SUMMARY
Clinical isolates from three microsporidia species, Encephalitozoon intestinalis and Encephalitozoon hellem, and the insect parasite Anncaliia (Brachiola, Nosema) algerae, were used in spore germination and enterocyte-like (C2Bbe1) cell infection assays to determine the effect of a panel of antimicrobial peptides. Spores were incubated with lactoferrin (Lf), lysozyme (Lz), and human beta defensin 2 (HBD2), human alpha defensin 5 (HD5), and human alpha defensin 1 (HNP1), alone and in combination with Lz, prior to germination. Of the Encephalitozoon species only E. hellem spore germination was inhibited by HNP1, while A. algerae spore germination was inhibited by Lf, HBD2, HD5 and HNP1, although HBD2 and HD5 inhibition required the presence of Lz. The effects of HBD2 and HD5 on microsporidia enterocyte infection paralleled their effects on spore germination. Lysozyme alone only inhibited infection with A. algerae, while Lf inhibited infection by E. intestinalis and A. algerae. HNP1 significantly reduced enterocyte infection by all three parasite species and a combination of Lf, Lz and HNP1 caused a further reduced infection with A. algerae. These data suggest that intestinal antimicrobial peptides contribute to the defense of the intestine against infection by luminal microsporidia spores and may partially determine which parasite species infects the intestine.
Keywords: Microsporidia, Encephalitozoon intestinalis, Encephalitozoon hellem, Anncaliia algerae, lactoferrin, lactoferricin B, lysozyme, human beta defensin 2, human alpha defensin 5, human neutrophil peptide 1
INTRODUCTION
Microsporidia are obligate intracellular fungal parasites, approximately 14 species of which infect humans (Didier and Weiss. 2006). The 1,200 species that make up the phylum, Microsporidia, share a common stage, a spore that contains a unique coiled tube used to impale target cells and inject infectious sporoplasm (Keohane and Weiss, 1999). Deployment of the tube is known as germination. Spores may also be phagocytized, with germination taking place within the cell (Couzinet et al. 2000; Franzen, 2004). In the gastrointestinal tract, where the majority of human microsporidia infections occur (Kotler and Orenstein, 1998; Didier and Weiss, 2006), infection of differentiated mucosal epithelial cells most likely results from impalement via spores that germinate in the lumen in close proximity to the target cells (Leitch et al. 2005), and inhibition of this germination process is an attractive target for therapy (Leitch et al, 1993; Keohane and Weiss, 1999).
In humans, environmental spores infect the gastrointestinal tract, nasopharynx and lungs, eyes and skin (Mathis et al. 2005; Didier and Weiss, 2006), all of which are protected by an arsenal of antimicrobial peptides (Hancock and Scott, 2000). While these peptides have been extensively studied for their antimicrobial effects against bacteria, some protozoa, viruses and other fungi, they have not been studied in the microsporidia. The present study employed spores from clinical isolates of three microsporidia species, Encephalitozoon intestinalis, the second most common cause of intestinal microsporidiosis (Kotler and Orenstein, 1998), a second Encephalitozoon species, E. hellem, known to cause ocular and disseminated infections (Didier and Weiss, 2006), and Anncaliia (Brachiola, Nosema) algerae, an insect parasite that has caused several human infections (Coyle et al. 2004; Visvesvara et al. 2005).
A germination assay and a cultured intestinal epithelial cell infection assay were used to determine if antimicrobial peptides that are typically found in the gastrointestinal tract inhibited infection, if any inhibition could be attributed to an inhibition of spore germination, and if antimicrobial peptides account for the fact that only certain microsporidia species cause intestinal microsporidiosis.
MATERIALS AND METHODS
Microsporidia culture and spore isolation
Clinical isolates of Encephalitozoon intestinalis, Encephalitozoon hellem and Anncaliia algerae were used in this study. The E. intestinalis and E. hellem isolates were obtained from American Type Culture Collection (ATCC 50651 and 50451 respectively), while the A. algerae isolate (CDC:V422) was obtained from the Centers for Disease Control and Prevention, Atlanta, GA. The microsporidia cultures were maintained as described elsewhere using green monkey kidney, Vero cells, cultured at 37°C (Visvesvara et al. 1999, Leitch and Ceballos, 2008), except that the A. algerae—infected cultures were maintained at 33°C to improve spore yield and because we found that human isolates of this species cultured at 30°C readily infected mammalian cells maintained at 37°C (Kucerova et al. 2004) Spores were purified as described previously (Leitch et al. 2005) and maintained in water at 4°C until used within 1 week of purification.
