Abstract
Secretoglobin (SCGB) 3A2 is a downstream target gene for the thyroid transcription factor-1 (TITF1). SCGB3A2 plays a role as an anti-inflammatory agent, however, its role in primary pulmonary carcinomas has not been examined. We assessed immunohistochemical expression of SCGB3A2 in primary pulmonary carcinomas and evaluated the correlation between the expression and histopathological phenotypes and prognosis. One hundred and fifty-six primary lung cancers undergone for surgical resection were examined. The percentages of SCGB3A2 positive cells were scored and tumors had immunoreactivity in more than 10% of tumor cells were considered positive for SCGB3A2. Overall reactivity for SCGB3A2 was observed in 116 (74.4%) of 156 primary lung cancers. SCGB3A2 was predominantly expressed in adenocarcinomas (86.5%), compared with squamous cell carcinomas (50.0%) and small cell carcinomas (42.9%). The expression in papillary adenocarcinomas was seen at higher frequency than that in tubular adenocarcinomas. There was no significant relationship between SCGB3A2 expression and tumor differentiation, and pathological stage. Positive expression of SCGB3A2 was not associated with better survival rate. SCGB3A2 expression in primary pulmonary carcinomas is high, especially in adenocarcinomas. Our results indicate that SCGB3A2 has a potential to be a specific and useful marker for primary pulmonary adenocarcinomas.
Keywords: Secretoglobin 3A2 (SCGB3A2), UGRP1 (Uteroglobin-related protein 1), Pulmonary carcinoma, Immunohistochemistry, TITF-1 (thyroid transcription factor-1)
INTRODUCTION
Secretoglobin (SCGB) 3A2, also called uteroglobin-related protein 1 (UGRP1), was originally identified as a downstream target gene for the homeodomain transcription factor, also known as T/EBP (thyroid-specific enhancer-binding protein), thyroid transcription factor (TITF1) or NKX2.11). TITF1 regulates the expression of thyroid- and lung-specific genes such as those encoding surfactant proteins -A, -B, and -C, and Clara cell secretory protein (CCSP, also referred to as uteroglobin, Clara cell specific 10-kDa protein (CC10), secretoglobin 1A1 (SCGB1A1))1–5). Targeted disruption of TITF1 gene in mice results in immediate postnatal death due to respiratory failure caused by severely hypoplastic lungs, in addition to a deficiency of the thyroid and pituitary glands, demonstrating that TITF1 plays an essential role in lung development6). SCGB3A2 mRNA is detected in the lungs of mouse embryos as early as embryonic day 12.5 right after the onset of TITF1 expression1). In murine lung, SCGB3A2 is expressed mainly in the airway epithelial cells and is considered as an early molecular marker for Clara cells7). Human SCGB3A2 is expressed throughout the epithelium of the conducting airways and submucosal glands of the neonatal lung in a pattern similar to human SCGB1A1 (CCSP)7).
SCGB3A2 is a member of the SCGB gene superfamily. Based on amino acid sequence similarity between SCGB3A2 and SCGB1A1, the prototypical member of the family, particularly in the antiflammin domain that is responsible for anti-inflammatory and immunomodulatory activities of SCGB1A1, it was suggested that SCGB3A2 may possess anti-inflammatory function1,8). Several studies suggested that SCGB3A2 has an association with bronchial asthma1,9–12) and plays a role in regulating immune response in the lung13,14). A recent report demonstrated that SCGB3A2 suppresses lung inflammation when recombinant adenovirus expressing SCGB3A2 was administered to a mouse model for allergic airway inflammation15). However, little is known about the functional role of SCGB3A2, especially in the area of malignant neoplasm.
Recently, the role of other secretoglobin proteins, namely SCGB3A1 and SCGB1A1 in carcinogenesis has been extensively studied. SCGB3A1, also called High in normal-1 (HIN-1) or UGRP216), was identified as a tumor suppressor gene; its expression is silenced by methylation in the majority of breast carcinomas while it is highly expressed in normal breast epithelium17–19). Further, SCGB1A1 might be a novel cytokine that functions as a tumor suppressor20,21). SCGB1A1 antagonizes neoplastic phenotype, thus suggesting that down-regulation of SCGB1A1 may contribute to carcinogenesis22,23).
Moreover, TITF1 has recently been used as a diagnostic marker for primary lung cancer, especially for identification of pulmonary adenocarcinomas24–30). A meta-analysis showed that TITF1 expression correlates with a better patient outcome31). Since SCGB3A2 is a downstream target of TITF1 and is highly enriched in lung, it is attractive to examine the expression and functional role of SCGB3A2 in primary pulmonary carcinomas. The aim of this present study is 1) to examine the prevalence of SCGB3A2 expression in primary pulmonary carcinomas, 2) to evaluate its relation with clinico-pathological parameters, and 3) to assess its role as a prognostic marker for primary pulmonary carcinomas.
