Abstract
c-Cbl and Cbl-b are highly conserved adaptor proteins that participate in integrin signaling, regulating cytoskeletal organization, motility, and bone resorption. Deletion of both c-Cbl and Cbl-b in mice leads to embryonic lethality, indicating that the two proteins perform essential redundant functions. To examine the redundant actions of c-Cbl and Cbl-b in osteoclasts, we depleted c-Cbl in Cbl-b−/− osteoclasts by using a short hairpin RNA. Depleting both Cbl proteins disrupted both the podosome belt and the microtubule network and decreased bone-resorbing activity. Stabilizing the microtubules with paclitaxel or inhibiting histone deacetylase 6 (HDAC6), which destabilizes microtubules by deacetylating β-tubulin, protected both the microtubule network and the podosome belt. Examination of the mechanism involved demonstrated that the conserved four-helix bundle of c-Cbl's tyrosine kinase binding domain bound to β-tubulin, and both c-Cbl and Cbl-b displaced HDAC6. In addition to the effects on microtubules and the podosome belt, depleting both Cbls significantly increased the levels of the proapoptotic protein Bim and apoptosis relative to the levels induced by eliminating either protein alone. Thus, both c-Cbl and Cbl-b promote bone resorption via the stabilization of microtubules, allowing the formation of the podosome belt in osteoclasts, and by promoting osteoclast survival.
INTRODUCTION
c-Cbl and Cbl-b are two widely expressed mammalian members of a family of adaptor proteins with ubiquitin ligase activity that down-regulate signaling from several receptor and nonreceptor tyrosine kinases by targeting the ubiquitinating machinery to the activated kinases or associated effector proteins and/or by promoting endocytosis (Horne et al., 2005; Tsygankov, 2008). c-Cbl also ubiquitylates the proapoptotic BH3-only Bcl-2 family member Bim in osteoclasts (OCs), thereby promoting Bim's degradation and OC survival (Akiyama et al., 2003). In addition to these ubiquitylation-dependent activities, c-Cbl and Cbl-b also promote a variety of positive signaling mechanisms by assembling signaling complexes downstream of several receptors, including the macrophage colony stimulating factor (M-CSF) receptor (c-Fms), receptor activator of nuclear factor-κB (RANK) and integrins (Sanjay et al., 2001; Dikic and Giordano, 2003). In addition, c-Cbl regulates the actin cytoskeleton (Meng and Lowell, 1998; Scaife and Langdon, 2000), and stabilizes microtubules (MTs) and induces their polymerization in vitro by binding to tubulin (Teckchandani et al., 2005).
The domain structures of c-Cbl and Cbl-b are the most similar among the mammalian Cbl proteins (Thien and Langdon, 2005). Like other family members, c-Cbl and Cbl-b share a tyrosine kinase binding (TKB) domain that binds phosphorylated tyrosine residues and a RING domain that binds ubiquitin-conjugating enzymes (E2s). The TKB and RING domains and the short linker region that connects them constitute the highly conserved N-terminal halves of Cbl family proteins. The C-terminal halves of c-Cbl and Cbl-b are much less conserved, with high homology only in a subset of the several protein binding sites that mediate interactions with other signaling proteins (Horne et al., 2005; Swaminathan and Tsygankov, 2006), including short proline-rich motifs that bind Src homology (SH)3 domains, phosphorylated tyrosine residues that bind SH2 domains, binding sites for 14-3-3 proteins, and a C-terminal UBA/leucine zipper domain that allows the dimerization of these two Cbl proteins (Bartkiewicz et al., 1999; van Leeuwen et al., 1999; Lill et al., 2000; Ota et al., 2000).
Both c-Cbl and Cbl-b are expressed in OCs (Chiusaroli et al., 2003), in which they are in part associated with podosomes (Bruzzaniti et al., 2005), the specialized highly dynamic F-actin–containing attachment structures that are formed by OCs (Destaing et al., 2003; Luxenburg et al., 2006) and other highly motile cells (Linder and Aepfelbacher, 2003). The dynamic formation and disassembly of podosomes and their organization in a peripheral ring (the podosome belt) (Zambonin Zallone et al., 1989; Destaing et al., 2003) are critically important for normal OC migration and bone resorption (Sanjay et al., 2001; Destaing et al., 2003, 2008; Tehrani et al., 2006; Gil-Henn et al., 2007). The formation of the podosome belt is at least in part dependent on peripheral MTs that are stabilized by acetylation of the tubulin subunits and destabilized by the deacetylation of tubulin by histone deacetylase 6 (HDAC6) (Destaing et al., 2003, 2005; Gil-Henn et al., 2007).
Single deletions of either the c-Cbl or Cbl-b genes in mice results in relatively mild and different phenotypes in various cells, including immune cells (Murphy et al., 1998; Naramura et al., 1998; Bachmaier et al., 2000; Chiang et al., 2000), mast cells (Zhang et al., 2004), hematopoietic stem cells (Rathinam et al., 2008), adipose cells (Hirasaka et al., 2007), and OCs and osteoblasts (Murphy et al., 1998; Chiusaroli et al., 2003; Suzue et al., 2006; Nakajima et al., 2009). In contrast, deleting both genes results in lethality before day 10 of embryonic development (Naramura et al., 2002), indicating that the two proteins play required redundant roles in at least some developmentally important processes that have not yet been identified. To identify and characterize redundant functions of c-Cbl and Cbl-b in OCs, we removed both Cbl proteins in vitro by depleting c-Cbl in Cbl-b−/− OC-like cells (OCLs) by using short hairpin RNA (shRNA). Our results indicate that the absence of both c-Cbl and Cbl-b leads to the disruption of MTs and the podosome belt, resulting in decreased bone-resorbing activity, and independently, to increased levels of the proapoptotic Bcl-2 family member Bim and increased apoptosis.
MATERIALS AND METHODS
Antibodies and Reagents
Minimal essential medium, alpha modification (α-MEM), fetal bovine serum (FBS), fluorescein-conjugated secondary antibodies, and TO-PRO-3 were purchased from Invitrogen (Carlsbad, CA). Mouse monoclonal anti-c-Cbl antibody was obtained from BD Biosciences (San Diego, CA). Mouse anti-tubulin total, mouse anti-α-tubulin, mouse anti-acetyl-tubulin, and paclitaxel were purchased from Sigma-Aldrich (St. Louis, MO). Mouse anti-β-tubulin, mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), mouse anti-HDAC6 and mouse anti-actin antibodies were obtained from Millipore Bioscience Research Reagents (Temecula, CA). Mouse anti-Cbl-b antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG and HRP-conjugated anti-rabbit IgG antibodies were obtained from Fisher Scientific (Fairlawn, NJ). HRP-conjugated anti-Myc and HRP-conjugated anti-hemagglutinin (HA) was purchased from Roche Applied Sciences (Indianapolis, IN). zVAD-FMK and FluorSave were purchased from Calbiochem (San Diego, CA). Trichostatin A was purchased from EMD Chemicals (Gibbstown, NJ).
Transient Transfections
293VnR cells (human embryonic kidney 293 cells stably expressing the αvβ3 integrin, the major vitronectin receptor) (Sanjay et al., 2001) were transiently transfected with increasing amounts of myc-c-Cbl or HA-Cbl-b DNA by using FuGENE 6 (Roche Applied Sciences) according the manufacturer's specifications. Total DNA (10 μg/100-mm2 tissue culture plate) was kept constant by the addition of empty pBK plasmid. Transfected cells were maintained in α-MEM containing 10% FBS for 24 h after transfection, lysed, and used for coimmunoprecipitation assay.
Coimmunoprecipitation
293VnR cells were lysed in modified radioimmune precipitation assay (mRIPA) buffer (50 mM Tris-Cl, 150 mM NaCl, 1% Nonidet P-40, and 0.25% sodium deoxycholate) containing protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride; 1 mM NaF; 1 mM sodium orthovanadate; and 10 μg/ml each of leupeptin, aprotinin, and pepstatin). Lysates were incubated for 20 min at 4°C, and the supernatant was clarified by centrifugation. Then, 3 μg of primary antibody was used for 500 μg of cell lysates, and tubes were incubated at 4°C for 2 h, rotating. Immunoprecipitations were performed using protein G-agarose beads (Roche Applied Sciences) for 1 h. Immunoprecipitation of β-tubulin was performed using a monoclonal antibody against β-tubulin. Nonspecific proteins were removed by washing three times in mRIPA buffer. Samples were boiled in SDS-containing sample buffer under reducing conditions and proteins were resolved by 4–12% gradient Nu-PAGE gel, and followed by Western blot analyses.