Germination assay
Purified spores were pretreated with test solutions for 30 minutes at 37°C. Test solutions contained Lf (human milk), lactoferricin B or Lz (human milk) (Sigma Chemical Co., St. Louis, MO) or recombinant human defensins (Peptides International, Louisville, KY). Peptide concentrations are given in μg/ml, except Lz which is given in thousand units/ml (concentration used, ≥ 30μg protein/ml). This pretreatment was followed by exposure to a germination solution for an additional 30 minutes at 37°C and fixation with 5% neutral formalin. Fixed spores samples were then placed on chambered coverslips, the spores allowed to settle overnight, and the percentage of germinated spores determined by phase contrast microscopy as described previously (Leitch and Ceballos, 2008). Two germination solutions were used, most commonly a 140 mM NaCl solution buffered to pH 7.5 with 20 mM Hepes to which was added H2O2 to a final concentration of 5%. In some A. algerae experiments parallel assays were performed in which a 140 mM NaCl, pH 9.5, glycine buffered solution was used. The high pH germination solution was previously used to germinate Brachiola (Anncaliia) algerae spores (Frixione et al. 1997). However, we found no qualitative difference between the two germination solutions and have used the 5% H2O2 solution whenever we compared germination in A. algerae and the two Encephalitozoon species.
Infection assay
The Caco2 clone, C2Bbel (ATCC CRL-2102), was used in all infection assays as described elsewhere (Leitch and Ceballos, 2008). Briefly, cells were cultured on collagen coated chamber slides and at 7 days post-confluence monolayers were infected with 4 × 105 spores per well of the 8 well-chamber slides. After 24 hours post-infection (PI) excess spores were removed by two washes with Opti-MEM containing 1mg/ml chondroitin sulfate, and the wells refilled with medium. At 3 days PI cells were fixed with paraformaldehyde and stained with calcofluor to visualize parasite sporogonial stages (Didier et al. 1995) and propidium iodide to detect host cell and parasite nuclei. Fluorescent microscopy was used to visualize 2,300 μm2 fields and images captured and analyzed as described previously (Leitch and Ceballos, 2008).
Statistial evaluation
In all germination assays differences between control and experimental samples from the same stock of purified spores were evaluated using paired t tests of a minimum of 4 replicate experiments for each treatment. Results are given as percentage change in germination, negative values indicating inhibition. In infection assays an average of 15 fields were evaluated for the number of infected cells and the significance of differences between means determined by one-way ANOVA of repeated measures using aggregate data sets of a minimum of 4 replicate experiments. Post hoc analyses were performed using Tukey’s tests, and p values of <0.05 were considered significant.
RESULTS
Effects of antimicrobial peptides on microsporidia spore germination
Preliminary spore germination experiments were performed using A. algerae spores stimulated to germinate with the pH 9.5 germination buffer (Frixione et al. 1997). No qualitative differences were found between A. algerae spore germination assays when the high pH germination buffer was used or when 5% H2O2 was added to the pH 7.5 germination buffer. In all experiments where germination was compared between the three microsporidia species the H2O2-containing germination buffer was used.
