Abstract
The canine double hemorrhage model is an established model to study cerebral vasospasm, the late sequelae of subarachnoid hemorrhage (SAH). The present study uses magnetic resonance imaging (MRI) to examine the recently reported early brain injury after SAH. Double hemorrhage SAH modeling was obtained by injecting 0.5 mL/kg of autologous arterial blood into the cisterna magna of five adult mongrel dogs on day 0 and day 2, followed by imaging at day 2 and day 7 using a 4.7-Tesla (T) scanner. White matter (WM) showed a remarkable increase in T2 values at day 2 which resolved by day 7, whereas gray matter (GM) T2 values did not resolve. The apparent diffusion coefficient (ADC) values progressively increased in both WM and GM after SAH, suggestive of a transition from vasogenic to cytotoxic edema. Ventricular volume also increased dramatically. Prominent neuronal injury with Nissl's staining was seen in the cortical GM and in the periventricular tissue. Multimodal MRI reveals acute changes in the brain after SAH and can be used to non-invasively study early brain injury and normal pressure hydrocephalus post-SAH. MR can also predict tissue histopathology and may be useful for assessing pharmacological treatments designed to ameliorate SAH.
Key words: apparent diffusion coefficient, brain edema, canine double hemorrhage model, magnetic resonance imaging, subarachnoid hemorrhage, T2, ventricular volume
Introduction
Subarachnoid hemorrhage (SAH) is a type of hemorrhagic stroke with devastating consequences that are well known; however, the underlying pathophysiology is still poorly understood. Many types of experimental animal models are used to study SAH and have been reviewed elsewhere (Megyesi et al., 2000). The canine double hemorrhage model is a well-established experimental model frequently employed to study the late sequelae of SAH such as cerebral vasospasm as it resembles the vasospasm observed in clinical SAH (Megyesi et al., 2000; Saito et al., 1989; Varsos et al., 1983). To date, cerebral vasospasm is thought to be responsible for the morbidity and mortality resulting from delayed neurological deterioration that occurs after SAH. However, recent studies have demonstrated that other acute events after SAH, such as global ischemia, the breakdown of the blood-brain barrier (BBB) subsequent to apoptotic and inflammatory triggers leading to brain edema, can contribute to poor outcomes (Hansen-Schwartz et al., 2007 ;Macdonald et al., 2007).
Magnetic resonance imaging (MRI) is an extremely useful non-invasive modality routinely employed to detect temporal and spatial alterations in the brain, not only in the clinical setting, but also in experimental animal studies. MRI can provide visual information about BBB rupture, tissue edema, as well as necrosis that can be readily seen using T1-, T2-, and diffusion-weighted imaging (DWI). Often the abnormal signal intensity seen on MR images is the result of alterations in tissue physical parameters induced by a variety of histological, pathologic and physiological factors. Quantitative MR can disclose pathological processes in specific brain regions by describing the functional characteristics of the injured tissue. T2 relaxation times are considered to relate to the dynamic state of water at microscopic tissue levels and are sensitive to water binding (Mathur-De Vre, 1984). Evaluation of relaxation times offers a method for investigating neurophysiology and neuropathology in vivo (Pfefferbaum et al., 1994). The DWI sequence complements the classical anatomical MRI techniques and is sensitive to the changes in intracerebral structure and function at early stages of brain injury (Ashwal et al., 2007a). The quantitative DWI parameter, apparent diffusion coefficient (ADC), is able to characterize water mobility in local tissues for a variety of injury models (Moseley et al., 1990).
Presently, there are no reports on the use of multimodal MRI in the canine double hemorrhage SAH model. This project investigated the temporal and spatial alterations within the brain before and after SAH using MRI.
Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee at Loma Linda University.