Glutathione Transferase (GST) Pull-Down Assay
Plasmids encoding chimeric proteins of GST and the complete c-Cbl TKB domain (GST-TKB, amino acids 1–362), the nonconserved N-terminal 50 residues (GST-50), the four-helix subdomain (GST-4H, 1–184), the four-helix and EF hand subdomains (GST-4H/EF, 1–265), the EF hand and SH2 subdomains (GST-EF/SH, 184–478), and the SH2 subdomain (GST-SH, 266–362) were generated by amplifying sequences from the plasmid containing the myc-tagged full-length c-Cbl by polymerase chain reaction (PCR) and subcloning them into pGEX-4T (GE Healthcare, Pittsburgh, PA). Fusion proteins were expressed in Escherichia coli BL21 cells and purified with glutathione-Sepharose beads as described previously (Sanjay et al., 2006). Purified GST fusion proteins were incubated with 500 μg of whole cell extract from 293VnR cells at 4°C for 2 h in binding buffer (60 mM NaCl, 1 mM EDTA, 20 mM Tris-HCl, 0.05% Nonidet P-40, 6 mM MgCl2, and 8% glycerol). The beads were then washed three times with wash buffer (500 mM NaCl, 1 mM EDTA, 20 mM Tris-HCl, 0.05% Nonidet P-40, 6 mM MgCl2, and 8% glycerol). The bound proteins were eluted with SDS sample buffer, loaded onto a 4–12% polyacrylamide gel, and detected by Western blotting.
Preparation of Osteoclast-like Cells
Mouse OC-like cells (OCLs) were generated by two methods. 1) For bone resorption assays, immunofluorescence microscopy, and tartrate-resistant acid phosphatase (TRAP) staining, murine bone marrow macrophages (BMMs) were cocultured with primary calvarial osteoblasts obtained from CD1 mice (as the source of M-CSF and RANK ligand [RANKL]) in α-MEM containing 10 nM 1,25-dihydroxyvitamin D3 and 1 μM prostaglandin E2 on a collagen gel (Tanaka et al., 1996). Functionally active OCLs were recovered by digesting the collagen with 0.1% collagenase and replated onto coverslips or dentin for an additional 24 h. 2) For biochemical analysis, BMMs were cultured on tissue culture plastic in α-MEM for 2 d with M-CSF (10 ng/ml) and for an additional 4–5 d in the same medium with M-CSF (10 ng/ml) and RANKL (100 ng/ml) to avoid contamination with osteoblasts, which contain c-Cbl and Cbl-b.
Generation of Adenovirus with shRNA
DNA sequences that encoded short hairpin RNA to c-Cbl (c-Cbl-shRNA) 5′-CACCGCAGATGGCTCAAGAGACACGAATGTCTCTTGAGCCATCTGC-3′ or an inactive randomized control RNA (Cntr-shRNA) 5′-CACCGCCTCCAGCACTGTCGTTAACGAATTAACGACAGTGCTGGAGG-3′ were cloned into pENTRU6 vector, recombined into pAd/BLOCKit vector (Gateway system; Invitrogen), and packaged into recombinant adenoviruses expressing c-Cbl-shRNA or Cntr-shRNA by using U293 cells (Invitrogen) following the manufacturer's instructions. Adenovirus was added to immature mouse OCLs after 3 d of coculturing with osteoblasts on collagen or 2 d of culture with RANKL and M-CSF. Cells were infected with a multiplicity of infection (MOI) of 200, which resulted in a 50–75% down-regulation of c-Cbl in Cbl-b−/− OCLs relative to uninfected or control-shRNA-infected OCLs. The following day, cells were washed and differentiation was continued for an additional 3–4 d before the cells were replated onto coverslips, plastic plates, or dentin slices for 24 h and then either processed for confocal immunofluorescence microscopy, TRAP staining, or bone resorption assay or harvested for biochemical analyses.
Confocal Microscopy
OCLs plated on FBS-coated coverslips were incubated for 24 h at 37°C and then fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min. Cells were permeabilized in 0.05% saponin for 30 min and acetone for 1 min. Coverslips for actin labeling were extracted in ice-cold acetone for 3–5 min, incubated in a 1:40 dilution of rhodamine phalloidin stock solution (Invitrogen) for 1 h, washed with PBS, and mounted in FluorSave (Calbiochem, San Diego, CA). All coverslips were blocked in 5% normal goat serum (Roche Applied Sciences) for 30 min and incubated in appropriate primary antibodies, washed, incubated with fluorescent secondary antibody (Alexa Fluor 488, green; 568, red; and 647, blue), washed again, and mounted in FluorSave. Nuclei were labeled with TO-PRO-3 (1:1000) in the secondary antibody solution. Fixed cells were visualized using a 510 Meta laser scanning confocal microscope (Carl Zeiss, Thornwood, NY). Images were recorded, and composites compiled and total image enhancements were performed using Photoshop 6.0 (Adobe Systems, San Jose, CA). The intensities of acetyl-tubulin staining in the total cell area and the peripheral and central regions (outer, 40% and inner, 60%, respectively) and mean pixel intensity values were normalized to the mean pixel intensity of the Cbl-b−/− OCLs.
TRAP Staining
Cytological demonstration of TRAP in OCs was performed using the Leukocyte Acid Phosphatase labeling kit (Sigma-Aldrich), according to the manufacturer's instructions.
OC Resorption Assay
Infected OCLs were plated onto dentin slices and cultured for 24–48 h. Dentin slices were washed, incubated in 1 N ammonium hydroxide for 5 min, and sonicated for 10 s to remove cells. Resorption pits were stained with a solution containing 1% toluidine blue and 1% sodium borate for 3–4 min, washed with water, and air-dried. Pit surface area was quantified using the OsteoMeasure cell counting program (Osteometrics, Decatur, GA). Results were then normalized for OC number, as measured by staining parallel cultures for TRAP activity and counting cells with more than three nuclei.
Apoptosis Assay
Apoptosis was quantified by measuring the level of DNA fragmentation in wild-type (WT), c-Cbl−/−, Cbl-b−/−, c-Cbl-shRNA–treated Cbl-b−/−, and Cntr-shRNA–treated Cbl-b−/− OCLs, by using the Cellular DNA Fragmentation enzyme-linked immunosorbent assay (ELISA) assay (Roche Applied Sciences), following the manufacturer's instructions. The results were normalized to the number of cells in the culture.
Statistical Analysis
Each experiment was repeated at least three times. The results obtained from a typical experiment were expressed as the means ± SDs. Significant differences were determined using analysis of variance followed by Student's t test. p < 0.05 was considered significant.
RESULTS
Depleting Both c-Cbl and Cbl-b Causes the Loss of the Podosome Belt and Decreased Bone Resorption
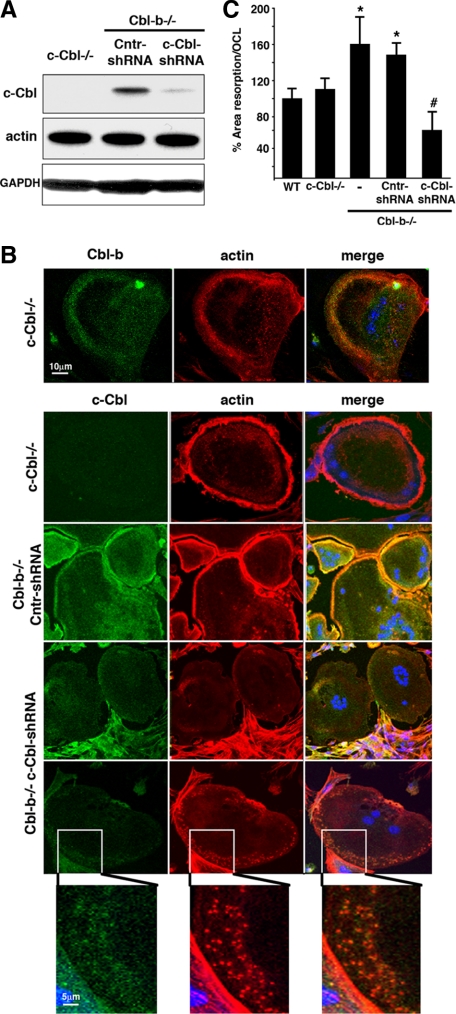
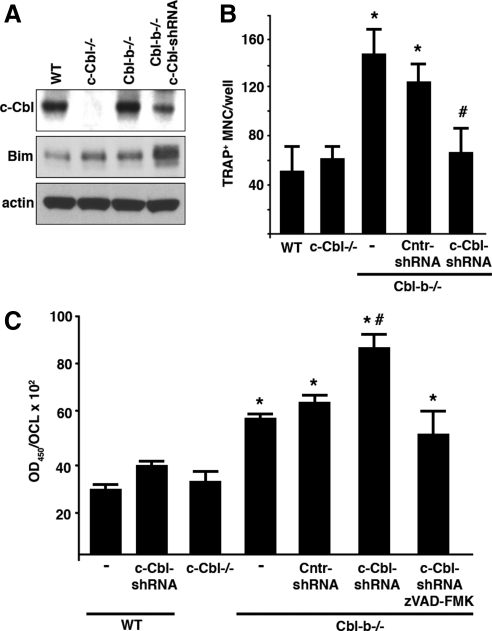
Because double-knockout animals die before embryonic day 10, i.e., before bone development and OC formation in vivo, we used an adenovirally encoded shRNA to deplete c-Cbl in Cbl-b−/− OCLs in vitro to identify and characterize the redundant functions of c-Cbl and Cbl-b in OCs. Analysis by Western blot, confirmed by confocal immunofluorescence microscopy, indicated successful depletion of c-Cbl protein synthesis (∼75% of control) after 3 d of adenoviral infection (Figure 1, A and B).