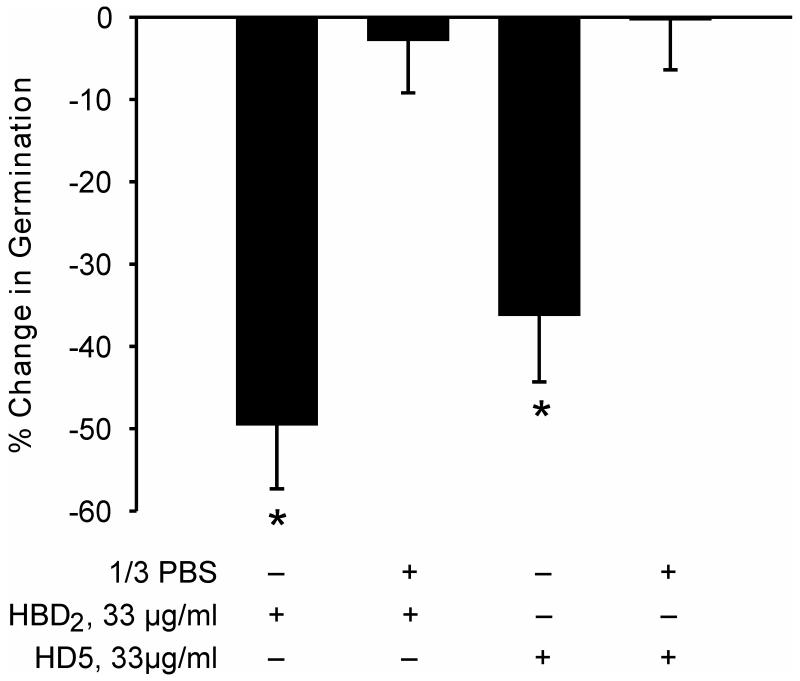
The antimicrobial effects of many peptides are notoriously salt sensitive (e.g. Vylkova et al. 2007). To test whether this was the case with inhibition of microsporidia spore germination a preliminary experiment was undertaken in which A. algerae germination assays were performed with the defensin pretreatment taking place in either water or in 1/3 PBS. Exposure to 33μg/ml HBD2 or HD5 in water significantly inhibited germination, but even 1/3 PBS completely eliminated this inhibition (Figure 1). Because the electrolyte composition of the intestinal lumen is sodium-rich all subsequent germination experiments were performed with the spore defensin exposure occurring in PBS.
Figure 1.
Inhibition of Anncaliia algerae spore germination in response to pH 9.5 germination solution. Spores were pretreated with HBD2 or HD5 in water or in 1/3PBS. Mean and SEM values. * Different from control spore germination, p < 0.05.
Preliminary experiments showed that A. algerae spore germination was significantly inhibited by exposure to Lf. Table 1 summarizes minimum inhibitory concentration (MIC) data obtained when spores of the three microsporidia species under study were exposed to Lf and its pepsin-digestion product, lactoferricin B. This exposure took place in water or in PBS. The A. algerae germination was stimulated by the high pH germination solution or by 5% H2O2 in pH 7.5 germination solution, while the Encephalitozoon species spore germination assays only employed the H2O2 stimulation. The peptide concentration used in these experiments was in the range 2 μg/ml to 2 mg/ml, the higher concentration of Lf being in the range of that reported in tears (Levay and Viljoen, 1995). The A. algerae MIC for Lf was 10 μg/ml using peptide exposure in either water or PBS, while the Encephalitozoon species were unaffected by Lf up to a concentration of 2 mg/ml. With the smaller lactoferricin B the MIC for A. algerae germination was in the range of 5 – 10 μg/ml.
Table 1.
Microsporidia spore germination lactoferrin and lactoferricin B minimum inhibitory concentrations (MIC).
| Lactoferrin | Lactoferricin B | |||
|---|---|---|---|---|
| Water | PBS | Water | PBS | |
| E. intestinalis | NI | NI | NI | NI |
| E. hellem | NI | NI | NI | NI |
| B. algerae (H2O2) | 10μg/ml | 10μg/ml | 5μg/ml | 10μg/ml |
| B. algerae (pH 9.5) | 10μg/ml | 10μg/ml | 5μg/ml | 10μg/ml |
Spores were exposed to agents in water or PBS at concentrations ranging from 2μg/ml to 2 mg/ml prior to exposure to germination solution. NI, no inhibition at the concentration range tested.