MATERIALS AND METHODS
Patients
Surgical specimens from 156 primary lung cancers were analyzed for this study. All patients were operated at Fukushima Medical University Hospital from 1991 to 2000. They consisted of 100 males and 56 females (mean age of 64.0 years). The histology of the disease was determined based on hematoxylin and eosin-stained preparations according to the criteria of the World Health Organization. The results revealed 96 adenocarcinomas (Ad), 44 squamous cell carcinomas (Sq), 9 large cell carcinomas (LC) and 7 small cell carcinomas (SCLC). It was determined that 69 patients were in stage I, 18 were in stage II, 63 were in stage III, and 3 were in stage IV. The post-operative stages of 3 patients were not recorded. This study was approved by the ethical committee of our institute. Written informed consent was obtained from all the patients.
Production of human SCGB3A2 polyclonal antibody
A cDNA sequence encoding the mature human SCGB3A2 protein (excluding signal sequence) was prepared by PCR and subcloned into bacterial expression vector pET-32a(+) (EMD Biosciences-Novagen, San Diego, CA), placing the SCGB3A2 sequence in frame downstream of a hexahistidine tag. The tagged SCGB3A2 protein was expressed in bacteria BL21 (DE3) by incubation with 1 mM isopropylthio-β-galactoside for 5 hours. Cells were collected and lysed in native conditions, and tagged protein was purified on a nickel-nitrilotriacetic acid agarose column followed by SDS-PAGE. The purified protein was used to prepare SCGB3A2 antibody in rabbits (Macromolecular Resources, Fort Collins, CO).
In order to determine the specificity of anti-SCGB3A2 antibody, SCGB3A2 as well as SCGB3A1 and SCGB1A1 proteins were expressed in COS-1 cells using pEGFP expression plasmid (see the section of Western blot analysis below). Whole cell lysates were subjected to western blotting using antibodies against GFP and SCGB3A2 (Fig. 1). SCGB3A1 and SCGB1A1 were examined because they share amino acid sequence similarity to SCGB3A2 and the site of their expression partially overlaps with SCGB3A21,16). Western blot results revealed that anti-SCGB3A2 antibody only detected SCGB3A2 protein, but not SCGB3A1 and SCGB1A1, demonstrating the specificity of the antibody. This antibody was used by immunohistochemistry to examine the expression of SCGB3A2 in the present study.
Fig. 1. Characteristics of anti-SCGB3A2 antibody.
Western blotting using antibodies against GFP and SCGB3A2. Western blot results revealed that anti- SCGB3A2 antibody only detected SCGB3A2 protein.
Western blot analysis
Complementary DNAs encoding human SCGB3A1, SCGB3A2 and SCGB1A1 except those corresponding to signal peptide were amplified and inserted into Eco RI and Sal I sites of pEGFP-C vector (Clontech, Mountain View, CA). Transient transfection into COS-1 cells was carried out using Effectene Transfection Reagent (Qiagen, Valencia, CA). After 2 days, cells were collected, and cell lysates were subjected to 13% SDS-polyacrylamide gels (PAGE) under reducing condition, followed by transfer to Hybond-ECL nitrocellulose membrane (GE Healthcare Bio-Sciences, Piscataway, NJ). The filter was incubated in PBS containing 5% skim milk and then for 1 hour with anti-GFP antibody (sc-8334; Santa Cruz) or anti-human SCGB3A2 antibody. The filter was washed in PBS containing 0.1% Tween 20, incubated with horseradish peroxidase-conjugated anti-rabbit IgG (GE Healthcare Bio-Sciences). After washing with the same buffer, protein bands were detected using ECL Plus Western Blotting Detection Reagents (GE Healthcare Bio-Sciences).
Immunohistochemistry and Evaluation
Immunohistochemical staining for SCGB3A2 was performed on 4 µm paraffin embedded sections by the avidin-biotin-peroxidase complex method. Distribution of peroxidase was revealed by incubating the sections in a solution containing 3,3′-diaminobenzidine tetrahydrochloride (DAB). Hematoxylin staining was applied as a counter stain. Cases were evaluated independently by two observers. In each case, at least 200 tumor cells were quantified by microscopy using a × 40 magnification objective, and a score (range, 0–100) for a percentage of positive cells expressing SCGB3A2 was obtained. Subsequently, we semi-quantified the degree of SCGB3A2 staining into 3 categories according to the following criteria, negative (−)=0–9% of tumor cells are positive for SCGB3A2; weakly positive (+)=10–59% of tumor cells are positive for SCGB3A2; and strongly positive (2+)=60–100% of tumor cells are positive for SCGB3A2. Both weakly and strongly positive cases were considered positive for SCGB3A2.
Statistical Analysis
Statistical analysis was performed using the SPSS software system (SPSS for Windows, 11.0J). The χ2 test was used to analyze correlations between categorical clinico-pathological variables. To analyze the relationship between SCGB3A2 positive score and clinico-pathological characteristics, one-way analysis of variance (ANOVA) was used. Survival curves were calculated using Kaplan-Meier method, and the log-rank test was used to assess a statistical significance of differences between groups. Data are presented as the mean±SD. P values less than 0.05 was regarded as significant.
RESULTS
In normal human lung, SCGB3A2 expression was immunohistochemically found in the cytoplasm of epithelial cells of the bronchioles, alveoli, and serous gland (Fig. 2). In 156 specimens of primary pulmonary carcinomas, the expression of SCGB3A2 was examined (Fig. 3). Positive reactivity for SCGB3A2 was clearly observed in 116 of 156 (74.4%) carcinomas (Table 1). Among them, fifty-three patients (34.0%) strongly expressed SCGB3A2.