Canine double-hemorrhage model for experimental SAH
Five adult mongrel dogs (15.0–20.6 kg) were used for this investigation; four were used for MR analysis, after using the first to set up pilot scans. Surgical procedures were performed as previously described (Yamaguchi et al., 2004; Yatsushige et al., 2005; Zho, et al., 2004). Briefly, general anesthesia was induced with an intramuscular injection of acepromazine (0.1–0.5 mg/kg), atropine (0.05 mg/kg), and xylazine (1.1 mg/kg), which was followed by endotracheal delivery of isoflurane and O2 using mechanical ventilation. Autologous blood (0.5 mL/kg) obtained from femoral artery was injected percutaneously into the cisterna magna using an 18 gauge needle with aseptic precautions and after sterile preparation of parts. The injection was made manually over 2–3 min in the midline 3 cm distal to the inion at the occiput-C1 junction with the neck in a flexed position after withdrawing cerebrospinal fluid (CSF; 0.25 mL/kg). The needle was then withdrawn, and the animal was placed prone in the Trendelenberg 30° position for 15 min to facilitate settling of blood into the basal cisterns. The autologous blood injections were done on days 0 and 2 in an identical manner. All vital parameters and physiological variables such as respiration, pulse, blood gases, and temperature were continuously monitored throughout the procedure. The core body temperature was monitored throughout the procedure with a rectal probe and maintained at 36–37°C with the aid of heating blankets and “bear huggers.” The systolic blood pressure was recorded at 100–120 mm Hg, and SaO2 (arterial oxygen saturation, V60046 monitor; SurgiVet, Waukesha, Wisconsin) was maintained above 95% throughout the procedure.
Angiography
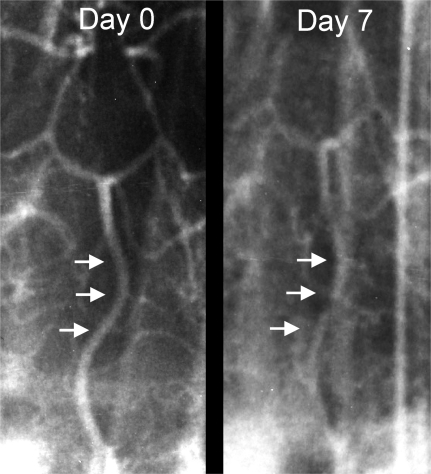
Angiograms were obtained as previously described (Yamaguchi et al., 2004; Yatsushige et al., 2005; Zhou et al., 2004), on day 0 (baseline) before the first autologous blood injection and on day 7 (ideal time for cerebral vasospasm) prior to euthanization using contrast medium (7 mL; Visipaque). To minimize the parallax error, cerebral angiograms were performed by c-arm fluoroscopy with constant magnification and exposure factors. The arterial diameters (Fig. 1) were measured in a blinded fashion at the following five portions: the distal portion (just before bifurcation of basilar top), the proximal portion (just after vertebrobasilar union), and three equidistant points between the first two points using the National Institutes of Health image analyzer ImageJ (version 1.32). The mean of these measurements was calculated, and the mean diameter of the basilar artery on day 7 was quantified as a percentage of the mean on day 0.
FIG. 1.
Angiographic evidence of cerebral vasospasm. The figure shows a baseline angiogram obtained at day 0 before the first injection and another obtained at day 7 prior to sacrifice that depict vasospastic changes in the form of decreased basilar arterial diameter. The basilar artery is pointed out with arrows.
Magnetic resonance imaging
Animals were lightly anesthetized with isoflurane, intubated and blood gases were monitored by LLU veterinary staff prior, during and after imaging for animal health. MRI was performed prior (pre-scan) to SAH induction, and at 2 and 7 days after the initial SAH injection. Animals were imaged on a Bruker Avance 4.7-Tesla (T) MRI (Bruker Biospin, Billerica, MA) equipped with a 30 -cm gradient. Two imaging data sets were acquired: (1) a 10-echo T2, and (2) a diffusion-weighted sequence where each sequence collected 25 coronal slices (3 mm thickness, interleaved by 3 mm). The T2 sequence had the following parameters: repetition time (TR)/echo time (TE) = 3740 msec/20.5 msec; matrix = 256 × 256; field of view (FOV) = 8 cm; number of excitation (NEX) = 2; and acquisition time = 31 min. The spin echo diffusion sequence parameters were TR/TE = 3000 msec/25 msec; diffusion weighting = 2.83 and 190.34 sec/mm2; matrix = 128 × 128; FOV = 8 cm; NEX = 2; and acquisition time = 25 min.
