Abstract
In vivo molecular imaging with target-specific activatable “smart” probes, which only yield fluorescence at the intended target, enables sensitive and specific cancer detection because of high target to background ratios (TBR). Dimerization and fluorescence quenching has been shown to occur in concentrated aqueous solutions of various fluorophores. Here, we hypothesized that fluorophore dimerization and quenching after conjugation to targeting proteins can occur at low concentration, which is reasonable for in vivo imaging probes, because protein molecules can stabilize the fluorophore dimers based on physico-chemical interactions. This dimerization can be exploited as a mechanism for fluorescence activation. Rhodamine derivatives were conjugated to the cancer targeting molecules, avidin and trastuzumab, which target D-galactose receptor and HER2/neu antigen, respectively. After conjugation, a large proportion of R6G and TAMRA formed H-type dimers, even at low concentrations, but could be fully dequenched upon dissociation of the dimers to monomers. Lipophilicity was a potential factor in promoting H-dimer formation. To demonstrate the fluorescence activation effect during in vivo fluorescence endoscopic molecular imaging, a highly quenched probe, avidin-TAMRA or a minimally quenched probe, avidin-Alexa488 was administered into mice with ovarian metastases to the peritoneum. The tumors were clearly visualized with avidin-TAMRA, with low background fluorescence; in contrast, the background fluorescence was high for avidin-Alexa488. Thus, H-dimer formation as a mechanism of fluorescence quenching could be used to develop fluorescence activatable probes for in vivo molecular imaging. Effective activatable optical probes can be designed by focusing on the H-dimer formation of fluorophores.
INTRODUCTION
Molecular imaging enables the visualization of specific molecules in vivo. Molecular probes for in vivo imaging typically consist of a targeting ligand, linker molecule and imaging “beacon” (1,2). Among the imaging beacons, optical fluorescent molecules have the advantages of higher sensitivity and lower cost than other imaging modalities, , but suffer from the well-recognized limitation of poor tissue penetration which restricts their application to surface or near-surface phenomena. However, a desirable property of fluorescent signals is that they can be “switchable”, enabling the development of target-specific activatable “smart” probes which only become fluorescent at the intended target (1,3). “Smart” probes have the potential to detect pathology in vivo with high sensitivity and specificity because background signal is minimized and thus, target to background ratios are high. There are a number of mechanisms for generating activatable probes but all require efficient quenching in the “off” state. These include photon-induced electron transfer (PeT) (4,5), and homo- and hetero-fluorescence resonance energy transfer (FRET) (6–8).
Some fluorophores, such as rhodamine- and BODIPY-delivatives, form homo-dimers at relatively high concentrations, and the photophysical chemistry underlying quenching by dimerization in concentrated aqueous solutions has been well studied (9–12). This homo-dimer formation induces short (H-dimer) or long (J-dimer) shifts of absorbance spectra, which completely quench the emission fluorescence signal (13). Since the dissociation constants of these dimers are large (~10−4–10−5 M) (13,14), dimer formation of fluorophores alone is not expected to occur at the concentrations (nano to picomolar) typically used for in vivo molecular imaging.
However, when target-specific molecular probes are synthesized, fluorophores are covalently conjugated with target-specific proteins such as antibodies or receptor-ligands, which have distinct, stable conformations under physiological conditions. Indeed, it is common that a fluorophore produces less light after conjugation with a protein than it does in its pure state (8,15,16). We speculated that protein molecules can facilitate H-dimer formation at low fluorophore concentrations, because each fluorophore theoretically has only a few preferential reaction sites on a protein based on physico-chemical characteristics such as charge and hydrophilicity. These characteristics might lead to crowding of fluorophores at specific sites on the protein, resulting in homo-dimer formation and quenching.
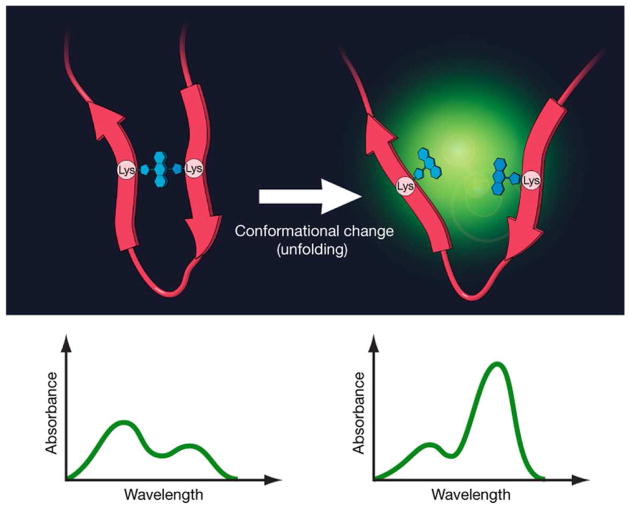
Furthermore, we hypothesized that fluorophore dimer formation, after protein binding, is potentially dissociable after conformational change or catabolism of the bound protein resulting in activation of the released fluorophore (Fig. 1). In the case of cancer imaging, dimer formation of fluorophores bound to target-specific proteins leads to quenching that is dissociable after internalization and catabolism of the probe within the cell but not outside the cell, leading to high tumor to background ratios (TBR). In this study, we evaluate the efficacy of various fluorophores (listed in Fig. 2) to form homo (H)-dimers on cancer targeting proteins, and then demonstrate that efficient H-dimer formation is a practical mechanism to generate activatable optical molecular imaging probes for in vivo molecular imaging of cancer.
Figure 1.
A schema of quenching and activation mechanism employing H-dimer formation after binding to the protein.
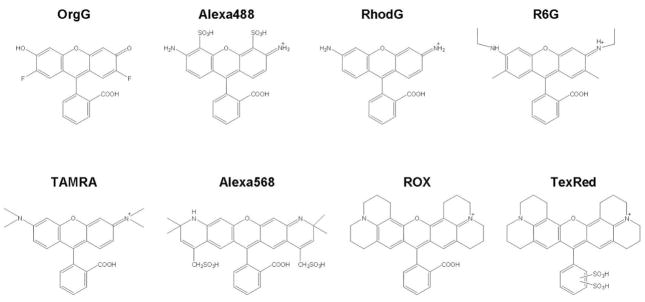
Figure 2.
Chemical structures of several rhodamine fluorophores Oregon Green (OrgG), Alexa Fluor488 (Alexa488), Rhodamine Green (RhodG), Rhodamine 6G (R6G), TAMRA, Alexa Fluor568 (Alexa568), ROX or Texas Red-X (TexRed)
RESULTS AND DISCUSSION
Mass spectral analysis of H-dimer formed Av-TAMRA
Although the TAMRA-avidin (tetramer) ratio was approximately 3:1, each avidin monomer is frequently conjugated with 2 fluorophores as shown on the Mass spectral analysis, (Supplemental Figure 2). This data suggests fluorophores tended to cluster in proximate pairs on monomers after conjugation with avidin. In addition, despite random conjugation, we found the lysine residue located at site 90 was a preferential conjugation site, although we could not determine the full spectrum of conjugation sites on the avidin molecule.