Figure 1.
Depletion of both Cbl proteins leads to the loss of the podosome belt and decreased bone resorption. (A) c-Cbl−/− and Cbl-b−/− OCLs were generated in the presence of M-CSF and RANKL. Cbl-b−/− OCLs were infected with control (Cntr)-shRNA or c-Cbl-shRNA adenovirus as described in Materials and Methods. OCLs were lysed and whole cell extracts (WCE) were analyzed for expression of c-Cbl, actin and GAPDH by Western blot. (B) c-Cbl−/− and Cbl-b−/− OCLs were generated by coculture with osteoblasts and the Cbl-b−/− OCLs were infected with Cntr-shRNA and c-Cbl-shRNA as indicated. The OCLs were replated on the glass coverslips for 24 h and the presence of c-Cbl, Cbl-b (both green), and actin (red) analyzed by confocal immunofluorescence. Depleting both c-Cbl and Cbl-b led to the disruption of the podosome belt. Isolated podosomes colocalized with areas of low residual c-Cbl staining in some OCLs (bottom). (C) WT, c-Cbl−/−, Cbl-b−/−, and Cbl-b−/− OCLs infected with Cntr-shRNA or c-Cbl-shRNA were replated onto dentin slices and cultured for 24 h. Resorption pits were stained and quantified. *p < 0.05 relative to WT; #p < 0.05 relative to Cbl-b−/−. Depleting both c-Cbl and Cbl-b decreased the bone resorbing activity of OCLs.
The characteristic peripheral podosome belts were disrupted in doubly Cbl-depleted OCLs, whereas single c-Cbl−/− and Cbl-b−/− OCLs showed no changes (Figure 1B). The extent of the loss of the podosome belt was variable and depended on the degree of c-Cbl depletion. In some cells, the belt was completely absent, whereas in others (bottom), a few isolated podosomes with reduced actin cloud (Destaing et al., 2003) were still observed in the cell periphery, coincident with patches of low amounts of residual c-Cbl.
OCLs generated from c-Cbl−/− mice have essentially normal bone-resorbing activity (Chiusaroli et al., 2003), whereas the bone-resorbing activity of Cbl-b−/− OCLs is increased (Nakajima et al., 2009). Because the ability of OCs to form podosomes and organize them into a mature belt is critically important for normal bone resorption (Destaing et al., 2003; Tehrani et al., 2006; Gil-Henn et al., 2007), reduced bone-resorbing activity is a predicted consequence of the defective podosome belt that we observed in the Cbl-deficient OCLs. Comparison of the bone resorbing activities of OCLs from WT, c-Cbl−/−, Cbl-b−/−, and doubly Cbl-depleted OCLs (Figure 1C) revealed that the resorbing activity was indeed decreased when both Cbl proteins were depleted, by ∼70% relative to the high bone-resorbing activity of the Cbl-b−/− OCLs, and by nearly 40% relative to the bone-resorbing activity of WT and c-Cbl−/− OCLs, confirming the previously reported importance of podosome formation and organization for bone resorption.
Depleting c-Cbl from Cbl-b−/− OCLs Disrupts Microtubule Organization
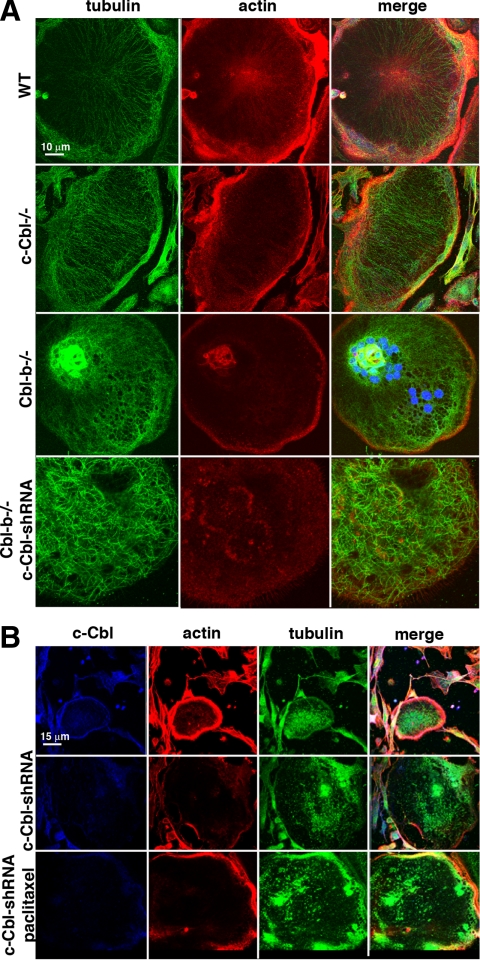
Because podosome belt formation is controlled by the microtubule network in OCs (Destaing et al., 2003, 2005; Gil-Henn et al., 2007), and c-Cbl stabilizes MTs and induces tubulin polymerization by an as yet uncharacterized mechanism (Teckchandani et al., 2005), we hypothesized that the depletion of both Cbl proteins could result in the depolymerization of MTs, which in turn would lead to the disorganization of the podosome belt. Indeed, immunofluorescent staining of tubulin revealed that the microtubule network was not significantly affected by the loss of either c-Cbl or Cbl-b but that depleting both proteins disrupted the organization of the OCL microtubule network, and in particular the peripheral circumferential MTs (Figure 2A), indicating that the two Cbl proteins act redundantly to stabilize MTs.
Figure 2.
c-Cbl–depleted Cbl-b−/− OCLs exhibit alterations in microtubule organization. (A) WT, Cbl-b−/−, and c-Cbl-shRNA–infected Cbl-b−/− OCLs were fixed and analyzed by confocal immunofluorescence for tubulin (green) and actin (red). The microtubular network of the doubly Cbl-depleted OCLs was disrupted. (B) The localization of c-Cbl (blue), actin (red), and tubulin (green) was analyzed in Cbl-b−/− OCLs (top), and c-Cbl-shRNA–infected Cbl-b−/− OCLs generated in the presence (middle) or absence (bottom) of 1 nM paclitaxel. Paclitaxel prevented the loss of both the peripheral MTs and the podosome belt.
To determine whether the loss of the podosome belt was due only to the loss of the normal microtubule network or if another redundant action of the Cbl proteins also promoted podosome formation and the organization of the peripheral belt, we tested the effect of paclitaxel, which binds to β-tubulin and stabilizes MTs (Wilson and Forer, 1997; Dowdy et al., 2006). We found that adding paclitaxel to the Cbl-b−/− OCL cultures at the time of infection with the c-Cbl-shRNA adenovirus prevented both the loss of the microtubule network and the disruption of the podosome belt (Figure 2B), indicating that the loss of the podosome belt when both Cbl proteins were depleted was primarily due to the disruption of the circumferential microtubule network.