Fig. 2. Immunohistochemical staining of SCGB3A2. (in normal human lung).
From the upper side a) epithelial cells of the bronchioles; b) type II epithelial cells of alveoli; and c) serous gland. a), c) Magnification; ×100. b) Magnification; ×400.
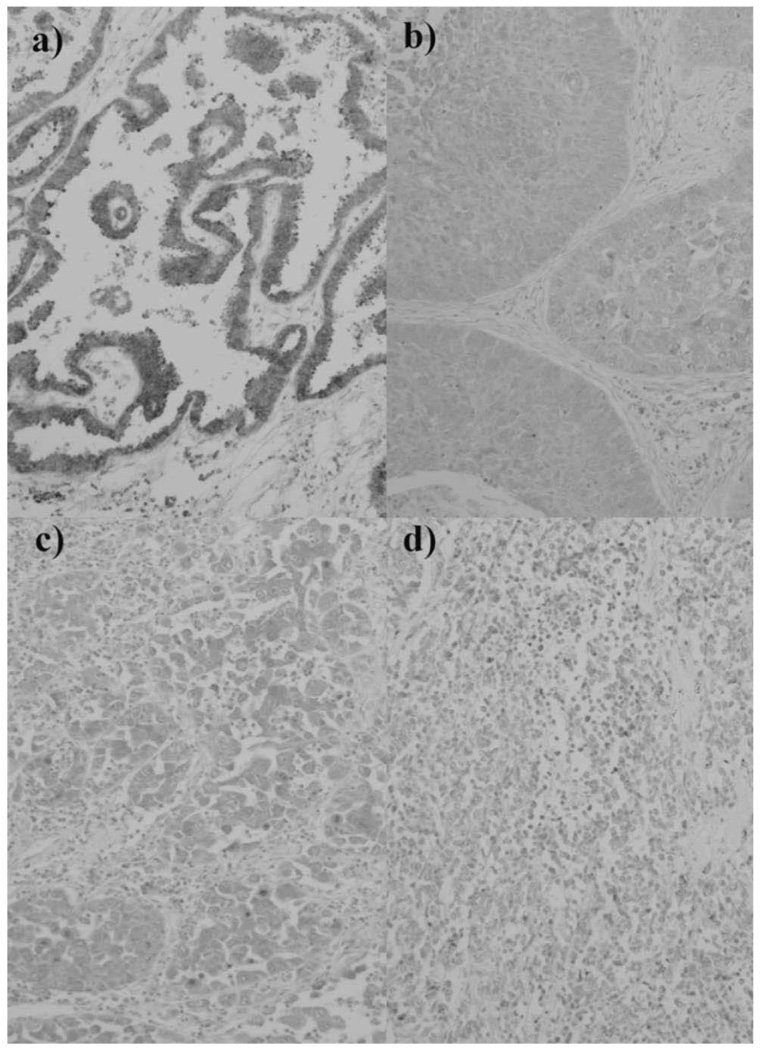
Fig. 3. Immunohistochemical staining of SCGB3A2 in human lung carcinoma.
From the left side a) adenocarcinomas (Ads); b) squamous cell carcinomas (Sqs); c) Large cell carcinomas (LCs); and d) small cell carcinomas (SCLCs). Magnification; ×100.
Table 1.
Association of SCGB3A2 With Clinicopathological Variables
| Variables | Negative (%) | Positive (%) |
Total | P value | |||
|---|---|---|---|---|---|---|---|
| (−) | (+) | (2+) | total (+, 2+) | ||||
| Age | < Median | 27 (28.7) | 39 (41.5) | 28 (29.8) | 67 (71.3) | 94 | 0.340 |
| ≤ Median | 13 (21.0) | 24 (38.7) | 25 (40.3) | 49 (79.0) | 62 | ||
| Sex | Male | 31 (31.0) | 39 (39.0) | 30 (30.0) | 69 (69.0) | 100 | 0.100 |
| Female | 9 (16.1) | 24 (42.9) | 23 (41.1) | 47 (83.9) | 56 | ||
| Histology* | Ad | 13 (13.5) | 40 (41.7) | 43 (44.8) | 83 (86.5) | 96 | <0.001 |
| Sq | 22 (50.0) | 18 (40.9) | 4 (9.1) | 22 (50.0) | 44 | ||
| LC | 1 (11.1) | 3 (33.3) | 5 (55.6) | 8 (88.9) | 9 | ||
| SCLC | 4 (57.1) | 2 (28.6) | 1 (14.3) | 3 (42.9) | 7 | ||
| Differentiation† | Well | 7 (12.5) | 21 (37.5) | 28 (50.0) | 49 (87.5) | 56 | 0.720 |
| Moderately | 5 (14.7) | 17 (50.0) | 12 (35.3) | 29 (85.3) | 34 | ||
| Poor | 1 (16.7) | 2 (33.3) | 3 (50.0) | 5 (83.3) | 6 | ||
| Subtype‡ | Pap | 6 (9.1) | 26 (39.4) | 34 (51.5) | 60 (90.9) | 66 | 0.040 |
| Tub | 5 (38.5) | 4 (30.8) | 4 (30.8) | 8 (61.5) | 13 | ||
| BAC | 2 (12.5) | 9 (56.3) | 5 (31.3) | 14 (87.5) | 16 | ||
| Morphology§ | Clara + typeII | 4 (9.5) | 17 (40.5) | 21 (50.0) | 38 (90.5) | 42 | 0.006 |
| Ciliated | 2 (5.3) | 17 (44.7) | 19 (50.0) | 36 (94.7) | 38 | ||
| Goblet | 3 (60.0) | 1 (20.0) | 1 (20.0) | 2 (40.0) | 5 | ||
| Bronchial gland | 4 (36.4) | 5 (45.5) | 2 (18.2) | 7 (63.6) | 11 | ||
| Stage | I, II | 18 (20.7) | 40 (46.0) | 29 (33.3) | 69 (79.3) | 87 | 0.178 |
| III, IV | 22 (33.3) | 23 (34.8) | 21 (31.8) | 44 (66.7) | 66 | ||
| Total | 40 (25.6) | 63 (40.4) | 53 (34.0) | 116 (74.4) | 156 | ||
Ad, adenocarcinoma; Sq, squamous cell carcinoma; LC, large cell carcinoma; SCLC, small cell carcinoma.
