Summary
Aneurismal subarachnoid haemorrhage (SAH) is a devastating disease that is associated with significant morbidity and mortality. The mortality is approximately 50%, with 30% of survivors having significant morbidity. There is substantial evidence to suggest that oxidative stress is significant in the development of acute brain injury and cerebral vasospasm following SAH. There are several sources for the excessive generation of free radicals following SAH, including disrupted mitochondrial respiration and extracellular hemoglobin. There is also the upregulation of free radical producing enzymes such as inducible nitric oxide synthase (iNOS), xanthine oxidase, NADPH oxidase (NOX), as well as enzymes involved in the metabolism of arachidonic acid. Additionally, intrinsic antioxidant systems such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) are inhibited. Experiments have linked free radicals to the apoptosis of neurons and endothelial cells, BBB breakdown and the altered contractile response of cerebral vessels following SAH. Antioxidant therapy has provided neuroprotection and antispasmotic effects in experimental SAH and some therapies have demonstrated improved outcomes in clinical trials. These studies have laid a foundation for the use of antioxidants in the treatment of aneurismal SAH.
Keywords: Subarachnoid haemorrhage, oxidative stress, acute brain injury, cerebral vasospasm
Introduction
Spontaneous rupture of a cerebral aneurysm gives rise to subarachnoid haemorrhage (SAH), a disease that carries significant morbidity and mortality, and affects a significant percentage of the population worldwide. Autopsy studies show that roughly 5% of the population harbors intracranial aneurisms and 10/100,000 people suffer from aneurismal SAH [72]. SAH carries an initial mortality of 15–20% and a 40% mortality at one month with roughly one-third of survivors harboring significant morbidity in the form of cognitive and/or motor deficits [95, 96]. Research has concentrated primarily on vasospasm and its sequelae, in an attempt to combat the high morbidity and mortality associated with SAH [43]. More recently, treatment modalities have also focused on acute brain injury following SAH, as this has also been linked to significant morbidity and mortality [11]. The mechanisms of acute brain injury are still poorly understood and the study of vasospasm has still not yielded a therapy that effectively eliminates the problem. Many studies have provided evidence that oxidative stress plays a significant role in the processes of acute brain injury as well as cerebral vasospasm, and these will be reviewed in this paper. Normal mammalian cellular respiration results in the production of free radicals, and the brain, as a result of its high metabolic demands, is especially susceptible to free radical injury when cellular respiration becomes disrupted. An imbalance that favors the production of reactive oxygen species (ROS) versus their neutralization by intrinsic antioxidant systems has been demonstrated in the brain following SAH in both experimental models and humans [23, 46, 64, 66], and the most common free radicals involved in oxidative stress are superoxide anion, hydroxyl radical, nitric oxide, and peroxynitrite [98]. This mini-review will discuss the production of excessive free radicals in SAH and their connections to acute brain injury as well as cerebral vasospasm.
Sources of free radicals in subarachniod haemorrhage
Mitochondrial oxidative stress
The foremost considered sources of free radicals following SAH are the leakage of superoxide anions from mitochondria due to an ischemic disruption of the electron transfer chain, and the cascade of free radicals produced from the auto-oxidation of hemoglobin [1, 64, 68]. Electron transfer during normal mitochondrial respiration is accompanied by the leakage of electrons from the transport chain and their subsequent reaction with O2 to produce superoxide. This free radical is normally cleared by superoxide dismutase, but following periods of ischemia, such as those that follow SAH [9, 28, 40], the mitochondria becomes a source of excessive free radical production that cannot be cleared by antioxidant enzymes before they have the potential of causing significant lipid, protein, and DNA damage [19]. The mechanisms for ROS production by mitochondria are under intensive investigation, but in general, the production of reactive oxygen species is maximal when the components of the transport chain are maximally reduced [78, 80]. Excessive mitochondrial Ca2+ accumulation following ischemia interrupts the electron transport chain and collapses the mitochondria membrane potential by formation of the membrane permeability transition, which represents the opening of nonspecific pores allowing solutes of less than 1500 daltons to equilibrate across the membrane. Opening the high-conductance pore induces a maximal rate of substrate oxidation and O2 consumption in an attempt by the mitochondria to establish an electrochemical gradient, leaving more free electrons to interact with oxygen and create superoxide [78, 80, 122]. Specific investigations into mitochondrial activity following SAH have found disrupted mitochondrial respiration that favors the production of ROS [7, 63, 65]. Marzatico and Baena et al. have each recorded increased levels of state 4 mitochondrial respiration and decreased respiratory control ratios following SAH [7, 65], a state that is associated with the increased production of ROS. Future approaches to inhibiting the excessive generation of mitochondrial ROS in SAH include the uncoupling of the electron transport chain and the inhibition of membrane permeability transition.
Hemoglobin free radical generation
The liberation of oxyhemoglobin (oxyHb) into the CSF following SAH is a major producer of superoxide anion (O2•) and hydrogen peroxide (H2O2) as it undergoes auto-oxidation to methemeglobin [3]. The iron or ferrous ion liberated from oxyHb then catalyzes the generation of the more damaging hydroxyl radical (OH•) from O2•− and H2O2 (metHb) [30, 33, 75]. Methemoglobin and oxyhemoglobin react with hydrogen peroxide to generate ferrylhemoglobins (Fe4+), which is also a strong oxidizing agent [26]. Ferryl haeme protein can initiate a cycle of lipid oxidation which can react with further lipids in an auto-catalytic cycle [90]. Many studies demonstrate the oxidizing capacity of hemoglobin on lipid membranes, proteins, and DNA [26, 91, 94]. Subarachnoid hemolysate also increases cytochorome c mediated DNA fragmentation and apoptosis in mouse brains [69, 71]. This damage correlates inversely with superoxide dismutase expression, implicating free radicals in the mechanism [69–71]. Studies have also linked hemoglobin generated free radicals to vasospasm and have found iron chelators to successfully inhibit vasospasm [1, 2, 37, 38, 114]. Iron chelation has not been studied in the acute brain injury of SAH, but it is worth investigating because hemoglobin free radial production has been linked to neuronal cytotoxity [10, 107, 116] and damage to vascular endothelium [26]. Iron chelators have also shown neuroprotection in other CNS injuries [92].
Enzymatic sources of free radicals
In addition to the mitochondria and hemoglobin, a number of other enzymatic pathways to free radical production have been investigated. The accumulation of intracellular Ca2+ in neurons through voltage-sensitive and glutamate-sensitive channels due to the extracellular hemoglobin and the substantial ischemic insult of SAH [9, 28, 40] has been found to produce free radicals through the activation of several pro-oxidant pathways: phospholipases, xanthine oxidase, and nitric oxide synthase.