Cbl Proteins Enhance Microtubule Stability by Antagonizing the HDAC6-catalyzed Deacetylation of Tubulin
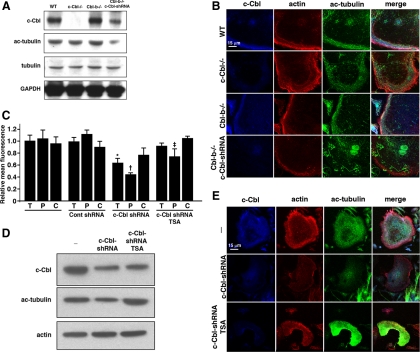
MTs in OCs and other cells are destabilized in part by the HDAC6-catalyzed deacetylation of tubulin (Destaing et al., 2005; Thompson et al., 2007). Because overexpression of c-Cbl leads to increased acetylated tubulin and microtubule stabilization in human pulmonary artery endothelial cells (Teckchandani et al., 2005), we hypothesized that depleting both Cbl proteins could increase HDAC6-catalyzed deacetylation of tubulin and consequently destabilize the MTs. Consistent with this hypothesis, the acetylated tubulin level in the doubly Cbl-depleted OCLs was decreased (Figure 3, A and B). Interestingly, the decrease occurred largely in the peripheral region of the OCLs, where c-Cbl prominently colocalized with acetylated tubulin and the podosome belt in the WT and Cbl-b−/− OCLs (Figure 3, B and C), and the peripheral band of acetylated tubulin observed in WT and Cbl-b−/− OCLs was often completely absent when both Cbl proteins were depleted (Figure 3B), consistent with the hypothesized protection of tubulin acetylation by the Cbl proteins.
Figure 3.
Depleting both c-Cbl and Cbl-b reduces tubulin acetylation, and inhibiting HDACs partially prevents the loss of the peripheral podosome belt. (A) WT, c-Cbl−/−, Cbl-b−/−, and c-Cbl-shRNA–infected Cbl-b−/− OCLs were lysed and whole cell extracts (WCEs) were analyzed for c-Cbl, acetylated tubulin, and total tubulin by Western blot. GAPDH was blotted to confirm equal loading. The level of acetylated tubulin was decreased in the c-Cbl–depleted Cbl-b−/− OCLs. (B) WT, c-Cbl−/−, Cbl-b−/−, and c-Cbl-shRNA–infected Cbl-b−/− OCLs were fixed and analyzed by confocal immunofluorescence for c-Cbl (blue), actin (red), and acetylated tubulin (green). Acetylated tubulin was decreased in the doubly Cbl-depleted OCLs. (C) The mean pixel intensities of confocal images of Cbl-b−/− OCLs treated as indicated and stained for acetyl-tubulin were determined in total cells (T), cell peripheries (P), and cell centers and normalized to the mean total pixel intensity of Cbl-b−/− OCLs. Data are presented as mean ± SEM; significance was determined relative to the comparable area in the untreated Cbl-b−/− OCLs; *p < 0.05 versus total uninfected Cbl-b−/− fluorescence; †p < 0.001 versus peripheral uninfected Cbl-b−/− fluorescence; ‡p = 0.0498 versus peripheral uninfected Cbl-b−/− fluorescence. (D) Cbl-b−/− OCLs and Cbl-b−/− OCLs infected with c-Cbl-shRNA adenovirus in the presence or absence of 100 nM TSA were lysed, and WCEs were analyzed by Western blot for c-Cbl, acetylated tubulin and actin, confirming the inhibition of tubulin deacetylation by TSA. (E) Cbl-b−/− (top) and Cbl-b−/− OCLs infected with c-Cbl-shRNA in the presence (middle) or absence (bottom) of 100 nM TSA were fixed and analyzed by confocal immunofluorescence for c-Cbl (blue), actin (red), and acetylated tubulin (green). Inhibiting histone deacetylase with TSA prevented the loss of the podosome belt.
To determine whether HDAC6-catalyzed deacetylation of tubulin contributed to the loss of the MTs and the consequent disruption of the podosome belt when both Cbl proteins were depleted, we treated the Cbl-b−/− OCL cultures with the histone deacetylase inhibitor trichostatin A (TSA) (Matsuyama et al., 2002; Dowdy et al., 2006) at the time of infection with the c-Cbl-shRNA adenovirus. TSA prevented the deacetylation of tubulin (Figure 3, D and E) and not only the loss of the microtubule network but also the loss of the podosome belt (Figure 3E), demonstrating that Cbl proteins prevent HDAC6 from deacetylating tubulin and indicating that tubulin deacetylation contributes to the loss of both the microtubules and the podosome belt in the doubly Cbl-depleted OCLs.
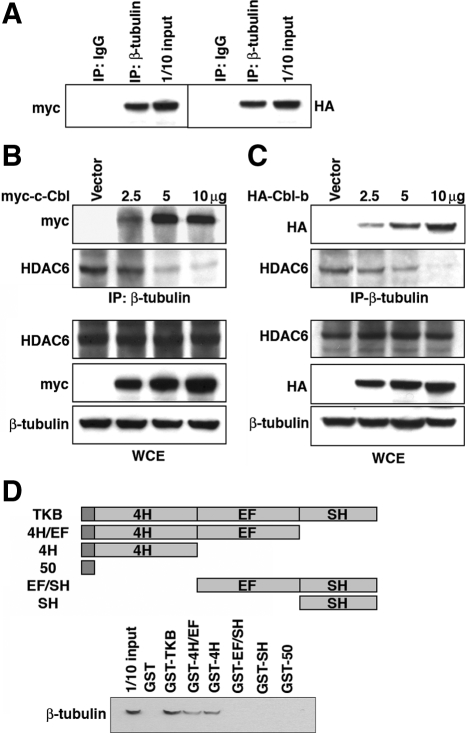
Because both c-Cbl and HDAC6 bind tubulin (Zhang et al., 2003; Teckchandani et al., 2005), Cbl proteins might antagonize HDAC6-catalyzed microtubule deacetylation by competitively displacing HDAC6 from the MTs. To test this hypothesis, we analyzed the effect of the Cbl proteins on the HDAC6-tubulin interaction. Increasing amounts of c-Cbl or Cbl-b were transfected into 293VnR cells for 24 h, the cells were lysed, and β-tubulin was immunoprecipitated. The amounts of c-Cbl, Cbl-b, and HDAC6 in the immune complexes were analyzed by Western blot. Both c-Cbl and Cbl-b bound to β-tubulin (Figure 4A), consistent with the near-identity of the TKB domains of the two Cbl proteins. Furthermore, both c-Cbl and Cbl-b competitively displaced HDAC6 from β-tubulin (Figure 4, B and C, respectively), suggesting that c-Cbl and Cbl-b redundantly prevent the binding of HDAC6 to and the subsequent deacetylation of polymerized tubulin, thereby stabilizing both the MTs and the podosome belt in OCs.
Figure 4.
c-Cbl and Cbl-b displace HDAC6 from β-tubulin. (A) 293VnR cells were transfected with 5 μg of myc-c-Cbl or HA-Cbl-b. The cells were lysed and immunoprecipitated with anti-β-tubulin antibody or normal IgG. Bound c-Cbl (left) and Cbl-b (right) were detected with anti-myc or anti-HA antibodies, respectively. Both c-Cbl and Cbl-b bound to β–tubulin. (B and C) 293VnR cells were transfected with 5 μg of empty vector or 2, 5, and 10 μg of myc-c-Cbl (B) or HA-Cbl-b (C). β-Tubulin was immunoprecipitated, and the amounts of HDAC6, c-Cbl, and Cbl-b in the immune complexes were quantified by Western blot. The expression of HDAC6, the Cbl proteins, and β-tubulin in WCEs were determined. Both c-Cbl and Cbl-b dose-dependently reduced the amount of β-tubulin–associated HDAC6. (D) Immobilized GST, GST-TKB, GST-50, GST-4H/EF, GST-4H, GST-EF/SH, and GST-SH fusion proteins were incubated with 500 μg of whole cell extract from 293VnR cells, washed, and eluted with SDS sample buffer. The bound proteins were resolved by electrophoresis on a 4–12% polyacrylamide gel and bound β-tubulin was detected by Western blot by using anti-β-tubulin antibody.
Finally, to further map the site on the c-Cbl TKB domain that interacts with β-tubulin, we generated constructs of GST fused to the c-Cbl TKB domain or overlapping fragments and determined which bound to β-tubulin when incubated with 293VnR cell lysates (Figure 4D). We found that only the fusion proteins that contained the four-helix bundle bound β-tubulin.