Only adenocarcinomas were included.
Only adenocarcinomas were included. Pap, papillary adenocarcinoma; Tub, tubular adenocarcinoma; BAC, bronchiolo-alveolar carcinoma
Only adenocarcinomas were included.
Clara + type II, Clara cell/ type II alveolar epithelial cell type; Ciliated, ciliated epithelial cell type; Goblet, goblet cell type; Bronchial gland, bronchial gland cell type.
Based on histological criteria, SCGB3A2 expression was found in 86.5% of adenocarcinomas, 50.0% of squamous cell carcinomas, 88.9% of large cell carcinomas, and 42.9% of small cell carcinomas. The SCGB3A2 positive score was significantly higher in adenocarcinomas than squamous cell carcinomas (P < 0.001) and small cell carcinomas (P < 0.005) (Fig. 4).
Fig. 4. SCGB3A2 Expression According to Histology.
SCGB3A2 positive scores according to histological classification. The SCGB3A2 positive score in adenocarcinomas was significantly higher than that in squamous cell carcinomas and small cell carcinomas.
In adenocarcinomas, the relationships between SCGB3A2 expression and tumor differentiation and pathological subtypes were analyzed. There was no association between SCGB3A2 expression and tumor differentiation, while the expression in papillary adenocarcinomas was found at higher frequency than that in tubular adenocarcinomas.
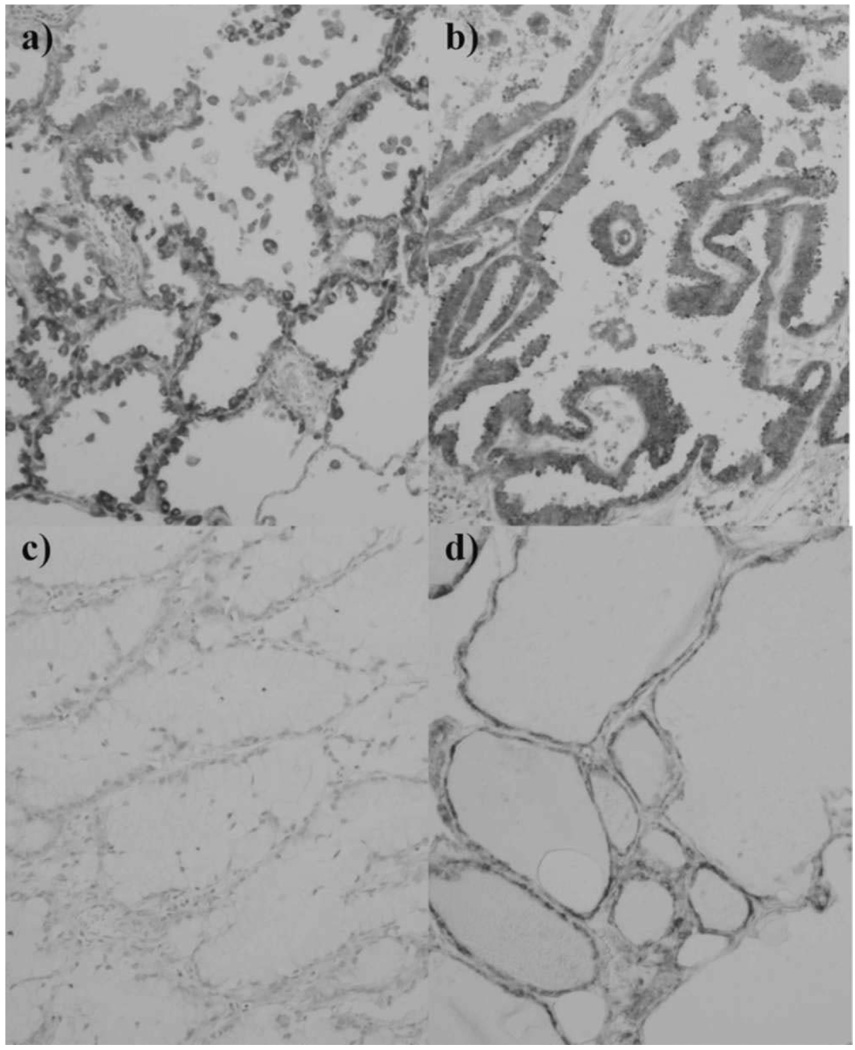