Effect of H-dimer formation on absorption spectrum and fluorescence intensity
The dimerization and resulting fluorescence quenching of rhodamine fluorophores in concentrated aqueous solutions are known phenomena in photochemistry (9–14). We sought to determine if this concept could be applied to macromolecular conjugation chemistry in the design of targeted activatable fluorescent probes for in vivo molecular imaging. The fluorophores were conjugated to the cancer targeting molecules, avidin and a humanized monoclonal antibody, trastuzumab, which bind D-galactose receptor and human epidermal growth factor receptor type 2 (HER2/neu) antigen, respectively.
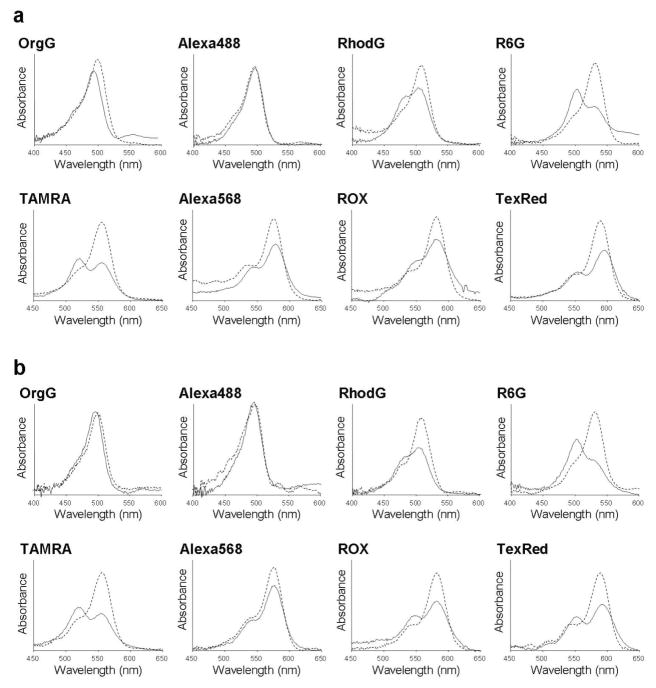
The absorption spectra of each conjugate without (solid lines) and with (dashed lines) SDS-induced unfolding are shown in Fig. 3. Without SDS, the spectra showed H-dimer formation with the appearance of a blue shifted peak (except for OrgG and Alexa488) both in avidin and trastuzumab conjugates. J-Dimer was not observed with any fluorophore. With SDS, the H-dimers were separated into monomers and H dimer formation could be expressed as follows;
Figure 3.
Absorbance spectrum of rhodamine fluorophores without SDS (solid line) and with SDS (dashed line). The blue shifted peak without SDS represents H-dimer formation of fluorophores. Both avidin and trastuzumab conjugates showed a similar tendency for each fluorophore tested.
Here, diAbs and moAbs represent the absorbance at the H-dimer peak and at the monomer peak, respectively. The results are summarized in Table 1. The changes in fluorescence intensity after SDS administration, that is, the “quenching capacities” are also shown in Table 1. The strongest fluorescence activation or quenching capacity was seen for TAMRA, R6G and TexRed in both avidin and trastuzumab conjugates. It is well-known that pyrenes form excimers at the high concentration, and emit longer and broader wavelength fluorescence than do monomers, because of complex ground state and excited state molecules (17). This is a useful characteristic for making molecular beacons for in vitro studies (18). In this study, we could not detect excimer formation based on the specific shape of the emission profile of all rhodamine-based probes tested in this study.
Table 1.
H-Dimer forming extents and fluorescence quenching capacities of avidin or trastuzumab-rhodamine conjugates.
| Avidin conjugates | Trastuzumab conjugates | |||
|---|---|---|---|---|
| H-dimer extent | Quenching capacity | H-dimer extent | Quenching capacity | |
| OregG | 0.86 | 2.6 | 0.97 | 1.1 |
| Alexa488 | 0.69 | 2.7 | 0.91 | 1.0 |
| RhodG | 1.70 | 2.5 | 1.89 | 3.7 |
| R6G | 3.58 | 13.5 | 3.63 | 8.9 |
| TAMRA | 2.74 | 10.0 | 2.79 | 7.4 |
| Alexa568 | 1.41 | 3.1 | 1.16 | 1.9 |
| ROX | 1.72 | 4.1 | 2.13 | 7.2 |
| TexRed | 1.67 | 10.1 | 2.00 | 12.0 |
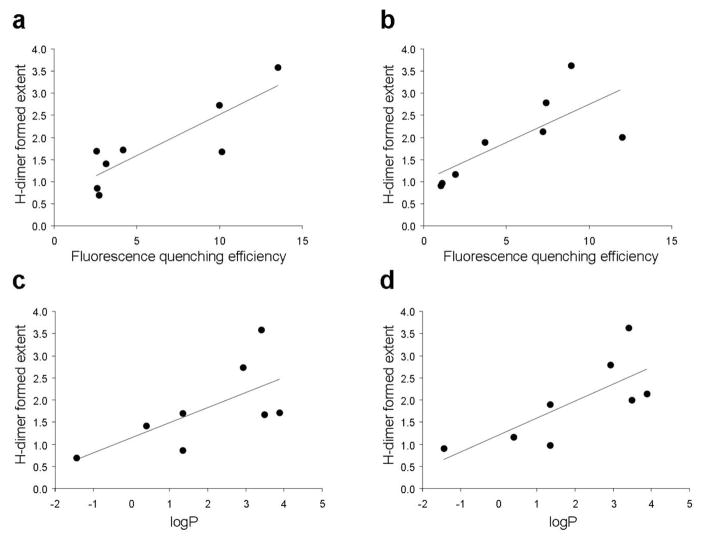
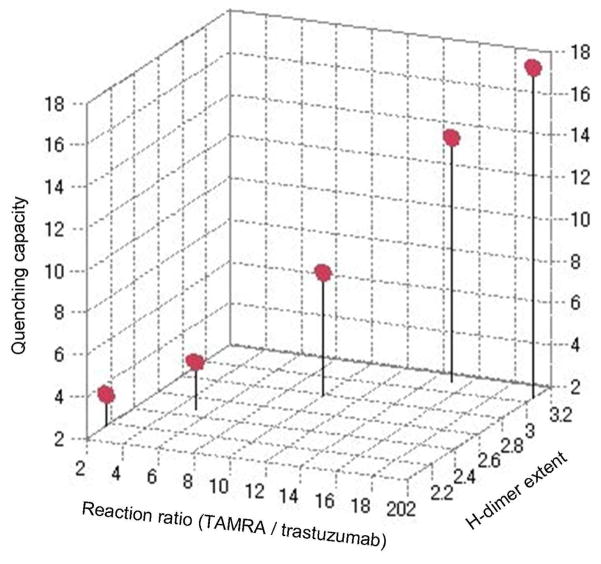
The correlation between the extent of H-dimer formation and fluorescence quenching efficiency is plotted in Fig. 4(a, b) and shows a strong positive relationship in both avidin and trastuzumab conjugates (r=0.86 for avidin, r=0.74 for trastuzumab). The one exception was TexRed conjugated proteins, which showed a lower dimerization rate despite high quenching efficiency. This might be explained by the overlapping involvement of other fluorescence quenching mechanisms such as fluorescence resonance energy transfer (FRET) between fluorophores. To clarify the contribution of the non-absorbance shifted fraction, which represents the monomer form, in fluorescence quenching (mainly FRET), we calculated that fraction based on the difference in absorbance value and fluorescence yield at the monomer wavelength with and without SDS As shown in Table 2, longer emission wavelength fluorophores tend to have a larger proportion of FRET-induced quenching that contributed to increasing the quenching capacity for ROX and TexRed. In addition, there were good relationships between the reaction ratio (TAMRA and trastuzumab), quenching efficiency and H-dimer formation (Fig. 5). This suggests that the higher fluorophore-protein ratios are more likely to form H-dimer; however, too much fluorophore on one targeting protein might lead to a decrease in binding affinity with the targeting molecule.