Depleting Both c-Cbl and Cbl-b Increases the Amount of the Proapoptotic Bcl-2 Family Member Bim and Increases Apoptosis of the OCLs
In addition to its roles in modulating signaling from αvβ3 integrin and the M-CSF receptor, c-Cbl also promotes the ubiquitylation and degradation of the proapoptotic Bcl-2 family member Bim (Akiyama et al., 2003). The high degree of homology of the TKB and RING domains of c-Cbl and Cbl-b suggested that Cbl-b might have a similar effect on Bim and, if so, that the two proteins might act redundantly to maintain a low level of Bim in OCs and thereby promote OC survival. We therefore compared the expression level of Bim in WT, c-Cbl−/−, Cbl-b−/−, and doubly Cbl-depleted OCLs (Figure 5A). The levels of Bim in the c-Cbl−/− and Cbl-b−/− OCLs were modestly increased over the level in the WT OCLs, consistent with our earlier findings in the c-Cbl−/− OCLs. Despite these slight increases in Bim levels in the c-Cbl−/− and Cbl-b−/− OCLs, the WT and c-Cbl−/− cultures contained similar numbers of OCLs the WT and the absence of Cbl-b significantly increased the number of OCLs generated in culture (Figure 5B), confirming our previous observation (Nakajima et al., 2009). Thus, it seems that the small increases in Bim in the OCLs that lack only one of the Cbl proteins has little negative effect on OCL survival.
Figure 5.
Depleting both c-Cbl and Cbl-b increases OCL apoptosis. (A) WT, c-Cbl−/−, Cbl-b−/−, and Cbl-b−/− OCLs infected with Cntr-shRNA or c-Cbl-shRNA adenovirus were lysed and whole cell extracts (WCEs) were analyzed for c-Cbl and Bim expression by Western blot. Actin was blotted to confirm equal loading. The absence of either c-Cbl or Cbl-b increased Bim levels slightly, but depleting OCLs of both c-Cbl and Cbl-b markedly increased the level of Bim protein. (B) WT, c-Cbl−/−, Cbl-b−/−, and Cbl-b−/− OCLs infected with Cntr-shRNA or c-Cbl-shRNA were generated by culturing BMMs for 4 d M-CSF and RANKL as described in Materials and Methods, and then they were fixed and stained for TRAP and the multinucleated TRAP+ cells were counted. *p < 0.05 relative to WT; #p < 0.05 relative to Cbl-b−/−. Depleting both c-Cbl and Cbl-b reduced the number of OCLs relative to the number when only Cbl-b was eliminated. (C) Apoptosis in WT, c-Cbl−/−, Cbl-b−/−, and Cbl-b−/− OCLs infected with Cntr-shRNA or c-Cbl-shRNA in the presence or absence of 100 μM zVAD-FMK was quantified by measuring the fragmentation of 5-bromo-2′-deoxyuridine-labeled DNA by ELISA, as described in Materials and Methods, and normalizing the values to the number of OCLs present in the cultures. *p < 0.05 relative to WT; #p < 0.05 relative to Cbl-b−/−. Depleting only Cbl-b increased apoptosis, in contrast to the lack of effect of deleting or depleting only c-Cbl. Depleting both c-Cbl and Cbl-b further increased the level of apoptosis. Inhibiting caspase activity reduced the amount of apoptosis.
In contrast to the small increases in Bim in the OCLs that lacked only one of the Cbl proteins, much more Bim was present when both Cbl proteins were depleted, consistent with the hypothesized redundant ability of the two Cbl proteins to maintain Bim at low levels. Based on the known proapoptotic effect of Bim, we hypothesized that the doubly Cbl-depleted OCLs would undergo increased apoptosis and suffer reduced survival. DNA fragmentation, a characteristic feature of apoptosis, was not changed by deleting the c-Cbl gene (Figure 5C), providing further evidence that the small increase in Bim that was induced by eliminating only c-Cbl (or Cbl-b) was not sufficient to increase apoptosis. However, and notwithstanding the similar small increases in Bim in the c-Cbl−/− and Cbl-b−/− OCLs and the larger numbers of OCLs in the Cbl-b−/− cultures, deleting the Cbl-b gene increased DNA fragmentation, normalized to the number of cells, by approximately twofold relative to the levels found in the WT and c-Cbl−/− cells, indicating that Cbl-b protects OCs from apoptosis by both Bim-dependent and Bim-independent mechanisms. Depleting c-Cbl in the Cbl-b−/− OCLs further increased DNA fragmentation to threefold higher than in WT or c-Cbl−/− cells, indicating that the two Cbl proteins do indeed act redundantly to protect against apoptosis, as hypothesized. Consistent with the increased Bim and DNA fragmentation in the doubly Cbl-depleted OCLs, the number of OCLs in these cultures was reduced to ∼50% of the elevated level observed in the Cbl-b−/− cultures (Figure 5B). Finally, treating the doubly Cbl-depleted OCLs with the pan-caspase inhibitor zVAD-FMK reduced the amount of DNA fragmentation in the doubly Cbl-depleted OCLs to a level approaching that in the untreated WT OCLs, indicating that the increased DNA fragmentation was indeed the consequence of increased apoptosis (Nagata, 2005).
The Loss of Podosomes in the Doubly Depleted OCLs Is Not Due to the Increased Apoptosis
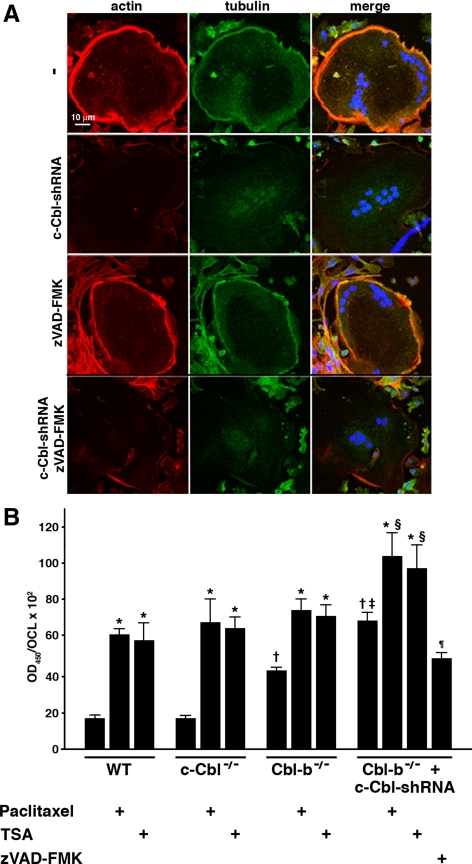
Altering microtubule stability, either positively or negatively, can induce apoptosis (Dumontet and Sikic, 1999; Bhalla, 2003), as can the loss of cell attachment or altered integrin signaling (Chiarugi and Giannoni, 2008; Marastoni et al., 2008), Conversely, some elements in the apoptosis cascade have been reported to modulate cell attachment and motility (Frisch, 2008), Thus, the loss of the peripheral MTs and the podosome belt and the increased apoptosis that result from the depletion of both c-Cbl and Cbl-b could be causally related. We first determined whether inhibiting apoptosis would protect against the disruption of the podosome belt. We found that treating the doubly Cbl-depleted cells with zVAD-FMK did not prevent the loss of the podosome belt (Figure 6A), although DNA fragmentation was reduced to a level similar to that in the Cbl-b−/− cells (Figure 5C), which form normal podosome belts. Thus, the loss of the podosome belt in the doubly Cbl-depleted OCLs does not seem to be due to the increased apoptosis of the cells.
Figure 6.
The increased apoptosis does not cause the disruption of the podosome belt in the c-Cbl–depleted Cbl-b−/− OCLs. (A) Cbl-b−/− OCLs were infected with Cbl-shRNA in the presence or absence of 100 μM zVAD-FMK. Cells were fixed and analyzed by confocal immunofluorescence for actin (red) and tubulin (green). The pan-caspase inhibitor zVAD-FMK failed to prevent the loss of the podosome belt. (B) Cbl-b−/− OCLs infected with Cbl-shRNA were incubated with zVAD-FMK (100 μM), paclitaxel (1 nM), or TSA (100 nM) or vehicle as indicated. Apoptosis was quantified as in Figure 5. Paclitaxel and TSA increased apoptosis to similar levels in the WT, c-Cbl−/−, and Cbl-b−/− OCLs, despite the higher apoptosis in the untreated Cbl-b−/− OCLs. Apoptosis in the c-Cbl-depleted Cbl-b−/− OCLs increased by similar amounts relative to the Cbl-b−/− OCLs, regardless of the treatment. *p < 0.05 versus matched untreated; †p < 0.05 versus untreated WT; ‡p < 0.05 versus untreated Cbl-b−/−; §p < 0.05 versus paclitaxel-treated or TSA-treated WT, c-Cbl−/−, and Cbl-b−/−; ¶p < 0.05 versus untreated doubly Cbl-depleted.