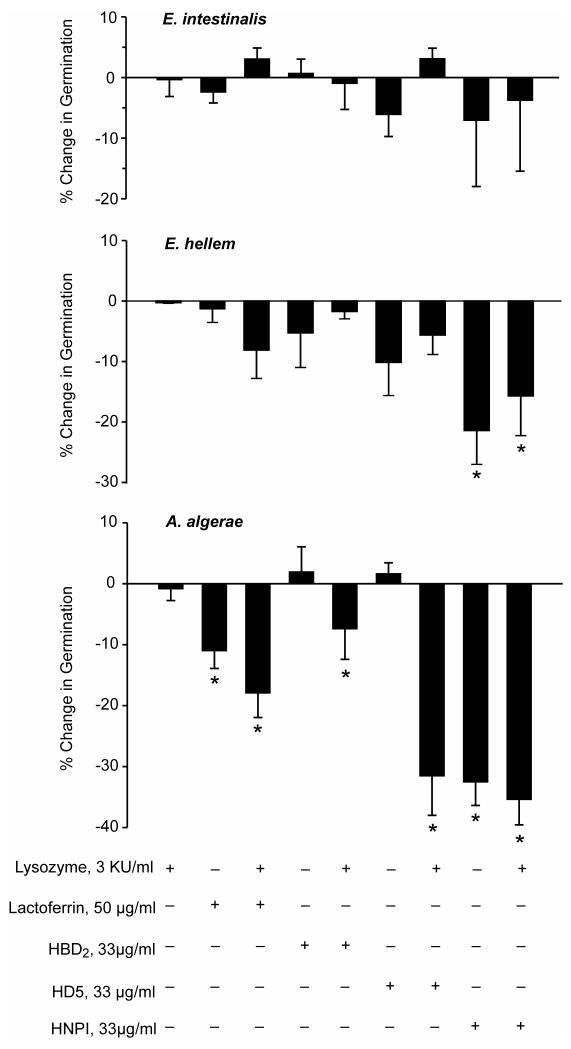
Figure 2 sumarizes data obtained when assessing the effects of Lz, Lf and the three defensins on spore germination. Lysozyme alone had no effect on germination in the three microsporidia species. Because there are reports that Lz has synergistic antimicrobial effects with other peptides (Hancock and Scott, 2000; Chen et al, 2005) experiments were conducted in which the Lf and defensin exposure occurred in the presence and absence of Lz. All peptide exposure took place in PBS and the germination solution used contained H2O2. At the concentrations used, Lf, HBD2, HD5 and HNP1, and these peptides in combination with Lz were without effect on E. intestinalis spore germination, while E. hellem spore germination was significantly inhibited by HNP1, with or without Lz. In the case of A. algerae, HBD2 and HD5 significantly inhibited germination, but only in the presence of Lz, while both Lf and HNP1 significantly inhibited germination in both the presence or absence of Lz.
Figure 2.
Effect of prior exposure of E. intestinalis, E. hellem and A. algerae spores to a panel of antimicrobial peptides in the presence and absence of lysozyme on 5% H2O2-stimulated spore germination. Mean and SEM values. * Different from control spore germination, p < 0.05.
Effect of antimicrobial peptides on microsporidia infection of enterocytes
Figures 3 illustrate the effects of Lz and Lf alone, and HBD2 and HD5 in the presence or absence of Lz, and HNP1 alone and in the presence of both Lz and Lf on the infection of differentiated enterocytes by the three microsporidia species studied. The results of these infection assay experiments are similar but not identical to the germination assay results illustrated in Figure 2. In these infection assays the variance was high because infection tended to be greater in those randomly chosen fields with more differentiated enterocytes that formed villus-like crests. Trends were observed with all three parasite species that suggested that all the antimicrobial peptides used reduced infection. However, as with the germination assay, there were no statistically significant effects of Lz alone or when combined with HBD2 or HD5 on E. intestinalis or E hellem enterocyte infection.
Figure 3.
Effect of antimicrobial peptides on the infection of differentiated C2Bbe1 cells 3 days after exposure to E. intestinalis, E. hellem or A. algerae spores in the presence or absence of antimicrobial peptides, alone or in combination. The peptide concentrations were the same as those in the germination experiment (Figure 2). Mean and SEM values. * Different from control, p < 0.05.
In the case of A. algerae infections HBD2 and HD5 also had no statistically significant effect on infection while, unlike spore germination, Lz alone inhibited infection. Combining either of these two defensins with Lz further reduced infection, but this effect was only statistically significant in the case of HD5. With all three microsporidia species HNP1 alone inhibited enterocyte infection, as did Lf alone. However the Lf inhibition was not statistically significant in the case of E. hellem. Combining HNP1 with both Lf and Lz resulted in a greater decrease in infection than with HNP1 alone. This additional effect was only statistically significant with A. algerae.
DISCUSSION
Multiple microsporidia species can be cultured in mammalian cells (Visvesvara et al, 1999), yet only two species, Enterocytozoon bieneusi and Encepahalitozoon intestinalis, generally infect the human gastrointestinal tract (Didier and Weiss, 2006). Although relatively rare, there have been reports of other microsporidia species being recovered from patient stool samples (e.g. del Aguila et al. 2001; Muller et al. 2001). Franzen and colleagues (2005) concluded that the route of infection was a key factor in determining the target organ(s) in clinical microsporidiosis.