Figure 4.
Correlation between fluorescence quenching efficiency and H-dimer formation (a, b), and fluorophore lipophilicity (logP) and H-dimer formation (c, d) for various rhodamine-avidin (a, c) and rhodamine-trastuzumab conjugates (b, d). Strong correlation was observed between H-dimer formation and fluorescence quenching. A relationship was also found between lipophilicity and H-dimer formation. This indicates that fluorophore lipophilicity is one of the causes for H-dimer formation on protein molecule.
Table 2.
Extents of FRET contribution in fluorescence quenching
| FRET (%) | Quenching capacity (by FRET)* | |
|---|---|---|
| OregG | < 0 | < 0 |
| Alexa488 | < 0 | < 0 |
| RhodG | 45.8 | 1.8 |
| R6G | < 0 | < 0 |
| TAMRA | 14.5 | 1.1 |
| Alexa568 | 32.9 | 0.6 |
| ROX | 60.9 | 4.4 |
| TexRed | 78.1 | 9.3 |
Data were calculated by (quenching capacity in Table 1) ×(FRET(%))/100.
Figure 5.
Relationships between the reaction ratio, quenching efficiency and H-dimer formation in trastuzumab-TAMRA conjugates are shown. The higher the labeling density per target molecule (called reaction ratio) well correlated to the higher the quenching capacity (called quenching efficiency) and the shorter absorbance shift (H-dimer formation). Good relationships were observed in these three parameters.
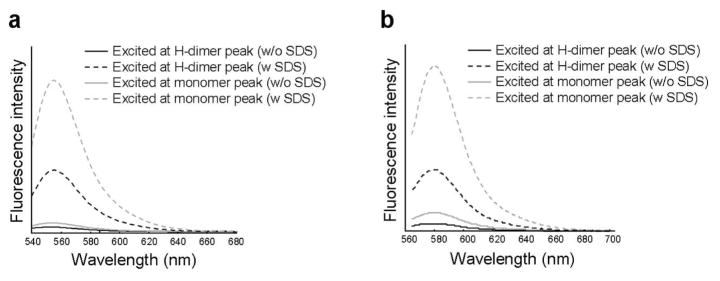
To confirm the effect of H-dimer formation on fluorescence emission, fluorescence intensities were measured both at H-dimer and monomer absorption peaks for Av-R6G and Av-TAMRA. The fluorescence intensity was higher when the conjugates were excited at the monomer peak than when excited at the H-dimer peak whether or not they were treated with SDS (Fig. 6). The intensity ratios (monomer/dimer) were almost the same with or without SDS (ratio=2 for Av-R6G and ratio=3 for Av-TAMRA), that is, fluorescence was proportionally increased when excited at the monomer peak regardless of the degree of H-dimer formation These results support the concept that dimerized fluorophores conjugated to proteins are minimally fluorescent.
Figure 6.
Fluorescence spectra of Av-TAMRA (a) and Av-Alexa488. The conjugates were excited at either the dimer peak (black line) or the monomer peak (gray line) without SDS (solid line) and with SDS (dashed line). The fluorescence intensity was higher with the monomer peak excitation than with the dimer peak excitation with and without SDS. This means there is less H-dimer formation with these conjugates.
Then, the logP values of each fluorophore molecule were measured to investigate the effect of lipophilicity on H-dimer formation (Table 3). The lipophilicity was highest in ROX and lowest in Alexa488. There was a correlation between lipophilicity and the degree of H-dimer formation (Fig. 4(c, d)), r = 0.66 for avidin, r = 0.75 for trastuzumab). This indicates that lipophilicity is one of the causes of H-dimer formation within these probes. Hydrophilicity shows moderate correlation with HOMO values of the free dyes calculated with the B3LYP/6-31G method, which, however, might be altered after conjugation with protein. Therefore, other chemical characteristics (e.g. HOMO/LUMO and charge distribution) in the conjugates might also affect H-dimer formation.
Table 3.
Estimated logP and HOMO/LUMO values of rhodamine fluorophores.
| Lipophilicity (logP) | |
|---|---|
| OregG | 1.34 |
| Alexa488 | −1.44 |
| RhodG | 1.35 |
| R6G | 3.4 |
| TAMRA | 2.92 |
| Alexa568 | 0.39 |
| ROX | 3.88 |
| TexRed | 3.48 |
ND: Not determined because of opposite charges on fluorophores
Furthermore, the interaction between the fluorophores and generic aromatic amino acids in targeting proteins might provide additional quenching effects. Despite very different conformational and chemical characteristics of the two targeting ligands, only slight differences in H-dimer formation were observed between avidin and trastuzumab conjugated with the same fluorophore. This slight difference between avidin and trastuzumab might derive from this fluorophore-protein interaction.