Determining whether the loss of the MTs and podosome belt contributed to the increase in apoptosis is complicated by the fact that preventing the loss of the cytoskeletal structures involves treating the cells with paclitaxel and HDAC inhibitors, which themselves induce apoptosis (Dowdy et al., 2006). Consistent with their known proapoptotic effects, paclitaxel and TSA both increased apoptosis by three- to fourfold in the WT and c-Cbl−/− cells (Figure 6B). The amounts of apoptosis in the paclitaxel- and TSA-treated Cbl-b−/− OCLs were also similar to the levels in the treated WT and c-Cbl−/− cells, despite the increased apoptosis in the untreated Cbl-b−/− cultures, suggesting that the absence of Cbl-b and the MT-stabilizing agents activate the same apoptotic mechanism(s) and that the effects are not additive. In contrast, depleting both Cbl proteins both increased constitutive apoptosis and further sensitized the OCLs to the effects of paclitaxel and TSA. Interestingly, the increases in apoptosis in the paclitaxel- and TSA-treated cultures were similar to the increase in the doubly Cbl-depleted cells over the level in the Cbl-b−/− OCLs. Because paclitaxel-induced apoptosis is significantly Bim-dependent in some systems (Bouillet et al., 1999; Li et al., 2005, 2007), this suggests that the increased apoptosis in the doubly depleted OCLs is at least partly due to the increased amount of Bim in those cells. Thus, depleting both c-Cbl and Cbl-b seems to increase apoptosis by multiple mechanisms and not simply by reducing microtubule stability or the consequent loss of the podosome belt, and conversely, the loss of the podosome belt in the doubly Cbl-depleted OCLs is not simply a consequence of the increased apoptosis.
DISCUSSION
The widely expressed c-Cbl and Cbl-b proteins regulate multiple cellular functions, including intracellular signaling, motility and survival. Single c-Cbl or Cbl-b gene deletions induce different and sometimes subtle effects in various cells and tissues (Murphy et al., 1998; Naramura et al., 1998; Bachmaier et al., 2000; Chiang et al., 2000; Zhang et al., 2004; Hirasaka et al., 2007; Rathinam et al., 2008), including OCs in which c-Cbl promotes motility and Cbl-b down-regulates OC differentiation and bone resorption (Sanjay et al., 2001; Chiusaroli et al., 2003; Nakajima et al., 2009). Thus, c-Cbl and Cbl-b perform isoform-specific functions that the other does not compensate. In addition, the early embryonic lethality caused by the simultaneous deletion of both c-Cbl and Cbl-b genes (Naramura et al., 2002) demonstrates that the two closely related proteins play essential redundant roles during development, consistent with their high homology. We now report that c-Cbl and Cbl-b act redundantly in OCs to promote podosome formation and organization, stabilize MTs and antagonize apoptosis.
The formation of the characteristic peripheral podosome belt, a prerequisite for normal bone-resorbing activity (Destaing et al., 2003; Tehrani et al., 2006; Gil-Henn et al., 2007), required the presence of at least one of the Cbl proteins (Figure 1B); consequently, the bone-resorbing activity of the doubly depleted OCLs was reduced (Figure 1C). The extent of the loss of the belt depended on the degree of c-Cbl depletion in individual Cbl-b−/− cells. The podosome belt and individual podosomes were absent when no c-Cbl was detected, whereas OCLs with small areas of residual c-Cbl staining exhibited scattered discrete podosome cores and faint actin cloud coincident with the residual c-Cbl (Figure 1B).
The loss of the podosome belt was due to the disruption of MTs, because stabilizing the MTs with paclitaxel preserved the podosome belt (Figure 2B). Previous studies demonstrated that the existence of the podosome belt requires the presence of a circumferential band of MTs (Babb et al., 1997; Destaing et al., 2003, 2005; Gil-Henn et al., 2007). Moreover, it has been reported that podosome formation also requires the presence of MTs (Linder et al., 2000), although this has been disputed (Jurdic et al., 2006). Deacetylation of tubulin by HDAC6, when activated downstream of active Rho and mDia, destabilizes the OC MTs (Destaing et al., 2005), and we have shown that Pyk2 promotes the transition of small podosome rings to the mature peripheral belt by antagonizing Rho activation (Gil-Henn et al., 2007). Because overexpressing c-Cbl in endothelial and COS-7 cells stabilizes MTs and increases microtubule acetylation via a mechanism that involves the binding of c-Cbl's highly conserved TKB domain to tubulin (Teckchandani et al., 2005), we hypothesized that the Cbl proteins could be promoting podosome belt formation by antagonizing the HDAC6-catalyzed deacetylation of tubulin and thereby stabilizing the OC MTs. Consistent with this hypothesis, we found that depleting both Cbl proteins led to decreased tubulin acetylation (Figure 3, A and B), for the most part in the periphery of the OCLs where the Cbl proteins prominently colocalize with the circumferential MTs and the podosome belt (Figure 3, B and C), and disrupted MTs, especially the circumferential MTs (Figure 2, A and B). Because the loss of the podosome belt was prevented by either stabilizing the MTs with paclitaxel (Figure 2B), which does not interfere with HDAC6 binding to microtubules (Zhang et al., 2003) or by inhibiting HDAC6-catalyzed tubulin deacetylation (Figure 3, D and E), we conclude that the loss of the MTs as a consequence of increased HDAC6-catalyzed deacetylation of tubulin when both c-Cbl and Cbl-b are depleted is sufficient to cause the loss of the podosome belt, although other shared activities of the two Cbl proteins may also promote podosome formation.
We found that c-Cbl bound tubulin via a binding site in the TKB domain's conserved four-helix bundle, as predicted by Teckchandani et al. (2005) based on sequence similarity with the motor domain of a Chlamydomonas reinhardtii kinesin homologue, and that both Cbl proteins dose-dependently reduced the amount of tubulin-associated HDAC6 (Figure 4), indicating that the Cbl proteins promote microtubule acetylation and stability by antagonizing the HDAC6–tubulin interaction. Consistent with the report that the c-Cbl–induced protection of tubulin acetylation requires only the TKB domain and is not affected by disabling the phosphotyrosine binding activity (Teckchandani et al., 2005), this mechanism seems to be independent of Pyk2, because Pyk2 protein and autophosphorylation were not altered by depleting the Cbl proteins, and the deletion of Pyk2 did not affect the association of HDAC6 with tubulin (data not shown).
Depleting both Cbl proteins also increased apoptosis, which could explain the early embryonic lethality of the c-Cbl−/−·Cbl-b−/− mice (Naramura et al., 2002) if Cbl proteins exert similar redundant antiapoptotic effects in developmentally critical cells that express both proteins. Consistent with the need for tight regulation of cell survival in widely varying circumstances, apoptosis is induced by two converging mechanisms, an intrinsic mitochondria-dependent mechanism that is activated by various types of increased cell stress and an extrinsic mechanism that is activated by the binding of humoral factors to some members of the tumor necrosis factor receptor family (Strasser et al., 2000). These signaling cascades are modulated in turn by several important signaling effectors, including the extracellular signal-regulated kinase (Erk) and c-Jun NH2-terminal kinase mitogen-activated protein kinases and Akt (Miyazaki et al., 2000; Glantschnig et al., 2003; Lu et al., 2006; Bradley et al., 2008).