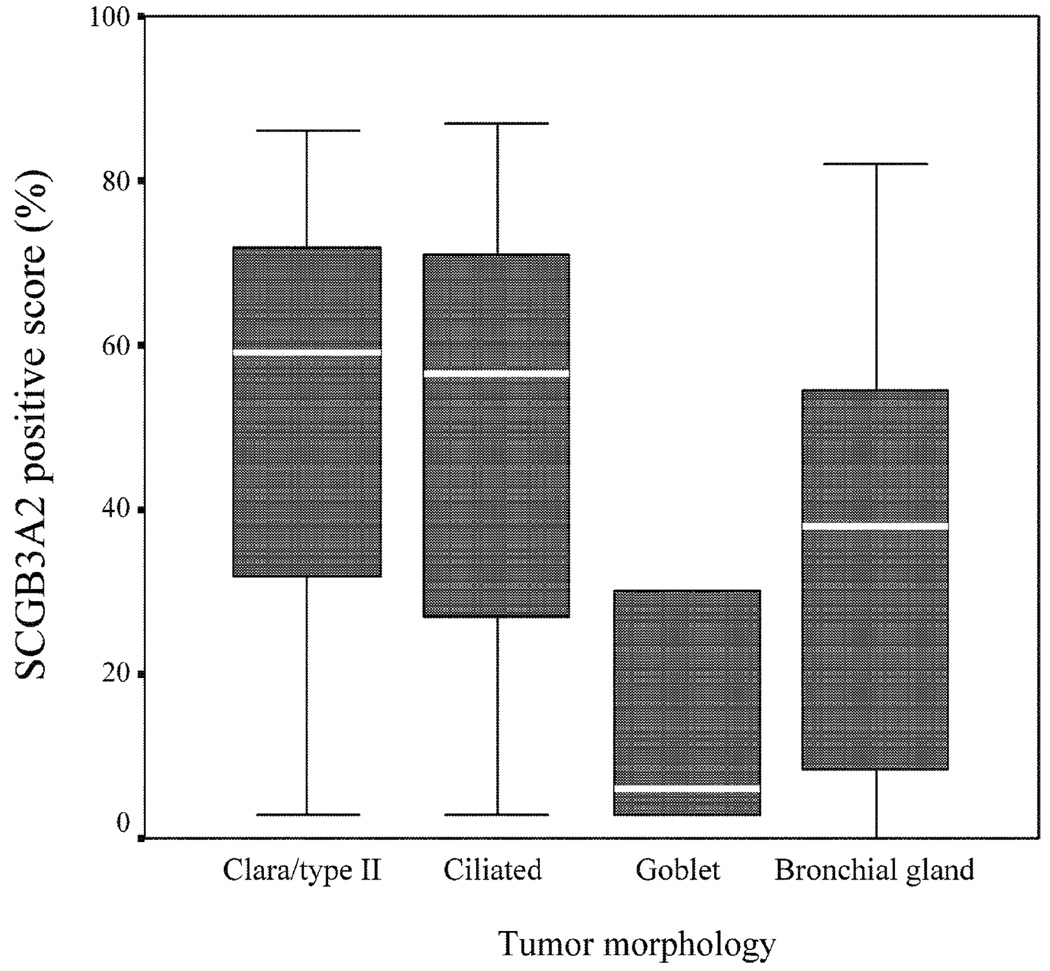
Moreover, we classified adenocarcinomas into 4 categories according to tumor cellular morphology: 1) Clara cell/type II alveolar epithelial cell type (42 cases), 2) ciliated epithelial cell type (38 cases), 3) goblet cell type (5 cases), and 4) bronchial gland cell type (11 cases) (Fig. 5). There was a significant difference in SCGB3A2 expression depending on tumor cellular morphology. The expression of SCGB3A2 tended to be higher in Clara cell/type II alveolar epithelial cell type and ciliated epithelial cell type than goblet cell type (Fig. 6).
Fig. 5. SCGB3A2 expression in adenocarcinomas.
Tumor cellular morphology of adenocarcinomas is classified as follows: a) Clara cell/type II alveolar epithelial cell type; b) ciliated epithelial cell type, c) goblet cell type; and d) bronchial gland cell type. Magnification; ×100.
Fig. 6. Tumor Morphology and SCGB3A2 Expression.
SCGB3A2 positive scores according to tumor morphology. The expression of SCGB3A2 is higher in Clara cell/ type II alveolar epithelial cell type and ciliated epithelial cell type than that of goblet cell type (P=0.1).
On the other hand, no significant correlation was found between SCGB3A2 expression and pathological stage: it was detected in 79.7% of stage I patients, 77.8% of stage II patients, 68.3% of stage III patients, and 33.3% of stage IV patients.