Interestingly, H-dimers were formed at lower fluorophore concentrations than has been reported previously with free fluorophores. The dissociation constant of R6G H-dimer was reported to be 590 μM (14). The fluorophore concentrations after protein conjugation used in this study were 0.22 and 0.10 μM (absorbance), and 0.11 and 0.10 μM (fluorescence) for avidin and trastuzumab, respectively. These results support the hypothesis that the protein molecules can promote and stabilize H-dimer formation for rhodamine fluorophores. The practical implication of these results is that H-dimer formation in molecular imaging probes can behave as “activatable probes” for in vivo imaging because the quenching is stable even after dilution in the body circulation and can be retained until the probes bind to the target, internalize, and are catabolized in the lysosome.
In vitro fluorescent microscope studies in cancer cells
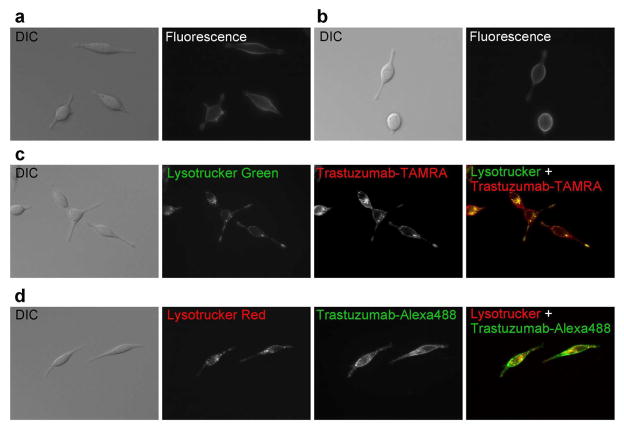
In order to demonstrate the system in cancer cells, in vitro cell experiments were performed using fluorescence microscopy. Both trastuzumab-TAMRA and trastuzumab-Alexa488 showed fluorescent signal on the surface of 3T3/HER2+ cells with 8hr 4°C incubation, trastuzumab-Alexa488 resulting in somewhat brighter signal (Fig. 7(a, b)). Fluorescence was observed inside the cell after 8hrs of incubation at 37°C with both conjugates, and the intracellular fluorescent dots were brighter with trastuzumab-TAMRA (Fig. 7(c, d)). This observation shows the fluorescence activation after the temperature-dependent internalization for trastuzumab-TAMRA, which has higher quenching efficiency. In addition, the fluorescent dots were co-localized to the endo-lysosome marker for both conjugates (Fig. 7(c, d)). These data supported our hypothesis that both trastuzumab-Alexa488 and trastuzumab-TAMRA bound to the cell surface receptor, were then internalized into the cells, and then could be denatured in the endo-lysosomes.
Figure 7.
Fluorescence microscopy as well as differential interference contrast (DIC) images in 3T3/HER2+ tumor cells. Trastuzumab-TAMRA (a) and trastuzumab-Alexa488 (b) showed fluorescent signal on the surface with 8hr incubation at 4°C. Fluorescent dots were detected within the cytoplasm 8hrs after incubation at 37°C with both conjugates (trastuzumab-TAMRA (c), trastuzumab-Alexa488 (d)), and temperature dependent internalization was observed. The fluorescent dots from TAMRA and Alexa488 were matched with lysosomal marker fluorescence.
In vitro receptor blocking studies
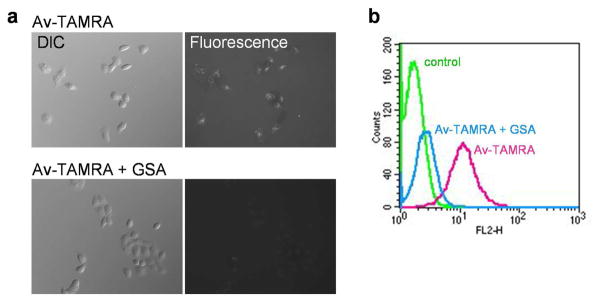
The receptor blocking experiments were performed to investigate whether the binding of Av-TAMRA and subsequent fluorescence activation is receptor dependent. As shown in Figure 8(a), fluorescence accumulation in SHIN3 cells was blocked by GSA treatment. FACS flow cytometry results are shown in Figure 8(b). The mean values were 1.6, 10.9 and 2.6 for control, Av-TAMRA and Av-TAMRA+GSA, respectively. These observations show that the internalization and fluorescence activation of Av-TAMRA is mediated by the D-galactose receptor.
Figure 8.
The results of in vitro blocking studies in SHIN3 cells. Fluorescence microcopy shows decrease in Av-TAMRA accumulation within cells after blocking with the unlabled D-galactose receptor ligand, GSA (a). FACS flow cytometry results are shown in (b). The fluorescence accumulation of Av-TAMRA was blocked by GSA. This suggests that the internalization and subsequent fluorescence activation of Av-TAMRA arises from the D-galactose receptor mediated pathway.
In vivo fluorescence endoscopy and in situ fluorescence spectral imaging of tumor implants
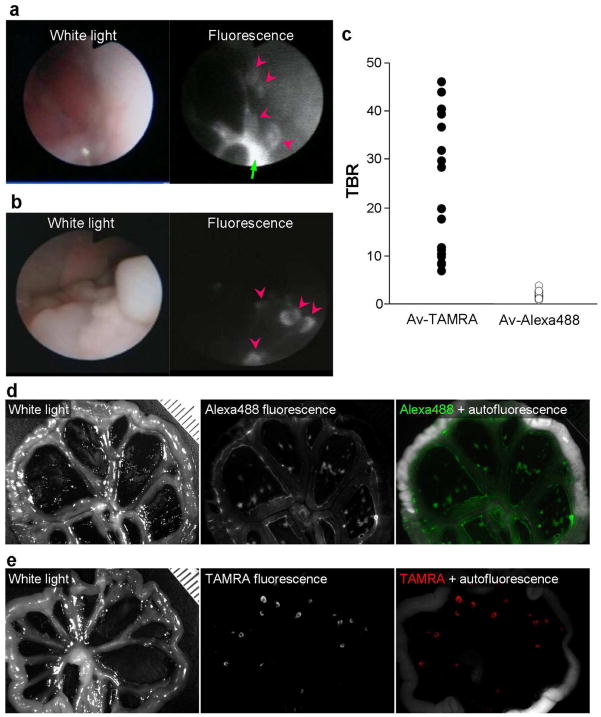
To demonstrate the fluorescence activation effect of H-dimer formed molecular target probes during in vivo molecular imaging of tumor implants, fluorescence endoscopy was performed using the highly quenched probe, Av-TAMRA and the minimally quenched probe, Av-Alexa488 (Fig 9(a, b)). The tumors were visualized with Av-TAMRA, with low background fluorescence indicating dequenching primarily within the targeted tumors. In contrast, the background fluorescent signal was high for Av-Alexa488. The calculated tumor-to-background ratio was 23.3 ± 14.1 and 1.8 ± 0.5 for Av-TAMRA and Av- Alexa488, respectively, a statistically significant difference (P < 0.0001, Mann-Whitney U-test, Fig. 9(c)). To validate the in vivo results, we performed tissue biopsy under fluorescence guided endoscopy with trastuzumab-TAMRA. The sampled tissues (n=50; 25 from fluorescent tissue, 25 from non-fluorescent tissue) were histologically analyzed, and demonstrated perfect sensitivity/specificity for tumor/non-tumor (100/100 %.)
