Abstract
Purpose
Gleason grade 4/5 prostate cancer is a determinant for recurrence following radical prostatectomy. Monoamine oxidase-A is over expressed in grade 4/5 compared to grade 3 cancer. Monoamine oxidase-A is also expressed by normal basal cells and in vitro studies suggest that its function is to repress secretory differentiation. Therefore, monoamine oxidase-A in grade 4/5 cancer might reflect dedifferentiation to a basal cell-like phenotype. We investigated whether monoamine oxidase-A expression correlates with another basal cell protein, CD44, in high grade cancer and whether either is associated with an aggressive phenotype.
Materials and Methods
A total of 133 grade 4/5 archival cancers from a cohort previously used to evaluate the prognostic significance of histomorphological variables were scored for monoamine oxidase-A and CD44 immunohistochemical labeling. Spearman rank correlations of the proteins, and histomorphological and clinical variables were determined. The univariate and multivariate value of each variable as a determinant of biochemical recurrence was assessed by logistic regression.
Results
Monoamine oxidase-A expression correlated with CD44. Neither was prognostic for biochemical recurrence. However, monoamine oxidase-A expression positively correlated with preoperative serum prostate specific antigen and the percent of grade 4/5 cancer.
Conclusions
Concurrent expression of monoamine oxidase-A and CD44 suggests that grade 4/5 cancer may be basal cell-like in nature, despite the absence of other classic basal cell biomarkers such as cytokeratins 5 and 14, and p63. The correlation of monoamine oxidase-A expression with prostate specific antigen and the percent of grade 4/5 cancer suggests that monoamine oxidase-A may contribute to growth of high grade cancer and that antidepressant drugs that target monoamine oxidase-A may have applications in treating prostate cancer.
Keywords: prostate; monoamine oxidase; neoplasm recurrence, local; adenocarcinoma; CD44 protein, human
The presence of Gleason grades 4 and/or 5 cancer in primary adenocarcinoma of the prostate is associated with a poor outcome following radical prostatectomy.1 The primary determinant of failure to cure prostate cancer by surgery is the percent of Gleason grade 4/5 present in the largest (index) cancer.2 Individuals in whom cancer is composed entirely of Gleason grade 3 have a greater than 95% chance of being cured by surgery. In contrast, for approximately every 10% increase in the percent of grade 4/5 cancer present at surgery there is a 10% biochemical failure rate, as measured by detectable and increasing serum PSA. Therefore, understanding the biological basis of the aggressive behavior of grade 4/5 cancer is of considerable clinical relevance.
The Gleason grading system is based on tumor microarchitecture and, although grade 4/5 cancer is described as poorly differentiated, the molecular and cellular characteristics of its phenotype are not well-known. A recent study by True et al provided new insight into molecular characteristics that distinguish grade 4/5 cancer from well differentiated grade 3 cancer.3 After collecting cancer cells by laser capture microdissection they performed cDNA microarray analysis to profile gene expression in cancers of different grades. In this study MAO-A, a mitochondrial enzyme that degrades neurotransmitters, including serotonin and norepinephrine,4 was identified as one of the most highly over expressed genes in grade 4/5 vs grade 3 cancer.3
The functions of MAO-A in the nervous system have been extensively studied and inhibitors of its activity have therapeutic value in neurological diseases.4 However, MAO-A actions in other tissues are not as well understood. In studies to reveal the biological role of MAO-A in prostate cancer we observed that MAO-A is also highly expressed in basal but not in secretory cells of the normal prostatic epithelium.5 Furthermore, using a primary cell culture model we noted that MAO-A inhibition suppressed the basal cell phenotype and induced secretory differentiation.5 These observations led us to hypothesize that MAO-A expression by high grade prostate cancer is indicative of dedifferentiation from a secretory to a basal cell phenotype.