Esterified arachidonic acid (AA) is released through the breakdown of membrane phospholipids by phospholipase A2 activity. AA is metabolized by cyclooxygenase, lipoxygenase, and cytochrome P450. Each of these pathways produces O2•− as a byproduct [17, 122]. This mechanism of free radical production is considered a significant source of free radicals in models of traumatic brain injury and ischemia [17, 25, 79, 89, 122], and may also be a significant source of free radicals in subarachnoid haemorrhage. Neuronal cell culture studies suggest that the cellular damage mediated by AA metabolism is through free radicals, as antioxidants were able to prevent AA induced damage [45, 110]. Increased phospholipase A2 activity follows SAH [107, 113], and numerous studies demonstrate increased expression of cyclooxygenase [86, 87, 111] and lipoxygenase [6, 97, 102, 119] following SAH. Inhibiting these pathways has been effective at reducing cerebral vasospasm, but it is unclear if reducing the oxidative stress or decreasing the eicasanoids produced by these pathways was responsible for the therapeutic effect [8, 97, 105]. Several studies show that cytochrome P450 is a substantial source of arachidonic acid derived metabolites and ROS in several disease processes [13], but its significance in SAH remains to be determined. Miyata et al. has shown that the inhibition of this enzyme following SAH reduced cerebral vasospasm in rats [76].
Xanthine dehydrogenase (XDH) is an enzyme present in cerebral endothelium and is required for the metabolism of purines to uric acid. XDH does not produce free radicals but is converted to xanthine oxidase (XO) during ischemia, hypoxia, and excitotoxity by Ca2+ activated proteases [83]. XO in turn catalyzes the oxidation of hypoxanthine to xanthine, resulting in uric acid, superoxide and hydrogen peroxide. XO inhibition has resulted in a reduction in oxidative damage in several ishemic brain injury models [83, 118], and studies have suggested an increase in the activity of this enzyme following SAH [50, 62, 117]. The study of XO inhibition in SAH is limited, and there is some speculation that this pathway may not be significant owing to the many other pathways for free radical production. Kim showed that XO inhibition had no effect on free radical mediated vasospasm in dogs following experimental SAH [50].
Nitric oxide (NO•) is a free radical generated from L-arginine, NADPH and oxygen by nitric oxide synthase (NOS) which has three isoforms: endothelial NOS (eNOS), neuronal NOS (nNos) and inducible NOS (iNOS) [12, 106]. Neuronal and inducible nitric oxide synthases are upregulated following SAH [99, 125], and levels of NO• metabolites are significantly elevated in the days following SAH [47, 82, 106]. It is debated whether the production of NO leads to either toxicity or neuroprotection. Some investigators believe NO• might reduce toxicity by modifying the NMDA receptor response [57, 58], in addition to having beneficial actions on cerebral blood flow immediately following SAH [57, 121]. Sehba and Bederson hypothesized a three phase change in cerebral NO• levels after SAH, each with different effects on the ischemic brain that helps to explain the conflicting actions attributed to NO•. In their model, the oxidative damage caused by NO• occurs 6 h after injury when NO• can no longer augment blood flow. The major producer of NO• at this time is iNOS [98]. With regards to free radical damage, it is known that once synthesized, NO• can interact with Superoxide (O2•) to form peroxynitrite (ONOO−), which can also decompose to form hydroxyl radical (OH•−) [25]. NO• and peroxynitrite are neurotoxic free radicals [15, 20] which exert significant DNA and mitochondrial damage leading to cell death. [55]. Yatsushige investigated the role of iNOS inhibition in acute brain injury following SAH and found that there was no significant reduction in edema or BBB integrity [125]. In constrast, Yang et al. looked at increasing NO• following SAH by the administration of its precursor L-arginine. This group found significant reductions in brain edema [124], suggesting that NO• is neuroprotective rather than cytotoxic in acute brain injury after SAH.
NADPH oxidase is a membrane-bound enzyme expressed in neurons [100, 108, 109, 126] which may produce superoxide anions directed toward the neuronal cell’s interior [54]. NADPH oxidase has been found to directly contribute to oxidative stress and neuronal apoptosis in in vitro studies [108], and increased expression of neuronal NOX been associated with oxidative stress in rat models of SAH [84, 85]. Increased NOX expression in cerebral vasculature has been associated with free radical mediated vasospasm following SAH, and NOX inhibition in these studies was found to prevent arterial contraction and improve cerebral blood flow [49, 74, 88, 103, 104, 127]. Neuronal NOX activity in acute brain injury after SAH has been conducted more recently. Ostrowski et al. found that the neuroprotection provided by hyperbaric oxygen in SAH involves the reduction of neuronal NOX and associated free radical mediated cell damage. These reductions in NOX activity were associated with significant reductions in mortality, neurological deficits, and neuronal cell death [84, 85]. Significant reductions in acute brain injury and neurological deficits were also observed in NOX knockout mice following intracerebral haemorrhage [109], but the direct inhibition of NOX in SAH has not been studied.
Disrupted antioxidant protection
In the brain there are several enzymatic protective systems that are in place against free radical production, and during normal cellular respiration, superoxide dis-mutases, glutathione peroxidases, and catalases are the significant enzymatic scavengers in brain tissue [56]. However, following SAH these enzymatic systems become downregulated or modulated in a way that reduces their antioxidant capabilities [21, 23, 56, 64]. Decreases in the activity of Zn and Cu–SOD have been demonstrated following SAH in rats [21, 64], and investigation of human SAH has shown significant increases in the ratio of SOD/GSH-Px activity. Under normal physiological conditions, hydrogen peroxide (H2O2) is produced from O2•− by SOD which is scavenged by GSH-Px, preventing the formation of the potent OH•− free radical [17, 23, 34]. However, an increase in SOD activity relative to GSH-Px activity creates a state of excess OH•− production. An increase in the SOD/GSH-Px activity ration following SAH correlates with the incidence of vasospasm [23] and has the potential of being a significant source of free radical mediated damage.