Cbl proteins directly regulate several factors that are known or predicted to play important roles, both positive and negative, in modulating osteoclast survival, including c-Fms (Lee et al., 1999); the receptor for M-CSF, Bim (Akiyama et al., 2003; El Chami et al., 2005); the proapoptotic BH3-only Bcl2 family member; and, as we report here, microtubule stability (Dumontet and Sikic, 1999; Bhalla, 2003). Thus, the increased apoptosis of the doubly Cbl-depleted OCLs is likely to be the cumulative effect of changes in multiple Cbl-modulated activities. For example, the two Cbl proteins mediate the M-CSF–induced ubiquitylation and degradation of Bim (Akiyama et al., 2003; this study), but they also down-regulate M-CSF-induced signaling generally (Lee et al., 1999), and depleting both Cbl proteins is therefore likely to increase both the proapoptotic action of Bim and the antiapoptotic action of Erks. The increased amount of Bim may also synergize with an apoptotic effect of microtubule disruption, because Bim is implicated in the induction of apoptosis by both MT-stabilizing and MT-depolymerizing agents (Bouillet et al., 1999; Li et al., 2007; Anderton et al., 2008).
Bim exerts its proapoptotic effect via the intrinsic mitochondria-dependent pathway in multiple cell types (Bouillet et al., 1999), including osteoclasts, which exhibit markedly increased survival, both in vivo and in vitro, when Bim is absent (Akiyama et al., 2003). c-Cbl associates with and ubiquitylates Bim downstream of M-CSF–activated c-Fms in osteoclasts, inducing its degradation (Akiyama et al., 2003), and the amount of Bim increases in OCs and testis when c-Cbl is eliminated (Akiyama et al., 2003; El Chami et al., 2005). The importance of the regulation of Bim by c-Cbl has been questioned, however, in part due to the relatively small increase in Bim in the c-Cbl−/− OCLs (Ewings et al., 2007). Here, we confirmed that the amount of Bim is slightly increased in c-Cbl−/− OCLs and in addition we demonstrated a similar small increase in the Cbl-b−/− OCLs. More importantly, we observed a greater than additive increase in Bim when both Cbl proteins were depleted, indicating that c-Cbl and Cbl-b each largely compensate for the other's absence in promoting Bim degradation.
The small increase in Bim that occurred in OCLs that lacked one or the other Cbl protein apparently has little effect on osteoclast survival, because similar amounts of DNA fragmentation was measured in the WT and c-Cbl−/− OCLs. Furthermore, because similar increases in Bim occurred in both the c-Cbl−/− and the Cbl-b−/− OCLs, the increased apoptosis in the Cbl-b−/− OCLs is apparently the consequence of a second, Bim-independent, change that was induced by the absence of Cbl-b and not of the small increase in Bim. Further investigation will be required to characterize the mechanism responsible for the increased apoptosis in the Cbl-b−/− OCLs and to determine whether it is truly Bim-independent or whether the increased apoptosis is the result of synergy between the small increase in Bim and the Cbl-b–specific change. Regardless, the increased apoptosis is more than compensated by the increased differentiation of the c-Cbl−/− OCLs that we observed previously (Nakajima et al., 2009), resulting in increased numbers of OCLs in the c-Cbl−/− cultures despite the increased amount of apoptosis. In contrast, the marked increase in Bim in the doubly depleted OCLs may be an important factor in the increased constitutive apoptosis that occurred in those cells, because depleting c-Cbl in the Cbl-b−/− OCLs induced similar increases (relative to the the Cbl-b−/− OCLs) in constitutive apoptosis and in paclitaxel-induced apoptosis, which is largely dependent on the presence of Bim (Bouillet et al., 1999; Li et al., 2005, 2007). Interestingly, Bim−/− OCLs lack well-formed podosome belts (Akiyama et al., 2003), suggesting that the modulation of Bim levels by the Cbl proteins might also affect podosome belt formation in some way, although elucidation of this hypothetical mechanism would require significant additional research.
The apoptotic effects of the paclitaxel and TSA that we used to stabilize the MTs and thereby preserve the belt prevented determination of whether the loss of the podosome belt itself also contributed to the increased apoptosis of the doubly Cbl-depleted OCLs. Alternatively, the loss of the podosome belt was apparently not a consequence of the increased apoptosis, because the broad-spectrum caspase inhibitor reduced apoptosis to the level seen in the Cbl-b−/− OCLs, which form podosome belts, but failed to preserve the belt.
In conclusion, although c-Cbl and Cbl-b clearly play specific roles in OCs that promote motility and down-regulate bone resorption, respectively (Chiusaroli et al., 2003; Nakajima et al., 2009), they also act redundantly to promote at least two critically important functions. First, both proteins promote the formation of the podosome belt and bone resorption by stabilizing the OC microtubule system, apparently by preventing HDAC6 from binding and deacetylating the MTs. Second, both Cbl proteins promote the down-regulation of the proapoptotic Bim protein, contributing to the protection of OCs from undergoing apoptosis and enhancing their survival. Thus, Cbl proteins are important pleiotropic regulators of multiple aspects of OC function.
ACKNOWLEDGMENTS
We thank the Harvard NeuroDiscovery Optical Imaging Center for assistance with quantification of confocal images, Peter Renehan for assistance in generating GST fusion proteins, Karen Ford for assistance with animal care, and Cecile Itzstein for helpful discussions. This study was supported by National Institutes of Health grants DE-04724 and AR-42927 (to R. B.) and the James Hudson Brown-Alexander Brown Coxe Postdoctoral Fellowship in the Medical Sciences (to E. P.).
Abbreviations used:
- OC
osteoclast
- OCL
osteoclast-like cell
- MT
microtubule.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-03-0248) on July 29, 2009.
REFERENCES
- Akiyama T., et al. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 2003;22:6653–6664. doi: 10.1093/emboj/cdg635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton E., Yee J., Smith P., Crook T., White R. E., Allday M. J. Two Epstein-Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor Bim: clues to the pathogenesis of Burkitt's lymphoma. Oncogene. 2008;27:421–433. doi: 10.1038/sj.onc.1210668. [DOI] [PubMed] [Google Scholar]
- Babb S. G., Matsudaira P., Sato M., Correia I., Lim S. S. Fimbrin in podosomes of monocyte-derived osteoclasts. Cell Motil. Cytoskeleton. 1997;37:308–325. doi: 10.1002/(SICI)1097-0169(1997)37:4<308::AID-CM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bachmaier K., et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- Bartkiewicz M., Houghton A., Baron R. Leucine zipper-mediated homodimerization of the adaptor protein c-Cbl. A role in c-Cbl's tyrosine phosphorylation and its association with epidermal growth factor receptor. J. Biol. Chem. 1999;274:30887–30895. doi: 10.1074/jbc.274.43.30887. [DOI] [PubMed] [Google Scholar]
- Bhalla K. N. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- Bouillet P., Metcalf D., Huang D.C.S., Tarlinton D. M., Kay T.W.H., Kontgen F., Adams J. M., Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bradley E. W., Ruan M. M., Oursler M. J. Novel pro-survival function of the Kruppel-like transcription factor Egr2 in promotion of M-CSF-mediated osteoclast survival downstream of the MEK/ERK pathway. J. Biol. Chem. 2008;283:8055–8064. doi: 10.1074/jbc.M709500200. [DOI] [PubMed] [Google Scholar]
- Bruzzaniti A., Neff L., Sanjay A., Horne W. C., De Camilli P., Baron R. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol. Biol. Cell. 2005;16:3301–3313. doi: 10.1091/mbc.E04-12-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y. J., Kole H. K., Brown K., Naramura M., Fukuhara S., Hu R.-J., Jang I. K., Gutkind J. S., Shevach E., Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- Chiarugi P., Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Chiusaroli R., Sanjay A., Henriksen K., Engsig M. T., Horne W. C., Gu H., Baron R. Deletion of the gene encoding c-Cbl alters the ability of osteoclasts to migrate, delaying resorption and ossification of cartilage during the development of long bones. Dev. Biol. 2003;261:537–547. doi: 10.1016/s0012-1606(03)00299-9. [DOI] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Geminard J.-C., Jurdic P., Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Gilquin B., Chabadel A., Khochbin S., Ory S., Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 2005;118:2901–2911. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- Destaing O., Sanjay A., Itzstein C., Horne W. C., Toomre D., De Camilli P., Baron R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I., Giordano S. Negative receptor signalling. Curr. Opin. Cell Biol. 2003;15:128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- Dowdy S. C., Jiang S., Zhou X. C., Hou X., Jin F., Podratz K. C., Jiang S.-W. Histone deacetylase inhibitors and paclitaxel cause synergistic effects on apoptosis and microtubule stabilization in papillary serous endometrial cancer cells. Mol. Cancer Ther. 2006;5:2767–2776. doi: 10.1158/1535-7163.MCT-06-0209. [DOI] [PubMed] [Google Scholar]