Image analysis
Volumetric MR image analysis methods have been previously published (Ashwal et al., 2007c). Briefly, using Amira software (Mercury Computer Systems, Inc.) T2-weighted images (T2WI) were analyzed to extract the brain and ventricular volumes using regions of hyperintensity to delineate the ventricles. Brain and ventricular volume were extracted at each imaging time point.
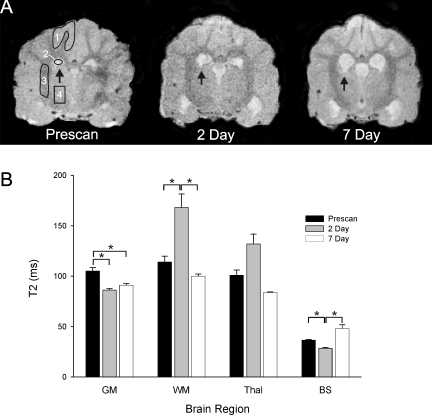
T2 and ADC values are quantitative measurements that respectively reflect the evolution of vasogenic and cytotoxic edema. All MR data sets were quantified using standard protocols published previously (Ashwal et al., 2007b). T2 relaxation rates were determined for each pixel and T2 maps generated. ADC maps were calculated using a linear two point fit. Bilateral regions of interest (ROIs) included gray matter (GM) and white matter (WM), thalamus (Thal), ventricles (Vent), and brainstem (BS) (Fig. 2A). These ROIs were then overlaid onto corresponding T2 and ADC maps and the mean, standard deviation, number of pixels, and area for each ROI was extracted. MRI analysis was performed without knowledge of the time point.
FIG. 2.
T2-weighted imaging (T2WI) changes within the canine brain after subarachnoid hemorrhage (SAH). (A) Sample T2WI illustrating the changes within the brain after SAH. Regions of interest (ROIs) included gray matter (GM, 1), ventricles (2), white matter (WM, 3), and thalamus (Thal, 4). Also note the increase in the area of the ventricles after SAH induction (arrows). (B) Quantitative T2 analysis showed decreased T2 values in gray matter and brainstem. However, at 2 days, there was a significant increase in T2 values within the WM, which then returned to pre-scan values by 7 days. *p < 0.05.
Statistical analysis
T2, ADC, and ventricular volumes were compared using a repeated-measures analysis of variance (ANOVA). When significance (p < 0.05) was found, a pair-wise multiple comparison procedure (Holm-Sidak method) was performed for each time point to isolate differences.
Clinical evaluation
The clinical behavior scores were recorded daily from day 0 to day 7, using the modified scoring table published previously (Yamaguchi et al., 2004; Yatsushige et al., 2005; Zhou et al., 2004). This scoring table had three categories: (1) appetite; (2) activity; and (3) neurological deficit. Points were given using the following criteria: appetite: finished meal = 2, left meal unfinished = 1, scarcely ate = 0; activity: active, barking, or standing = 2, lying down, will stand and walk with some stimulation = 1, almost always lying down = 0; neurological deficit: no deficit = 2, unstable walk because of ataxia or paresis = 1, impossible to walk and stand because of ataxia or paresis = 0.
Morphological evaluation of brain injury
After euthanasia with Beuthanasia-D, the dogs were perfused via both common carotid arteries with 2000 mL of phosphate-buffered saline (PBS), pH 7.4, followed by 2000 mL of 10% formalin. The brain tissues were carefully harvested and post-fixed in the same fixative. Ten-μm-thick sections of paraffin embedded tissue were used for Nissl's staining using 0.1% cresyl violet solution, and hematoxylin and eosin (H&E) staining to assess neuronal damage and luxol fast blue staining to assess WM injury using standard techniques as previously described (Wu et al., 2008).