Free radical mediated acute brain injury
At a cellular level, free radicals lead to neuronal damage by promoting, lipid peroxidation, protein breakdown, and DNA damage that in turn leads to cellular apoptosis, endothelial injury, and blood brain barrier (BBB) permeability [17, 56, 69, 71]. Lipid peroxidation of cell membranes can lead to the formation of many lipid peroxides altering membrane fluidity and permeability [17, 56, 69, 71]. Protein oxidation affects the functions of enzymes and cell receptors. Free radicals can also initiate apoptotic cascades or send the cells into necrosis through mitochondrial mediated mechanisms [18, 81]. Oxidative stress has been shown to induce apoptosis by increasing p53, inducing cytochrome c release and activating caspase-9 and caspase-3 [18]. Other studies have shown oxidative stress to activate p38 mitogen-activated protein (MAP) kinase and signal-regulated kinase (ERK) mediated apoptosis [81]. Reductions in oxidative stress have been shown to inhibit apoptosis. SOD overexpression in transgenic rats has shown significant reductions in apoptosis following experimental SAH [16, 69], and this reduction may be mediated by the activation of the Akt/glycogen synthase kinase-3β survival pathway [16]. Other studies show the efficacy of the systemic administration of antioxidants in SAH. Imperatore et al. and Germano et al. showed that nicaraven, a hydroxyl radical scavenger, improved neurobehavior following SAH. Turner et al. showed that the administration of tirilazad-like antioxidants U101033E and U74389G prevented the induction of heat shock proteins in the brain following SAH [24, 39, 112]. Endothelial cells are also susceptible to oxidative stress [26, 36, 61, 107], and oxidative stress has been shown to disrupt the BBB in various CNS injuries [29, 36, 101] while free radical induced BBB breakdown is likely to be important in SAH. Antioxidant therapy has been shown to protect the BBB in several animal models [24, 32, 39, 128].
Oxidative stress and vasospasm
Vasospasm is a frequent complication of subarchnoid haemorrhage and is critical to the prognosis of patients following SAH [59]. There are still many unanswered questions about the pathogenesis of cerebral vasospasm following SAH, and effective therapies are still being sought. Evidence suggests that oxidative stress is one of the factors contributing to post-hemorrhagic vasospasm [21, 48, 60]. Elevated superoxide anion levels in the cerebrospinal fluid after SAH have been reported to correlate to cerebral vasospasm [77]. Free radical scavengers such as iron chelators, ebselen, tirilazad, nicaracen, and inhibitors of free radical generating enzymes have been shown to reduce cerebral vasospasm in animal models of SAH [8, 22, 35, 38, 41, 42, 67, 73, 114, 115, 120, 127, 129]. Oxidative stress stimulates the proliferation and hypertrophy of smooth muscle cells [27], and induces endothelial apoptosis. These alterations are associated with changes in the contractile response of the cerebral vasculature. Maeda et al. showed that isolated strips of the bovine middle cerebral artery exposed to oxidative stress inhibited bradykinin-induced endothelium-dependent relaxation [61]. These contractile changes were prevented by free radical scavengers, as well as by the inhibition of either p38 MAP kinase or tyrosine kinase. It is also likely that the lipid peroxidation of phospholipids is connected to the production of diacylglycerol and the subsequent activation of protein kinase C (PKC), a key element to the mechanism of smooth muscle contraction [4]. Investigation continues to further elucidate how oxidative stress alters cerebral vascular contractile responses.
Clinical implications and future directions
Given the numerous sources of free radical production and evidence of significant oxidative stress in SAH, there is substantial support for the use of free radical scavengers for the treatment of brain injury and cerebral vasospasm in this disease. Many free radical scavenging compounds have been tested in clinical trials for the treatment of SAH with variable results. Ebselen is an organic antioxidant which poses glutathione peroxidase-like activity, has been successful at reducing vasospasm in animal models [35, 120], and has modest effects in clinical trials. Although ebeselen adminstration within 96 h of SAH failed to show differences in Glasgow Outcome Scale (GOS) versus the control, subgroup analysis showed that patients with delayed ischemic neurological deficits (DINDS) had better outcomes with treatment [93]. Tirilazad mesylate is another free radical scavenger with pre-clinical success, but this drug failed to demonstrate efficacy in clinical trials. Four randomized controlled trials, two conducted across Europe, Australia, and New Zealand, and two in North America, failed to consistently show improvements in mortality, GOS, or symptomatic vasospasm [31, 44, 52, 53]. Nicaraven, or AVS ((+/−)-N, N′-propylenedinicotinamide), a synthetic compound capable of scavenging hydroxyl radicals, has prevented vasospasm shown neuroprotective properties in experimental SAH [24, 39, 123]. Nicaraven treatment also demonstrated a significant reduction in symptomatic vasospasm and cumulative mortality in a randomized controlled trial [5]. In summary, the results of clinical trials with antioxidants have been mixed, but the potential for therapeutic efficacy still exists and is worth investigating. The failure of antioxidants in clinical trials may be attributed to the fact that oxidative stress is only one parameter of the injury. It is also possible that inappropriate dosing, or the severity and heterogeneity of the brain injury made it difficult to obtain statistically significant results. However, it is more likely that antioxidant therapy will be most effective as one component in a treatment regime that addresses the many pathways to brain injury and vasospasm following SAH.
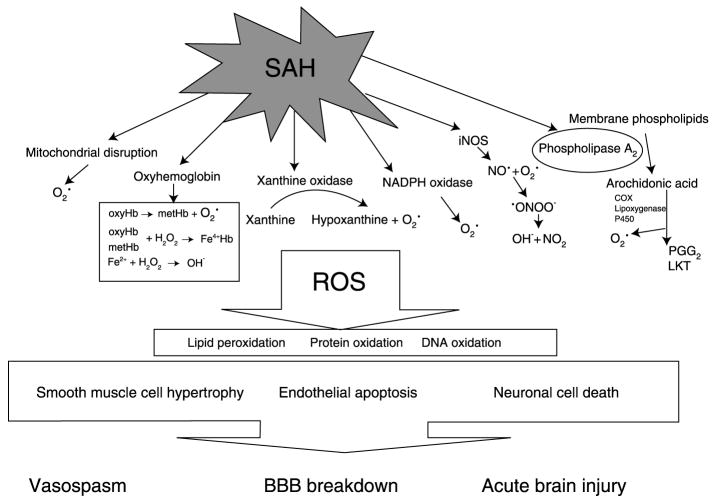
Fig. 1.
Schematic representation of major intracellular pathway in the generation of reactive oxygen species radicals in subarachnoid haemorrhage. ROS Reactive oxygen species; SAH subarachonoid haemorrhage
Acknowledgments
This study is partially supported by grants from NIH NS53407, NS45694, NS43338, and HD43120 to J. H. Zhang.