Of the three parasite species used in the present study only E. intestinalis commonly infects the human gastrointestinal tract of immunodeficient individuals. In a previous study we used the same parasite species and found that modeled gastric and duodenal chemical environments did not inhibit either spore germination or infection of C2Bbe1 cells (Leitch and Ceballos, 2008). The turnover time for human intestinal epithelial cells averages about 3 days (Jones and Gore, 1997). The two Encephalitozoon species completed their life cycles well within this time frame. However, the insect microsporidia, A. algerae, required 3 – 5 days to complete its life cycle in C2Bbe1 cells, suggesting that the mammalian body temperature is a limiting factor in the production of a sustained intestinal infection by this parasite species (Leitch and Ceballos, 2008).
Antimicrobial peptides constitute a major part of the innate immune system (Dann and Eckman, 2007) and could potentially explain why only Enterocytozoon bieneusi and E. intestinalis infect the human gastrointestinal tract. Two peptides, Lf and Lz, were chosen that are ubiquitous in the GI tract, and three defensins were chosen to represent those found on the intestinal villus surface (HBD2), in the lumen of the crypt of Lieberkuhn (HD5), and in lamina propria neutrophils (HNP1) (Hancock and Scott, 2000; Cunliffe, 2003; Ouelette,2006).
A total of six antimicrobial peptides were tested for their effect on spore germination and enterocyte infection using concentrations that were chosen based on antimicrobial data in the literature (Ganz et al, 1985; Nagoak et al, 2000; Newman et al, 2000; Samaranayke et al, 2001; Joly et al 2004; Chen et al, 2005; Vylkova et al, 2007). The peptides were the multifunctional Lf, lactoferricin B, HBD2, HD5, HNP1 and Lz. Lactoferrin was employed at a concentration above that generally reported in saliva and duodenum (Abrink et al, 2000; Jentsch et al, 2004), but less than that reported in human or bovine milk, or in tears (Levay and Viljoen, 1995; Bard et al, 2003). The Lz concentration used was also in excess of that of saliva (Rudney and Smith, 1985), but less than that found in human milk (Montagne et al, 1998). It is difficult to know the concentrations of defensins in the mucus unstirred layer of the intestine (Lievin-Le Moal and Servin, 2006), but in the confined volume of the mouse crypt lumen the alpha defensin concentration has been estimated to be as high as 10 mg/ml following Paneth cell stimulation (Ayabe et al, 2000).
The germination assay pointed to significant differences between the three parasite species in the response of spores to the antimicrobial peptides tested. E. intestinalis spore germination was not affected by any of the peptides and E. hellem spore germination was only inhibited by HNP1. Anncaliia algerae spore germination was inhibited by Lf and lactoferricin B at concentrations above 10 μg/ml and by HNP1 when peptide exposure occurred in PBS. The pepsin digest product of Lf, lactoferricin B, lacks the iron binding domain of the parent peptide, suggesting that the N terminal cationic antimicrobial domains were responsible for the observed inhibition (Gifford et al. 2005). Human β defensin 2 and HD5 only inhibited A. algerae spore germination when exposure occurred in water, but could occur in PBS if Lz was present. Lysozyme has been shown to have additive or synergistic antimicrobial effects with other antimicrobial peptides (Chen et al. 2005; Nagoaka et al. 2000), and salt sensitivity is a common feature of the effect of many antimicrobial peptides (Vylkova et al. 2007).
Infection assays were conducted using differentiated C2Bbe1 cells in an assay we believe favored infection via spore germination and target cell impalement (Leitch et al. 2005). All three microsporidia species infected differentiated enterocytes in culture, although the A. algerae infection was only about 10% that of the two Encephalitozoon species (Leitch and Ceballos, 2008).