Results
All of the dogs survived the imaging and surgical intervention for the full experimental period of 7 days. None of the five dogs showed any neurological deficits except for immediately after the procedures and during recovery from the effects of anesthesia. After the recovery from anesthesia, the animals had adequate appetite and normal activity. Previous studies by others (Kaoutzanis et al., 1993) have shown that although this model has minimal neurological deficits it produces excellent cerebral arterial vasoconstriction that parallels the progression of the disease in humans.
Angiographic analysis
All five dogs subjected to experimental SAH had cerebral vasospasm at day 7. The mean basilar arterial diameter decreased by approximately 24%. The percent diameter decrease in each of the individual animals was as follows: 8.3%, 32.5%, 21.7%, 29.4%, and 26%. Figure 1 depicts a representative angiogram that shows basilar arterial narrowing consistent with previous results (Yamaguchi et al., 2004; Yatsushige et al., 2005).
MR imaging findings
Gray matter
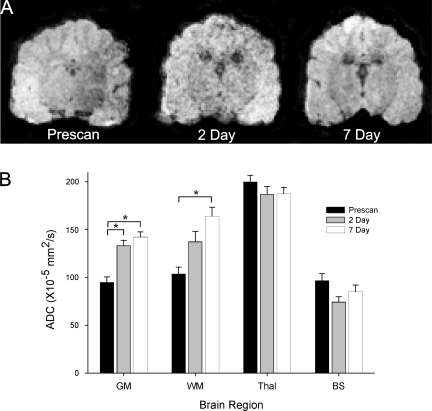
Quantitative analysis showed a progressive decline in T2 values within the cortex when compared to the pre-scan values (Fig. 2). At 2 and 7 days after SAH, there was a significant (p < 0.05) 82% and 87% decrease in T2 values respectively compared to pre-scan values. But there was no significant difference between day 2 and day 7 values, suggesting that the tissue changes within the GM are already present by the second SAH injection. Interestingly, the ADC, a reflection of water mobility, was significantly increased at 2 and 7 days with a peak increase of 150% by 7 days (Fig. 3).
FIG. 3.
Temporal changes in water mobility following subarachnoid hemorrhage (SAH). (A) Sample images from a dog brain prior to and following SAH induction. (B) Apparent diffusion coefficient (ADC) values were significantly increased in a temporal fashion in both white matter (WM) and gray matter (GM) reaching a maximum at 7 days. No significant ADC changes were found in the thalamus or brainstem.
White matter
In dramatic contrast to the GM, WM exhibited a large significant increase in T2 from 113 msec to 168 msec, a 148% increase (p < 0.05; Fig. 2). This large increase in presumed water content (edema) resolved and disappeared by 7 days. Similar to GM, the ADC progressively increased until day 7, when it reached significance (p < 0.05; Fig. 3). The ADC and increased water content at 2 days could be due to pressure from increasing CSF within the ventricles into the neighboring WM. It may also reflect a transition from vasogenic to cytotoxic edema.
Thalamus
Similar to the WM, the thalamus had an increase in T2 values at 2 days that also resolved by 7 days (Fig. 2). The 131% increase in water content within the thalamus was similar to that observed in WM but did not reach significance. Similarly, the ADC values were not significantly different at either time point after SAH induction (Fig. 3).
Brainstem
While this brain region is close to the SAH injection site, there was an initial significant decrease in T2 values at 2 days to 79% of pre-scan values (Fig. 2). However, a much larger increase in T2 values from 28 to 48 msec was observed at 7 days that was not significant. These changes within the brainstem could reflect initial tissue swelling and dephasing (reduced T2) due to local blood products (SAH), which is then followed by increased water mobility that could be indicative of ongoing neuropathology. While there was a decrease in ADC at 2 days that returned to pre-scan levels, these changes were not significant (Fig. 3).