Figure 9.
In vivo fluorescence endoscopic images in tumor bearing mice enhanced by Av-Alexa488 (a) and Av-TAMRA (b). The pink arrow heads show the tumor nodules. The tumors were clearly visualized with the activatable probe, Av-TAMRA. In contrast, Av-Alexa488, an always-on probe, showed high background signal and high fluorescence from excess injectate in the peritoneal cavity (green arrow). The measured tumor-to-background ratio (TBR) was higher for Av-TAMRA than Av-Alexa488 (c). Fluorescence spectral images of the peritoneal membranes for Av-Alexa488 (d) and Av-TAMRA (e). The results were consistent with endoscopic images. Tumors were detected with low background signal for Av-TAMRA, but the background was high for Av-Alexa488.
After the endoscopic imaging, fluorescence spectral imaging was performed. Again, the tumor nodules were clearly imaged by Av-TAMRA with fairly low background signal, in contrast, the background signal was high for Av-Alexa488 (Fig. 9(d, e)). The results corroborated the in vivo endoscopic images. Such high target-to-background fluorescence ratios lead to improved detection of small cancers. Ideally, high target to background ratios are achieved by minimizing signal from non-tumor tissue while maximizing signal from target tumor cells. This can be achieved by switching on the fluorophore only once it is inside the target cell. H-dimer formation results in high tumor-to-background ratios, so that, Av-TAMRA H-dimers depicted tiny cancer nodules clearly with low background signal (Fig. 8(b, e)). In contrast, the non-dimerized, “always on” probe, Av-Alexa488 showed high background fluorescence making detection of all but the largest tumors quite difficult (Fig. 9(a, d)). These observations are consistent with a previous study in which several rhodamine fluorophores were conjugated to a tumor targeting molecule, galactosyl serum albumin, and investigated both in vitro and in vivo (19). Among the rhodamine fluorophores used, TAMRA had best fluorescent properties as an in vivo molecular imaging probe.
Effective activatable optical probes can be designed by focusing on the chemical features of fluorophores which are predisposed to H-dimer formation. Furthermore, the concept of dimerization may be applicable to inter-molecular interactions between different molecules, such as fluorophore-quencher pairs. For instance, TAMRA and QSY7 are an efficient quenching/dequenching pair, which likely suppress emission by a combination of hetero-FRET effects and H-dimer formation (7). Although we successfully synthesized activatable imaging probes with a TAMRA and QSY7 fluorophore-quencher pair, 11 other fluorophore-quencher pairs, which were thought to be appropriate FRET pairs, were found to produce only “always on” or even “always off” imaging probes (20). Thus, there are only a limited number of successful fluorophore-quencher combinations which limit the applicability of this method. Additionally, it is thought that fluorophore-quencher combinations form significant amounts of both hetero and homo H-dimers after conjugating to protein, whereas, the H-dimer mechanism is based on homo dimer formation. We acknowledge that it is difficult to actually determine the amount of hetero dimer formation. In contrast to fluorophore-quencher combinations, most of the H-dimer-forming rhodamine-derivative conjugates, which we tested, were successful as target cell-specific activatable probes with the exception of Tex-Red. Therefore, unlike the fluorophore-quencher strategy, which is quite unpredictable, quenched targeted reagents based on H-dimer formation reliably activated in accordance with the amount of H-dimer present as determined by absorbance. Thus, probes based on H-dimer quenching are likely to be more generalizable than fluorophore-quencher combinations. Since these different quenching mechanisms can operate independently, it may be possible to design probes based on the combinational use of more than one mechanism including PeT, FRET, and H-dimer, to develop even more effective activatable molecular imaging probes.
It should be noted that there are already a number of targeted, activatable small molecule probes. Jiang et al. for instance, synthesized molecular probes that bind to the cell in the presence of specific cleaved enzymes which could be useful for in vivo molecular imaging (21). Hendrickson et al. successfully synthesized the intramoleculary quenched probe to image phospholipase A2 (PLA2) activities in vivo in zebrafish (22,23). They employed BODIPY and a quencher, and combined them with phospholipids substrate analogs. This probe showed better quenching and dequenching (activation) efficacy than our macromolecular probes based on quencher/fluorophore pairs (7). These small molecular imaging probes generally produced specificity by cleavage with a specific enzyme. From a chemistry perspective, these probes can be perfectly designed and synthesized, to amplify the signal from a single enzyme molecule. However, from a biological perspective, there are very few enzymes specifically expressed in cancer cells that are not also expressed in normal cells and tissues. The strategy for targeting cell surface molecules can produce better specificity, however, this can be successfully achieved only with targeted macromolecules including antibodies or receptor ligands. Therefore, although the chemical function or purity in macromolecular probes is not as good as that of small molecular probes, macromolecular probes might have the advantage of biological specificity in molecular imaging probes for cancer (5).
Optical imaging represents a powerful technology especially for cancer research. Currently, in vivo imaging applications include: 1) the study of cancer biology in vivo through expression and imaging of endogenously expressed fluorescent proteins and 2) the development of clinical applications for guiding the diagnosis/treatment of cancer with disease-specific fluorescent probes. Early studies involving imaging of fluorescent proteins revealed the potential of optical imaging in cancer biology, such as for the repeated imaging of cancer invasion and metastasis in longitudinal studies (24). One limitation to the use of fluorescent proteins for cell labeling in humans is that this process necessitates gene transfection, which may present problems for clinical imaging (25). Injectable fluorescent molecules chemically conjugated with organic fluorophores, however, are well-suited to the development of optical probes for clinical applications as they do not require manipulation of the host genome.
In conclusion, we demonstrate that H-dimer formation-derived fluorescence quenching occurs when fluorophores are conjugated to some proteins, which, in the case of molecular imaging probes, may be targeting ligands. After internalization and dissociation of the H-dimer in the target cell, dequenching occurs permitting high target to background imaging. Thus, H-dimer formation is a promising mechanism for activation of target-specific macromolecular optical probes for molecular imaging.
METHODS
Reagents
The NHS esters of Oregon Green (OrgG), Alexa Fluor488 (Alexa488), Rhodamine Green (RhodG), Rhodamine 6G (R6G), TAMRA, Alexa Fluor568 (Alexa568), ROX and Texas Red-X (TexRed) were purchased from Invitrogen Corporation (Carlsbad, CA). Avidin was purchased from Pierce Biochemical, Inc. (Milwaukee, WI). Trastuzumab (Tra), an FDA-approved humanized anti-HER-2 antibody, was purchased from Genentech Inc. (South San Francisco, CA). All other chemicals used were of reagent grade.