References
- 1.Arai T, Takeyama N, Tanaka T. Glutathione monoethyl ester and inhibition of the oxyhemoglobin-induced increase in cytosolic calcium in cultured smooth-muscle cells. J Neurosurg. 1999;90:527–532. doi: 10.3171/jns.1999.90.3.0527. [DOI] [PubMed] [Google Scholar]
- 2.Arthur AS, Fergus AH, Lanzino G, Mathys J, Kassell NF, Lee KS. Systemic administration of the iron chelator deferiprone attenuates subarachnoid hemorrhage-induced cerebral vasospasm in the rabbit. Neurosurgery. 1997;41:1385–1391. doi: 10.1097/00006123-199712000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Asano T. Oxyhemoglobin as the principal cause of cerebral vasospasm: a holistic view of its actions. Crit Rev Neurosurg. 1999;9:303–318. doi: 10.1007/s003290050147. [DOI] [PubMed] [Google Scholar]
- 4.Asano T, Matsui T. Antioxidant therapy against cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Cell Mol Neurobiol. 1999;19:31–44. doi: 10.1023/A:1006908422937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano T, Takakura K, Sano K, Kikuchi H, Nagai H, Saito I, Tamura A, Ochiai C, Sasaki T. Effects of a hydroxyl radical scavenger on delayed ischemic neurological deficits following aneurysmal subarachnoid hemorrhage: results of a multicenter, placebo-controlled double-blind trial. J Neurosurg. 1996;84:792–803. doi: 10.3171/jns.1996.84.5.0792. [DOI] [PubMed] [Google Scholar]
- 6.Baena R, Gaetani P, Marzatico F, Benzi G, Pacchiarini L, Paoletti P. Effects of nicardipine on the ex vivo release of eicosanoids after experimental subarachnoid hemorrhage. J Neurosurg. 1989;71:903–908. doi: 10.3171/jns.1989.71.6.0903. [DOI] [PubMed] [Google Scholar]
- 7.Baena R, Gaetani P, Silvani V, Spanu G, Marzatico F. Effect of nimodipine on mitochondrial respiration in different rat brain areas after subarachnoid haemorrhage. Acta Neurochir Suppl (Wien) 1988;43:177–181. doi: 10.1007/978-3-7091-8978-8_38. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa MD, Arthur AS, Louis RH, MacDonald T, Polin RS, Gazak C, Kassell NF. The novel 5-lipoxygenase inhibitor ABT-761 attenuates cerebral vasospasm in a rabbit model of subarachnoid hemorrhage. Neurosurgery. 2001;49:1205–1212. doi: 10.1097/00006123-200111000-00032. [DOI] [PubMed] [Google Scholar]
- 9.Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins AL, III, Vallabhajosyula P. Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery. 1998;42:352–360. doi: 10.1097/00006123-199802000-00091. [DOI] [PubMed] [Google Scholar]
- 10.Bilgihan A, Turkozkan N, Aricioglu A, Aykol S, Cevik C, Goksel M. The effect of deferoxamine on brain lipid peroxide levels and Na-K ATPase activity following experimental subarachnoid hemorrhage. Gen Pharmacol. 1994;25:495–497. doi: 10.1016/0306-3623(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 11.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 12.Calvert JW, Zhang JH. Pathophysiology of an hypoxic-ischemic insult during the perinatal period. Neurol Res. 2005;27:246–260. doi: 10.1179/016164105X25216. [DOI] [PubMed] [Google Scholar]
- 13.Caro AA, Cederbaum AI. Role of cytochrome P450 in phospholipase A2- and arachidonic acid-mediated cytotoxicity. Free Radic Biol Med. 2006;40:364–375. doi: 10.1016/j.freeradbiomed.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Clark JF, Sharp FR. Bilirubin oxidation products (BOXes) and their role in cerebral vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1223–1233. doi: 10.1038/sj.jcbfm.9600280. [DOI] [PubMed] [Google Scholar]
- 15.Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3beta survival signaling. J Cereb Blood Flow Metab. 2006;▪:▪–▪. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18:667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueroa S, Oset-Gasque MJ, Arce C, Martinez-Honduvilla CJ, Gonzalez MP. Mitochondrial involvement in nitric oxide-induced cellular death in cortical neurons in culture. J Neurosci Res. 2006;83:441–449. doi: 10.1002/jnr.20739. [DOI] [PubMed] [Google Scholar]
- 19.Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–352. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- 20.Forman LJ, Liu P, Nagele RG, Yin K, Wong PY. Augmentation of nitric oxide, superoxide, and peroxynitrite production during cerebral ischemia and reperfusion in the rat. Neurochem Res. 1998;23:141–148. doi: 10.1023/a:1022468522564. [DOI] [PubMed] [Google Scholar]
- 21.Gaetani P, Lombardi D. Brain damage following subarachnoid hemorrhage: the imbalance between anti-oxidant systems and lipid peroxidative processes. J Neurosurg Sci. 1992;36:1–10. [PubMed] [Google Scholar]
- 22.Gaetani P, Marzatico F, Lombardi D, Adinolfi D, Baena R. Effect of high-dose methylprednisolone and U74006F on eicosanoid synthesis after subarachnoid hemorrhage in rats. Stroke. 1991;22:215–220. doi: 10.1161/01.str.22.2.215. [DOI] [PubMed] [Google Scholar]
- 23.Gaetani P, Pasqualin A, Baena R, Borasio E, Marzatico F. Oxidative stress in the human brain after subarachnoid hemorrhage. J Neurosurg. 1998;89:748–754. doi: 10.3171/jns.1998.89.5.0748. [DOI] [PubMed] [Google Scholar]
- 24.Germano A, Imperatore C, d’Avella D, Costa G, Tomasello F. Antivasospastic and brain-protective effects of a hydroxyl radical scavenger (AVS) after experimental subarachnoid hemorrhage. J Neurosurg. 1998;88:1075–1081. doi: 10.3171/jns.1998.88.6.1075. [DOI] [PubMed] [Google Scholar]
- 25.Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- 26.Goldman DW, Breyer RJ, III, Yeh D, Brockner-Ryan BA, Alayash AI. Acellular hemoglobin-mediated oxidative stress toward endothelium: a role for ferryl iron. Am J Physiol. 1998;275:H1046–H1053. doi: 10.1152/ajpheart.1998.275.3.H1046. [DOI] [PubMed] [Google Scholar]