Normal secretory and basal epithelial cells, which perhaps include stem and/or progenitor cells, express cell type specific proteins.6 Most prostate cancers are acinar adenocarcinoma and they have many features in common with normal secretory cells.7 This is especially true of well differentiated cancer, as typified by Gleason grade 3. However, although grade 4 or 5 prostate cancer is less differentiated, it is not obviously basal cell-like in nature. In this regard it is unlike breast cancer, which includes an aggressive subtype classified as basal cell carcinoma. Breast cancers of this type have many features in common with normal basal cells of the mammary epithelium. They express cytokeratins 5 and 14, and p63, and lack estrogen receptor, progesterone receptor and HER2. In contrast, high grade prostate cancers rarely, if ever, express the basal cell proteins cytokeratin 5, cytokeratin 14 or p638–10 and they express androgen receptor, a biomarker of differentiated secretory cells. However, certain basal cell associated proteins, such as CD44 and Sox9, are often expressed in high grade prostate cancer.11 Why some basal cell proteins but not others are expressed in high grade cancer is unclear. A possibility is that the basal cell proteins expressed in high grade cancer are those that are also associated with stem or progenitor cells, such as CD44.12
Accordingly we investigated the relationship between the expression of MAO-A and CD44 in high grade cancer. We reasoned that co-expression of the 2 would be suggestive of a basal cell-like and/or progenitor cell-like phenotype of grade 4/5 cancer. The association of MAO-A and CD44 expression with several histomorphological and clinical parameters, including biochemical recurrence, was also evaluated. These analyses were done with Gleason grade 4/5 peripheral zone cancers from 133 archival specimens that were surgically removed by radical prostatectomy at our institution between 1984 and 1992.2 These cancers were selected from a larger set of specimens that had been previously characterized and used to identify morphological variables predicting biochemical recurrence.
MATERIALS AND METHODS
Clinical and Histopathological Characteristics of Patients and Tumor Specimens
The 133 cases selected for study were obtained from a larger set of 379 that was previously used to evaluate prognostic value of histomorphological variables.2 Cases were chosen by excluding all 72 patients with 100% Gleason grade 3 cancer since such men had had almost no incidence of recurrent disease in 5 or more years. An additional 174 cases were excluded from this study because tissue blocks were inaccessible or exhausted. In each case the prostate removed by radical prostatectomy had been subjected to a comprehensive histopathological review by a single pathologist, as previously described.2 The percent of cancer classified as Gleason grade 4 or 5 was determined in each case.
Patient characteristics and histopathological/morphological variables were obtained from an existing database (table 1 and table 2). PSA failure (biochemical failure) was used as the outcome in logistic regression analysis. PSA failure was defined as 2 consecutive PSA values above a cutoff point of 0.07 ng/ml for PSA measured by the Tosoh method (Tosoh Bioscience, Tessenderlo, Belgium) and 0.2 ng/ml for measurements by less sensitive methods. Time to failure on longitudinal analysis was calculated as the number of days between the date of surgery and the date of the first of 2 consecutive PSA tests that exceeded the cutoff point. Median followup was 2,696 days.
TABLE 1.
Quartile distribution of all variables
| Min | Quartile 1 | Median | Mean | Quartile 3 | Max | |
|---|---|---|---|---|---|---|
| Cure (61 pts):* | ||||||
| Age | 35 | 62 | 65 | 64.84 | 70 | 74 |
| PSA (ng/ml) | 2 | 8.4 | 11.3 | 14.3 | 17.6 | 41.7 |
| Index Ca vol (cc) | 0.35 | 1.33 | 2.04 | 3.82 | 5.09 | 24.4 |
| % Gleason grade 4/5 | 2 | 10 | 20 | 28.36 | 40 | 80 |
| Followup (yrs) | 1.99 | 6.36 | 8.64 | 8.19 | 9.91 | 14.59 |
| Prostate wt (gm) | 23 | 37 | 42 | 48.41 | 56 | 115 |
| Total Ca vol (cc) | 0.41 | 1.53 | 3.18 | 4.34 | 5.59 | 24.4 |
| Fail (72 pts): | ||||||
| Age | 44 | 61 | 65 | 64.44 | 69 | 74 |
| PSA (ng/ml) | 3.3 | 11.88 | 18.25 | 30.14 | 34.25 | 266 |
| Index Ca vol (cc) | 0.77 | 3.44 | 5.95 | 8.19 | 10.59 | 45.4 |
| % Gleason grade 4/5 | 5 | 28.75 | 40 | 47.64 | 70 | 95 |
| Yrs to recurrence | 0 | 0.25 | 0.7 | 1.65 | 2.58 | 8.35 |
| Followup (yrs) | 0.43 | 3.57 | 6.18 | 6.39 | 9.29 | 14.34 |
| Prostate wt (gm) | 20 | 35 | 46 | 47.06 | 53.25 | 127 |
| Total Ca vol (cc) | 1.42 | 3.83 | 6.52 | 8.54 | 10.69 | 45.4 |
Recurrence not applicable.