In both the germination and infection assays A. algerae was clearly more sensitive to the actions of antimicrobial peptides than were either of the Encephalitozoon species. However there were some differences between the effects of these peptides on germination and on infection. Lactoferrin did not inhibit. E. intestinalis spore germination but it significantly inhibited enterocyte infection. The large variance seen with the infection assay may have obscured any Lf effect on E. hellem infection. Given the multifunctional nature of this and other antimicrobial peptides (Levay and Viljoen, 1995; Gifford et al, 2005) it is perhaps not surprising that there might be some limited uncoupling of the antimicrobial effects when comparing a short-term germination assay and a cell infection assay assessed 72 hours after an initial 24 hour spore exposure to peptide. Lactoferrin increases Caco2 cell proliferation (Buccigrossi et al. 2007), which might have contributed to a reduction in the count of infected cells, and the binding of Lf to enterocyte glycosaminoglycans ( El Yazidi-Belkoura et al. 2001) may have interfered with spore adherence to the target cells (Hayman et al. 2005). Additionally, Lz had no effect on A. algerae germination in our standard germination assay, yet it unmasked the salt-sensitive inhibition by HBD2 and HD5, and inhibited infection by this species. It is not uncommon for Lz to lack antimicrobial effects on it own, yet increase the effect of other antimicrobial peptides (e.g. Singh et al. 2000).
The neutrophil defensin, HNP1, alone or in combination with Ly and Lz, two peptides also produced by neutrophils (Peeters and Vantrappen, 1975; Pryzwansky et al. 1979), inhibited enterocyte infection by the three microsporidia species tested. Thus neutrophil peptides could constitute an important line of defense against microsporidia infection in the intestinal lamina propria.
Antimicrobial peptides have multiple ways of killing or disabling microorganisms, in addition to a porin action (Brogden, 2005; Vylkova et al. 2007). We have shown that the inhibition of spore germination is one way in which some antimicrobial peptides can protect the intestine against microsporidia infection by some parasite species. Differences in spore, germination apparatus, and sporoplasm ultrastructure (Vavra and Larsson, 1999; Cali et al. 2002) may underlie some of the species-specific effects we observed in peptide inhibition of germination and infection.
This study suggests that intestinal antimicrobial peptides play a role in determining if an ingested microsporidia spore will infect the intestinal epithelium, with a principal effect of the peptides being on the spore germination process. The greater susceptibility of A. algerae spores to the antimicrobial peptides tested may help account for the fact that this species has not been implicated in intestinal microsporidiosis. However E. intestinalis and E. hellem were similarly affected by the peptides used in this study, suggesting that other peptides, peptide concentrations, or other factors are responsible for the fact that only the former Encephalitozoon species is important in infecting the gastrointestinal tract, particularly in immunocompromised individuals.
ACKNOWLEDGEMENTS
This work was supported by U.S. PHS grant R21 DK64573-A3.
REFERENCES
- Abrink M, Larsson E, Gobl A, Hellman L. Expression of lactoferrin in the kidney: Implications for innate immunity and iron metabolism. Kidney International. 2000;57:2004–2010. doi: 10.1046/j.1523-1755.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nature Immunology. 2000 August;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- Bard E, Laibe S, Bettinger D, Riethmuller D, Buchle S, Seilles E, Meillet D. New sensitive method for the measurement of lysozyme and lactoferrin for assessment of innate mucosal immunity. Part II: time-resolved immunofluorometric assay in serum and mucosal secretions. Clinical Chemistry and Laboratory Medicine. 2003;41:127–133. doi: 10.1515/CCLM.2003.021. [DOI] [PubMed] [Google Scholar]
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature Reviews Microbiology. 2005 March;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Buccigrossi V, de Marco G, Bruzzese E, Ombrato L, Bracale I, Polito G, Guarino A. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatric Research. 2007;61:410–414. doi: 10.1203/pdr.0b013e3180332c8d. [DOI] [PubMed] [Google Scholar]