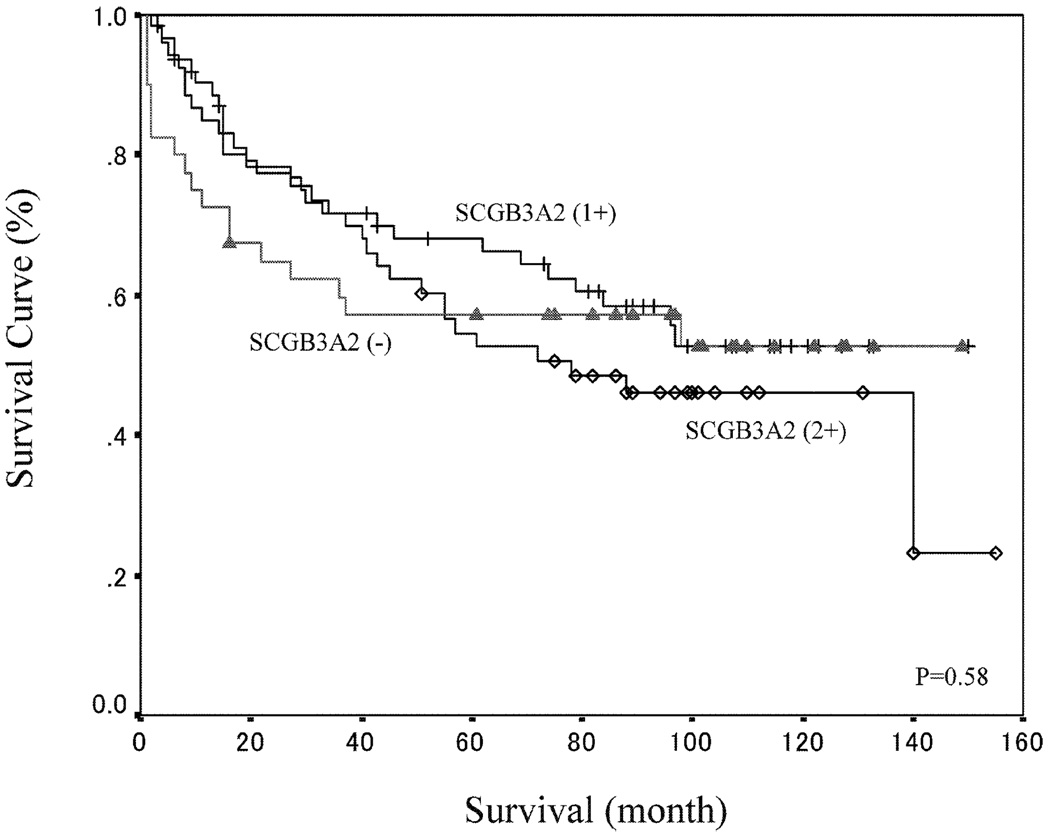
Further, SCGB3A2 expression didn’t make any significant contribution to survival rate (Fig. 7). There was no significant correlation according to SCGB3A2 expression at each pathological stage (data not shown). The same analysis using only patients with adenocarcinomas did not reveal any significant relationship between SCGB3A2 expression and survival rate.
Fig. 7. Survival Curve According to SCGB3A2 Expression.
DISCUSSION
In this manuscript, we report for the first time the expression of SCGB3A2 in primary pulmonary carcinomas and the association with histopathological phenotypes and prognosis. Recent studies have established that SCGB3A2 has an anti-inflammatory activity in lung1,9–15). The results presented here may provide additional role for SCGB3A2 in primary pulmonary carcinomas.
The expression of SCGB3A2 was found in approximately 75% of primary pulmonary carcinomas. Especially, the immunoreactivity was significantly higher in adenocarcinomas (86.5%) than squamous cell carcinomas and small cell carcinomas. In contrast, the frequency of TITF1 expression, which is used as a marker for primary lung cancer, was reported to be 39–63% in non small cell lung cancer and 62.5–96% in primary lung adenocarcinomas24–30). In this study, the prevalence of SCGB3A2 reactivity was higher than TITF1 in primary lung cancer and was similar to that in lung adenocarcinomas. Moreover, SCGB3A2 immunoreactivity in small cell carcinoma was low compared with TITF1 (53–92.7%)24–30). While TITF1 is expressed in the lung, thyroid, and ventral forebrain, the expression of SCGB3A2 is predominantly localized in the lung, with very low level expression also found in the thyroid1). Thus, SCGB3A2 appears to be specific to lung as compared to TITF1. Consequently, the sensitivity of SCGB3A2 in primary pulmonary carcinomas, especially in pulmonary adenocarcinomas, may be higher than TITF1.
In this study, there were relationships between SCGB3A2 expression and tumor subtypes and cellular morphology, but no association was found with tumor differentiation. Concerning the tumor cellular morphology, adenocarcinomas consisting of Clara cell/type II alveolar cell type or ciliated epithelial cell type showed a higher prevalence of SCGB3A2-positive cells. It may be due to the fact that SCGB3A2 expression is localized in the epithelial cells of airways and Clara cells in the bronchioles in origin1). The UGRP1 expression may be related to the sites of tumor origin.