TABLE 2.
Other variables
| Cure (61 pts) | Fail (72 pts) | |||
|---|---|---|---|---|
| No. Pts | Not Available |
No. Pts | Not Available |
|
| Pos lymph nodes | 1 | 0 | 11 | 0 |
| Seminal vesicle invasion | 5 | 1 | 29 | 0 |
| Capsular penetration | 10 | 2 | 18 | 0 |
| Pos surgical margin | 16 | 1 | 28 | 0 |
| MAO-A: | ||||
| Less than 1 | 13 | 0 | 12 | 0 |
| 1 | 14 | 0 | 14 | 0 |
| Greater than 1–less than 2 | 17 | 0 | 21 | 0 |
| 2 | 17 | 0 | 25 | 0 |
| CD44: | 10 | 10 | ||
| Less than 1 | 25 | 27 | ||
| 1 | 8 | 21 | ||
| Greater than 1–less than 2 | 3 | 3 | ||
| 2 | 15 | 11 | ||
Immunohistochemistry
One block containing grade 4/5 cancer was selected from each of the 133 formalin fixed, paraffin embedded radical prostatectomy specimens and tissue sections were cut at 5 µm. Tissues were deparaffinized with xylene and rehydrated with ethanol. Endogenous peroxidase activity was blocked by incubation in methanol containing 0.3% hydrogen peroxide. After pre-incubation with horse serum the tissues were incubated overnight at 4C with antibodies against MAO-A (SC-20156, Santa Cruz Biotechnology, Santa Cruz, California) (1:500) or CD44 (clone F10-44-2, Abcam®) (1:200). Antigen retrieval was applied to tissue sections before labeling with anti-CD44 using universal decloaker (Biocare Medical, Concord, California). Peroxidase substrate (Biocare Medical) was used in combination with secondary biotinylated antibody and peroxidase conjugated streptavidin to visualize labeling. After counterstaining with hematoxylin the sections were mounted and coverslipped. The intensity of cancer cell labeling with each antibody, based on expression in greater than 50% of each cancer, was independently scored by 2 observers according to an arbitrary scale of 0, 1 or 2 in order of increasing intensity. The scores of the 2 observers were averaged to create a composite score for each cancer.
Statistical Methods
Correlations were tested using Spearman’s rank correlation against a permutation null. Logistic regressions were used to test CD44, MAO-A and other variables as predictors of PSA failure. Cox proportional hazards models were used to test these for an association with recurrence time. The log rank test was also applied to dichotomized copies of MAO-A and CD44 at each possible level but none yielded significant findings. Statistical analysis was done using the R statistical computing environment (http://www.R-project.org).
RESULTS
MAO-A and CD44 Expression in Grade 4/5 Cancer
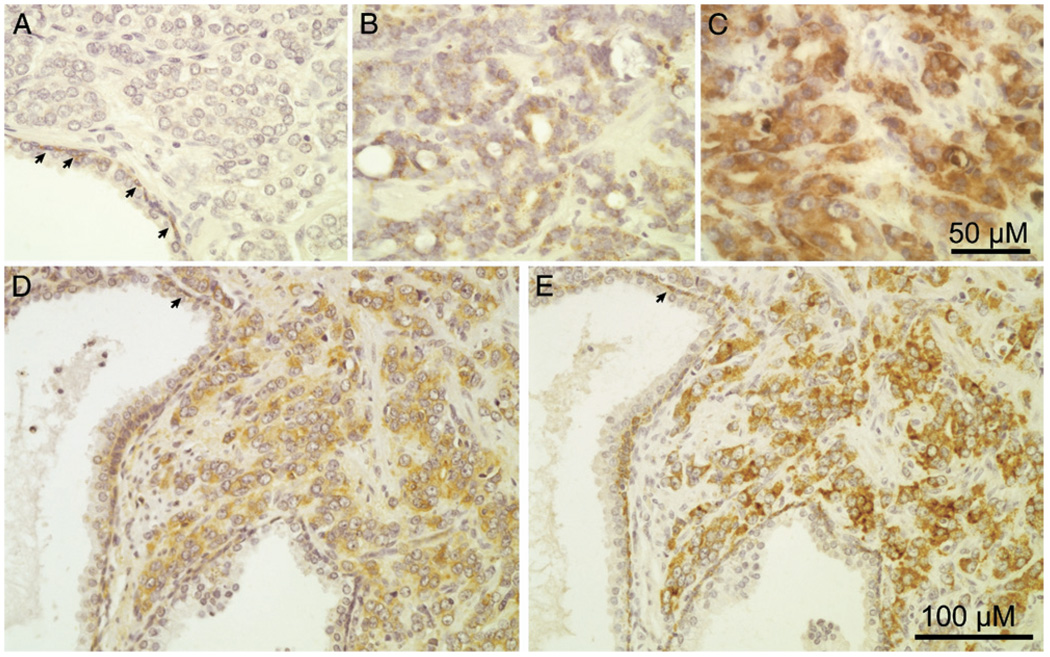
MAO-A expression in whole sections of 133 archival, formalin fixed, Gleason grades 4 and/or 5 cancers was evaluated by immunohistochemistry. Staining intensity in greater than 50% of cancer cells in each section was scored as 0, 1 or 2 in order of increasing intensity by 2 independent observers and scores were averaged to create a composite score. The figure shows representative images of cancers scored as 0, 1 and 2. Of these cancers 39% were scored as 1 or less, 29% were scored as greater than 1 and less than 2, and 32% were scored as 2 (table 2).