- Cali A, Weiss LM, Takvorian PM. Brachiola algerae spore membrane systems, their activity during extrusion, and a new structural entity, the multilayered interlaced network, associated with the polar tube and the sporoplasm. Journal of Eukaryotic Microbiology. 2002;49:164–174. doi: 10.1111/j.1550-7408.2002.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. Journal of Dermatological Science. 2005;40:123–132. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli SS. Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infection and Immunity. 2000;68:6939–6945. doi: 10.1128/iai.68.12.6939-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Weiss LM, Rhodes V, Cali A, Takvorian PM, Brown F, Visvesvara GS, Xiao L, Naktin J, Young E, Gareca M, Colasante G, Wittner M. Fatal myositis due to the microsporidian Brachiola algerae, a mosquito pathogen. New England Journal of Medicine. 2004;351:42–47. doi: 10.1056/NEJMoa032655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe RN. Alpha-defensins in the gastrointestinal tract. Molecular Immunology. 2003;40:463–467. doi: 10.1016/s0161-5890(03)00157-3. [DOI] [PubMed] [Google Scholar]
- Dann S,M, Eckmann L. Innate immune defenses in the intestinal tract. Current Opinions in Gastroenterology. 2007;23:115–120. doi: 10.1097/MOG.0b013e32803cadf4. [DOI] [PubMed] [Google Scholar]
- De Leeuw E, Burks SR, Li X, Kao JP, Lu W. Structure-dependent functional properties of human defensin 5. FEBS Letters. 2007;581:515–520. doi: 10.1016/j.febslet.2006.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Aguila C, Moura H, Fenoy S, Navajas R, Lopez-Velez R, Li L, Xiao L, Leitch GJ, da Silva A, Pieniazek NJ, Lal AA, Visvesvara GS. In vitro culture, ultrastructure, antigenic and molecular characterization of Encephalitozoon cuniculi isolated from urine and sputum samples from a Spanish patient with AIDS. Journal of Clinical Microbiology. 2001;39:1105–1108. doi: 10.1128/JCM.39.3.1105-1108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier ES, Weiss LM. Microsporidiosis: current status. Current Opinions in Infectious Diseases. 2006;19:485–492. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA. Comparison of three staining methods for detecting microsporidia in fluids. Journal of Clinical Microbiology. 1995;33:3138–3145. doi: 10.1128/jcm.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yazidi-Belkoura I, Legrand D, Nuijens J, Slomiany M-C, van Berkel P, Spik G. The binding of lactoferrin to glycosaminoglycans on enterocyte-like HT 29-18-C1 cells is mediated through basic residues located on the N terminus. Biochimica et Biophysica Acta. 2001;1568:197–204. doi: 10.1016/s0304-4165(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Franzen C. Microsporidia: how can they invade other cells? Trends in Parasitology. 2004;20:275–279. doi: 10.1016/j.pt.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Franzen C, Hosl M, Salzberger B, Hartmann P. Uptake of Encephalitozoon spp. and Vittaforma corneae (microsporidia) by different cells. Journal of Parasitology. 2005;91:745–749. doi: 10.1645/GE-468R.1. [DOI] [PubMed] [Google Scholar]
- Frixione E, Ruiz L, Cerbon J, Undeen AH. Germination of Nosema algerae (Microspora) spores: conditional inhibition by D2O, ethanol and Hg2+ suggests dependence of water influx upon membrane hydration and specific transmembrane pathways. Journal of Eukaryotic Microbiology. 1997;44:109–116. doi: 10.1111/j.1550-7408.1997.tb05946.x. [DOI] [PubMed] [Google Scholar]
- Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RL. Defensins. Natural peptide antibiotics of human neutrophils. Journal of Clinical Investigation. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford JL, Hunter HN, Vogel HJ. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cellular and Molecular Life Sciences. 2005;62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock REW, Scott MG. The role of antimicrobial peptides in animal defenses. Proceedings of the National Academy of Sciences (USA) 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman JR, Southern R, Nash TE. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infection and Immunity. 2005;73:841–848. doi: 10.1128/IAI.73.2.841-848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch H, Sievert Y, Gocke R. Lactoferrin and other markers from gingival crevicular fluid and saliva before and after periodontal treatment. Journal of Clinical Periodontology. 2004;31:511–514. doi: 10.1111/j.1600-051X.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- Joly S, Maze C, McCray PB, Jr., Guthmiller JM. Human β-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. Journal of Clinical Microbiology. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BA, Gore GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas and intestine. American Journal of Physiology. 1997;273:G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- Keohane EM, Weiss LM. The structure, function, and composition of the microsporidian polar tube. In: Whittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. American Society for Microbiology Press; Washington, D.C.: 1999. pp. 196–224. [Google Scholar]
- Kotler DP, Orenstein JM. Clinical syndromes associated with microsporidiosis. Advances in Parasitology. 1998;40:321–341. doi: 10.1016/s0065-308x(08)60126-8. [DOI] [PubMed] [Google Scholar]