Synthesis of fluorophore conjugated avidin and trastuzumab
Two proteins, avidin (targeting D-galactose receptor) and trastuzumab (a humanized monoclonal antibody against the human epithelial growth factor receptor type2 (HER2/neu) were selected as tumor targeting agents.
Avidin (14 nmol) was incubated with NHS esters of OrgG, Alexa488, RhodG, R6G, TAMRA, Alexa568, ROX and TexRed (70 nmol) in 0.1M Na2HPO4 (pH 8.5) at room temperature for 30 min. Each mixture was purified with a Sephadex G50 column (PD-10; GE Healthcare, Piscataway, NJ). For antibody conjugates, trastuzumab (6.5 nmol) was treated with NHS esters (65 nmol) and purified in the same manner as above. The number of fluorophore molecules per trastuzumab was approximately 3.
Measurement of absorption spectrum of the synthesized conjugates
The absorption spectrum was measured with UV-Vis system (8453 Value UV-Visible Value System; Agilent Technologies, Santa Clara, CA) by diluting the resulting solution to 73.6 and 34.3 nM as protein concentrations for avidin and trastuzumab conjugates, respectively. The concentrations, which were calculated based on fluorophores, were 0.22 and 0.10 μM for avidin and trastuzumab conjugates, respectively.
To dissociate the dimerized fluorophores, SDS (5%) was added to the solution, and the absorption spectrum was measured and compared to non-SDS treated solutions at the same concentrations.
Fluorescent intensity measurements
The fluorescence intensity was measured with a fluorescence spectrometer (Perkin-Elmer LS55, Perkin-Elmer, Shelton, CT, USA) both without and with SDS at concentrations of 36.8 and 34.3 nM, for avidin and trastsuzumab conjugates, respectively. The concentrations calculated based on fluorophores were 0.11 and 0.10 μM for avidin and trastuzumab conjugates, respectively. The excitation wavelength used was based on the intrinsic absorption maximum for each fluorophore in its monomeric form.
The non-absorbance shifted fraction, which represents the existence of monomer, (mainly FRET fraction) in fluorescence quenching was estimated by the following equation, where A or B is a fluorescence intensity without or with SDS, at the same monomer absorbance value;
To eliminate the dimer contribution from the monomer absorbance, we used the linear approximation at the overlapping spectra to adjust the base line and obtained the area underneath the curve.
For the H-dimer conjugates, Av-R6G and Av-TAMRA, the fluorescence intensities were also measured at a blue-shifted peak (H-dimer peak) and intrinsic absorption maxima (monomer peak) with and without SDS to investigate the effect of H-dimer formation on fluorescence emission.
Estimation of logP value of each fluorophore molecule
The estimates of the logarithm of partition coefficient in n-octanol/water (log P) were obtained by use of Crippen’s fragmentation method (26).
Fluorescence microscopy studies with antibody conjugates
3T3/HER2+ cells (1 × 104) were plated on a cover glass-bottomed culture well and incubated for 16 h. Then, trastuzumab-TAMRA or trastuzumab-Alexa488 was added to the medium (30 μg/mL), and the cells were incubated for either 8hr on ice or at 37°C. After incubation, cells were washed once with PBS, and fluorescence microscopy was performed using an Olympus BX51 microscope (Olympus America, Inc., Melville, NY) equipped with the following filters: green filter sets were used for TAMRA fluorescence (excitation wavelength 530 to 585 nm, emission wavelength 605 to 680 nm), and blue filter sets were used for Alexa488 fluorescence (excitation 470 to 490 nm, emission wavelength 515 to 550 nm). Transmitted light differential interference contrast images were also acquired. To investigate the intracellular localization of the conjugates, co-localization studies were performed using a lysosomal marker (LysoTracker® Green for trastuzumab-TAMRA, or LysoTracker® RED for trastuzumab-Alexa488, Invitrogen Co., Carlsbad, CA). LysoTracker® was added 30 min prior to imaging. The blue or green filter sets were additionally employed for LysoTracker® Green or LysoTracker® RED, respectively.
In vitro receptor blocking studies
To investigate receptor specificity, in vitro blocking studies were performed. SHIN3 cells (1 × 104) were plated on a cover glass-bottomed culture well and incubated for 16 h. Av- TAMRA was added to the medium (3 μg/mL), and the cells were incubated for 8 hr at 37°C. After incubation, cells were washed once with PBS, and fluorescence microscopy was performed as described above with the green filter sets. For the blocking study, the D-galactose receptor ligand, galactosyl serum albumin (GSA, 30 μg/mL) was added to the medium.
One-color flow cytometry studies were also performed. SHIN3 cells were placed on a 12-chamber well and incubated for 16 hours. Av-TAMRA was added to the medium (10 μg/mL) and cells were incubated for 12 hr at 37°C. GSA (100 μg/mL) was also added to the medium for blocking. After the incubation, the cells were washed with ice-cold PBS and flow cytometry was performed employing 488 nm laser for excitation. Signals from cells were collected using 585/42 nm band-pass filter.
In vivo fluorescence imaging in peritoneal tumor bearing mice
All procedures were carried out in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), National Research Council, and approved by the National Cancer Institute Animal Care and Use Committee. Intraperitoneal (i.p.) tumor implants were introduced by an i.p. injection of 2 × 106 SHIN3 (D-galactose receptor positive ovarian cancer cells) in female nude mice (National Cancer Institute Animal Production Facility, Frederick, MD). The imaging studies were performed 3 weeks after the injection of tumor cells. Alexa488 conjugated avidin (Av-Alexa488) or TAMRA conjugated avidin (Av-TM) was injected into the SHIN3 peritoneal tumor bearing mice (50 μg, i.p., n =3). These probes were tested in live animals after i.p injection of the probes into the abdomen of mice with peritoneal dissemination of ovarian cancer. Intraperitoneal dosing was less likely to be affected by pharmacokinetics than intravenously injected molecular probes. Two hours after the conjugates were injected, the mice were anesthetized with pentobarbital (30 mg/kg i.v., with 0.1 % scopolamine butyl bromide), and fluorescence endoscopy was performed using the Olympus EVIS ExERA-II CLV-180 system (Olympus Corp., Tokyo, Japan). The excitation wavelengths used were 482/35 nm and 543/25 nm for Av-Alexa488 and Av-TAMRA, respectively. The emission filters were 536/40 nm and 597.5/55 nm for Av-Alexa488 and Av-TAMRA, respectively. The fluorescence intensities of tumor and background were measured on the images (n = 20) using Image-J software (National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/) and the tumor to background ratio was calculated. In addition, we performed tissue biopsy from 50 sites with or without fluorescence signal (25 in each) under fluorescence guided endoscopy (as shown in Supplement video C). The tissues were embedded in paraffin and stained with H&E.