Figure 1.
Immunohistochemical staining of grade 4/5 cancer by antibodies against MAO-A or CD44 was independently scored by 2 observers according to arbitrary scale of 0(A), 1 (B) or 2 (C) in order of increasing intensity. Individual cancer stained with intensity of 2 for CD44 (D) and MAO-A (E). Arrows indicate labeling of normal basal cells with antibodies against MAO-A or CD44.
CD44 expression was evaluated in 113 cancers previously stained for MAO-A. The tissue blocks were exhausted in the remaining cases. As for MAO-A, CD44 staining was scored by 2 independent observers according to the intensity of staining in greater than 50% of each cancer on a scale of 0, 1 or 2 and a composite score was determined. Of these cancers 72% were scored as 1 or less, 5% were scored as greater than 1 and less than 2, and 23% were scored as 2 (table 2).
MAO-A and CD44 Expression Correlated in Gleason Grade 4/5 Cancer
Composite scores for MAO-A and CD44 correlated positively (ρ = 0.24, Spearman’s rank correlation p = 0.01), confirming an association between MAO-A and CD44 expression in grade 4/5 cancers (table 3). The figure shows a representative cancer with high MAO-A and CD44 expression.
TABLE 3.
Spearman rank correlations
| Recurrence (yes/no) |
MAO-A | CD44 | Gleason Grade 4/5 |
Prostate Wt |
PSA | Ca Vol | Age | Pos Lymph Nodes |
Seminal Vesicle Invasion |
Capsular Penetration |
Pos Surgical Margin |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | Total | ||||||||||||
| Recurrence (yes/no) | 1.00 | 0.08 | 0.01 | 0.42‡ | 0.01 | 0.33‡ | 0.46‡ | 0.43$ | −0.04 | 0.24‡ | 0.36‡ | 0.10 | 0.13 |
| MAO-A (0–2) | 1.00 | 0.24* | 0.25† | 0.19* | 0.26† | 0.01 | 0.01 | 0.06 | 0.00 | 0.02 | −0.11 | −0.04 | |
| CD44 (0–2) | 1.00 | 0.01 | −0.02 | 0.01 | −0.06 | −0.09 | 0.12 | −0.02 | 0.09 | −0.19 | −0.10 | ||
| Gleason grade 4/5 | 1.00 | 0.04 | 0.26† | 0.17 | 0.15 | 0.05 | 0.20* | 0.21* | 0.06 | 0.04 | |||
| Prostate wt | 1.00 | 0.32‡ | 0.22* | 0.26† | 0.18* | 0.10 | 0.05 | 0.17 | 0.03 | ||||
| PSA | 1.00 | 0.57‡ | 0.58‡ | 0.11 | 0.37‡ | 0.39‡ | 0.20* | 0.27† | |||||
| Ca vol: | |||||||||||||
| Index | 1.00 | 0.98‡ | 0.09 | 0.31‡ | 0.45‡ | 0.37‡ | 0.41‡ | ||||||
| Total | 1.00 | 0.10 | 0.30‡ | 0.45‡ | 0.42‡ | 0.44‡ | |||||||
| Age | 1.00 | −0.03 | 0.18 | 0.11 | −0.02 | ||||||||
| Pos lymph nodes | 1.00 | 0.36‡ | 0.09 | 0.06 | |||||||||
| Seminal vesicle invasion | 1.00 | 0.25‡ | 0.17 | ||||||||||
| Capsular penetration | 1.00 | 0.75‡ | |||||||||||
| Pos surgical margin | 1.00 | ||||||||||||
Statistically significant (p <0.05).