- Kucerova Z, Moura H, Visvesvara GS, Leitch GJ. Differences between Brachiola (Nosema) algerae isolates of human and insect origin when tested using an in vitro spore germination assay and a cultured cell infection assay. Journal of Eukaryotic Microbiology. 2004;51:339–343. doi: 10.1111/j.1550-7408.2004.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Leitch GJ, Visvesvara GS, He Q. Inhibition of microsporidian spore germination. Parasitology Today. 1993;9:422–424. doi: 10.1016/0169-4758(93)90052-h. [DOI] [PubMed] [Google Scholar]
- Leitch GJ, Ward TL, Shaw AP, Newman G. Apical spore phagocytosis is not a significant route of infection of differentiated enterocytes by Encephalitozoon intestinalis. Infection and Immunity. 2005;73:7697–7704. doi: 10.1128/IAI.73.11.7697-7704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch GJ, Ceballos C. Effects of host temperature, and gastric and duodenal environments on microsporidia spore germination and infectivity of intestinal epithelial cells. Parasitology Research. 2008 doi: 10.1007/s00436-008-1156-4. (in press). 10.1007/s00436-008-1156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levay PF, Viljoen MM. Lactoferrin: A general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clinical Microbiology Reviews. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Weber R, Deplazes P. Zoonotic potential of the microsporidia. Clinical Microbiology Reviews. 2005;18:423–445. doi: 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne P, Cuilliere ML, Mole C, Rene MC, Faure G. Microparticle-enhanced nephelometric immunoassay of milk and other human body fluids. Clinical Chemistry. 1998;44:1610–1615. [PubMed] [Google Scholar]
- Muller A, Bialek R, Kamper A, Fatkenheuer G, Salzberger B, Franzen C. Detection of microsporidia in travelers with diarrhea. Journal of Clinical Microbiology. 2001;39:630–1632. doi: 10.1128/JCM.39.4.1630-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka I, Hirota S, Yamogida S, Ohwada A, Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflammation Research. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- Newman SL, Gootee L, Gabay JE, Selsted ME. Identification of constituents of human neutrophil azurophil granules that mediate fungistasis against Histoplasma capsulatum. Infection and Immunity. 2000;68:5668–5672. doi: 10.1128/iai.68.10.5668-5672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette AJ. Paneth cell alpha-defensin synthesis and function. Current Topics in Microbiology and Immunology. 2006;306:1–25. doi: 10.1007/3-540-29916-5_1. [DOI] [PubMed] [Google Scholar]
- Peeters T, Vantrappen G. The Paneth cell: a source of intestinal lysozyme. Gut. 1975;16:553–558. doi: 10.1136/gut.16.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryzwansky KB, Rausch PG, Spitznagel JK, Herion JC. Immunocytochemical distinction between primary and secondary granule formation in developing human neutrophils: correlations with Romanowsky stains. Blood. 1979;53:179–185. [PubMed] [Google Scholar]
- Rudney JD, Smith QT. Relationship between levels of lysozyme, lactoferrin, salivary peroxidase and secretory immunoglobulin A in stimulated parotid saliva. Infection and Immunity. 1985;49:469–475. doi: 10.1128/iai.49.3.469-475.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayke YH, Samaranayake LP, Pow EHN, Beena VT, Yeung KWS. Antifungal effects of lysozyme and lactoferrin against genetically similar, sequential Candida albicans isolates from a human immunodeficiency virus-infected southern Chinese cohort. Journal of Clinical Microbiology. 2001;39:3296–3302. doi: 10.1128/JCM.39.9.3296-3302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Tack BF, McCray PB, Jr., Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. American Journal of Physiology. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- Vavra J, Larsson JIR. Structure of the microsporidia. In: Whittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. American Society for Microbiology Press; Washington, D.C.: 1999. pp. 7–84. [Google Scholar]
- Visvesvara GS, Moura H, Leitch GJ, Schwartz DA. Culture and propagation of microsporidia. In: Whittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. American Society for Microbiology Press; Washington, D.C.: 1999. pp. 363–392. [Google Scholar]
- Visvesvara GS, Moura H, Leitch GJ, Schwartz DA, Xiao LX. Public health importance of Brachiola algerae (Microsporidia) — an emerging pathogen of humans. Folia Parasitologica. (Praha) 2005;52:83–94. doi: 10.14411/fp.2005.011. [DOI] [PubMed] [Google Scholar]
- Vylkova S, Nayyar N, Li W, Edgerton M. Human beta defensins kill Candida albicans in an energy-dependent and salt—sensitive manner without causing membrane disruption. Antimicrobial Agents and Chemotherapy. 2007;51:154–161. doi: 10.1128/AAC.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]