- 27.Griendling KK, Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. J Lab Clin Med. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 28.Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22:654–661. doi: 10.1227/00006123-198804000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Gursoy-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- 30.Gutteridge JM. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986;201:291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- 31.Haley EC, Jr, Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86:467–474. doi: 10.3171/jns.1997.86.3.0467. [DOI] [PubMed] [Google Scholar]
- 32.Hall ED, Andrus PK, Smith SL, Oostveen JA, Scherch HM, Lutzke BS, Raub TJ, Sawada GA, Palmer JR, Banitt LS. Neuroprotective efficacy of microvascularly-localized versus brain-penetrating antioxidants. Acta Neurochir Suppl. 1996;66:107–113. doi: 10.1007/978-3-7091-9465-2_19. [DOI] [PubMed] [Google Scholar]
- 33.Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978;92:321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- 34.Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 35.Handa Y, Kaneko M, Takeuchi H, Tsuchida A, Kobayashi H, Kubota T. Effect of an antioxidant, ebselen, on development of chronic cerebral vasospasm after subarachnoid hemorrhage in primates. Surg Neurol. 2000;53:323–329. doi: 10.1016/s0090-3019(00)00168-3. [DOI] [PubMed] [Google Scholar]
- 36.Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood–brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 37.Harada T, Mayberg MR. Inhibition of delayed arterial narrowing by the iron-chelating agent deferoxamine. J Neurosurg. 1992;77:763–767. doi: 10.3171/jns.1992.77.5.0763. [DOI] [PubMed] [Google Scholar]
- 38.Horky LL, Pluta RM, Boock RJ, Oldfield EH. Role of ferrous iron chelator 2,2′-dipyridyl in preventing delayed vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg. 1998;88:298–303. doi: 10.3171/jns.1998.88.2.0298. [DOI] [PubMed] [Google Scholar]
- 39.Imperatore C, Germano A, d’Avella D, Tomasello F, Costa G. Effects of the radical scavenger AVS on behavioral and BBB changes after experimental subarachnoid hemorrhage. Life Sci. 2000;66:779–790. doi: 10.1016/s0024-3205(99)00651-7. [DOI] [PubMed] [Google Scholar]
- 40.Jarus-Dziedzic K, Czernicki Z, Kozniewska E. Acute decrease of cerebrocortical microflow and lack of carbon dioxide reactivity following subarachnoid haemorrhage in the rat. Acta Neurochir Suppl. 2003;86:473–476. doi: 10.1007/978-3-7091-0651-8_97. [DOI] [PubMed] [Google Scholar]
- 41.Kanamaru K, Weir BK, Findlay JM, Grace M, Macdonald RL. A dosage study of the effect of the 21-aminosteroid U74006F on chronic cerebral vasospasm in a primate model. Neurosurgery. 1990;27:29–38. doi: 10.1097/00006123-199007000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Kanamaru K, Weir BK, Simpson I, Witbeck T, Grace M. Effect of 21-aminosteroid U-74006F on lipid peroxidation in subarachnoid clot. J Neurosurg. 1991;74:454–459. doi: 10.3171/jns.1991.74.3.0454. [DOI] [PubMed] [Google Scholar]
- 43.Kaptain GJ, Lanzino G, Kassell NF. Subarachnoid haemorrhage: epidemiology, risk factors, and treatment options. Drugs Aging. 2000;17:183–199. doi: 10.2165/00002512-200017030-00003. [DOI] [PubMed] [Google Scholar]
- 44.Kassell NF, Haley EC, Jr, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- 45.Katsuki H, Akino N, Okuda S, Saito H. Antioxidants, but not cAMP or high K+, prevent arachidonic acid toxicity on neuronal cultures. Neuroreport. 1995;6:1101–1104. doi: 10.1097/00001756-199505300-00007. [DOI] [PubMed] [Google Scholar]
- 46.Kaynar MY, Tanriverdi T, Kemerdere R, Atukeren P, Gumustas K. Cerebrospinal fluid superoxide dismutase and serum malondialdehyde levels in patients with aneurysmal subarachnoid hemorrhage: preliminary results. Neurol Res. 2005;27:562–567. doi: 10.1179/016164105X17288. [DOI] [PubMed] [Google Scholar]
- 47.Khaldi A, Zauner A, Reinert M, Woodward JJ, Bullock MR. Measurement of nitric oxide and brain tissue oxygen tension in patients after severe subarachnoid hemorrhage. Neurosurgery. 2001;49:33–38. doi: 10.1097/00006123-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Kim DE, Suh YS, Lee MS, Kim KY, Lee JH, Lee HS, Hong KW, Kim CD. Vascular NAD(P)H oxidase triggers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke. 2002;33:2687–2691. doi: 10.1161/01.str.0000033071.99143.9e. [DOI] [PubMed] [Google Scholar]
- 49.Kim DE, Suh YS, Lee MS, Kim KY, Lee JH, Lee HS, Hong KW, Kim CD. Vascular NAD(P)H oxidase triggers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke. 2002;33:2687–2691. doi: 10.1161/01.str.0000033071.99143.9e. [DOI] [PubMed] [Google Scholar]
- 50.Kim P, Yaksh TL, Romero SD, Sundt TM., Jr Production of uric acid in cerebrospinal fluid after subarachnoid hemorrhage in dogs: investigation of the possible role of xanthine oxidase in chronic vasospasm. Neurosurgery. 1987;21:39–44. doi: 10.1227/00006123-198707000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Kranc KR, Pyne GJ, Tao L, Claridge TD, Harris DA, Cadoux-Hudson TA, Turnbull JJ, Schofield CJ, Clark JF. Oxidative degradation of bilirubin produces vasoactive compounds. Eur J Biochem. 2000;267:7094–7101. doi: 10.1046/j.1432-1327.2000.01812.x. [DOI] [PubMed] [Google Scholar]
- 52.Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90:1018–1024. doi: 10.3171/jns.1999.90.6.1018. [DOI] [PubMed] [Google Scholar]
- 53.Lanzino G, Kassell NF, Dorsch NW, Pasqualin A, Brandt L, Schmiedek P, Truskowski LL, Alves WM. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part I: A cooperative study in Europe, Australia, New Zealand, and South Africa. J Neurosurg. 1999;90:1011–1017. doi: 10.3171/jns.1999.90.6.1011. [DOI] [PubMed] [Google Scholar]