Statistically significant (p <0.01).
Statistically significant (p <0.001).
MAO-A Expression Was Associated With Preoperative Serum PSA and Percent of Grade 4/5 Cancer
By Spearman’s rank correlation CD44 expression did not correlate with any histomorphological or clinical variables except for a marginally significant association with capsular penetration (table 3). However, MAO-A expression positively correlated with preoperative serum PSA and the percent of grade 4/5 cancer (ρ = 0.26 and 0.25, respectively, each p = 0.003, table 3). A correlation between MAO-A and prostate weight was of lesser significance (ρ = 0.19, p = 0.03). These findings show that increased MAO-A expression was associated with characteristics of grade 4/5 cancer that predict clinical behavior. Table 3 also shows correlations between the other variables.
MAO-A Expression Did Not Predict PSA Failure
We then investigated whether the staining intensity of MAO-A or CD44 predicted biochemical (PSA) failure following radical prostatectomy. Of the 133 men in this study 72 experienced failure with a median time to recurrence of 256 days during the median followup of 2,696 days, of whom 45 experienced failure within 2 years after prostatectomy. The latter is considered to be most clinically meaningful because such failures are associated with lower progression-free survival than failures occurring later.13 Neither MAO-A nor CD44 expression significantly correlated with failure within 2 years after prostatectomy by Cox proportional hazards modeling (data not shown). Spearman’s rank correlations showed that neither MAO-A nor CD44 correlated with recurrence (table 3). Univariate logistic regression was used to test each histomorphological and clinical variable as well as MAO-A and CD44 expression as determinants of failure during followup (table 4). As seen previously in the larger set of 379 cases from which the subset used in this study was derived, the percent of grade 4/5 was highly predictive of failure (p < 0.0001).2 Other significant variables were preoperative serum PSA, index cancer volume, total cancer volume and seminal vesicle invasion. The expression of neither MAO-A nor CD44 was a significant determinant of recurrence.
TABLE 4.
Univariate logistic regression as failure determinants
| Variable | Coefficient | SE | t Value | p Value |
|---|---|---|---|---|
| Gleason grade 4/5 | 0.035 | 0.008 | 4.141 | 3.46e–05 |
| Prostate wt | −0.004 | 0.010 | −0.434 | 0.664 |
| Log10 PSA | 2.260 | 0.606 | 3.731 | 1.9e–04 |
| Log10 Ca vol: | ||||
| Index | 2.728 | 0.564 | 4.837 | 1.32e–06 |
| Total | 2.752 | 0.598 | 4.606 | 4.10e–06 |
| Age | −0.009 | 0.027 | −0.341 | 0.733 |
| Pos lymph nodes | 2.381 | 1.060 | 2.247 | 0.025 |
| Seminal vesicle invasion | 2.004 | 0.525 | 3.815 | 1.36e–04 |
| Capsular penetration | 0.491 | 0.441 | 1.113 | 0.266 |
| Pos surgical margin | 0.560 | 0.379 | 1.476 | 0.140 |
| MAO-A (score 1.5 or greater) | 0.340 | 0.356 | 0.955 | 0.339 |
| CD44 (score 0.5 or greater) | 0.618 | 0.408 | 1.514 | 0.130 |
Table 5 lists the results of multivariate logistic regression for all variables, including MAO-A and CD44 expression. As seen previously in the larger set of 379 cases from which the subset used in this study was derived, the percent of Gleason grade 4/5 and index cancer volume were the primary determinants of failure to cure prostate cancer by radical prostatectomy.2 MAO-A and CD44 were insignificant for independently adding to the probability of failure.
TABLE 5.