The SCGB3A2 expression was not associated with pathological stages. Furthermore, SCGB3A2 positive reactivity was not associated with survival rate in overall lung carcinomas and adenocarcinomas. These facts suggest that SCGB3A2 may have some roles in carcinogenesis, not in the degree of tumor progression, while TITF1 was considered as a good prognostic factor for survival in non-small cell lung cancers, especially in adenocarcinomas31). In this regard, it is interesting that both SCGB3A1 and SCGB1A1 are considered as tumor suppressor17–23). More studies are required to address these questions.
In summary, we carried out for the first time detailed analysis on SCGB3A2 expression in primary pulmonary carcinomas and evaluated the association with histopathological phenotypes and the prognosis. Our results suggest that the specificity and utility of SCGB3A2 may be higher than TITF1, the currently used marker, for the diagnosis of primary lung adenocarcinomas.
REFERENCES
- 1.Niimi T, Keck-Waggoner CL, Popescu NC, Zhou Y, Levitt RC, Kimura S. UGRP1, a uteroglobin/Clara cell secretory protein-related protein, is a novel lung-enriched downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor. Mol Endocrinol. 2001;15:2021–2036. doi: 10.1210/mend.15.11.0728. [DOI] [PubMed] [Google Scholar]
- 2.Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno MD, Bohinski RJ, Huelsman KM, Whitsett JA, Korfhagen TR. Lung cell-specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SP-A promoter and thyroid transcription factor-1. J Biol Chem. 1995;270:6531–6536. doi: 10.1074/jbc.270.12.6531. [DOI] [PubMed] [Google Scholar]
- 4.Kelly SE, Bachurski CJ, Burhans MS, Glasser SW. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J Biol Chem. 1996;271:6881–6888. doi: 10.1074/jbc.271.12.6881. [DOI] [PubMed] [Google Scholar]
- 5.Ray MK, Chen CY, Schwartz RJ, DeMayo FJ. Transcriptional regulation of a mouse Clara cell-specific protein (mCC10) gene by the NKx transcription factor family members thyroid transciption factor 1 and cardiac muscle-specific homeobox protein (CSX) Mol Cell Biol. 1996;16:2056–2064. doi: 10.1128/mcb.16.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds SD, Reynolds PR, Pryhuber GS, Finder JD, Stripp BR. Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am J Respir Crit Care Med. 2002;166:1498–1509. doi: 10.1164/rccm.200204-285OC. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee AB, Chilton BS. The uteroglobin/Clara cell protein family. Ann NY Acad Sci. 2000;923:147–155. [Google Scholar]
- 9.Chiba Y, Srisodsai A, Supavilai P, Kimura S. Interleukin-5 reduces the expression of uteroglobin-related protein (UGRP) 1 gene in allergic airway inflammation. Immunol Lett. 2005;97:123–129. doi: 10.1016/j.imlet.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba Y, Kusakabe T, Kimura S. Decreased expression of uteroglobin-related protein 1 in inflamed mouse airways is mediated by IL-9. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1193–L1198. doi: 10.1152/ajplung.00263.2004. [DOI] [PubMed] [Google Scholar]
- 11.Niimi T, Munakata M, Keck-Waggoner CL, Popescu NC, Levitt RC, Hisada M, Kimura S. A polymorphism in the human UGRP1 gene promoter that regulates transcription is associated with an increased risk of asthma. Am J Hum Genet. 2002;70:718–725. doi: 10.1086/339272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinzmann A, Dietrich H, Deichmann KA. Association of uteroglobin-related protein 1 with bronchial asthma. Int Arch Allergy Immunol. 2003;131:291–295. doi: 10.1159/000072141. [DOI] [PubMed] [Google Scholar]
- 13.Srisodsai A, Kurotani R, Chiba Y, Sheikh F, Young HA, Donnelly RP, Kimura S. Interleukin-10 induces uteroglobin-related protein (UGRP) 1 gene expression in lung epithelial cells through homeodomain transcription factor T/EBP/NKX2.1. J Biol Chem. 2004;279:54358–54368. doi: 10.1074/jbc.M405331200. [DOI] [PubMed] [Google Scholar]
- 14.Bin LH, Nielson LD, Liu X, Mason RJ, Shu HB. Identification of iteroglobin-related protein 1 and macrophage scavenger receptor with collagenous structure as a lung-specific ligand-receptor pair. J Immunol. 2003;171:924–930. doi: 10.4049/jimmunol.171.2.924. [DOI] [PubMed] [Google Scholar]
- 15.Chiba Y, Kurotani R, Kusakabe T, Miura T, Link BW, Misawa M, Kimura S. Uteroglobin-related protein 1 expression suppresses allergic airway inflammation in mice. Am J Respir Crit Care Med. 2006;173:958–964. doi: 10.1164/rccm.200503-456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niimi T, Copeland NG, Gilbert DJ, Jenkins NA, Srisodsai A, Zimonjic DB, Keck-Waggoner CL, Popescu NC, Kimura S. Cloning, expression, and chromosomal localization of the mouse gene (Scgb3a1, alias Ugrp2) that encodes a member of the novel uteroglobin-related protein gene family. Cytogenet Genome Res. 2002;97:120–127. doi: 10.1159/000064067. [DOI] [PubMed] [Google Scholar]