- 54.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 55.Leist M, Nicotera P. Apoptosis, excitotoxicity, and neuropathology. Exp Cell Res. 1998;239:183–201. doi: 10.1006/excr.1997.4026. [DOI] [PubMed] [Google Scholar]
- 56.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 57.Lipton SA, Singel DJ, Stamler JS. Nitric oxide in the central nervous system. Prog Brain Res. 1994;103:359–364. doi: 10.1016/s0079-6123(08)61149-8. [DOI] [PubMed] [Google Scholar]
- 58.Lipton SA, Stamler JS. Actions of redox-related congeners of nitric oxide at the NMDA receptor. Neuropharmacology. 1994;33:1229–1233. doi: 10.1016/0028-3908(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 59.Longstreth WT, Jr, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology. 1993;43:712–718. doi: 10.1212/wnl.43.4.712. [DOI] [PubMed] [Google Scholar]
- 60.Macdonald RL, Weir BK. Cerebral vasospasm and free radicals. Free Radic Biol Med. 1994;16:633–643. doi: 10.1016/0891-5849(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 61.Maeda Y, Hirano K, Nishimura J, Sasaki T, Kanaide H. Endothelial dysfunction and altered bradykinin response due to oxidative stress induced by serum deprivation in the bovine cerebral artery. Eur J Pharmacol. 2004;491:53–60. doi: 10.1016/j.ejphar.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 62.Marklund N, Ostman B, Nalmo L, Persson L, Hillered L. Hypoxanthine, uric acid and allantoin as indicators of in vivo free radical reactions. Description of a HPLC method and human brain microdialysis data. Acta Neurochir (Wien) 2000;142:1135–1141. doi: 10.1007/s007010070042. [DOI] [PubMed] [Google Scholar]
- 63.Marzatico F, Gaetani P, Baena R, Silvani V, Paoletti P, Benzi G. Bioenergetics of different brain areas after experimental subarachnoid hemorrhage in rats. Stroke. 1988;19:378–384. doi: 10.1161/01.str.19.3.378. [DOI] [PubMed] [Google Scholar]
- 64.Marzatico F, Gaetani P, Cafe C, Spanu G, Baena R. Antioxidant enzymatic activities after experimental subarachnoid hemorrhage in rats. Acta Neurol Scand. 1993;87:62–66. doi: 10.1111/j.1600-0404.1993.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 65.Marzatico F, Gaetani P, Silvani V, Lombardi D, Sinforiani E, Baena R. Experimental isobaric subarachnoid hemorrhage: regional mitochondrial function during the acute and late phase. Surg Neurol. 1990;34:294–300. doi: 10.1016/0090-3019(90)90004-9. [DOI] [PubMed] [Google Scholar]
- 66.Marzatico F, Gaetani P, Tartara F, Bertorelli L, Feletti F, Adinolfi D, Tancioni F, Baena R. Antioxidant status and alpha1-antiproteinase activity in subarachnoid hemorrhage patients. Life Sci. 1998;63:821–826. doi: 10.1016/s0024-3205(98)00338-5. [DOI] [PubMed] [Google Scholar]
- 67.Matsui T, Asano T. Effects of new 21-aminosteroid tirilazad mesylate (U74006F) on chronic cerebral vasospasm in a “two-hemorrhage” model of beagle dogs. Neurosurgery. 1994;34:1035–1039. doi: 10.1227/00006123-199406000-00012. [DOI] [PubMed] [Google Scholar]
- 68.Matz P, Weinstein P, States B, Honkaniemi J, Sharp FR. Subarachnoid injections of lysed blood induce the hsp70 stress gene and produce DNA fragmentation in focal areas of the rat brain. Stroke. 1996;27:504–512. doi: 10.1161/01.str.27.3.504. [DOI] [PubMed] [Google Scholar]
- 69.Matz PG, Copin JC, Chan PH. Cell death after exposure to subarachnoid hemolysate correlates inversely with expression of CuZn-superoxide dismutase. Stroke. 2000;31:2450–2459. doi: 10.1161/01.str.31.10.2450. [DOI] [PubMed] [Google Scholar]
- 70.Matz PG, Fujimura M, Chan PH. Subarachnoid hemolysate produces DNA fragmentation in a pattern similar to apoptosis in mouse brain. Brain Res. 2000;858:312–319. doi: 10.1016/s0006-8993(99)02454-3. [DOI] [PubMed] [Google Scholar]
- 71.Matz PG, Fujimura M, Lewen A, Morita-Fujimura Y, Chan PH. Increased cytochrome c-mediated DNA fragmentation and cell death in manganese-superoxide dismutase-deficient mice after exposure to subarachnoid hemolysate. Stroke. 2001;32:506–515. doi: 10.1161/01.str.32.2.506. [DOI] [PubMed] [Google Scholar]
- 72.McCormick WF, Nofzinger JD. Saccular intracranial aneuryms: an autopsy study. J Neurosurg. 1965;22:155–159. doi: 10.3171/jns.1965.22.2.0155. [DOI] [PubMed] [Google Scholar]
- 73.McGirt MJ, Parra A, Sheng H, Higuchi Y, Oury TD, Laskowitz DT, Pearlstein RD, Warner DS. Attenuation of cerebral vasospasm after subarachnoid hemorrhage in mice overexpressing extracellular superoxide dismutase. Stroke. 2002;33:2317–2323. doi: 10.1161/01.str.0000027207.67639.1e. [DOI] [PubMed] [Google Scholar]
- 74.Miller AA, Drummond GR, Sobey CG. Novel isoforms of NADPH-oxidase in cerebral vascular control. Pharmacol Ther. 2006;111:928–948. doi: 10.1016/j.pharmthera.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Misra HP, Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972;247:6960–6962. [PubMed] [Google Scholar]
- 76.Miyata N, Seki T, Tanaka Y, Omura T, Taniguchi K, Doi M, Bandou K, Kametani S, Sato M, Okuyama S. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther. 2005;314:77–85. doi: 10.1124/jpet.105.083964. [DOI] [PubMed] [Google Scholar]
- 77.Mori T, Nagata K, Town T, Tan J, Matsui T, Asano T. Intracisternal increase of superoxide anion production in a canine subarachnoid hemorrhage model. Stroke. 2001;32:636–642. doi: 10.1161/01.str.32.3.636. [DOI] [PubMed] [Google Scholar]
- 78.Moro MA, Almeida A, Bolanos JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med. 2005;39:1291–1304. doi: 10.1016/j.freeradbiomed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 79.Muralikrishna AR, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 80.Murphy AN, Fiskum G, Beal MF. Mitochondria in neuro-degeneration: bioenergetic function in cell life and death. J Cereb Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Naoi M, Maruyama W, Shamoto-Nagai M, Yi H, Akao Y, Tanaka M. Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Mol Neurobiol. 2005;31:81–93. doi: 10.1385/MN:31:1-3:081. [DOI] [PubMed] [Google Scholar]