- Dumontet C., Sikic B. I. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J. Clin. Oncol. 1999;17:1061–1070. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- El Chami N., et al. Androgen-dependent apoptosis in male germ cells is regulated through the proto-oncoprotein Cbl. J. Cell Biol. 2005;171:651–661. doi: 10.1083/jcb.200507076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewings K. E., Wiggins C. M., Cook S. J. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle. 2007;6:2236–2240. doi: 10.4161/cc.6.18.4728. [DOI] [PubMed] [Google Scholar]
- Frisch S. M. Caspase-8, fly or die. Cancer Res. 2008;68:4491–4493. doi: 10.1158/0008-5472.CAN-08-0952. [DOI] [PubMed] [Google Scholar]
- Gil-Henn H., et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/− mice. J. Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantschnig H., Fisher J. E., Wesolowski G., Rodan G. A., Reszka A. A. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- Hirasaka K., et al. Deficiency of Cbl-b gene enhances infiltration and activation of macrophages in adipose tissue and causes peripheral insulin resistance in mice. Diabetes. 2007;56:2511–2522. doi: 10.2337/db06-1768. [DOI] [PubMed] [Google Scholar]
- Horne W. C., Sanjay A., Bruzzaniti A., Baron R. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol. Rev. 2005;208:106–125. doi: 10.1111/j.0105-2896.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- Jurdic P., Saltel F., Chabadel A., Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur. J. Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lee P.S.W., Wang Y., Dominguez M. G., Yeung Y.-G., Murphy M. A., Bowtell D.D.L., Stanley E. R. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Moudgil T., Ross H. J., Hu H.-M. Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 2005;12:292–303. doi: 10.1038/sj.cdd.4401554. [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang J., Liu Z., Woo C.-W., Thiele C. J. Downregulation of Bim by brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from paclitaxel but not etoposide or cisplatin-induced cell death. Cell Death Differ. 2007;14:318–326. doi: 10.1038/sj.cdd.4401983. [DOI] [PubMed] [Google Scholar]
- Lill N. L., Douillard P., Awwad R. A., Ota S., Lupher M. L., Miyake S., Meissner-Lula N., Hsu V. W., Band H. The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 2000;275:367–377. doi: 10.1074/jbc.275.1.367. [DOI] [PubMed] [Google Scholar]
- Linder S., Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Linder S., Hufner K., Wintergerst U., Aepfelbacher M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J. Cell Sci. 2000;113:4165–4176. doi: 10.1242/jcs.113.23.4165. [DOI] [PubMed] [Google Scholar]
- Lu C., Zhu F., Cho Y.-Y., Tang F., Zykova T., Ma W., Bode A. M., Dong Z. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006;23:121–132. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C., Parsons J. T., Addadi L., Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J. Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- Marastoni S., Ligresti G., Lorenzon E., Colombatti A., Mongiat M. Extracellular matrix: a matter of life and death. Connect. Tissue Res. 2008;49:203–206. doi: 10.1080/03008200802143190. [DOI] [PubMed] [Google Scholar]
- Matsuyama A., et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Lowell C. A. A β1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., et al. Reciprocal role of ERK and NF-κB pathways in survival and activation of osteoclasts. J. Cell Biol. 2000;148:333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. A., Schnall R. G., Venter D. J., Barnett L., Bertoncello I., Thien C. B., Langdon W. Y., Bowtell D. D. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol. Cell. Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S. DNA degradation in development and programmed cell death. Annu. Rev. Immunol. 2005;23:853–875. doi: 10.1146/annurev.immunol.23.021704.115811. [DOI] [PubMed] [Google Scholar]
- Nakajima A., Sanjay A., Chiusaroli R., Adapala N. S., Neff L., Itzstein C., Horne W. C., Baron R. The loss of Cbl-b increases osteolcast bone-resorbing activity and induces osteopenia. J. Bone Miner. Res. 2009;24:1162–1172. doi: 10.1359/JBMR.090205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramura M., Jang I.-K., Kole H., Huang F., Haines D., Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- Naramura M., Kole H. K., Hu R. J., Gu H. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95:15547–15552. doi: 10.1073/pnas.95.26.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota S., Hazeki K., Rao N., Lupher M. L., Jr, Andoniou C. E., Druker B., Band H. The RING finger domain of Cbl is essential for negative regulation of the Syk tyrosine kinase. J. Biol. Chem. 2000;275:414–422. doi: 10.1074/jbc.275.1.414. [DOI] [PubMed] [Google Scholar]
- Rathinam C., Thien C.B.F., Langdon W. Y., Gu H., Flavell R. A. The E3 ubiquitin ligase c-Cbl restricts development and functions of hematopoietic stem cells. Genes Dev. 2008;22:992–997. doi: 10.1101/gad.1651408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjay A., et al. Cbl associates with Pyk2 and Src to regulate Src kinase activity, aVb3 integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 2001;152:181–195. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjay A., Miyazaki T., Itzstein C., Purev E., Horne W. C., Baron R. Identification and functional characterization of an Src homology domain 3 domain-binding site on Cbl. FEBS J. 2006;273:5442–5456. doi: 10.1111/j.1742-4658.2006.05535.x. [DOI] [PubMed] [Google Scholar]
- Scaife R. M., Langdon W. Y. c-Cbl localizes to actin lamellae and regulates lamellipodia formation and cell morphology. J. Cell Sci. 2000;113:215–226. doi: 10.1242/jcs.113.2.215. [DOI] [PubMed] [Google Scholar]
- Strasser A., O'Connor L., Dixit V. M. Apoptosis signaling. Annu. Rev. Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Suzue N., et al. Ubiquitin ligase Cbl-b downregulates bone formation through suppression of IGF-I signaling in osteoblasts during denervation. J. Bone Miner. Res. 2006;21:722–734. doi: 10.1359/jbmr.060207. [DOI] [PubMed] [Google Scholar]
- Swaminathan G., Tsygankov A. Y. The Cbl family proteins: ring leaders in regulation of cell signaling. J. Cell. Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Amling M., Neff L., Peyman A., Uhlmann E., Levy J. B., Baron R. c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- Teckchandani A. M., Birukova A. A., Tar K., Verin A. D., Tsygankov A. Y. The multidomain protooncogenic protein c-Cbl binds to tubulin and stabilizes microtubules. Exp. Cell Res. 2005;306:114–127. doi: 10.1016/j.yexcr.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Tehrani S., Faccio R., Chandrasekar I., Ross F. P., Cooper J. A. Cortactin has an essential and specific role in osteoclast actin assembly. Mol. Biol. Cell. 2006;17:2882–2895. doi: 10.1091/mbc.E06-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien C.B.F., Langdon W. Y. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem. J. 2005;391:153–166. doi: 10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D., Pusch M., Whistler J. L. Changes in G protein-coupled receptor sorting protein affinity regulate postendocytic targeting of G protein-coupled receptors. J. Biol. Chem. 2007;282:29178–29185. doi: 10.1074/jbc.M704014200. [DOI] [PubMed] [Google Scholar]
- Tsygankov A. Y., editor. Cbl Proteins. New York: Nova Science Publishers, Inc.; 2008. [Google Scholar]
- van Leeuwen J.E.M., Paik P. K., Samelson L. E. The oncogenic 70Z Cbl mutation blocks the phosphotyrosine binding domain-dependent negative regulation of ZAP-70 by c-Cbl in Jurkat T cells. Mol. Cell. Biol. 1999;19:6652–6664. doi: 10.1128/mcb.19.10.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. J., Forer A. Effects of nanomolar Taxol on crane-fly spermatocyte spindles indicate that acetylation of kinetochore microtubules can be used as a marker of poleward tubulin flux. Cell Motil. Cytoskeleton. 1997;37:20–32. doi: 10.1002/(SICI)1097-0169(1997)37:1<20::AID-CM3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Zambonin Zallone A., Teti A., Grano M., Rubinacci A., Abbadini M., Gaboli M., Marchisio P. C. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a β3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp. Cell Res. 1989;182:645–652. doi: 10.1016/0014-4827(89)90266-8. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chiang Y. J., Hodes R. J., Siraganian R. P. Inactivation of c-Cbl or Cbl-b differentially affects signaling from the high affinity IgE receptor. J. Immunol. 2004;173:1811–1818. doi: 10.4049/jimmunol.173.3.1811. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li N., Caron C., Matthias G., Hess D., Khochbin S., Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22:1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]