- 17.Porter D, Lahti-Domenici J, Torres-Arzayus M, Chin L, Polyak K. Expression of high in normal-1 (HIN-1) and uteroglobin related protein-1 (UGRP-1) in adult and developing tissues. Mech Dev. 2002;114:201–204. doi: 10.1016/s0925-4773(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 18.Krop IE, Sqroi D, Porter DA, Lunetta KL, LeVangie R, Seth P, Kaekin CM, Phei E, Bosenberg M, Schnitt S, Marks JR, Pagon Z, Belina D, Razumovic J, Polyak K, et al. HIN-1, a putative cytokine highly expressed in normal but not cancerous mammary epithelial cells. Proc Natl Acad Sci USA. 2001;98:9796–9801. doi: 10.1073/pnas.171138398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krop IE, Parker MT, Blousthtain-Qimron N, Porter D, Gelman R, Sasaki H, Maurer M, Terry MB, Parsons R, Polyak K. HIN-1, an inhibitor of cell growth, invasion, and AKT activation. Cancer Res. 2005;65:9659–9669. doi: 10.1158/0008-5472.CAN-05-1663. [DOI] [PubMed] [Google Scholar]
- 20.Kundu GC, Zhang Z, Mantile-Selvaggi G, Mandal A, Yuan CJ, Mukherjee AB. Uteroblobin binding proteins: regulation of cellular mortility and invasion in normal and cancer cells. Ann N Y Acad Sci. 2000;923:234–248. doi: 10.1111/j.1749-6632.2000.tb05533.x. [DOI] [PubMed] [Google Scholar]
- 21.Cowan MJ, Huang X, Yao XL, Shelhamer JH. Tumor necrosis factor alpha stimulation of human clara cell secretory protein production by human airway epithelial cells. Ann N Y Acad Sci. 2000;923:193–201. doi: 10.1111/j.1749-6632.2000.tb05530.x. [DOI] [PubMed] [Google Scholar]
- 22.Szabo E, Goheer A, Witschi H, Linnoila RI. Overexpression of CC10 modifies neoplastic potential in lung cancer cells. Cell Growth Differ. 1998;9:475–485. [PubMed] [Google Scholar]
- 23.Linnoila RI, Szabo E, Demayo F, Witschi H, Sabourin C, Malkinson A. The role of CC10 in pulmonary carcinogenesis: from a marker to tumor suppresion. Ann N Y Acad Sci. 2000;923:249–267. doi: 10.1111/j.1749-6632.2000.tb05534.x. [DOI] [PubMed] [Google Scholar]
- 24.Bejarano PA, Baughman RP, Biddinger PW, Miller MA, Fenoglio-Preiser C, al-Kafaji B, Di Lauro R, Whitsett JA. Surfactant proteins and thyroid transcription factor-1 in pulmonary and breast carcinomas. Mod Pathol. 1996;9:445–452. [PubMed] [Google Scholar]
- 25.Di Loreto C, Di Lauro V, Puglisi F, Damante G, Fabbro D, Beltrami CA. Immunocytochemical expression of tissue-specific transcription factor-1 in lung carcinoma. J Clin Pathol. 1997;50:30–33. doi: 10.1136/jcp.50.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohinski RJ, Bejarano PA, Balko G, Warnick RE, Whitsett JA. Determination of lung as the primary site of cerebral metastatic adenocarcinoma using monoclonal antibody to thyroid transcription factor-1. J Neurooncol. 1998;40:227–231. doi: 10.1023/a:1006102607697. [DOI] [PubMed] [Google Scholar]
- 27.Puglisi F, Barbone F, Damante G, Bruckbauer M, Di Lauro V, Beltrami CA, Di Loreto C. Prognostic value of thyroid transcription factor-1 in primary, resected, non-small cell lung carcinoma. Mod Pathol. 1999;30:318–324. [PubMed] [Google Scholar]
- 28.Haque AK, Syed S, Lele SM, Freeman DH, Adegboyega PA. Immunohistochemical study of thyroid transcription factor-1 and HER2/neu in non-small cell lung cancer: Strong thyroid transcription factor-1 expression predicts better survival. Appl Immunohistochem Mol Morphol. 2002;10:103–109. doi: 10.1097/00129039-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura N, Miyagi E, Murata S, Kawaoi A, Katoh R. Expression of thyroid transcription factor-1 in normal and neoplastic lung tissue. Mod Pathol. 2002;15:1058–1067. doi: 10.1097/01.MP.0000028572.44247.CF. [DOI] [PubMed] [Google Scholar]
- 30.Tan D, Li Q, Deeb G, Ramnath N, Slocum HK, Brooks J, Cheney R, Wiseman S, Anderson T, Loewen G. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer. A high-throughput tissue microarray and immunohistochemistory study. Hum Pathol. 2003;34:597–604. doi: 10.1016/s0046-8177(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 31.Berghmans T, Paesmans M, Mascaux C, Martin B, Meert AP, Haller A, Lafitte JJ, Sculier JP. Thyroid transcription factor 1-a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol. 2006;17:1673–1676. doi: 10.1093/annonc/mdl287. [DOI] [PubMed] [Google Scholar]