Ventricles
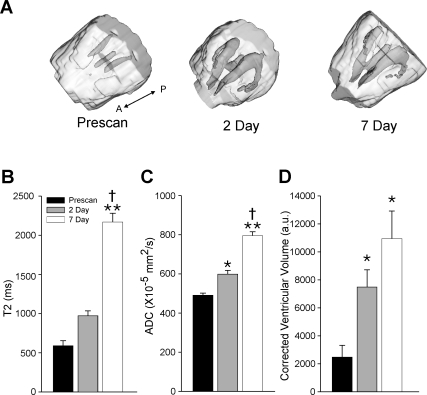
One of the more obvious imaging changes within the canine brain after SAH was the enlargement of the ventricles (Fig. 4). Three-dimensional reconstruction of the brain and the ventricular system dramatically underscores the progressive increase over the 7 day experimental period (Fig. 4A). The T2 values also increased in a similar stepwise fashion with T2 values rising from 580 msec to 971 msec and 2167 msec at 2 and 7 days, respectively (Fig. 4B). Only the 7-day time point was significantly different from both pre-scan and 2-day T2 values. The overall 370% increase in T2 relaxation is consistent with increased fluid accumulation within the ventricles. In further support of this conjecture, the ADC also increased from 4.91 to 5.99 and 7.95 (×10−5 mm2/sec) at 2 and 7 days after SAH (Fig. 4C). Thus, water mobility increased significantly within the ventricles in a stepwise fashion resulting in a 160% increase after SAH induction. Finally, ventricular volume also significantly increased over the 2- and 7-day time points in all of the canine subjects undergoing SAH (Fig. 4D), which is consistent with the quantitative MR changes observed with T2 and ADC measurements.
FIG. 4.
Ventricular volume becomes increasingly enlarged after subarachnoid hemorrhage (SAH). (A) An oblique three-dimensional reconstruction of a canine brain illustrates the dramatic increase in ventricular size over the 7 day experimental period. (A-P; anterior-posterior direction). (B) The T2 values also dramatically increase over the 7 days suggestive of increased free cerebrospinal fluid (CSF). (C) Further confirmation was found with the increasing apparent diffusion coefficient (ADC) values that demonstrate significantly increased water mobility within the ventricles due in part to the dramatic enlargement. (D) Ventricular volume increased over time reaching significance at 2 and 7 days. *p < 0.05 and **p < 0.001 from pre-scan; †p < 0.05 from day 2.
Cellular pathology
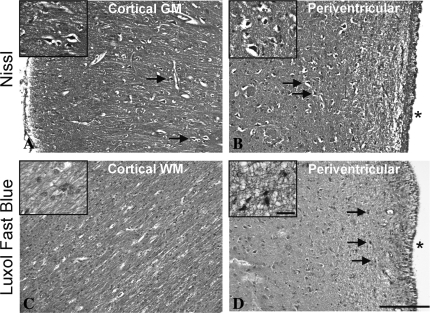
Neuronal damage assessed by Nissl's staining at day 7 was observed in varying degrees over the entire extent of the brain. The most prominent brain injury indicated by multiple pyknotic nuclei was seen in the GM of the cortex and in the periventricular tissue (Fig. 5A,B). H&E/luxol fast blue stained sections also showed pyknotic nuclei in the periventricular area (Fig. 5D); however, the cortical WM did not appear to be affected (Fig. 5C).
FIG. 5.
Figure shows representative pictures of Nissl's staining (A,B) and luxol fast blue co-staining with hematoxylin and eosin (H&E) (C,D) from the cortex (A,C) and the periventricular area (B,D) on paraffin-embedded histological sections. Shrunken, dense, pyknotic cells marked by arrowheads were seen with both Nissl's staining and H&E staining in the cortex and periventricular area. The white matter, on the other hand, did not show histological damage at day 7 after subarachnoid hemorrhage (SAH). The inset shows magnified cellular structures. Scale bar = 0.25 mm and 25 μm at lower and higher magnification, respectively. Asterisk in B and D indicates the ventricular space.