After endoscopy, the mice were sacrificed with carbon dioxide and ex vivo imaging of the peritoneal implants was performed on a Maestro In-Vivo Imaging System (CRi, Inc., Woburn, MA). A band-pass excitation filter from 445 to 490 nm and a long-pass emission filter over 515 nm were used for detecting Alexa488 fluorescence, and a band-pass excitation filter from 503 to 555 nm and a long-pass emission filter over 580 nm were used for detecting TAMRA fluorescence. The tunable emission filter was automatically stepped in 10-nm increments from 500 to 800 nm while the camera captured images at each wavelength interval with constant exposure. The spectral fluorescence images consisting of autofluorescence spectra and the spectra from Alexa488 or TAMRA were obtained and then unmixed based on their spectral patterns.
Supplementary Material
Supplemental Figure 1 To confirm that limiting conformational changes of the carrier protein supresses H-dimer dissociation and fluorescent activation, avidin tetramer was cross-linked by disuccinimidyl suberate (DSS; Pierce, Rockford, IL) to minimize the dissociation and the conformational changes of avidin in the lysosome. Absorbance spectrums of Av-TAMRA (a) and cross-linked Av-TAMRA (b) without SDS (solid line) and with SDS (dashed line) conditions indicate that H-dimer dissociation was inhibited by cross-linking of the avidin tetramer. Both quenching capacity and dissociable H-dimer formation were lower in the cross-linked probe. The results of quenching efficiency and H-dimer formation for cross-linked Av-TAMRA were plotted as a red dot on the correlation graph (c). The blue dot shows the results for Av-TAMRA. The slope of correlation line without the red dot is 0.186. This is similar to the slope of the line connecting the red and blue dots, 0.167. These results show that the inhibition of H-dimer dissociation by cross-linking of avidin significantly contributes to the decrease in fluorescence activation, in other words, the fluorescence activation is attributed to the conformational change of the carrier protein. FACS flow cytometry results are shown in (d). SHIN3 cells were incubated with Av- TAMRA (10 μg/mL) or cross-linked to Av-TAMRA (10 μg/mL) for 10hrs at 37°C. The mean values were 1.6, 10.9 and 1.8 for control, Av-TAMRA and cross-linked Av- TAMRA, respectively. The cross-linked Av-TAMRA showed minimum fluorescence, due to a failure of fluorescence activation, which is concordant with the results above.
Supplemental Figure 2 MALDI Mass spectra of Av-TAMRA was performed using a MALDI-TOF-TOF system (Axima Performance, Shimadzu, Columbia, MD), profiles 51-650, obtained with 7.8mV. Similar heights of peaks were obtained with TAMRA conjugated avidin monomer in differing ratios (1:1 and 2:1) peaks (a). In addition, TAMRA conjugated lysine residue (90) was detected by further analysis (b).
Supplemental Videos In vivo real-time endoscopic videos of the SHIN3 tumor visualized with either Av-Alexa488 (A; “always-on”, with minimal H-dimer formation) or Av-TAMRA (B; “quenched/activatable”, with H-dimer formation). Fluorescence-guided endoscopic tissue biopsy was performed with injection of Av-TAMRA (C).
These endoscopic videos show that peritoneal tumor implants, which were originated form the same ovarian cancer SHIN3 cell lines, emit different fluorescence signals from pre-injected Av-Alexa488 (A; “always-on”, with minimal H-dimer formation) and Av- TAMRA (B; “quenched/activatable”, with H-dimer formation) reagents 2 hrs before imaging. The tiny tumors were clearly visualized with the activatable probe, Av-TAMRA with H-dimer formation (see video B). In contrast, Av-Alexa488, an always-on probe with minimal H-dimer formation, showed high background signal and high fluorescence from unabsorbed injected fluid in the peritoneal cavity (see video A).
Left: image without emission filter, right: fluorescence image through an appropriate emission filter. First half: excitation light image specific for Alexa488 (A) and TAMRA (B), Second half: white light image.
In addition, we performed tissue biopsy from 50 places with or without fluorescence signal (25 in each) under fluorescence-guided endoscope (see video C).
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
We thank Prof. Yasuteru Urano, the University of Tokyo, for his kind suggestion and calculation of HOMO values. We also thank to Drs. Masatoshi Takahashi, Brian J. Field, and Masayuki Nishimura in the Shimadzu Scientific Instruments, Inc., Columbia, MD for their great assistance for performing the various mass spectroscopy analyses of the Av- TAMRA conjugate.