Multivariate logistic regression of 12 variables
| Log10 Index Ca Vol | Gleason Grade 4/5 | |
|---|---|---|
| Coefficient | 0.485 | 0.006 |
| SE | 0.086 | 0.001 |
| t Value | 5.613 | 4.252 |
| p Value | 1.15e–07 | 4.02e–05 |
Rejecting preoperative serum PSA, capsular penetration, seminal vesicle invasion, positive surgical margin, positive lymph nodes, total cancer volume, patient age, prostate weight, and CD44 and MAO-A expression as insignificant for independently adding to the probability of failure.
DISCUSSION
In our set of 133 Gleason grade 4/5 prostate cancers we found high MAO-A expression, defined as a score of greater than 1 on a scale of 0 to 2, in 60% of cases. This result was similar to that of True et al, who reported that MAO-A protein was highly expressed (score of 2 or 3 by their definition) in about 60% of grade 4/5 cancers in a tissue microarray containing 800 specimens.3 Therefore, increased expression of MAO-A, which is also expressed by normal basal epithelial cells of the prostate, occurs frequently in high grade prostate cancer.
We found that another basal cell associated protein, CD44, was also highly expressed in some grade 4/5 cancers, including 28% with a score of greater than 1. Furthermore, CD44 expression positively correlated with MAO-A expression, suggesting a relationship between these 2 proteins. In studies of primary cultures of cancer cells we found that MAO-A inhibition resulted in down-regulation of CD44 mRNA (unpublished data), suggesting that perhaps MAO-A activity regulates CD44 expression. CD44, a transmembrane glycoprotein that binds hyaluronan and modulates growth factor activity, has attracted a great deal of attention recently because of its association with stem and/or progenitor cells. Purified CD44+ cells from prostate cancer xenografts were found to have greater tumorigenic and metastatic capabilities than CD44−/low cells.12 Based on the association of CD44 with stem/progenitor cells we might speculate that co-expression of CD44 and MAO-A in high grade prostate cancer reflects a progenitor, rather than a basal, cell phenotype.
Establishing the clinical significance of CD44 expression in prostate cancer has been complicated by the existence of multiple splicing variants. In 1 study expression of the variant CD44v6 did not predict biochemical recurrence in patients who underwent radical prostatectomy for clinically localized cancer.14 In another study the variant CD44v3 but not total CD44 predicted PSA failure in a cohort of patients treated with radical prostatectomy in Finland.15 The specific epitope recognized by the anti-CD44 antibody that we used is not specified but we did not find that CD44 expression in our set of grade 4/5 cancers predicted biochemical failure.
MAO-A expression also did not predict recurrence in our study, suggesting that MAO-A is unlikely to be useful as a prognostic marker to distinguish grade 4/5 cancers that progress from those that do not. Despite the strong value of the presence of high grade cancer as a determinant of recurrence, additional biomarkers are needed.16 However, the absence of a predictive value of MAO-A in our study could be due to several limitations, including sample size and the semiquantitative evaluation of expression. The possibility of the predictive value of MAO-A is suggested by the fact that MAO-A expression positively correlated with preoperative levels of serum PSA and the percent of grade 4/5 in the cancers, which are significant risk factors for recurrence.
Relatively little is known about the role of MAO-A in malignancy, and cancer promoting and cancer inhibiting activities have been reported.17 Our finding of an association of increased MAO-A expression with known risk factors for progression suggests that MAO-A has cancer promoting activity in the prostate. Our previous studies of the role of MAO-A in normal prostatic epithelium suggest a biological basis for this association. Using cultured normal prostatic epithelial cells we noted that MAO-A prevented these basal-like cells from differentiating into secretory cells. Under differentiation promoting conditions clorgyline, an irreversible MAO-A inhibitor, induced the structural and functional characteristics of secretory cells.5 If MAO-A has a similar role in high grade cancer, we might propose that dedifferentiation related to MAO-A activity results in the depression of growth regulatory mechanisms, leading to an increase in the amount of grade 4/5 in cancers with high MAO-A expression. Alternatively other properties of MAO-A could lead to the increased growth of high grade cancer. For example, the enzymatic activity of MAO-A produces reactive oxygen species, which are associated with growth promoting activity.18 Regardless of the mechanism responsible for the correlation of MAO-A expression with the percent of grade 4/5, this association suggests that MAO-A is related to the aggressive behavior of prostate cancer at least indirectly by increasing the percent of grade 4/5 cancer, which in turn is a highly significant predictor of the inability to cure prostate cancer by surgery. This finding suggests that drugs that target MAO-A, which have been widely used to treat depression and other neurological disorders, might have a role in preventing or treating high grade prostate cancer.