Discussion
The present study shows for the first time the utility of using MRI as a tool in evaluating early brain injury after SAH in the canine double hemorrhage model. This animal model is well established and frequently employed to study cerebral vasospasm, which is a late sequelae of SAH (Megyesi et al., 2000). In the present study, cerebral vasospasm obtained as a measure of angiographic decrease in basilar arterial diameter at day 7 after SAH was seen in all canine subjects consistent with previous reports (Yamaguchi et al., 2004; Yatsushige et al., 2005).
Following failure of clazosentan, an endothelin receptor antagonist, in improving patient outcome in spite of attenuating vasospasm, recent authoritative opinions suggest moving the focus to early brain injury after SAH (Hansen-Schwartz et al., 2007; Macdonald et al., 2007). The acute events after SAH comprised of BBB disruption leading to brain edema, apoptotic cell death, global ischemia, and inflammatory changes not only impact the development of cerebral vasospasm but also contribute to ensuing morbidity and mortality (Hansen-Schwartz et al., 2007; Macdonald et al., 2007; Park et al., 2004).
The MRI data obtained in the present study show many acute changes after experimental SAH. The T2 lengthening seen at day 2 in cortical WM and thalamic tissues indicate edematous and/or inflammatory changes. Our previous studies, as well as studies by others, have provided experimental and clinical evidence of early inflammatory changes after SAH (Gaetani et al., 1992, 1998; Yatsushige et al., 2005). However, other studies have shown that inflammation also persists to 7 days after SAH. Thus, the transient T2 lengthening seen at day 2 in our studies and its resolution by day 7 may represent early edematous changes. The progressive temporal increase seen in the ADC values indicate increased water mobility in both cortical WM and GM. Taken together with the transient increase in T2 lengthening, the data suggests a shift from early vasogenic edema to a cytotoxic edema in the cortex at later time points.
This is supported by the histological data that shows neuronal damage at 7 days within the GM of the cortex. Nissl's staining showed the presence of shrunken pyknotic nuclei. However, the WM stained by luxol fast blue did not show any damage at 7 days, which may be due to resolution of the initial vasogenic edema. Other neuronal pathological damage was evident in the periventricular area where both Nissl's and H&E staining showed dense, pyknotic cells.
Ventricular pathology is not surprising in this model as clinical SAH can present with a high incidence of hydrocephalus (Sheehan et al., 1999). In the present study, we observed a progressive development of ventriculomegaly in all the canine subjects over a period of 7 days. However, there were no symptoms of gait impairment or urinary incontinence usually associated with hydrocephalus (Vanneste, 2000; Wilson et al., 2006). Although we did not measure the intracranial pressure (ICP) in this study, there were also no symptoms suggestive of raised ICP. While raised ICP itself could contribute to the initial changes in the brain after SAH, this animal model does not result in a sustained increase in ICP. A previous study using a double hemorrhage canine model showed that an early elevation of ICP induced changes in the cerebral arteries within the basal cisterns and was indicative of BBB breakdown within the first hour (Sasaki et al., 1985). However, the rise of ICP was short lived (minutes), and the most profound effects in the pathophysiology of SAH were due to the presence of blood in the subarachnoid space (Sasaki et al., 1985). We observed neuronal damage in cortex as well as periventricular tissue. Blood clotting after SAH is known to induce neuronal damage, and some studies have even suggested that magnetic resonance spectroscopy (MRS) could be an approach to detect the neuronal damage after SAH (Handa et al., 1997). Thus, the observed ventricular enlargement leading to hydrocephalus could be a result of decreased absorption of CSF due to inflammatory and apoptotic changes from blood in the subarachnoid space. The remarkable periventricular neuronal damage seen in this model is supportive of this hypothesis. Thus, the hydrocephalus encountered in this animal model is likely to be normal pressure hydrocephalus, which is similar to the clinical scenario.
