoro T, Matsui K, Higa S, Kitajima I. Pitavastatin at low dose activates endothelial nitric oxide synthase through PI3K-AKT pathway in endothelial cells. Life Sci. 2005;76:2257–2268. doi: 10.1016/j.lfs.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, Itter G, Rosen R, Bohm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 41.Wolfrum S, Dendorfer A, Schutt M, Weidtmann B, Heep A, Tempel K, Klein HH, Dominiak P, Richardt G. Simvastatin acutely reduces myocardial reperfusion injury in vivo by activating the phosphatidylinositide 3-kinase/akt pathway. J Cardiovasc Pharmacol. 2004;44:348–355. doi: 10.1097/01.fjc.0000137162.14735.30. [DOI] [PubMed] [Google Scholar]
- 42.Yu HY, Inoguchi T, Nakayama M, Tsubouchi H, Sato N, Sonoda N, Sasaki S, Kobayashi K, Nawata H. Statin attenuates high glucose-induced and angiotensin II-induced MAP kinase activity through inhibition of NAD(P)H oxidase activity in cultured mesangial cells. Med Chem. 2005;1:461–466. doi: 10.2174/1573406054864052. [DOI] [PubMed] [Google Scholar]
- 43.Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]