CONCLUSIONS
The progression of prostate cancer from Gleason grade 3 to 4/5 marks a critical transition from curable to lethal disease. The recent identification of MAO-A as one of the most highly over expressed genes in grade 4/5 compared to grade 3 cancers raises the possibility that the activity of this enzyme is a key factor in the aggressive behavior of high grade cancer. Although MAO-A expression was not predictive of biochemical recurrence in our cohort, it correlated with other powerful prognostic markers, including preoperative serum PSA and the percent of grade 4/5 cancer. These findings suggest that MAO-A may promote the growth of high grade cancer and it may be targeted for therapy by antidepressant drugs that inhibit MAO-A. Furthermore, the concurrent expression of MAO-A and CD44, which are biomarkers of normal prostatic basal epithelial cells, suggests a basal or possibly progenitor cell-like phenotype of high grade cancer.
ACKNOWLEDGMENTS
Dr. John McNeal performed the histopathological review of prostatectomy specimens.
Supported by National Institutes of Health Grants 5 R01 CA121460 and 1 K01 CA123532.
Abbreviations and Acronyms
- MAO-A
monoamine oxidase-A
- PSA
prostate specific antigen
REFERENCES
- 1.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 2.Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 3.True L, Coleman I, Hawley S, Huang CY, Gifford D, Coleman R, et al. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci U S A. 2006;103:10991. doi: 10.1073/pnas.0603678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Nolley R, Chen Z, Reese SW, Peehl DM. Inhibition of monoamine oxidase A promotes secretory differentiation in basal prostatic epithelial cells. Differentiation. 2008;76:820. doi: 10.1111/j.1432-0436.2007.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schalken JA, van Leenders G. Cellular and molecular biology of the prostate: stem cell biology. Urology. 2003;62:11. doi: 10.1016/s0090-4295(03)00758-1. [DOI] [PubMed] [Google Scholar]
- 7.Liu AY, Roudier MP, True LD. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. Am J Pathol. 2004;165:1543. doi: 10.1016/S0002-9440(10)63412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliai BR, Kahane H, Epstein JI. Can basal cells be seen in adenocarcinoma of the prostate? an immunohistochemical study using high molecular weight cytokeratin (clone 34betaE12) antibody. Am J Surg Pathol. 2002;26:1151. doi: 10.1097/00000478-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. Am J Surg Pathol. 2007;31:1246. doi: 10.1097/PAS.0b013e31802f5d33. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Leav I, Ibaragi S, Wegner M, Hu GF, Lu ML, et al. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 2008;68:1625. doi: 10.1158/0008-5472.CAN-07-5915. [DOI] [PubMed] [Google Scholar]
- 12.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 13.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 14.Ekici S, Cerwinka WH, Duncan R, Gomez P, Civantos F, Soloway MS, et al. Comparison of the prognostic potential of hyaluronic acid, hyaluronidase (HYAL-1), CD44v6 and microvessel density for prostate cancer. Int J Cancer. 2004;112:121. doi: 10.1002/ijc.20368. [DOI] [PubMed] [Google Scholar]
- 15.Aaltomaa S, Lipponen P, Viitanen J, Kankkunen JP, Ala-Opas M, Kosma VM. Prognostic value of CD44 standard, variant isoforms 3 and 6 and -catenin expression in local prostate cancer treated by radical prostatectomy. Eur Urol. 2000;38:555. doi: 10.1159/000020355. [DOI] [PubMed] [Google Scholar]
- 16.Carter HB, Isaacs WB. Improved biomarkers for prostate cancer: a definite need. J Natl Cancer Inst. 2004;96:813. doi: 10.1093/jnci/djh174. [DOI] [PubMed] [Google Scholar]
- 17.Toninello A, Pietrangeli P, De Marchi U, Salvi M, Mondovi B. Amine oxidases in apoptosis and cancer. Biochim Biophys Acta. 2006;1765:1. doi: 10.1016/j.bbcan.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]