Previous literature suggests that MRI can also be a valuable tool to assess the bleeding after SAH in small as well as large animal models (Perl et al., 1999; Woodcock, Jr. et al., 2001). Some small animal studies utilizing MRI have pointed out the ease of detection of acute events such as spreading depression (Busch et al., 1998). The canine double hemorrhage model, barring the disadvantage of working with large animals, is popular due to comparability with clinical SAH and ease of angiography (Megyesi et al., 2000). The present study amalgamates MRI, a routinely used clinical diagnostic tool, with this well-established animal model to study early brain injury, an exciting new direction in SAH research.
Conclusion
The MRI findings in conjunction with the histopathology of this study suggest that the canine double hemorrhage model shows acute changes in the brain after SAH and can be used to non-invasively study early brain injury and normal-pressure hydrocephalus post-SAH.
Acknowledgments
We gratefully acknowledge the assistance of the animal care staff, particularly Dr. D. Wolf, Dr. S. Matthews, and P. Bush for monitoring the canines during the imaging and postoperative care. This study was partially supported by the National Institutes of Health (NIH NS53407, NS45694, NS43338, and HD43120; to J.H.Z.). Neuroimaging support was provided in part by NASA (Cooperative Agreement NNJO4HD80G; to L.L.U.).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Ashwal S. Tone B. Tian H.R. Chong S. Obenaus A. Comparison of two neonatal ischemic injury models using magnetic resonance imaging. Pediatr. Res. 2007;61:9–14. doi: 10.1203/01.pdr.0000251612.16069.4b. [DOI] [PubMed] [Google Scholar]
- Busch E. Beaulieu C. de Crespigny A. Moseley M.E. Diffusion MR imaging during acute subarachnoid hemorrhage in rats. Stroke. 1998;29:2155–2161. doi: 10.1161/01.str.29.10.2155. [DOI] [PubMed] [Google Scholar]
- Gaetani P. Lombardi D. Brain damage following subarachnoid hemorrhage: the imbalance between anti-oxidant systems and lipid peroxidative processes. J. Neurosurg. Sci. 1992;36:1–10. [PubMed] [Google Scholar]
- Gaetani P. Tartara F. Pignatti P. Tancioni F. Baena R. De Benedetti F. Cisternal CSF levels of cytokines after subarachnoid hemorrhage. Neurol. Res. 1998;20:337–342. doi: 10.1080/01616412.1998.11740528. [DOI] [PubMed] [Google Scholar]
- Handa Y. Kaneko M. Matuda T. Kobayashi H. Kubota T. In vivo proton magnetic resonance spectroscopy for metabolic changes in brain during chronic cerebral vasospasm in primates. Neurosurgery. 1997;40:773–780. doi: 10.1097/00006123-199704000-00023. [DOI] [PubMed] [Google Scholar]
- Hansen-Schwartz J. Vajkoczy P. Macdonald R.L. Pluta R.M. Zhang J.H. Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol. Sci. 2007;28:252–256. doi: 10.1016/j.tips.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Kaoutzanis M. Yokota M. Sibilia R. Peterson J.W. Neurologic evaluation in a canine model of single and double subarachnoid hemorrhage. J. Neurosci. Methods. 1993;50:301–307. doi: 10.1016/0165-0270(93)90037-r. [DOI] [PubMed] [Google Scholar]
- Macdonald R.L. Pluta R.M. Zhang J.H. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat. Clin. Pract. Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- Mathur-De Vre R. Biomedical implications of the relaxation behaviour of water related to NMR imaging. Br. J Radiol. 1984;57:955–976. doi: 10.1259/0007-1285-57-683-955. [DOI] [PubMed] [Google Scholar]
- Megyesi J.F. Vollrath B. Cook D.A. Findlay J.M. In vivo animal models of cerebral vasospasm: a review. Neurosurgery. 2000;46:448–460. [PubMed] [Google Scholar]