Footnotes
Supporting Information Available. Tight cross-linking in vitro and cell experiment for inhibition of the conformational changes of avidin, and MALDI mass spectral analysis data of H-dimer formed within Av-TAMRA. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–33. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–9. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urano Y. Sensitive and selective tumor imaging with novel and highly activatable fluorescence probes. Anal Sci. 2008;24:51–3. doi: 10.2116/analsci.24.51. [DOI] [PubMed] [Google Scholar]
- 4.Kamiya M, Kobayashi H, Hama Y, Koyama Y, Bernardo M, Nagano T, Choyke PL, Urano Y. An enzymatically activated fluorescence probe for targeted tumor imaging. J Am Chem Soc. 2007;129:3918–29. doi: 10.1021/ja067710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, Nagano T, Watanabe T, Hasegawa A, Choyke PL, Kobayashi H. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15:104–9. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hama Y, Urano Y, Koyama Y, Gunn AJ, Choyke PL, Kobayashi H. A self-quenched galactosamine-serum albumin-rhodamineX conjugate: a “smart” fluorescent molecular imaging probe synthesized with clinically applicable material for detecting peritoneal ovarian cancer metastases. Clin Cancer Res. 2007;13:6335–43. doi: 10.1158/1078-0432.CCR-07-1004. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa M, Kosaka N, Longmire MR, Urano Y, Choyke PL, Kobayashi H. Fluorophore-Quencher Based Activatable Targeted Optical Probes for Detecting in Vivo Cancer Metastases. Mol Pharm. 2009;6:386–95. doi: 10.1021/mp800115t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa M, Regino CA, Choyke PL, Kobayashi H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8:232–9. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez Arbeloa I, Ruiz Ojeda P. Dimeric states of Rhodamine B. Chem Phys Lett. 1982;87:556–60. [Google Scholar]
- 10.Kemnitz K, Yoshihara K. Entropy-driven dimerization of xanthene dyes in nonpolar solution and temperature-dependent fluorescence decay of dimers. J Phys Chem. 1991;95:6095–104. [Google Scholar]
- 11.Bergstrom F, Mikhalyov I, Hagglof P, Wortmann R, Ny T, Johansson LB. Dimers of dipyrrometheneboron difluoride (BODIPY) with light spectroscopic applications in chemistry and biology. J Am Chem Soc. 2002;124:196–204. doi: 10.1021/ja010983f. [DOI] [PubMed] [Google Scholar]
- 12.Tleugabulova D, Zhang Z, Brennan JD. Characterization of Bodipy Dimers Formed in a Molecularly Confined Environment. J Phys Chem B. 2002;106:13133–13138. [Google Scholar]
- 13.Valdes-Aguilera O, Neckers DC. Aggregation phenomena in xanthene dyes. Acc Chem Res. 1989;22:171–7. [Google Scholar]
- 14.Selwyn JE, Steinfeld JI. Aggregation of equilibriums of xanthene dyes. J Phys Chem. 1972;76:762–74. [Google Scholar]
- 15.Hama Y, Urano Y, Koyama Y, Kamiya M, Bernardo M, Paik RS, Shin IS, Paik CH, Choyke PL, Kobayashi H. A target cell-specific activatable fluorescence probe for in vivo molecular imaging of cancer based on a self-quenched avidin-rhodamine conjugate. Cancer Res. 2007;67:2791–9. doi: 10.1158/0008-5472.CAN-06-3315. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69:1268–72. doi: 10.1158/0008-5472.CAN-08-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winnik FM. Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem Rev. 1993;93:587–614. [Google Scholar]
- 18.Fujimoto K, Shimizu H, Inouye M. Unambiguous detection of target DNAs by excimer-monomer switching molecular beacons. J Org Chem. 2004;69:3271–5. doi: 10.1021/jo049824f. [DOI] [PubMed] [Google Scholar]
- 19.Longmire MR, Ogawa M, Hama Y, Kosaka N, Regino CA, Choyke PL, Kobayashi H. Determination of optimal rhodamine fluorophore for in vivo optical imaging. Bioconjug Chem. 2008;19:1735–42. doi: 10.1021/bc800140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa M, Kosaka N, Urano Y, Choyke PL, Kobayashi H. Activatable optical imaging probes with various fluorophore-quencher combinations. Progress in Biomedical Optics and Imaging 10 (SPIE Proceedings); 2009. pp. 71900Z-1–71900Z-8. [Google Scholar]
- 21.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101:17867–72. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendrickson HS, Hendrickson EK, Johnson ID, Farber SA. Intramolecularly quenched BODIPY-labeled phospholipid analogs in phospholipase A(2) and platelet-activating factor acetylhydrolase assays and in vivo fluorescence imaging. Anal Biochem. 1999;276:27–35. doi: 10.1006/abio.1999.4280. [DOI] [PubMed] [Google Scholar]
- 23.Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, Mullins MC, Hendrickson HS, Hendrickson EK, Halpern ME. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292:1385–8. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, Teraishi F, Kyo S, Mizuguchi H, Hashimoto Y, Urata Y, Tanaka N, Fujiwara T. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med. 2006;12:1213–9. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 26.Ghose AK, Crippen GM. Atomic physicochemical parameters for three-dimensional-structure-directed quantitative structure-activity relationships. 2. Modeling dispersive and hydrophobic interactions. J Chem Inf Comput Sci. 1987;27:21–35. doi: 10.1021/ci00053a005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 To confirm that limiting conformational changes of the carrier protein supresses H-dimer dissociation and fluorescent activation, avidin tetramer was cross-linked by disuccinimidyl suberate (DSS; Pierce, Rockford, IL) to minimize the dissociation and the conformational changes of avidin in the lysosome. Absorbance spectrums of Av-TAMRA (a) and cross-linked Av-TAMRA (b) without SDS (solid line) and with SDS (dashed line) conditions indicate that H-dimer dissociation was inhibited by cross-linking of the avidin tetramer. Both quenching capacity and dissociable H-dimer formation were lower in the cross-linked probe. The results of quenching efficiency and H-dimer formation for cross-linked Av-TAMRA were plotted as a red dot on the correlation graph (c). The blue dot shows the results for Av-TAMRA. The slope of correlation line without the red dot is 0.186. This is similar to the slope of the line connecting the red and blue dots, 0.167. These results show that the inhibition of H-dimer dissociation by cross-linking of avidin significantly contributes to the decrease in fluorescence activation, in other words, the fluorescence activation is attributed to the conformational change of the carrier protein. FACS flow cytometry results are shown in (d). SHIN3 cells were incubated with Av- TAMRA (10 μg/mL) or cross-linked to Av-TAMRA (10 μg/mL) for 10hrs at 37°C. The mean values were 1.6, 10.9 and 1.8 for control, Av-TAMRA and cross-linked Av- TAMRA, respectively. The cross-linked Av-TAMRA showed minimum fluorescence, due to a failure of fluorescence activation, which is concordant with the results above.
Supplemental Figure 2 MALDI Mass spectra of Av-TAMRA was performed using a MALDI-TOF-TOF system (Axima Performance, Shimadzu, Columbia, MD), profiles 51-650, obtained with 7.8mV. Similar heights of peaks were obtained with TAMRA conjugated avidin monomer in differing ratios (1:1 and 2:1) peaks (a). In addition, TAMRA conjugated lysine residue (90) was detected by further analysis (b).
Supplemental Videos In vivo real-time endoscopic videos of the SHIN3 tumor visualized with either Av-Alexa488 (A; “always-on”, with minimal H-dimer formation) or Av-TAMRA (B; “quenched/activatable”, with H-dimer formation). Fluorescence-guided endoscopic tissue biopsy was performed with injection of Av-TAMRA (C).
These endoscopic videos show that peritoneal tumor implants, which were originated form the same ovarian cancer SHIN3 cell lines, emit different fluorescence signals from pre-injected Av-Alexa488 (A; “always-on”, with minimal H-dimer formation) and Av- TAMRA (B; “quenched/activatable”, with H-dimer formation) reagents 2 hrs before imaging. The tiny tumors were clearly visualized with the activatable probe, Av-TAMRA with H-dimer formation (see video B). In contrast, Av-Alexa488, an always-on probe with minimal H-dimer formation, showed high background signal and high fluorescence from unabsorbed injected fluid in the peritoneal cavity (see video A).
Left: image without emission filter, right: fluorescence image through an appropriate emission filter. First half: excitation light image specific for Alexa488 (A) and TAMRA (B), Second half: white light image.
In addition, we performed tissue biopsy from 50 places with or without fluorescence signal (25 in each) under fluorescence-guided endoscope (see video C).