- 82.Ng WH, Moochhala S, Yeo TT, Ong PL, Ng PY. Nitric oxide and subarachnoid hemorrhage: elevated level in cerebrospinal fluid and their implications. Neurosurgery. 2001;49:622–626. doi: 10.1097/00006123-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 83.Nishino T, Tamura I. The mechanism of conversion of xanthine dehydrogenase to oxidase and the role of the enzyme in reperfusion injury. Adv Exp Med Biol. 1991;309A:327–333. doi: 10.1007/978-1-4899-2638-8_74. [DOI] [PubMed] [Google Scholar]
- 84.Ostrowski RP, Colohan AR, Zhang JH. Neuroprotective effect of hyperbaric oxygen in a rat model of subarachnoid hemorrhage. Acta Neurochir Suppl. 2006;96:188–193. doi: 10.1007/3-211-30714-1_41. [DOI] [PubMed] [Google Scholar]
- 85.Ostrowski RP, Tang J, Zhang JH. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 2006;37:1314–1318. doi: 10.1161/01.STR.0000217310.88450.c3. [DOI] [PubMed] [Google Scholar]
- 86.Osuka K, Suzuki Y, Watanabe Y, Takayasu M, Yoshida J. Inducible cyclooxygenase expression in canine basilar artery after experimental subarachnoid hemorrhage. Stroke. 1998;29:1219–1222. doi: 10.1161/01.str.29.6.1219. [DOI] [PubMed] [Google Scholar]
- 87.Osuka K, Watanabe Y, Yamauchi K, Nakazawa A, Usuda N, Tokuda M, Yoshida J. Activation of the JAK-STAT signaling pathway in the rat basilar artery after subarachnoid hemorrhage. Brain Res. 2006;1072:1–7. doi: 10.1016/j.brainres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Paravicini TM, Sobey CG. Cerebral vascular effects of reactive oxygen species: recent evidence for a role of NADPH-oxidase. Clin Exp Pharmacol Physiol. 2003;30:855–859. doi: 10.1046/j.1440-1681.2003.03920.x. [DOI] [PubMed] [Google Scholar]
- 89.Phillis JW, O’Regan MH. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit Rev Neurobiol. 2003;15:61–90. doi: 10.1615/critrevneurobiol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 90.Reeder BJ, Sharpe MA, Kay AD, Kerr M, Moore K, Wilson MT. Toxicity of myoglobin and haemoglobin: oxidative stress in patients with rhabdomyolysis and subarachnoid haemorrhage. Biochem Soc Trans. 2002;30:745–748. doi: 10.1042/bst0300745. [DOI] [PubMed] [Google Scholar]
- 91.Rogers MS, Patel RP, Reeder BJ, Sarti P, Wilson MT, Alayash AI. Pro-oxidant effects of cross-linked haemoglobins explored using liposome and cytochrome c oxidase vesicle model membranes. Biochem J. 1995;310(Pt 3):827–833. doi: 10.1042/bj3100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sadrzadeh SM, Anderson DK, Panter SS, Hallaway PE, Eaton JW. Hemoglobin potentiates central nervous system damage. J Clin Invest. 1987;79:662–664. doi: 10.1172/JCI112865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saito I, Asano T, Sano K, Takakura K, Abe H, Yoshimoto T, Kikuchi H, Ohta T, Ishibashi S. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1998;42:269–277. doi: 10.1097/00006123-199802000-00038. [DOI] [PubMed] [Google Scholar]
- 94.Sarti P, Hogg N, Darley-Usmar VM, Sanna MT, Wilson MT. The oxidation of cytochrome-c oxidase vesicles by hemoglobin. Biochim Biophys Acta. 1994;1208:38–44. doi: 10.1016/0167-4838(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 95.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 96.Schievink WI, Riedinger M, Jhutty TK, Simon P. Racial disparities in subarachnoid hemorrhage mortality: Los Angeles County, California, 1985–1998. Neuroepidemiology. 2004;23:299–305. doi: 10.1159/000080096. [DOI] [PubMed] [Google Scholar]
- 97.Schulz R, Jancar S, Cook DA. Cerebral arteries can generate 5- and 15-hydroxyeicosatetraenoic acid from arachidonic acid. Can J Physiol Pharmacol. 1990;68:807–813. doi: 10.1139/y90-123. [DOI] [PubMed] [Google Scholar]
- 98.Sehba FA, Bederson JB. Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:381–398. doi: 10.1179/016164106X114991. [DOI] [PubMed] [Google Scholar]
- 99.Sehba FA, Chereshnev I, Maayani S, Friedrich V, Jr, Bederson JB. Nitric oxide synthase in acute alteration of nitric oxide levels after subarachnoid hemorrhage. Neurosurgery. 2004;55:671–677. doi: 10.1227/01.neu.0000134557.82423.b2. [DOI] [PubMed] [Google Scholar]
- 100.Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 101.Sharma HS, Drieu K, Westman J. Antioxidant compounds EGB-761 and BN-52021 attenuate brain edema formation and hemeoxygenase expression following hyperthermic brain injury in the rat. Acta Neurochir Suppl. 2003;86:313–319. doi: 10.1007/978-3-7091-0651-8_68. [DOI] [PubMed] [Google Scholar]
- 102.Shimizu T, Watanabe T, Asano T, Seyama Y, Takakura K. Activation of the arachidonate 5-lipoxygenase pathway in the canine basilar artery after experimental subarachnoidal hemorrhage. J Neurochem. 1988;51:1126–1131. doi: 10.1111/j.1471-4159.1988.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 103.Shin HK, Lee JH, Kim CD, Kim YK, Hong JY, Hong KW. Prevention of impairment of cerebral blood flow autoregulation during acute stage of subarachnoid hemorrhage by gene transfer of Cu/Zn SOD-1 to cerebral vessels. J Cereb Blood Flow Metab. 2003;23:111–120. doi: 10.1097/01.WCB.0000036561.60552.63. [DOI] [PubMed] [Google Scholar]
- 104.Shin HK, Lee JH, Kim KY, Kim CD, Lee WS, Rhim BY, Hong KW. Impairment of autoregulatory vasodilation by NAD(P)H oxidase-dependent superoxide generation during acute stage of subarachnoid hemorrhage in rat pial artery. J Cereb Blood Flow Metab. 2002;22:869–877. doi: 10.1097/00004647-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 105.Sippell G, Lehmann P, Hollmann G. Automation of multiple sephadex LH-20 column chromatography for the simultaneous separation of plasma corticosteroids. J Chromatogr. 1975;108:305–312. doi: 10.1016/s0021-9673(00)84673-7. [DOI] [PubMed] [Google Scholar]