- Moseley M.E. Cohen Y. Kucharczyk J. Mintorovitch J. Asgari H.S. Wendland M.F. Tsuruda J. Norman D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Park S. Yamaguchi M. Zhou C. Calvert J.W. Tang J. Zhang J.H. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–2417. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- Perl J. Tkach J.A. Porras-Jimenez M. Lieber M. Obuchowski N. Ross J.S. Ding X.P. Ruggieri P.M. Shearer D.M. Khajavi K. Masaryk T.J. Hemorrhage detected using MR imaging in the setting of acute stroke: an in vivo model. AJNR Am. J. Neuroradiol. 1999;20:1863–1870. [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A. Mathalon D.H. Sullivan E.V. Rawles J.M. Zipursky R.B. Lim K.O. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Saito A. Nakazawa T. Cerebral vasospasm model produced by subarachnoid blood injection in dogs. Jpn. J. Pharmacol. 1989;50:250–252. doi: 10.1254/jjp.50.250. [DOI] [PubMed] [Google Scholar]
- Sasaki T. Kassell N.F. Yamashita M. Fujiwara S. Zuccarello M. Barrier disruption in the major cerebral arteries following experimental subarachnoid hemorrhage. J. Neurosurg. 1985;63:433–440. doi: 10.3171/jns.1985.63.3.0433. [DOI] [PubMed] [Google Scholar]
- Sheehan J.P. Polin R.S. Sheehan J.M. Baskaya M.K. Kassell N.F. Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1999;45:1120–1127. doi: 10.1097/00006123-199911000-00021. [DOI] [PubMed] [Google Scholar]
- Vanneste J.A. Diagnosis and management of normal-pressure hydrocephalus. J. Neurol. 2000;247:5–14. doi: 10.1007/s004150050003. [DOI] [PubMed] [Google Scholar]
- Varsos V.G. Liszczak T.M. Han D.H. Kistler J.P. Vielma J. Black P.M. Heros R.C. Zervas N.T. Delayed cerebral vasospasm is not reversible by aminophylline, nifedipine, or papaverine in a “two-hemorrhage” canine model. J. Neurosurg. 1983;58:11–17. doi: 10.3171/jns.1983.58.1.0011. [DOI] [PubMed] [Google Scholar]
- Wilson R.K. Williams M.A. Normal pressure hydrocephalus. Clin. Geriatr. Med. 2006;22:935–951. doi: 10.1016/j.cger.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Woodcock R.J., Jr. Short J. Do H.M. Jensen M.E. Kallmes D.F. Imaging of acute subarachnoid hemorrhage with a fluid-attenuated inversion recovery sequence in an animal model: comparison with non-contrast-enhanced CT. AJNR Am. J. Neuroradiol. 2001;22:1698–1703. [PMC free article] [PubMed] [Google Scholar]
- Wu Q.Z. Yang Q. Cate H.S. Kemper D. Binder M. Wang H.X. Fang K. Quick M.J. Marriott M. Kilpatrick T.J. Egan G.F. MRI identification of the rostral-caudal pattern of pathology within the corpus callosum in the cuprizone mouse model. J. Magn. Reson. Imaging. 2008;27:446–453. doi: 10.1002/jmri.21111. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M. Zhou C. Nanda A. Zhang J.H. Ras protein contributes to cerebral vasospasm in a canine double-hemorrhage model. Stroke. 2004;35:1750–1755. doi: 10.1161/01.STR.0000129898.68350.9f. [DOI] [PubMed] [Google Scholar]
- Yatsushige H. Yamaguchi M. Zhou C. Calvert J.W. Zhang J.H. Role of c-Jun N-terminal kinase in cerebral vasospasm after experimental subarachnoid hemorrhage. Stroke. 2005;36:1538–1543. doi: 10.1161/01.STR.0000170713.22011.c8. [DOI] [PubMed] [Google Scholar]
- Zhou C. Yamaguchi M. Kusaka G. Schonholz C. Nanda A. Zhang J.H. Caspase inhibitors prevent endothelial apoptosis and cerebral vasospasm in dog model of experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2004;24:419–431. doi: 10.1097/00004647-200404000-00007. [DOI] [PubMed] [Google Scholar]