- 106.Suzuki H, Kanamaru K, Tsunoda H, Inada H, Kuroki M, Sun H, Waga S, Tanaka T. Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. J Clin Invest. 1999;104:59–66. doi: 10.1172/JCI5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takenaka K, Kassell NF, Foley PL, Lee KS. Oxyhemoglobin-induced cytotoxicity and arachidonic acid release in cultured bovine endothelial cells. Stroke. 1993;24:839–845. doi: 10.1161/01.str.24.6.839. [DOI] [PubMed] [Google Scholar]
- 108.Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:RC53–▪. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Neil GD, Zhang JH. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94:1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 110.Toborek M, Malecki A, Garrido R, Mattson MP, Hennig B, Young B. Arachidonic acid-induced oxidative injury to cultured spinal cord neurons. J Neurochem. 1999;73:684–692. doi: 10.1046/j.1471-4159.1999.0730684.x. [DOI] [PubMed] [Google Scholar]
- 111.Tran Dinh YR, Jomaa A, Callebert J, Reynier-Rebuffel AM, Tedgui A, Savarit A, Sercombe R. Overexpression of cyclooxygenase-2 in rabbit basilar artery endothelial cells after subarachnoid hemorrhage. Neurosurgery. 2001;48:626–633. doi: 10.1097/00006123-200103000-00037. [DOI] [PubMed] [Google Scholar]
- 112.Turner CP, Panter SS, Sharp FR. Anti-oxidants prevent focal rat brain injury as assessed by induction of heat shock proteins (HSP70, HO-1/HSP32, HSP47) following subarachnoid injections of lysed blood. Brain Res Mol Brain Res. 1999;65:87–102. doi: 10.1016/s0169-328x(98)00340-4. [DOI] [PubMed] [Google Scholar]
- 113.Vigne P, Frelin C. Endothelins activate phospholipase A2 in brain capillary endothelial cells. Brain Res. 1994;651:342–344. doi: 10.1016/0006-8993(94)90716-1. [DOI] [PubMed] [Google Scholar]
- 114.Vollmer DG, Hongo K, Ogawa H, Tsukahara T, Kassell NF. A study of the effectiveness of the iron-chelating agent deferoxamine as vasospasm prophylaxis in a rabbit model of subarachnoid hemorrhage. Neurosurgery. 1991;28:27–32. doi: 10.1097/00006123-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 115.Vollmer DG, Kassell NF, Hongo K, Ogawa H, Tsukahara T. Effect of the nonglucocorticoid 21-aminosteroid U74006F experimental cerebral vasospasm. Surg Neurol. 1989;31:190–194. doi: 10.1016/0090-3019(89)90115-8. [DOI] [PubMed] [Google Scholar]
- 116.Vollrath B, Chan P, Findlay M, Cook D. Lazaroids and deferoxamine attenuate the intracellular effects of oxyhaemoglobin in vascular smooth muscle. Cardiovasc Res. 1995;30:619–626. [PubMed] [Google Scholar]
- 117.von Holst H, Sollevi A. Increased concentration of hypoxanthine in human central cerebrospinal fluid after subarachnoid haemorrhage. Acta Neurochir (Wien) 1985;77:52–59. doi: 10.1007/BF01402306. [DOI] [PubMed] [Google Scholar]
- 118.Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 119.Watanabe T, Asano T, Shimizu T, Seyama Y, Takakura K. Participation of lipoxygenase products from arachidonic acid in the pathogenesis of cerebral vasospasm. J Neurochem. 1988;50:1145–1150. doi: 10.1111/j.1471-4159.1988.tb10585.x. [DOI] [PubMed] [Google Scholar]
- 120.Watanabe T, Nishiyama M, Hori T, Asano T, Shimizu T, Masayasu H. Ebselen (DR3305) ameliorates delayed cerebral vasospasm in a canine two-hemorrhage model. Neurol Res. 1997;19:563–565. doi: 10.1080/01616412.1997.11740859. [DOI] [PubMed] [Google Scholar]
- 121.Widenka DC, Medele RJ, Stummer W, Bise K, Steiger HJ. Inducible nitric oxide synthase: a possible key factor in the pathogenesis of chronic vasospasm after experimental subarachnoid hemorrhage. J Neurosurg. 1999;90:1098–1104. doi: 10.3171/jns.1999.90.6.1098. [DOI] [PubMed] [Google Scholar]
- 122.Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol. 2002;35:67–86. doi: 10.5483/bmbrep.2002.35.1.067. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto S, Teng W, Nishizawa S, Kakiuchi T, Tsukada H. Improvement in cerebral blood flow and metabolism following subarachnoid hemorrhage in response to prophylactic administration of the hydroxyl radical scavenger, AVS, (+/−)-N,N′-propylenedinicotinamide: a positron emission tomography study in rats. J Neurosurg. 2000;92:1009–1015. doi: 10.3171/jns.2000.92.6.1009. [DOI] [PubMed] [Google Scholar]
- 124.Yang MF, Sun BL, Xia ZL, Zhu LZ, Qiu PM, Zhang SM. Alleviation of brain edema by L-arginine following experimental subarachnoid hemorrhage in a rat model. Clin Hemorheol Microcirc. 2003;29:437–443. [PubMed] [Google Scholar]
- 125.Yatsushige H, Calvert JW, Cahill J, Zhang JH. Limited Role of Inducible Nitric Oxide Synthase in Blood Brain Barrier Function after Experimental Subarachnoid Hemorrhage. Journal of Neurotrauma. 2006;23:1874–▪. doi: 10.1089/neu.2006.23.1874. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 127.Zheng JS, Zhan RY, Zheng SS, Zhou YQ, Tong Y, Wan S. Inhibition of NADPH oxidase attenuates vasospasm after experimental subarachnoid hemorrhage in rats. Stroke. 2005;36:1059–1064. doi: 10.1161/01.STR.0000163102.49888.b7. [DOI] [PubMed] [Google Scholar]
- 128.Zuccarello M, Anderson DK. Protective effect of a 21-aminosteroid on the blood–brain barrier following subarachnoid hemorrhage in rats. Stroke. 1989;20:367–371. doi: 10.1161/01.str.20.3.367. [DOI] [PubMed] [Google Scholar]
- 129.Zuccarello M, Marsch JT, Schmitt G, Woodward J, Anderson DK. Effect of the 21-aminosteroid U-74006F on cerebral vasospasm following subarachnoid hemorrhage. J Neurosurg. 1989;71:98–104. doi: 10.3171/jns.1989.71.1.0098. [DOI] [PubMed] [Google Scholar]